Effect of Foliar Application of Silicon and Selenium on the Growth, Yield and Fruit Quality of Tomato in the Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment
2.2. Plant Growth and Fruit Yield
2.3. Measurement of Si and Se Concentrations
2.4. Determination of Carbohydrate and Organic Acid Concentrations
2.5. Determination of Concentrations of Soluble Protein, Free Amino Acid and Nitrate
2.6. Determination of Secondary Metabolites in Tomato Fruits
2.7. Statistical Analysis
3. Results
3.1. Plant Growth
| Treatment | Plant Height (cm) | Stem Diameter (mm) | Number of Leaves |
|---|---|---|---|
| CK | 208.1 ± 6.7 ab | 9.3 ± 0.3 b | 18.7 ± 0.7 ab |
| Na | 207.3 ± 5.6 b | 9.3 ± 0.2 b | 18.6 ± 0.7 b |
| Si | 206.4 ± 5.2 b | 10.0 ± 0.3 a | 19.3 ± 0.7 ab |
| Se | 211.4 ± 4.8 ab | 9.8 ± 0.3 a | 18.8 ± 0.6 ab |
| Si + Se | 213.2 ± 5.7 a | 9.8 ± 0.4 a | 19.3 ± 1.2 a |
| F test | ** | *** | * |
3.2. Yield-Related Traits
| Treatment | Yield per Plant (kg plant−1) | Fruit Number per Plant | Single Fruit Weight (g) | Fruit Shape Index |
|---|---|---|---|---|
| CK | 1.62 ± 0.40 bc | 17.3 ± 3.6 b | 93.2 ± 9.3 | 0.77 ± 0.06 |
| Na | 1.59 ± 0.30 c | 17.6 ± 3.6 ab | 91.6 ± 13.5 | 0.77 ± 0.06 |
| Si | 1.83 ± 0.36 ab | 19.7 ± 3.1 a | 93.5 ± 13.4 | 0.77 ± 0.05 |
| Se | 1.75 ± 0.30 abc | 19.1 ± 2.2 ab | 91.6 ± 9.8 | 0.75 ± 0.06 |
| Si + Se | 1.93 ± 0.29 a | 19.8 ± 3.2 a | 98.1 ± 9.8 | 0.76 ± 0.04 |
| F test | *** | ** | ns | ns |
3.3. Si and Se Concentrations in the Fruit
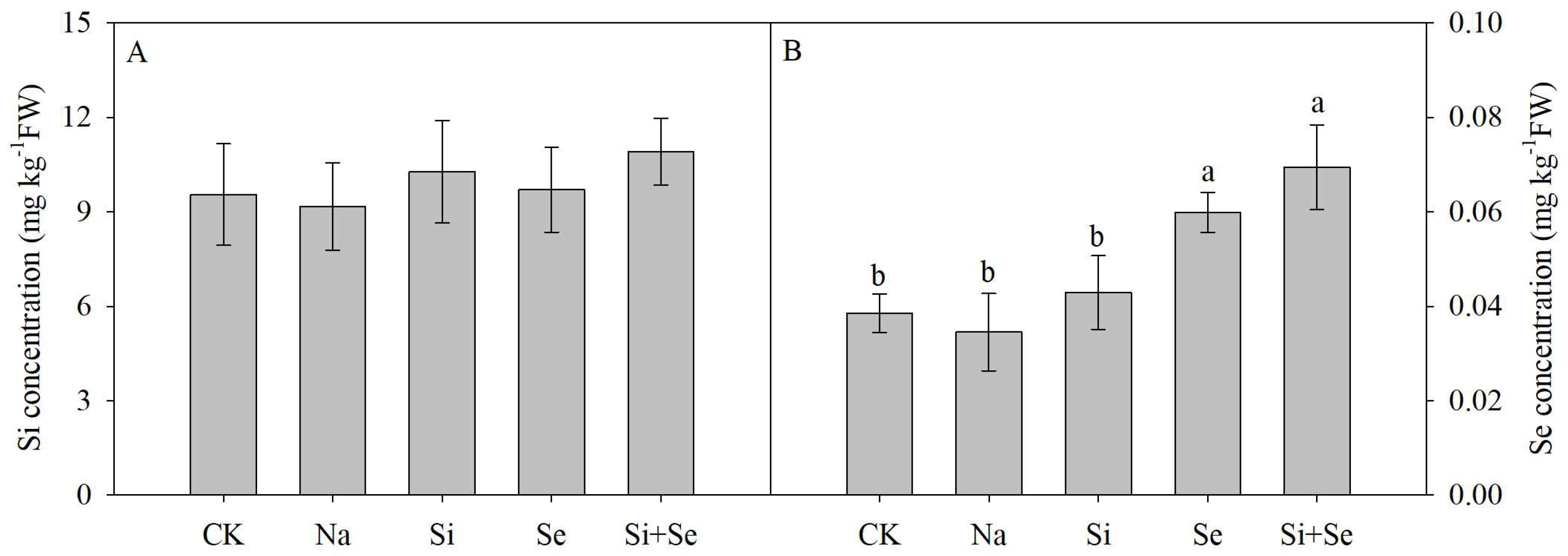
3.4. Carbohydrate Concentrations in the Fruit
| Treatment | Glucose (mg g−1) | Fructose (mg g−1) | Sucrose (mg g−1) | Starch (mg g−1) | Total Carbohydrates (mg g−1) |
|---|---|---|---|---|---|
| CK | 6.66 ± 0.41 ab | 3.39 ± 0.29 ab | 0.44 ± 0.07 c | 3.01 ± 0.11 | 13.5 ± 0.8 c |
| Na | 6.12 ± 0.14 b | 3.17 ± 0.08 b | 0.38 ± 0.03 c | 2.83 ± 0.30 | 12.5 ± 0.4 c |
| Si | 6.72 ± 0.54 ab | 3.57 ± 0.35 ab | 0.62 ± 0.03 b | 3.40 ± 0.25 | 14.3 ± 1.0 bc |
| Se | 7.19 ± 0.17 a | 3.83 ± 0.09 a | 0.78 ± 0.04 ab | 3.87 ± 0.72 | 15.7 ± 0.5 ab |
| Si + Se | 7.37 ± 0.19 a | 3.87 ± 0.18 a | 0.88 ± 0.10 a | 4.20 ± 1.08 | 16.3 ± 0.8 a |
| F test | ** | * | *** | ns | *** |
3.5. Organic Acid Concentrations in the Fruit
| Treatment | Citric Acid (mg g−1) | Malic Acid (mg g−1) | Tartaric Acid (mg g−1) | Acetic Acid (mg g−1) | Total Organic Acids (mg g−1) |
|---|---|---|---|---|---|
| CK | 4.07 ± 0.33 b | 0.88 ± 0.11 | 0.96 ± 0.08 | 0.59 ± 0.05 | 6.50 ± 0.52 |
| Na | 4.35 ± 0.27 ab | 0.75 ± 0.04 | 0.89 ± 0.04 | 0.57 ± 0.03 | 6.56 ± 0.31 |
| Si | 4.70 ± 0.08 a | 0.83 ± 0.12 | 0.99 ± 0.09 | 0.53 ± 0.18 | 7.05 ± 0.37 |
