Influence of Field and Storage Diseases and Pests on Tuber Yield and Quality of Exotic and Local Yam (Dioscorea spp.) Genotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Experimental Design and Cultural Practices
2.3. Data Collection
2.4. Statistical Analysis
3. Results and Discussion
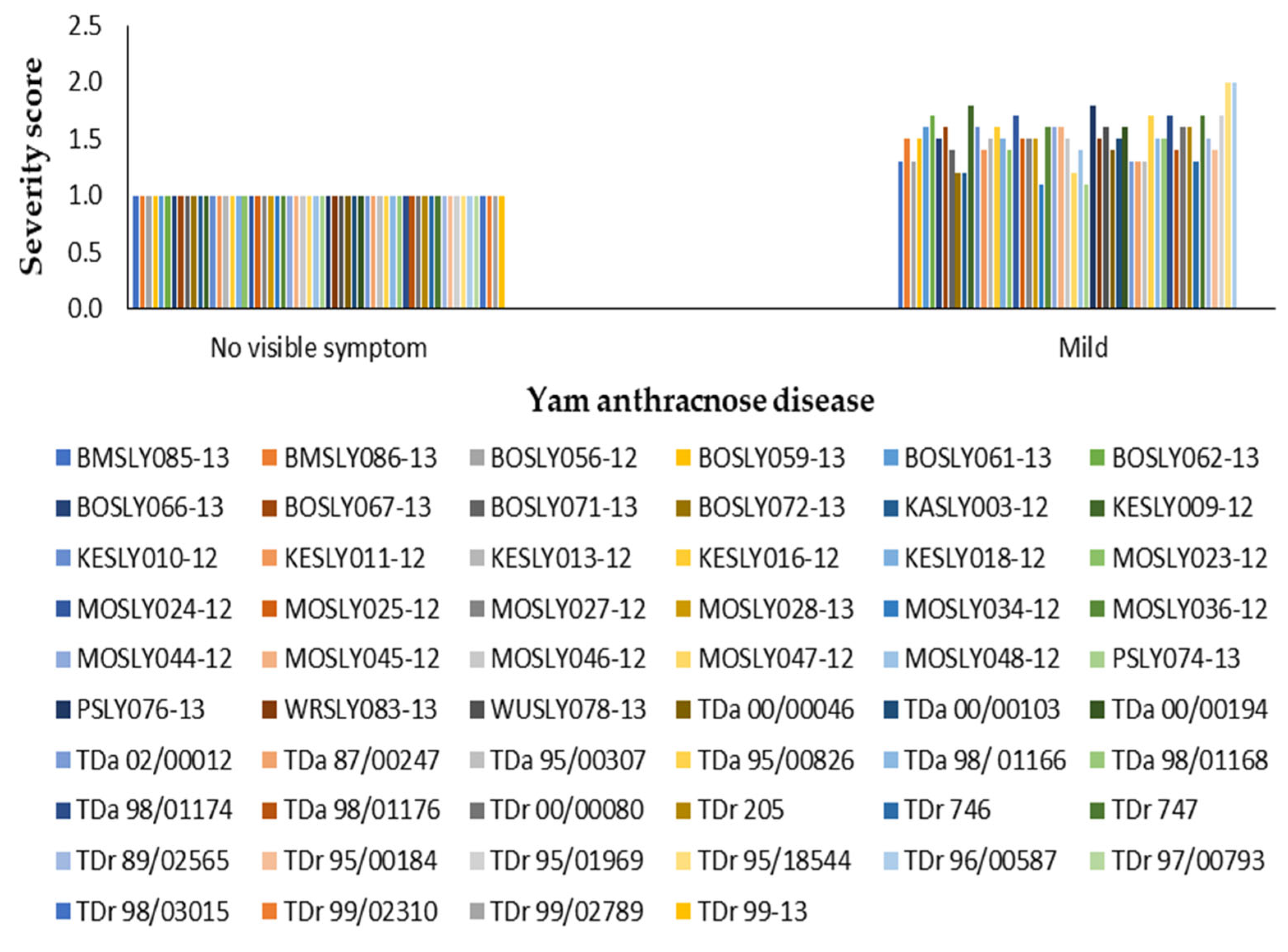
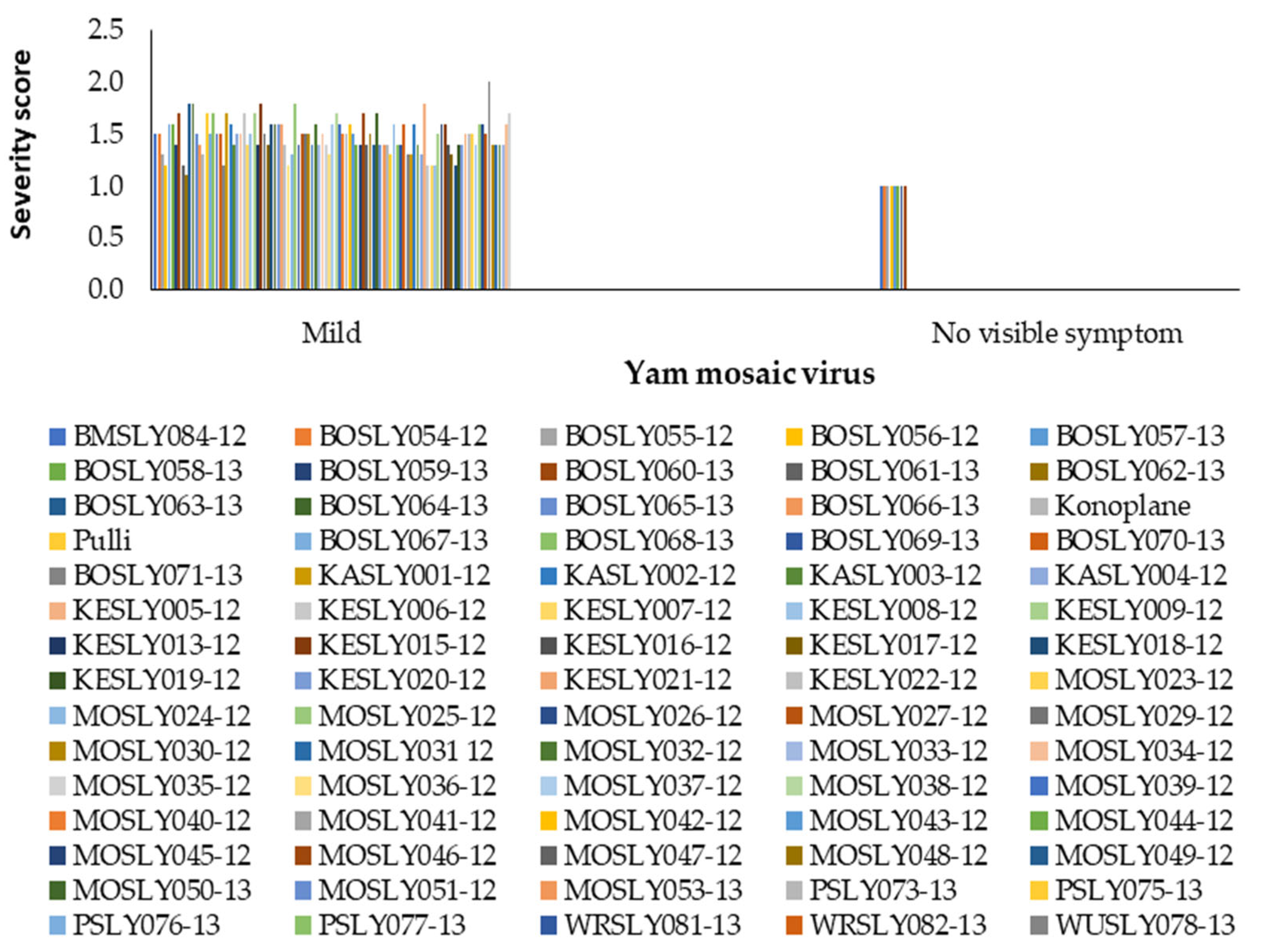
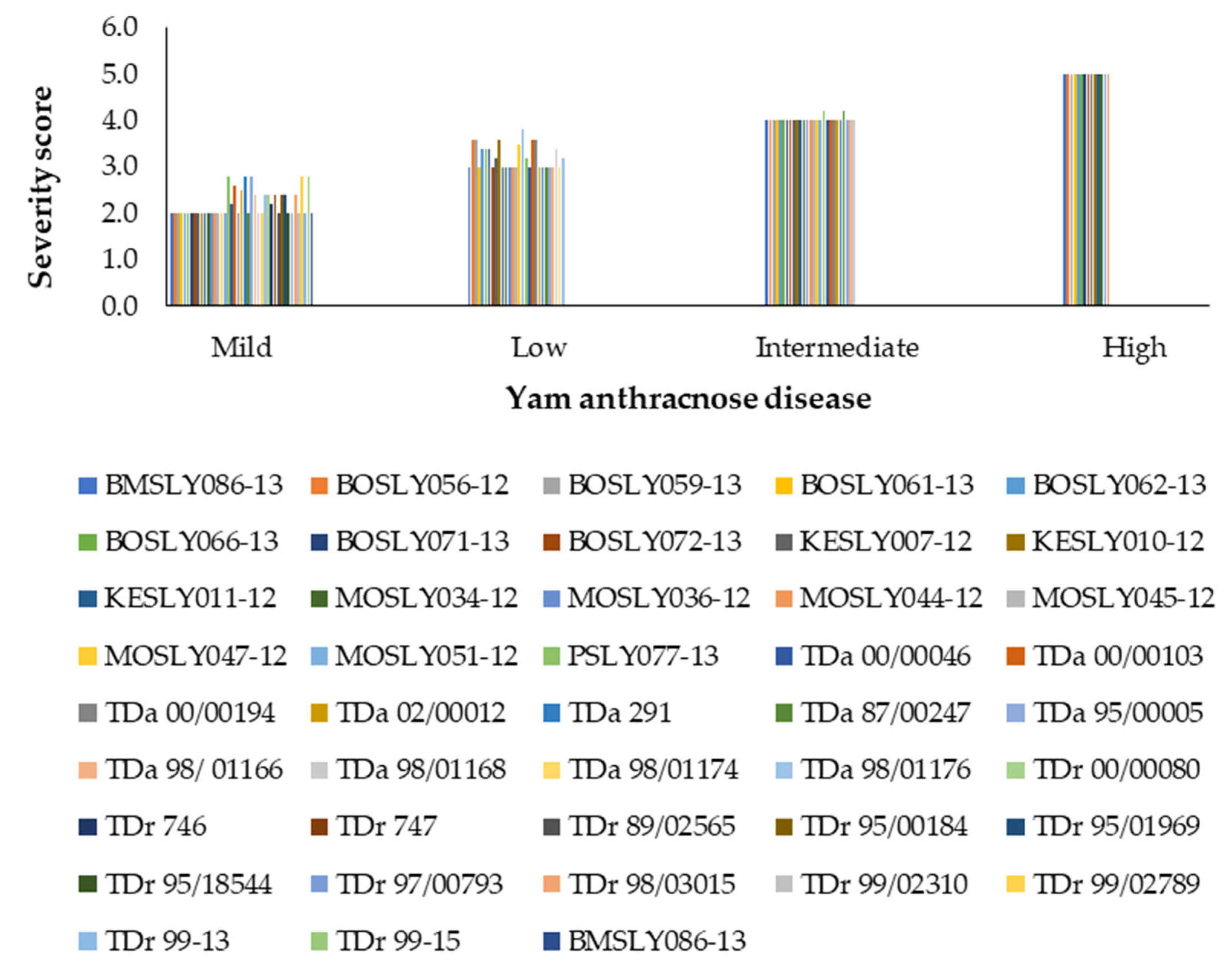
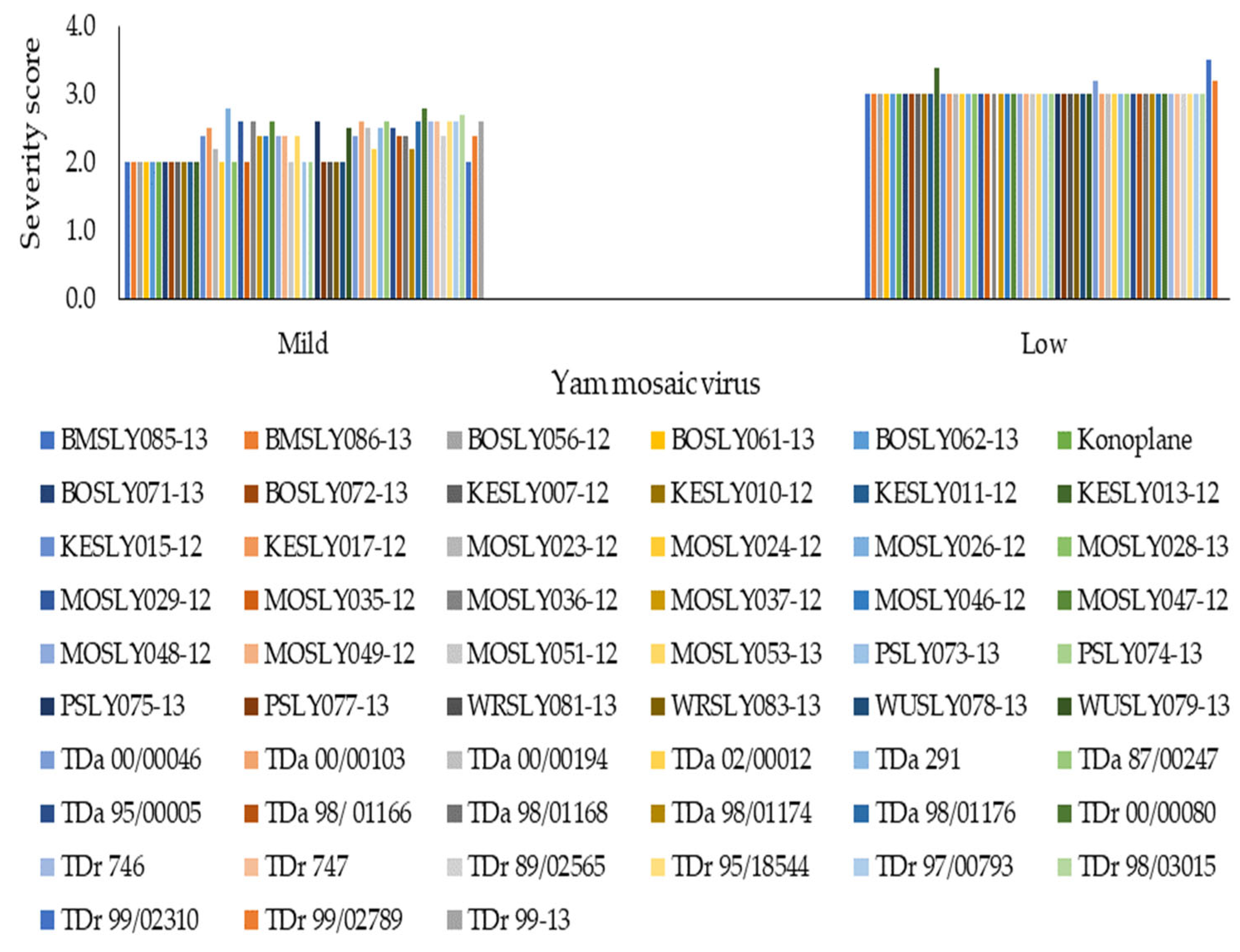
3.1. Effect of Field Disease Severity on Yield
3.2. Effect of Storage Disease Severity on Tuber Weight
