Abstract
Scopatizzo, belonging to the Cucumis melo L., is a local variety of Apulia (Southern Italy), which is consumed as unripe melon as an alternative of cucumber due to its better-quality profile and for the absence of cucurbitacins. The latter are tetracyclic triterpenes synthesized by some Cucurbitaceae species, known to confer an unpleasant taste to fruits and cause health problems. Following the discovery of Scopatizzo fruits with bitter taste, cucurbitacins were searched for in their ethanolic extract. Flow injection analysis with detection performed by atmospheric pressure chemical ionization-high resolution mass spectrometry provided evidence for the presence of at least four cucurbitacins, which were absent in typical, sweet-tasting fruits. Further insight into this discovery will be required in the near future to assess if the detection of cucurbitacins may mark the appearance of genotypes whose fruits have features not compatible with commercialization.
1. Introduction
Cucurbitacins constitute a group of plant secondary metabolites corresponding to tetracyclic triterpenoids found in various species of the Cucurbitaceae family. They are known for their bitterness and toxicity, and their presence in several species of vegetables is generally considered undesirable firstly for their unpleasant taste [1].
The consumption of food products containing high amounts of cucurbitacins can lead to gastrointestinal distress and other health issues [1]. For this reason, commercial Cucurbitaceae varieties are carefully bred to minimize cucurbitacin content [2]. Despite their toxicity, cucurbitacins have also been studied for their potential medicinal properties: some studies suggested that certain cucurbitacins may have anticancer and anti-inflammatory properties, but their use in this context is still in the early stages of research [3].
The Cucurbitaceae family stands out as one of the most genetically diverse plant families globally. Among its members, there are numerous significant vegetables and medicinal plants with a wide global distribution. The swift progress in sequencing technologies and bioinformatics algorithms has empowered the creation of genome sequences for many crucial Cucurbitaceae species [4]. This advancement has significantly eased research in areas like gene discovery, genome evolution, genetic diversity, and the molecular breeding of cucurbit crops. So far, genome sequences of 18 different cucurbit species belonging to tribes Benincaseae, Cucurbiteae, Sicyoeae, Momordiceae, and Siraitieae have been deciphered [4]. Understanding the genome information plays a vital role in aiding breeders to improve the quality of Cucurbitaceae vegetables through the application of biotechnological methods. In this regards, nonconventional breeding approaches can complement conventional breeding methods leading to savings in time, expenses, and improving selection efficiency [5]. Variability in breeding techniques and approaches is essential in both methods to develop new and improved Cucurbitaceae varieties with enhanced nutritional profiles [5].
Breeders have focused on developing Cucurbitaceae varieties with low or negligible cucurbitacin levels [4]. These efforts aim to remove the bitterness and toxicity associated with cucurbitacins. Research has been conducted to understand the genetic basis of cucurbitacin content in Cucurbitaceae fruits [2]. Genes associated with cucurbitacin production have been identified, and efforts have been made to develop markers for selective breeding [2].
Apulia region (Southern Italy) is an important secondary center of diversity for C. melo L. [6]. Several landraces of this species are still grown there including the so-called unripe melons, such as ‘Barattiere’, ‘Carosello’, ‘Scopatizzo’ (Figure 1), etc. [7,8,9,10].

Figure 1.
Plants of Scopatizzo with main stem vertically trained at full flowering stage (A). Side view of ermaphrodyte flower of Scopatizzo (B). Top view of male (top) and ermaphrodithe (bottom) flower of Scopatizzo (C).
Scopatizzo is characterized by its relatively small-sized fruits, which have a light and sparse fuzziness. They are light green in color, have an elliptical shape and an oval-shaped longitudinal section (Figure 2) [10,11]. At the commercial harvest, the Scopatizzo fruits do not have well-formed seeds. In fact, in addition to the flesh (mesocarp), the central part (placenta) of the fruit can be eaten like other genotypes of Apulian unripe melons [9,10,11]. The leaves are of medium size and have a pentalobed shape with slight leaf margin serration. The flowers, typically yellow in color for the species, exhibit an andromonoecious-male expression, with a predominance of male flowers [10,11]. Each Scopatizzo plant can produce more than 10 fruits with an average yield of around 2600 g plant−1 [10].