| Se | 4.62 ± 0.15 ab | 0.76 ± 0.08 | 1.02 ± 0.05 | 0.32 ± 0.10 | 6.72 ± 0.27 |
| Si + Se | 4.80 ± 0.19 a | 0.78 ± 0.11 | 1.07 ± 0.09 | 0.34 ± 0.09 | 6.99 ± 0.48 |
| F test | * | ns | ns | * | ns |
3.6. Concentrations of Soluble Protein, Free Amino Acid and Nitrate in the Fruit
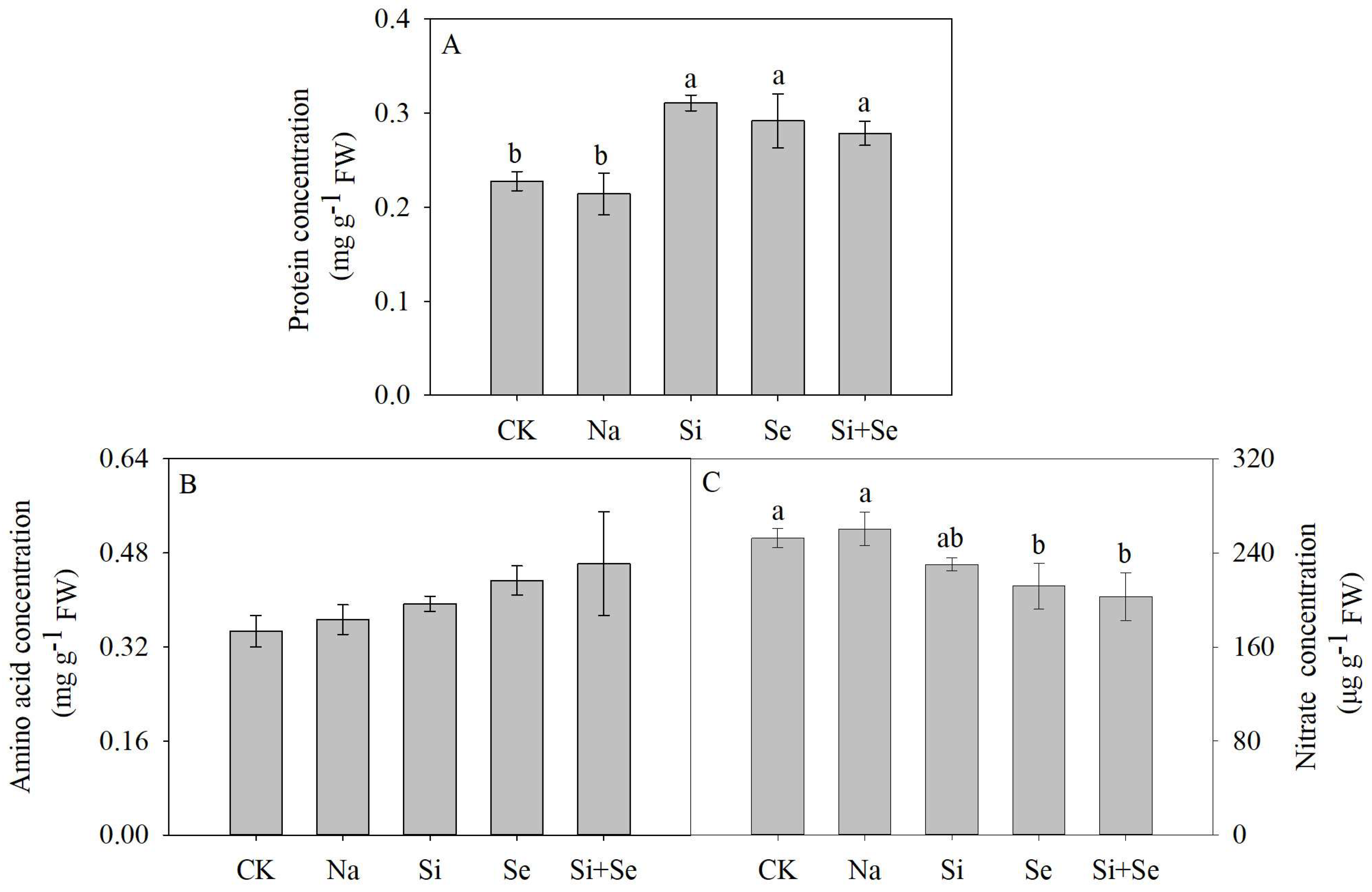
3.7. Concentrations of Secondary Metabolites in the Fruit
| Treatment | Vitamin C (μg g−1) | Total Phenols (μg g−1) | Flavonoids (μg g−1) | Anthocyanin (nmol g−1) | Lycopene (mg kg−1) | β-Carotene (mg kg−1) | Total Carotenoids (mg kg−1) |
|---|---|---|---|---|---|---|---|
| CK | 134.0 ± 9.9 b | 322.5 ± 14.1 c | 88.0 ± 14.0 ab | 11.6 ± 1.1 b | 14.8 ± 1.8 b | 7.43 ± 0.81 | 22.2 ± 1.3 b |
| Na | 131.1 ± 17.4 b | 335.7 ± 13.6 bc | 76.6 ± 12.8 b | 10.6 ± 0.8 b | 15.7 ± 2.0 b | 6.77 ± 0.52 | 22.4 ± 2.4 b |
| Si | 141.3 ± 5.9 ab | 351.0 ± 7.9 b | 86.7 ± 9.7 ab | 11.8 ± 0.4 b | 17.0 ± 1.4 ab | 6.70 ± 0.16 | 23.7 ± 1.5 b |
| Se | 169.4 ± 26.2 a | 384.2 ± 6.9 a | 82.9 ± 8.3 b | 14.2 ± 0.2 a | 21.7 ± 3.3 a | 7.85 ± 0.30 | 29.5 ± 3.0 a |
| Si + Se | 159.2 ± 9.0 ab | 390.1 ± 9.7 a | 110.6 ± 15.1 a | 11.6 ± 0.2 b | 19.7 ± 0.5 ab | 7.14 ± 0.14 | 26.9 ± 0.6 ab |
| F test | * | *** | * | *** | ** | ns | ** |
3.8. Comprehensive Evaluation of Plant Growth, Fruit Yield and Quality
| Treatment | D+ | D− | CI | Ranking |
|---|---|---|---|---|
| CK | 0.635 | 0.140 | 0.181 | 4 |
| Na | 0.704 | 0.048 | 0.064 | 5 |
| Si | 0.468 | 0.317 | 0.404 | 3 |
| Se | 0.215 | 0.587 | 0.732 | 2 |
| Si + Se | 0.149 | 0.664 | 0.817 | 1 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hernández-Hernández, H.; Quiterio-Gutiérrez, T.; Cadenas-Pliego, G.; Ortega-Ortiz, H.; Hernández-Fuentes, A.D.; Fuente, M.C.; Valdés-Reyna, J.; Juárez-Maldonado, A. Impact of selenium and copper nanoparticles on yield, antioxidant system, and fruit quality of tomato plants. Plants 2019, 8, 355. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Liu, J.; Chen, Y.; Zhang, X. Exploring the effects of selenium treatment on the nutritional quality of tomato fruit. Food Chem. 2018, 252, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, A.H.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of beneficial elements. Curr. Opin. Plant. Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Laane, H.M. The effects of foliar sprays with different silicon compounds. Plants 2018, 7, 45. [Google Scholar] [CrossRef]