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nweke, F.I. Yam in West Africa: Food, Money, and More; Michigan State University Press: East Lansing, MI, USA, 2015. [Google Scholar]
- Obidiegwu, J.E.; Lyons, J.B.; Chilaka, C.A. The Dioscorea Genus (Yam)—An appraisal of nutritional and therapeutic potentials. Foods 2020, 9, 1304. [Google Scholar] [CrossRef]
- Norman, P.E. Morphological and Cytological Diversity of Some Yams (Dioscorea spp.) in Sierra Leone. Ph.D. Thesis, University of KwaZulu-Natal, Durban, South Africa, 2010; 142p. [Google Scholar]
- FAOSTAT. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 1 May 2020).
- Sesay, L.; Norman, P.E.; Massaquoi, A.; Kobba, F.; Allieu, A.P.; Gboku, M.L.; Fomba, S.N. Assessment of farmers’ indigenous knowledge and selection criteria of yam in Sierra Leone. Sky J. Agric. Res. 2013, 2, 1–6. [Google Scholar]
- Bantilan, C. Nutrition. 11 Health and Nutrition Benefits of Yams; Healthline Media, Inc.: New York, NY, USA, 2019; Available online: Healthline.com (accessed on 13 July 2023).
- Mignouna, H.D.; Abang, M.M.; Asiedu, R. Genomics of yams, a common source of food and medicine in the tropics. In Plant Genetics and Genomics: Crops and Models; Moore, P., Ming, R., Eds.; Springer: Berlin, Germany, 2008; pp. 549–570. [Google Scholar]
- FAOSTAT. Food and Agriculture Organization Statistical Databases. 2016. Available online: https://www.fao.org/faostat/en/#home (accessed on 19 March 2017).
- Adeyinka, S.A.; Agre, P.A.; Asare, P.A.; Adu, M.O.; Taah, K.J.; Mondo, J.M.; Akaba, S. Exploring the bush yam (Dioscorea praehensilis Benth) as a source of agronomic and quality trait genes in white Guinea yam (Dioscorea rotundata Poir) breeding. Agronomy 2022, 12, 55. [Google Scholar]
- FAOSTAT. Online and Multilingual Database. 2021. Available online: https://www.fao.org/statistics/en/ (accessed on 30 November 2021).