Figure 2.
Fruits of Scopatizzo melon.
Although unripe melons, including Scopatizzo, are taxonomically C. melo, their fruits are harvested at the immature stage to be consumed fresh and raw, in salads or without dressings and are appreciated as an alternative to cucumber (C. sativus) due to their better-quality profile and for the absence of cucurbitacins [8,11]. Effectively, to our best knowledge, the literature lacks evidence with regards to cucurbitacins presence in these Apulian landraces of C. melo. Nevertheless, in the summer of 2022, the presence of bitter tasting ‘Scopatizzo’ fruits (Figure 2) was recognized during a genotype comparison trial in an experimental farm, thus suggesting the first-time presence of cucurbitacins in an Apulian landrace of C. melo. Notably, other warnings about bitter tasting ‘Scopatizzo’ fruits were received from local markets in 2022.
To further support the occurrence of cucurbitacins in bitter fruits, flow-injection analysis (FIA) with detection based on high-resolution Fourier Transform mass spectrometry with Atmospheric Pressure Chemical Ionization (APCI-FTMS) was exploited in this study for the characterization of the moderately polar fraction of the metabolome of sweet and bitter Scopatizzo fruits. As a result, the main difference observed between the corresponding APCI-FTMS spectra could be tentatively recognized as the remarkably higher content of cucurbitacin molecules in bitter-tasting fruits. The accurate m/z values were exploited for the tentative identification of cucurbitacins, and data were compared with those reported in the literature on cucurbitacins in C. melo fruits.
2. Materials and Methods
2.1. Cropping Details
The experimental trial from which the first bitter ‘Scopatizzo’ fruits emerged was carried out from May to July 2022 at the Experimental farm “La Noria” of the Institute of Sciences of Food Production, National Research Council [Mola di Bari, Italy (41.062156° N, 17.066914° E)], in open field conditions. Nine local varieties of C. melo L. were cultivated including ’Scopatizzo’. Seedlings were purchased from an Apulian plant nursery, and they were transplanted at a plant density of 2.5 plants·m−2, with a plant distance on the raw and between the raw respectively of 0.40 m and 1 m.
Plants were daily fertigated according to “Specification for integrated production of field cucumber, Apulia Region, 2022”, with a micro-irrigation system, to optimize water consumption. Plant stems were trained vertically to optimize leaves light interception, air flow through the canopy and to avoid fungal proliferation on leaves. Pollination was guaranteed by introduction of bumblebees (Bombus terrestris L.). Starting from 30 days after transplant (DAT) the fruits were harvested twice a week, at unripe stage, when the grooves on the epicarp of the fruits were barely noticeable and the seeds were barely outlined, and the placenta was not divided from the mesocarp.
2.2. Materials
LC-MS grade acetonitrile and water used for FIA, LC-MS grade formic acid used as the additive for the FIA carrier fluid, and ACS reagent grade ethanol used for the extraction of cucurbitacins were all purchased from Sigma Aldrich (Merck, Milan, Italy).
2.3. Sample Preparation
Once harvested, the ‘Scopatizzo’ unripe melons were subjected to an organoleptic essay to separate bitter from sweet-tasting fruits. Thereafter, the plant material was stored at −20 °C for 24 h prior to the freeze-drying process to occur in a ScanVac CoolSafe 55-9 Pro freeze dryer (LaboGene ApS, Lynge, Denmark).