- Farooq, M.A.; Dietz, K.J. Silicon as Versatile Player in Plant and Human Biology: Overlooked and Poorly Understood. Front. Plant Sci. 2015, 6, 994. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, D.; Huang, X.; Wang, S.; Qiu, R.; Zhang, Z.; Ming, J. Effect of Enterobacter sp. EG16 on selenium biofortification and speciation in pak choi (Brassica rapa ssp. chinensis). Sci. Hortic. 2023, 310, 111723. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Q.; Liao, X.; Yang, X.; Chao, W.; Cong, X.; Zhang, W.; Liao, Y.; Ye, J.; Qian, H.; et al. Exploring Effects of Exogenous Selenium on the Growth and Nutritional Quality of Cabbage (Brassica oleracea var. capitata L.). Horticulturae 2023, 9, 330. [Google Scholar] [CrossRef]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Luís, O.C.M.; de Mello Prado, R. Synergy of selenium and silicon to mitigate abiotic stresses: A review. Gesunde Pflanz. 2023, 75, 1461–1474. [Google Scholar] [CrossRef]
- Sun, H.; Duan, Y.; Mitani-Ueno, N.; Che, J.; Jia, J.; Liu, J.; Guo, J.; Ma, J.F.; Gong, H. Tomato roots have a functional silicon influx transporter but not a functional silicon efflux transporter. Plant Cell Environ. 2020, 43, 732–744. [Google Scholar] [CrossRef]
- Karagiannis, E.; Michailidis, M.; Skodra, C.; Molassiotis, A.; Tanou, G. Silicon influenced ripening metabolism and improved fruit quality traits in apples. Plant Physiol. Biochem. 2021, 166, 270–277. [Google Scholar] [CrossRef]
- Dann, E.K.; Le, D.P. Effects of silicon amendment on soilborne and fruit diseases of avocado. Plants 2017, 6, 51. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Z.; Li, Y.; Xie, W.; Li, W.; Tang, X.; Ashraf, U.; Kong, L.; Wu, L.; Wang, S.; et al. Selenium-silicon (Se-Si) induced modulations in physio-biochemical responses, grain yield, quality, aroma formation and lodging in fragrant rice. Ecotox. Environ. Saf. 2020, 196, 110525. [Google Scholar] [CrossRef] [PubMed]
- Segalin, S.R.; Huth, C.; Rosa, T.D.; Pahins, D.B.; Mertz, L.M.; Nunes, U.R.; Martin, T.N. Foliar application of silicon and the effect on wheat seed yield and quality. J. Seed Sci. 2013, 35, 86–91. [Google Scholar] [CrossRef]
- Gottardi, S.; Iacuzzo, F.; Tomasi, N.; Cortella, G.; Manzocco, L.; Pinton, R.; Römheld, V.; Mimmo, T.; Scampicchio, M.; Dalla Costa, L.; et al. Beneficial effects of silicon on hydroponically grown corn salad (Valerianella locusta (L.) Laterr) plants. Plant. Physiol. Biochem. 2012, 56, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Téllez, L.I.; García-Jiménez, A.; Escobar-Sepúlveda, H.F.; Ramírez-Olvera, S.M.; Bello-Bello, J.J.; Gómez-Merino, F.C. Silicon induces hormetic dose-response effects on growth and concentrations of chlorophylls, amino acids and sugars in pepper plants during the early developmental stage. PeerJ 2020, 8, e9224. [Google Scholar] [CrossRef]