- Ita, E.E.; Uyoh, E.A.; Nakamura, I.; Ntui, V.O. Efficient elimination of Yam mosaic virus (YMV) from white yam (Dioscorea rotundata Poir.) by cryotherapy of axillary buds. S. Afr. J. Bot. 2020, 130, 123–129. [Google Scholar] [CrossRef]
- Yeyeh, T.M.N.; Diallo, H.A.; Akinbade, S.A.; Séka, K.; Kumar, P.L. Distribution, incidence and severity of viral diseases of yam (Dioscorea spp.) in Côte d’Ivoire. Afr. J. Biotechnol. 2014, 13, 465–470. [Google Scholar]
- Njukeng, A.P.; Azeteh, I.N.; Mbong, G.A. Survey of the incidence and distribution of two viruses infecting yam (Dioscorea spp.) in two agro-ecological zones of Cameroon. Intern. J. Curr. Microbiol. Appl. Sci. 2014, 3, 1153–1166. [Google Scholar]
- Kenyon, L.; Lebas, B.S.M.; Seal, S.E. Yams (Dioscorea spp.) from the South Pacific Islands contain many novel badnaviruses: Implications for international movement of yam germplasm. Archives Virol. 2008, 153, 877–889. [Google Scholar] [CrossRef]
- Adeniji, M.O.; Shoyinka, S.A.; Ikotun, T.; Asiedu, R.; Hughes, J.A.; Odu, B.O. Yield loss in guinea yam (Dioscorea rotundata Poir.) due to infection by Yam mosaic virus (YMV) genus potyvirus. Ife J. Sci. 2012, 14, 237–244. [Google Scholar]
- Bayat, M.; Zargar, M.; Astarkhanova, T.; Pakina, E.; Ladan, S.; Lyashko, M.; Shkurkin, S.I. Facile Biogenic Synthesis and Characterization of SevenMetal-Based Nanoparticles Conjugated with Phytochemical Bioactives Using Fragaria ananassa Leaf Extract. Molecules 2021, 26, 3025. [Google Scholar] [CrossRef]
- Nigam, D.; LaTourrette, K.; Souza, P.F.N.; Garcia-Ruiz, H. Genome-wide variation in potyviruses. Front. Plant Sci. 2019, 10, 1439. [Google Scholar] [CrossRef]
- Maina, M. Yam (Dioscorea spp.). How will this crop be revived to enhance food security in East Africa? In Agricultural Biosciences, Proceedings of the 1st International e-Conference on Agricultural Biosciences, Online, 2–16 June 2008; F.a.C.T Limited: Nairobi, Kenya, 2008; Volume 1, Available online: https://www.e-conference.elewa.org/agriculture/ (accessed on 8 April 2009).
- Morse, S.; McNamara, N. Agronomic and economic performance of seed yam production using minisetts in the middle belt of Nigeria. J. Crop Improv. 2018, 32, 90–106. [Google Scholar] [CrossRef]
- Morse, S.; McNamara, N. Pesticide residues in seed yams produced using the adaptive Yam Minisett Technique. J. Crop Improv. 2020, 34, 644–653. [Google Scholar] [CrossRef]
- Morse, S.; McNamara, N. Factors influencing the agronomic performance of the adapted yam minisett technique in Nigeria–planting date and gender of the farmer. Exp. Agric. 2018, 54, 1–15. [Google Scholar] [CrossRef]
- Almekinders, C.J.; Walsh, S.; Jacobsen, K.S.; Andrade-Piedra, J.L.; McEwan, M.A.; de Haan, S.; Staver, C. Why interventions in the seed systems of roots, tubers and banana crops do not reach their full potential. Food Secur. 2019, 11, 23–42. [Google Scholar] [CrossRef]
- Bentley, J.; Andrade-Piedra, J.P.; Demo, P.; Dzomeku, B.M.; Jacobsen, K.S.; Kikulwe, E.; Kromann, P.; Kumar, L.; Mcewan, M.; Mudege, N.N.; et al. Understanding root, tuber, and banana seed systems and coordination breakdown: A multi-stakeholder framework. J. Crop Improv. 2018, 32, 599–621. [Google Scholar] [CrossRef]
- Bakherad, M.; Salimi, M.; Angaji, S.A.; Mahjoubi, F.; Majidizadeh, T. LRIG1 expression and colorectal cancer prognosis. BMC Med. Genom. 2021, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Mignouna, B.D.; Kumar, P.L.; Coyne, D.; Bandyopadhyay, R.; Ortega-Beltran, A.; Bhattacharjee, R.; De Koeyer, D. Identifying and managing plant health risks for key African crops: Yam, taro and cocoyam. In Critical Issues in Plant Health: 50 Years of Research in African Agriculture; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 213–228. [Google Scholar]