2.4. Extraction of Cucurbitacins from Lyophilized Plant Material
A slightly modified version of the protocol proposed by Chen et al. [12] was adopted for the extraction of cucurbitacins. Here, the lyophilized plant material was powdered in a ceramic mortar. The resulting powder was suspended into an ethanol/water 95:5 v/v mixture to a nominal concentration of 100 mg/mL. The suspension was subjected to ultrasound-assisted extraction in a DU-32 ultrasonic bath (Argo Lab, Carpi, Italy), operating at 40 kHz at maximum power (120 W). The liquid phase was clarified after centrifugation (4500 rpm) for 10 min. Once withdrawn, the supernatant was filtered using 0.2 μm nylon filters and diluted to a suitable volume by a 1:10 factor in pure ethanol prior to the FIA-APCI-FTMS analysis.
2.5. Instrumentation and Operating Conditions for FIA-APCI-FTMS Analysis
FIA-APCI(−)-FTMS analysis was performed using a LC-MS platform implementing an Ultimate 3000 HPLC quaternary chromatographic system and a Q Exactive high-resolution quadrupole-Orbitrap mass spectrometer (Thermo Fisher, West Palm Beach, CA, USA). Here, the chromatographic pump module was set to deliver the carrier phase at a 150 μL/min flow rate. The carrier was obtained by mixing acetonitrile (60% v/v) and water (40% v/v), both containing formic acid (0.1% v/v). The diluted ethanolic plant extract (see Section 2.4) was introduced into the flowing carrier phase at a 5 μL/min flow by using a Fusion 100T syringe pump (Chemyx, Stafford, TX, USA). A T-valve was used for the hydraulic connections. The resulting mixture was transferred into the Atmospheric Pressure Chemical Ionization (APCI) interface (Thermo Fisher, West Palm Beach, CA, USA) mounted on the Q Exactive mass spectrometer.
The operating parameters of the APCI interface and of the ion optics of the Q-Exactive spectrometer were set as follows:
- sheath gas flow rate: 40 a.u.;
- auxiliary gas flow rate: 20 a.u.;
- discharge current: 5 μA;
- capillary temperature: 320 °C;
- S-lens RF level: 55%;
- vaporizer temperature: 300 °C.
The mass spectrometer was calibrated on alternate days by infusing, at a flow rate of 5 μL/min, calibration solutions provided by the instrument manufacturer for negative polarity acquisitions. As a result, a mass accuracy always better than 5 ppm was achieved.
The orbitrap mass analyzer was operated at its maximum resolving power (140,000 at m/z 200) for full scan MS experiments. FTMS spectra were acquired in a 300–800 m/z interval. Here, the Automatic Gain Control (AGC) level was set to 1 × 106, with a maximum ion injection time of 100 ms. The total ion current (TIC) signal was constantly monitored and the FTMS spectra acquisition started when the TIC variation was stable below the 20% threshold.
3. Results
The presence of cucurbitacins is notoriously responsible for the unpleasant bitter taste of fruits produced by cucurbitaceous plants [1,13,14]. Hence, a rapid screening method based on FIA-APCI-FTMS was adopted to assess the presence of cucurbitacins in unexpectedly bitter ‘Scopatizzo’ melons.
Cucurbitacins, along with their glycosylated forms, can be detected as negatively charged adduct ions when both APCI and electrospray ionization (ESI) ion sources are employed [12,13]. Both formic acid and trifluoroacetic acid have been commonly used as adjuvants for the ionization process [14,15,16]. Notably, when compared to ESI, the use of APCI results in a more sensitive detection of cucurbitacins, although a more extensive in-source fragmentation of the analyte ion is expected [15].
Figure 3 shows a comparison between the APCI(−)-FTMS spectra acquired for the ethanolic extracts of sweet (plot A) and bitter-tasting (plot B) ‘Scopatizzo’ melons. Apart from the different intensity ratio of the dominating MS signals (i.e., those detected at nominal m/z 341 and m/z 387), the main discrepancy between the two FTMS spectra was represented by the presence of peaks at m/z 561.3095 and 603.3205. The latter were detected only in the case of bitter fruits. As reported in Table 1, these accurate m/z values are consistent, respectively, with the theoretical m/z values of [M−H+HCOOH]− adduct ions of cucurbitacins D and B (see structures reported in Figure 4), and, hypothetically, also of the respective isomeric forms reported so far in the literature, like cucurbitacin L and iso-cucurbitacin D, in the first case, and isocucurbitacin B and 23,24-dihydrocucurbitacin E in the second one.