- Hu, W.; Su, Y.; Zhou, J.; Zhu, H.; Guo, J.; Huo, H.; Gong, H. Foliar application of silicon and selenium improves the growth, yield and quality characteristics of cucumber in field conditions. Sci. Hortic. 2022, 294, 116770. [Google Scholar] [CrossRef]
- Xue, G.; Zhang, G.; Sun, Y.; Liao, S.; Chen, Y. Influences of spraying two different forms of silicon on plant growth and quality. Chin. Agric. Sci. Bull. 2012, 28, 272–276, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Gao, R.; Cao, B. Effects of silicon treatment on fruit development and silicon absorption of tomato. Shandong Agric. Sci. 2016, 48, 88–91, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Jalali, P.; Roosta, H.R.; Khodadadi, M.; Torkashvand, A.M.; Jahromi, M.G. Effects of brown seaweed extract, silicon, and selenium on fruit quality and yield of tomato under different substrates. PLoS ONE 2022, 17, e0277923. [Google Scholar] [CrossRef]
- Zhao, X.; Yao, Q.; Zhang, Y.; Hou, L.; Xing, G.; Shi, Y. Effects of foliar spraying exogenous silicon and iron fertilizers on tomato fruit quality. Shandong Agric. Sci. 2020, 52, 78–84. [Google Scholar] [CrossRef]
- Costan, A.; Stamatakis, A.; Chrysargyris, A.; Petropoulos, S.A.; Tzortzakis, N. Interactive effects of salinity and silicon application on solanum lycopersicum growth, physiology and shelf-life of fruit produced hydroponically. J. Sci. Food Agric. 2020, 100, 732–743. [Google Scholar] [CrossRef]
- Schiavon, M.; Dall’acqua, S.; Mietto, A.; Pilon-Smits, E.A.; Sambo, P.; Masi, A.; Malagoli, M. Selenium fertilization alters the chemical composition and antioxidant constituents of tomato (Solanum lycopersicon L.). J. Agric. Food Chem. 2013, 61, 10542–10554. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, M.; Malorgio, F.; Pezzarossa, B. Selenium enrichment of horticultural crops. Molecules 2017, 22, 933. [Google Scholar] [CrossRef]
- Xiang, J.; Rao, S.; Chen, Q.; Zhang, W.; Cheng, S.; Cong, X.; Zhang, Y.; Yang, X.; Xu, F. Research progress on the effects of selenium on the growth and quality of tea plants. Plants 2022, 11, 2491. [Google Scholar] [CrossRef]
- Pezzarossa, B.; Remorini, D.; Gentile, M.L.; Massai, R. Effects of foliar and fruit addition of sodium selenate on selenium accumulation and fruit quality. J. Sci. Food Agric. 2012, 92, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; He, L.; Du, B.; Pan, S.; Mo, Z.; Duan, M.; Tian, H.; Tang, X. Biofortification with chelating selenium in fragrant rice: Effects on photosynthetic rates, aroma, grain quality and yield formation. Field Crops Res. 2020, 255, 107909. [Google Scholar] [CrossRef]