- Mendoza, A.R.; Margaria, P.; Nagata, T.; Winter, S.; Blawid, R. Characterization of yam mosaic viruses from Brazil reveals a new phylogenetic group and possible incursion from the African continent. Virus Genes 2022, 58, 294–307. [Google Scholar] [CrossRef]
- Luo, G.-F.; Podolyan, A.; Kidanemariam, D.B.; Pilotti, C.; Houliston, G.; Sukal, A.C. A review of viruses infecting yam (Dioscorea spp.). Viruses 2022, 14, 662. [Google Scholar] [CrossRef]
- Odu, B.O.; Hughes, J.D.; Shoyinka, S.A.; Dongo, L.N. Isolation, characterization and identification of a potyvirus from Dioscorea alata L. (water yam) in Nigeria. Ann. Appl. Biol. 1999, 134, 65–71. [Google Scholar] [CrossRef]
- Wang, M.R.; Cui, Z.H.; Li, J.W.; Hao, X.Y.; Zhao, L.; Wang, Q.C. In vitro thermotherapy-based methods for plant virus eradication. Plant Methods 2018, 14, 87. [Google Scholar] [CrossRef]
- Panattoni, A.; Luvisi, A.; Triolo, E. Review. Elimination of viruses in plants: Twenty years of progress. Spanish J. Agric. Res. 2013, 11, 173–188. [Google Scholar] [CrossRef]
- Gong, H.; Igiraneza, C.; Dusengemungu, L. Major in vitro techniques for potato virus elimination and post eradication detection methods. A review. Am. J. Potato Res. 2019, 96, 379–389. [Google Scholar] [CrossRef]
- Shin, J.H.; Kang, D.K.; Sohn, J.K. Production of yam mosaic virus (YMV)-free Dioscorea opposita plants by cryotherapy of shoot-tips. CryoLetters 2013, 34, 149–157. [Google Scholar] [PubMed]
- Umber, M.; Filloux, D.; Gélabale, S.; Gomez, R.M.; Marais, A.; Gallet, S.; Gamiette, F.; Pavis, C.; Teycheney, P.Y. Molecular viral diagnosis and sanitation of yam genetic resources: Implications for safe yam germplasm exchange. Viruses 2020, 12, 1101. [Google Scholar] [CrossRef]
- González Ramírez, J.E.; Cabrera Jova, M.; Robaina, A.; Rodríguez Pérez, D.; González Cadalso, A.; Portal, O. Water-dissolved ozone mediates potyvirus sanitation during in vitro propagation of Dioscorea cayenensis subsp. rotundata (Poir.) Miège. Ozone Sci. Eng. 2020, 42, 89–94. [Google Scholar] [CrossRef]
- Bousalem, M.; Dallot, S.; Guyader, S. The use of phylogenetic data to develop molecular tools for the detection and genotyping of yam mosaic virus. Potential application in molecular epidemiology. J. Virol. Methods 2000, 90, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Mumford, R.A.; Seal, S.E. Rapid single-tube immunocapture RT-PCR for the detection of two yam potyviruses. J. Virol. Methods 1997, 69, 73–79. [Google Scholar] [CrossRef]
- Bayat, M.; Kavhiza, N.; Orujov, E.; Zargar, M.; Akhrarov, M.; Temewei, A.G. Integrated weed control methods utilizing planting pattern in sugar beet. Res. Crops 2019, 20, 413–418. [Google Scholar]
- Silva, G.; Bömer, M.; Nkere, C.; Lava Kumar, P.; Seal, S.E. Rapid and specific detection of yam mosaic virus by reverse-transcription recombinase polymerase amplification. J. Virol. Methods 2015, 222, 138–144. [Google Scholar] [CrossRef]
- Nkere, C.K.; Oyekanmi, J.O.; Silva, G.; Bömer, M.; Atiri, G.I.; Onyeka, J.; Maroya, N.G.; Seal, S.E.; Kumar, P.L. Chromogenic detection of yam mosaic virus by closed-tube reverse transcription loop-mediated isothermal amplification (CT-RT-LAMP). Arch. Virol. 2018, 163, 1057–1061. [Google Scholar] [CrossRef]