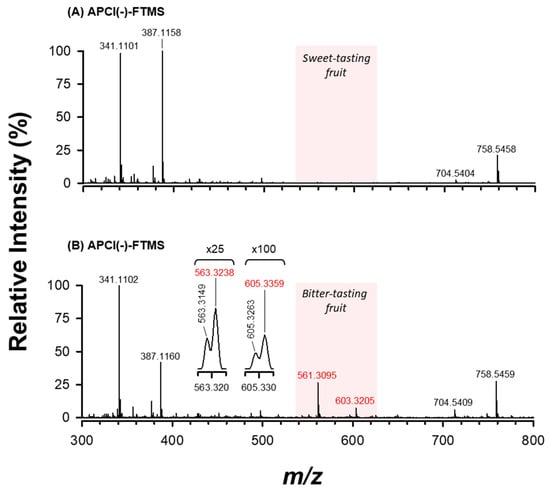
Figure 3.
APCI(−)-FTMS spectra of the ethanolic extracts of sweet (A) and bitter-tasting (B) ‘Scopatizzo’ melons (see Section 2.3 and 2.4 for additional information on sample preparation). The red-shaded m/z interval refers to the spectral region containing MS signals that discriminate the two samples due to the presence of cucurbitacins in bitter fruits. Highlighted in red are the accurate m/z values used for the tentative identification of cucurbitacins (see Table 1).

Table 1.
Cucurbitacins tentatively identified in bitter ‘Scopatizzo’ melons. The identification was based on accurate m/z values (see the main text for details).
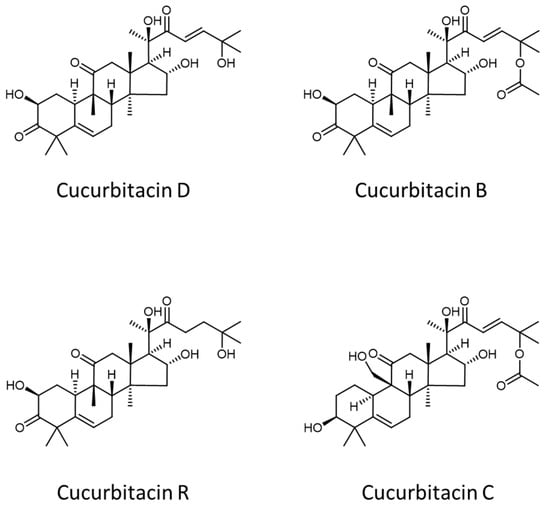
Figure 4.
Molecular structures of some of the cucurbitacins whose occurrence was tentatively assessed in the ethanolic extract of a bitter-tasting ‘Scopatizzo’ melon.
As shown in the insets of Figure 3B, a closer inspection of the isotope pattern of the two ions revealed the presence of two further signals, at m/z 563.3238 and 605.3359, that could be tentatively ascribed to [M−H+HCOOH]− ions of cucurbitacins R and C (see Figure 4), respectively, and their respective isomers (see Table 1 for details).
Notably, cucurbitacin D differs from the R one for the presence of an additional C=C bond in the side chain linked to the tetracyclic backbone (see Figure 4), thus its nominal molecular mass is 2 Da lower. As it can be inferred from Figure 4, this is the same difference observed between the molecular masses of cucurbitacins B and C. As a result, the M + 2 isotopic peaks of cucurbitacins D and B ions are expected to exhibit the same nominal m/z values than those of the monoisotopic (M + 0) peaks of cucurbitacins R and C, respectively. However, the available mass resolution was sufficiently high to observe the partial separation between those couples of peaks in mass spectra, thus enabling the distinct recognition of the corresponding compounds.