- Liang, K.H.; Liang, S.; Zhu, H. Comparative proteomics analysis of the effect of selenium treatment on the quality of foxtail millet. LWT-Food Sci. Technol. 2020, 131, 109691. [Google Scholar] [CrossRef]
- Businelli, D.; D’Amato, R.; Onofri, A.; Tedeschini, E.; Tei, F. Se-enrichment of cucumber (Cucumis sativus L.), lettuce (Lactuca sativa L.) and tomato (Solanum lycopersicum L. Karst) through fortification in pre-transplanting. Sci. Hortic. 2015, 197, 697–704. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium enrichment enhances the quality and shelf life of basil leaves. Plants 2020, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, K.M.; Gallardo-Williams, M.T.; Benson, R.F.; Martin, D.F. Effects of selenium supplementation on four agricultural crops. J. Agric. Food Chem. 2003, 51, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pang, J.; Li, H.; Qiang, X.; Zhang, Y.; Song, J. Effects of foliar-spraying selenium coupled with soil moisture on the yield and quality of tomato. Sci. Agric. Sin. 2022, 55, 4433–4444, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Neysanian, M.; Iranbakhsh, A.; Ahmadvand, R.; Ardebili, Z.O.; Ebadi, M. Comparative efficacy of selenate and selenium nanoparticles for improving growth, productivity, fruit quality, and postharvest longevity through modifying nutrition, metabolism, and gene expression in tomato; potential benefits and risk assessment. PLoS ONE 2020, 16, e0244207. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.I.M.; Al-Wasfy, M.M. The promotive impact of using silicon and selenium with potassium and boron on fruiting of valencia orange trees grown under minia region conditions. World Rural. Obs. 2014, 6, 28–36. [Google Scholar]
- Ahmed, M.M.A.A.; Ahmed, Y.M.A.; Ahmed, A.F.O. Yield and fruit quality of Ewaise mango trees grown under Upper Egypt conditions as affected by application of nutrients, plant extracts, selenium and silicon. Researcher 2018, 10, 11–20. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Yue, Z.; Wang, J.; Jin, L.; Xu, Z.; Jin, N.; Zhang, B.; Lyu, J.; Yu, J. Application of exogenous silicon for alleviating photosynthetic inhibition in tomato seedlings under low-calcium stress. Int. J. Mol. Sci. 2022, 23, 13526. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Moradtalab, N.; Eshaghi, Z.; Feizy, J. Effect of silicon supplementation on growth and metabolism of strawberry plants at three developmental stages. N. Z. J. Crop Hortic. Sci. 2018, 46, 144–161. [Google Scholar] [CrossRef]