- Bayat, M.; Zargar, M.; Chudinova, E.; Astarkhanova, T.; Pakina, E. In Vitro Evaluation of Antibacterial and Antifungal Activity of Biogenic Silver and Copper Nanoparticles: The First Report of Applying Biogenic Nanoparticles against Pilidium concavum and Pestalotia sp. Fungi. Molecules 2021, 26, 5402. [Google Scholar] [CrossRef] [PubMed]
- MAFFS/NARCC Ministry of Agriculture, Forestry and Food Security; National Agricultural Research Coordinating Council. Crop Production Guidelines for Sierra Leone; FAO: Rome, Italy, 2005; 104p. [Google Scholar]
- Egesi, C.N.; Onyeka, T.J.; Asiedu, R. Severity of anthracnose and virus diseases of water yam (Dioscorea alata L.) in Nigeria I: Effects of yam genotype and date of planting. Crop Prot. 2007, 26, 1259–1265. [Google Scholar] [CrossRef]
- Payne, R.E.; Harding, S.A.; Murray, D.A.; Soutar, D.M.; Baird, D.B.; Glaser, A.I.; Welham, S.J.; Gilmour, A.R.; Thompson, R.; Webster, R. The Guide to Genstat Release 14, Part 2: Statistics; V.S.N. International: Hemel Hempstead, UK, 2011. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics, a Biometrical Approach, 2nd ed.; McGraw-Hill Publishing Company: New York, NY, USA, 1980; 481p. [Google Scholar]
- Norman, P.E.; Asfaw, A.; Tongoona, P.B.; Danquah, A.; Danquah, E.Y.; De Koeyer, D.; Asiedu, R. Pollination success in some white yam genotypes under polycross and nested mating designs. Int. J. Biol. Sci. Appl. 2018, 5, 19–28. [Google Scholar]
- Ntui, V.O.; Uyoh, E.A.; Ita, E.E.; Markson, A.A.A.; Tripathi, J.N.; Okon, N.I.; Akpan, N.O.; Phillip, J.O.; Brisibe, E.A.; Ene-Obong, E.E.; et al. Strategies to combat the problem of yam anthracnose disease: Status and prospects. Mol. Plant Pathol. 2021, 22, 1302–1314. [Google Scholar] [CrossRef]
- Gauch, H.G., Jr.; Zobel, R.W. Identifying mega-environments and targeting genotypes. Crop Sci. 1997, 37, 311–326. [Google Scholar] [CrossRef]
- Van Loon, L.C. Disease induction by plant viruses. Adv. Virus Res. 1987, 33, 205–255. [Google Scholar]
- Egesi, C.N.; Onyeka, T.J.; Asiedu, R. Environmental stability of resistance to anthracnose and virus diseases of water yam (Dioscorea alata). Afr. J. Agric. Res. 2009, 4, 113–118. [Google Scholar]
- Morse, S. The role of plant health in the sustainable production of seed yams in Nigeria: A challenging nexus between plant health, human food security, and culture. Plant Pathol. 2022, 71, 43–54. [Google Scholar] [CrossRef]
- Ansah, I.G.K.; Tetteh, B.K.D. Determinants of yam postharvest management in the Zabzugu district of Northern Ghana. Adv. Agric. 2016, 16, 9. [Google Scholar] [CrossRef]


| Species | ||||
|---|---|---|---|---|
| D. alata | D. rotundata | D. esculenta | ||
| TDa 00/00103 | WUSLY079-13 | WRSLY082-13 | TDr 00/00080 | PSLY077-13 |
| TDa 00/00046 | MOSLY049-12 | BOSLY063-13 | TDr 205 | BMSLY086-13 |
| TDa 95/00247 | MOSLY053-13 | PSLY075-13 | TDr 746 | MOSLY045-12 |
| TDa 02/00012 | MOSLY041-12 | KESLY013-12 | TDr 747 | MOSLY034-12 |
| TDa 98/01168 | MOSLY029-12 | BOSLY070-13 | TDr 89/02565 | MOSLY047-12 |
| TDa 95/00005 | MOSLY040-12 | KESLY018-12 | TDr 95/00184 | BOSLY066-13 |
| TDa 95/00307 | WUSLY078-13 | PSLY073-13 | TDr 95/01969 | WRSLY083-13 |
| TDa 98/01166 | KESLY016-12 | BOSLY069-13 | TDr 95/18544 | KESLY011-12 |
| TDa 291 | BMSLY084-12 | KESLY019-12 | TDr 96/00587 | MOSLY052-13 |
| TDa 98/01176 | BOSLY067-13 | KESLY008-12 | TDr 97/00793 | |
| TDa 95/00826 | KESLY017-12 | BOSLY058-13 | TDr 98/03015 | D. prahensilis |
| TDa 00/00194 | MOSLY031-12 | MOSLY050-13 | TDr 99/02310 | BMSLY085-13 |
| TDa 98/01174 | MOSLY033-12 | KESLY020-12 | TDr 99/02789 | KESLY007-12 |