4. Discussion
In the present report we described for the first time the detection of cucurbitacins in an Apulian landrace of C. melo, an expression of the Mediterranean agrobiodiversity. ‘Scopatizzo’ is one of the several landraces of unripe Apulian melon that is establishing itself on the market for its peculiar quality traits, high fruitfulness, and good income for farmers. Moreover, it is listed as an item in the ‘List of Traditional Agri-Food Products’ of the Italian Department for Agriculture, since its processing, preservation and ageing methods are consolidated in time, harmonious for all the region involved, according to traditional rules, for a period not less than 25 years [11,17].
The discovery of ‘Scopatizzo’ fruits with a bitter taste represents a negative point even in the case of a low percentage of fruits with this characteristic, because consumers could associate this sporadic feature with a distinctive trait of the ‘Scopatizzo’, translating in a setback of its market rise.
Results of the present work show that bitter tasting ‘Scopatizzo’ fruits discovered during the genotypes comparison trial contained different types of cucurbitacins. As discussed in Section 3, FIA-APCI-FTMS analysis allowed the clear distinction between the ethanolic extracts of sweet and bitter ‘Scopatizzo’ melons. Indeed, four monoisotopic peaks, detected at m/z 561.3095, 563.3238, 603.3205, 605.3359, were considered as diagnostic for bitter-tasting fruits (see Figure 3). The corresponding accurate m/z values were compared with the theoretical m/z values calculated for the [M−H+HCOOH]− adduct ions of the main cucurbitacins reviewed by Cai et al. [18]. Here, only those matches showing a mass error lower than 5 ppm were considered to be reliable, as summarized in Table 1. Three isomeric species were detected among those reported by Cai et al. [18] for each of the matching theoretical m/z values. Notably, the presence of cucurbitacin D and B was previously reported in C. melo plants. Cucurbitacin B was found to be prevailing in the radicles and the cotyledons of the muskmelon vine [1].
The fruits of cultivated Cucurbitaceae have been selectively bred to eliminate the presence of cucurbitacins. It is generally believed that they possess a suppressor gene or a mutation responsible for the absence of cucurbitacins. However, back mutations can occur randomly, potentially resulting in plants with fruits containing cucurbitacins [1]. For examples, back-mutated watermelons and squash can produce between 930 and 3100 mg cucurbitacin E per kg fresh fruit and the offspring of such plants may also produce cucurbitacins [1]. Therefore, it is possible that even in the case of Scopatizzo, mutation phenomena have occurred in some plants. However, it must be considered that Scopatizzo is a local variety, that is, a crop propagated through self-production of seeds by farmers without any organized program of genetic improvement. Therefore, it is also possible that cross-pollination phenomena have occurred between Scopatizzo plants and other wild species belonging to the Cucurbitaceae family. Indeed, within the Scopatizzo cultivation region, squirting cucumber (Ecballium elaterium L.) plants are abundantly distributed. This particular wild species is renowned for its elevated levels of cucurbitacins [19].
A more extensive analytical investigation based on high-performance liquid chromatography coupled with high-resolution/multi-stage mass spectrometry will be performed in a future work to assess the effective presence of isomeric species, along with a more confident identification of cucurbitacins occurring in the ethanolic extracts of bitter ‘Scopatizzo’ melons.
5. Conclusions
Evidence for the occurrence of Cucurbitacins D, B, R, and C and/or their isomeric forms in a bitter-tasting fruit of ‘Scopatizzo’ melon, a landrace of C. melo typical of the Apulia region in Southern Italy, was obtained using flow-injection analysis with detection based on atmospheric pressure chemical ionization-Fourier transform mass spectrometry. To the best of our knowledge, this is the first report of the occurrence of such compounds, known for their potential toxicity, along with the related unpleasant organoleptic features, in a fruit of a C. melo Apulian landrace. An extended study will be performed in the near future on fruits harvested from C. melo plants grown in Puglia, to assess if the detection of cucurbitacins described in the present work may mark the appearance of genotypes whose fruits have features clearly not compatible with commercialization. Future research will also be conducted to identify genes associated with cucurbitacin production in ‘Scopatizzo’ fruits and develop markers for selective breeding, considering the possibility of back-mutations as well as cross-pollination between different genotypes of the Cucurbitaceae family.