- Gou, T.; Yang, L.; Hu, W.; Zhu, Y.; Guo, J.; Gong, H. Silicon improves the growth of cucumber under excess nitrate stress by enhancing nitrogen assimilation and chlorophyll synthesis. Plant Physiol. Biochem. 2020, 152, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ngamsuk, S.; Huang, T.C.; Hsu, J.L. Determination of phenolic compounds, procyanidins, and antioxidant activity in processed Coffea arabica L. leaves. Foods 2019, 8, 389. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Katsoulas, N.; Barros, L.; Ferreira, I.C. The effect of covering material on the yield, quality and chemical composition of greenhouse-grown tomato fruit. J. Sci. Food Agric. 2019, 99, 3057–3068. [Google Scholar] [CrossRef]
- Zhu, K.; Zhao, Y.; Ma, Y.; Zhang, Q.; Kang, Z.; Hu, X. Drip irrigation strategy for tomatoes grown in greenhouse on the basis of fuzzy Borda and K-means analysis method. Agric. Water Manag. 2022, 267, 107598. [Google Scholar] [CrossRef]
- Toresano-Sánchez, F.; Valverde-García, A.; Camacho-Ferre, F. Effect of the application of Silicon hydroxide on yield and quality of cherry tomato. J. Plant Nutr. 2012, 35, 567–590. [Google Scholar] [CrossRef]
- Chen, R.; Xue, L. Influences of selenium and copper nanopartcles on tomato growth, photosynthetic characteristics and yield of tomato under drought stress. Jiangsu Agric. Sci. 2022, 50, 127–134. [Google Scholar] [CrossRef]
- Sabatino, L.; La Bella, S.; Ntatsi, G.; Iapichino, G.; D’Anna, F.; De Pasquale, C.; Consentino, B.; Rouphael, Y. Selenium biofortification and grafting modulate plant performance and functional features of cherry tomato grown in a soilless system. Sci. Hortic. 2021, 285, 110095. [Google Scholar] [CrossRef]
- Buttaro, D.; Bonasia, A.; Minuto, A.; Serio, F.; Santamaria, P. Effect of silicon in the nutrient solution on the incidence of powdery mildew and quality traits in carosello and barattiere (Cucumis melo L.) grown in a soilless system. J. Hortic. Sci. Biotechnol. 2009, 84, 300–304. [Google Scholar] [CrossRef]
- Boldrin, P.F.; Faquin, V.; Ramos, S.J.; Boldrin, K.V.F.; Ávila, F.W.; Guilherme, L.R.G. Soil and foliar application of selenium in rice biofortifification. J. Food Compos. Anal. 2013, 31, 238–244. [Google Scholar] [CrossRef]
- Silva, D.L.; Mello, P.R.; Tenesaca, L.F.L.; Silva, J.L.F.; Mattiuz, B.H. Silicon attenuates calcium deficiency by increasing ascorbic acid content, growth and quality of cabbage leaves. Sci. Rep. 2021, 11, 1770. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Antoshkina, M.; Bondareva, L.; Sekara, A.; Campagna, E.; Caruso, G. Effect of Foliar Application of Sodium Selenate on Mineral Relationships in Brassicaceae Crops. Horticulturae 2023, 9, 535. [Google Scholar] [CrossRef]