| KASLY003-12 | BOSLY060-13 | MOSYL030-12 | TDr 99-13 | PSLY074-13 |
| MOSLY026-12 | MOSLY032-12 | BOSLY057-13 | TDr 99-15 | MOSLY036-12 |
| MOSLY042-12 | MOSLY038-12 | MOSLY035-12 | ||
| BOSLY065-13 | MOSLY046-12 | MOSLY043-12 | D. bulbifera | D. cayenensis |
| KESLY022-12 | KASLY002-12 | KESLY015-12 | MOSLY023-12 | MOSLY051-13 |
| MOSLY048-12 | MOSLY025-12 | WRSLY081-13 | BOSLY062-13 | BOSYL056-12 |
| PSLY076-13 | MOSLY039-12 | KESLY006-12 | BOSLY059-13 | BOSLY071-13 |
| KESLY021-12 | KASLY004-12 | KESLY014-12 | BOSLY061-13 | KESLY010-12 |
| KESLY005-12 | BOSLY064-13 | BOSLY068-13 | MOSLY044-12 | |
| WUSLY080-13 | KASLY001-12 | BOSLY072-13 | MOSLY024-12 | |
| MOSLY027-12 | MOSLY037-12 | MOSLY028-13 | KESLY009-12 | |
| KONOPLANE | PULLI | |||
| Estimate | Standard Error | t (108) | t pr. | |
|---|---|---|---|---|
| Intercept | 17.57 | 1.71 | 10.25 | <0.001 |
| YAD3MAP | −0.477 | 0.941 | −0.51 | 0.613 |
| YAD5MAP | −0.872 | 0.307 | −2.84 | 0.005 |
| YMV3MAP | 1.48 | 1.52 | 0.97 | 0.332 |
| YMV5MAP | −3.149 | 0.754 | −4.18 | <0.001 |
| Estimate | Standard Error | t (103) | t pr. | |
|---|---|---|---|---|
| Intercept | −6.5 | 12.0 | −0.54 | 0.591 |
| DRYR1MAH | −7.66 | 3.89 | −1.97 | 0.052 |
| DRYR2MAH | −0.84 | 3.12 | −0.27 | 0.788 |
| DRYR3MAH | 10.59 | 3.00 | 3.53 | <0.001 |
| MBUG1MAH | 3.21 | 3.82 | 0.84 | 0.402 |
| MBUG2MAH | −0.50 | 2.48 | −0.20 | 0.839 |
| MBUG3MAH | −0.44 | 1.99 | −0.22 | 0.826 |
| NEM1MAH | 3.73 | 4.46 | 0.84 | 0.405 |
| NEM2MAH | −5.21 | 3.64 | −1.43 | 0.155 |
| NEM3MAH | 2.50 | 2.56 | 0.98 | 0.333 |
| Month | Time | Temperature (°C) | Relative Humidity (%) | ||
|---|---|---|---|---|---|
| Indoor | Outdoor | Indoor | Outdoor | ||
| January | 09:00 a.m. | 27.9 | 28.4 | 71.0 | 74.0 |
| 12:00 p.m. | 29.5 | 30.2 | 69.0 | 69.0 | |
| 15:00 p.m. | 32.6 | 33.5 | 45.0 | 55.0 | |
| February | 09:00 a.m. | 27.6 | 29.4 | 59.0 | 63.0 |
| 12:00 p.m. | 29.3 | 32.5 | 57.0 | 59.0 | |
| 15:00 p.m. | 34.1 | 33.6 | 37.0 | 74.0 | |
| March | 09:00 a.m. | 28.4 | 28.6 | 65.0 | 70.0 |
| 12:00 p.m. | 30.0 | 31.5 | 40.0 | 46.0 | |
| 15:00 p.m. | 35.5 | 36.6 | 32.0 | 58.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saffa, M.D.; Saquee, F.S.; Norman, P.E.; Kavhiza, N.J.; Simbo, D.; Zargar, M.; Lyashko, M.; Pakina, E.; Vvedenskey, V. Influence of Field and Storage Diseases and Pests on Tuber Yield and Quality of Exotic and Local Yam (Dioscorea spp.) Genotypes. Horticulturae 2023, 9, 1183. https://doi.org/10.3390/horticulturae9111183
Saffa MD, Saquee FS, Norman PE, Kavhiza NJ, Simbo D, Zargar M, Lyashko M, Pakina E, Vvedenskey V. Influence of Field and Storage Diseases and Pests on Tuber Yield and Quality of Exotic and Local Yam (Dioscorea spp.) Genotypes. Horticulturae. 2023; 9(11):1183. https://doi.org/10.3390/horticulturae9111183
Chicago/Turabian StyleSaffa, Musa Decius, Francess Sia Saquee, Prince Emmanuel Norman, Nyasha John Kavhiza, Diakite Simbo, Meisam Zargar, Marina Lyashko, Elena Pakina, and Valentin Vvedenskey. 2023. "Influence of Field and Storage Diseases and Pests on Tuber Yield and Quality of Exotic and Local Yam (Dioscorea spp.) Genotypes" Horticulturae 9, no. 11: 1183. https://doi.org/10.3390/horticulturae9111183
APA StyleSaffa, M. D., Saquee, F. S., Norman, P. E., Kavhiza, N. J., Simbo, D., Zargar, M., Lyashko, M., Pakina, E., & Vvedenskey, V. (2023). Influence of Field and Storage Diseases and Pests on Tuber Yield and Quality of Exotic and Local Yam (Dioscorea spp.) Genotypes. Horticulturae, 9(11), 1183. https://doi.org/10.3390/horticulturae9111183