Author Contributions
Conceptualization, M.R. and P.S.; methodology, A.C., I.L., A.C., I.L., O.D.P. and P.S.; formal analysis, A.C. and I.L.; investigation, O.D.P., A.S., A.D., A.C. and M.R.; resources, I.L., P.S. and T.R.I.C.; data curation, O.D.P., A.S., A.D. and A.C.; writing—original draft preparation, M.R., A.C. and I.L.; writing—review and editing, O.D.P., A.C., A.S., A.D., M.R., I.L., C.D.C., T.R.I.C. and P.S.; supervision, I.L. and P.S.; project administration, I.L. and P.S.; funding acquisition, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
Project funded under the Regione Puglia Administration, Rural Development Program 2014–2020, Projects ‘Biodiversity of Apulian Fruit Vegetables (BiodiverSO Karpos, DDS n. 04250178565, CUP: B97H22003670009)—n. 4’, Measure 10, Sub-Measure 10.2, Operation 1, “Program for the Conservation and Valorisation of the Genetic Resources in Agriculture”.
Data Availability Statement
Not applicable.
Acknowledgments
This study was carried out also within: (1) Agritech National Research Center and received funding from the European Union Next-Generation EU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17 June 2022, CN00000022); (2) National Recovery and Resilience Plan (NRRP), Mission 4, Component 2, Investment 1.3—Call for proposals No. 341 of 15 March 2022 of the Italian Ministry of University and Research funded by the European Union—NextGenerationEU; Award Number: Project code PE0000003, Concession Decree No. 1550 of 11 October 2022 adopted by the Italian Ministry of University and Research, CUP D93C22000890001, Project title “ON Foods—Research and innovation network on food and nutrition Sustainability, Safety and Security—Working ON Foods”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gry, J.; Søborg, I.; Andersson, H.C. Cucurbitacins in Plant Food; Nordic Council of Ministers: Copenhagen, Denmark, 2006; ISBN 92-893-1381-1. [Google Scholar]
- Shang, J.; Kong, S.; Li, N.; Wang, J.; Zhou, D.; Li, N.; Ma, S. Genetic mapping and localization of major QTL for bitterness in melon (Cucumis melo L.). Sci. Hortic. 2020, 266, 109286. [Google Scholar] [CrossRef]
- Qing, Z.; Shi, Y.; Han, L.; Li, P.; Zha, Z.; Liu, C.; Liu, X.; Huang, P.; Liu, Y.; Tang, Q.; et al. Identification of seven undescribed cucurbitacins in Cucumis sativus (cucumber) and their cytotoxic activity. Phytochemistry 2022, 197, 113123. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, Q.; Zheng, Y.; Guo, J.; Yuan, S.; Fu, A.; Bai, C.; Zhao, X.; Zheng, S.; Wen, C.; et al. Cucurbitaceae genome evolution, gene function, and molecular breeding. Hortic. Res. 2022, 9, uhab057. [Google Scholar] [CrossRef] [PubMed]
- Wan Shafiin, W.N.S.S.; Ablah, N.L.; Nur Fatihah, H.N.; Alam, M.A.; Ma’arup, R.; Jahan, M.S.; Mustafa, K.A.; Alias, N. Breeding strategies for enhancing nutrient content and quality in Cucurbitaceae: A review. Int. J. Veg. Sci. 2021, 27, 415–438. [Google Scholar] [CrossRef]
- Pavan, S.; Marcotrigiano, A.R.; Ciani, E.; Mazzeo, R.; Zonno, V.; Ruggieri, V.; Lotti, C.; Ricciardi, L. Genotyping-by-sequencing of a melon (Cucumis melo L.) germplasm collection from a secondary center of diversity highlights patterns of genetic variation and genomic features of different gene pools. BMC Genom. 2017, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Renna, M.; Montesano, F.F.; Signore, A.; Gonnella, M.; Santamaria, P. BiodiverSO: A Case Study of Integrated Project to Preserve the Biodiversity of Vegetable Crops in Puglia (Southern Italy). Agriculture 2018, 8, 128. [Google Scholar] [CrossRef]
- Renna, M.; D’Imperio, M.; Gonnella, M.; Parente, A.; Santamaria, P.; Serio, F. Barattiere: An italian local variety of Cucumis melo L. with quality traits between melon and cucumber. Plants 2020, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Palmitessa, O.D.; Durante, M.; Leoni, B.; Montesano, F.; Renna, M.; Serio, F.; Somma, A.; Santamaria, P. Enhancement of a landrace of carosello (Unripe melon) through the use of light-emitting diodes (led) and nutritional characterization of the fruit placenta. Sustainability 2021, 13, 11464. [Google Scholar] [CrossRef]
- Somma, A.; Palmitessa, O.D.; Leoni, B.; Signore, A.; Renna, M.; Santamaria, P. Extraseasonal production in a soilless system and characterisation of landraces of carosello and barattiere (Cucumis melo L.). Sustainability 2021, 13, 11425. [Google Scholar] [CrossRef]
- Scopatizzo. Available online: https://www.patpuglia.it/it/12/Scopatizzo/5_352 (accessed on 30 October 2023).
- Chen, S.Y.; Zhou, Q.Y.J.; Chen, L.; Li, J.Y.; Xie, T.; Zhang, S.H. Screening and identifying cucurbitacins and cucurbitacin glycosides in Cucumis sativus using high-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry combined with in-source fragmentation and alkali adduct ions. Rapid Commun. Mass Spectrom. 2022, 36, e9323. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Chiu, M.H.; Nie, R.L.; Cordel, G.A.; Qiuz, S.X. Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat. Prod. Rep. 2005, 22, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Choi, D.; Cha, A.; Lee, Y.G.; Baek, N.I.; Rimal, S.; Sang, J.; Lee, Y.; Lee, S. Critical enzymes for biosynthesis of cucurbitacin derivatives in watermelon and their biological significance. Commun. Biol. 2020, 3, 444. [Google Scholar] [CrossRef] [PubMed]
- Sturm, S.; Stuppner, H. Analysis of cucurbitacins in medicinal plants by high-pressure liquid chromatography-mass spectrometry. Phytochem. Anal. 2000, 11, 121–127. [Google Scholar]
- Ul Haq, F.; Ali, A.; Khan, M.N.; Shah, S.M.Z.; Kandel, R.C.; Aziz, N.; Adhikari, A.; Choudhary, M.I.; ur-Rahman, A.; El-Seedi, H.R.; et al. Metabolite Profiling and Quantitation of Cucurbitacins in Cucurbitaceae Plants by Liquid Chromatography coupled to Tandem Mass Spectrometry. Sci. Rep. 2019, 9, 15992. [Google Scholar] [CrossRef] [PubMed]
- Didonna, A.; Renna, M.; Santamaria, P. Traditional Italian Agri-Food Products: A Unique Tool with Untapped Potential. Agriculture 2023, 13, 1313. [Google Scholar] [CrossRef]
- Cai, Y.; Fang, X.; He, C.; Li, P.; Xiao, F.; Wang, Y.; Chen, M. Cucurbitacins: A Systematic Review of the Phytochemistry and Anticancer Activity. Am. J. Chin. Med. 2015, 43, 1331–1350. [Google Scholar] [CrossRef] [PubMed]
- Attard, E. Rapid detection of cucurbitacins in tissues and in vitro cultures of Ecballium elaterium (L.) A. Rich. Report-Cucurbit Genet. Coop. 2002, 25, 71–75. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).