- Jarosz, Z. The effect of silicon application and type of medium on yielding and chemical composition of tomato. Acta Sci. Pol. Hortorum Cultus 2014, 13, 171–183. [Google Scholar]
- Kleiber, T.; Calomme, M.; Borowiak, K. The effect of choline-stabilized orthosilicic acid on microelements and silicon concentration, photosynthesis activity and yield of tomato grown under Mn stress. Plant Physiol. Biochem. 2015, 96, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A.; Watson, L.M.; Pathirana, R.; Joyce, N.I.; West, P.J.; Hunter, D.A.; McKenzie, M.J. Biofortification of tomato (Solanum lycopersicum) fruit with the anticancer compound methylselenocysteine using a selenocysteine methyltransferase from a selenium hyperaccumulator. J. Agric. Food Chem. 2011, 59, 10987–10994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chi, F.; Zhang, J.; Kuang, E.; Su, Q.; Liu, Y. Effects of spraying exogenous selenium on selenium content and quality of tomato and watermelon fruits. North. Hort. 2021, 2, 47–52. [Google Scholar] [CrossRef]
- Zhu, S.; Liang, Y.; Gao, D.; An, X.; Kong, F. Spraying foliar selenium fertilizer on quality of table grape (Vitis vinifera L.) from different source varieties. Sci. Hortic. 2017, 218, 87–94. [Google Scholar] [CrossRef]
- Li, L.; Tian, M.; Gao, Y.; Li, J. Effect of selenium fertilizer on growth and mineral element accumulation of tomato in substrate culture. Acta Agric. Zhejiang 2020, 32, 253–261. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Terry, L.A.; Tosetti, R.; Rosellini, I.; Pezzarossa, B. Effect of selenium enrichment on metabolism of tomato (Solanum lycopersicum) fruit during postharvest ripening. J. Sci. Food Agric. 2019, 99, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jin, N.; Jin, L.; Xiao, X.; Hu, L.; Liu, Z.; Wu, Y.; Xie, Y.; Zhu, W.; Lyu, J.; et al. Response of tomato fruit quality depends on period of LED supplementary light. Front. Nutr. 2022, 9, 833723. [Google Scholar] [CrossRef] [PubMed]
- Meucci, A.; Shiriaev, A.; Rosellini, I.; Malorgio, F.; Pezzarossa, B. Se-enrichment pattern, composition, and aroma profile of ripe tomatoes after sodium selenate foliar spraying performed at different plant developmental stages. Plants 2021, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zhang, Z.; Xu, K. Silicon improving water conservation, yield and quality of tomato under alternate wetting and drying condition. Trans. Chin. Soc. Agric. Eng. 2017, 33, 127–134. [Google Scholar] [CrossRef]
- Han, Y.; Han, Y.; Guo, W.; Li, J.; Fan, S.; Hao, J.; Huang, J.; Xu, X. The effect of application of selenate and selenite to soil on the fruit quality of tomato. Chin. Agric. Sci. Bull. 2014, 30, 220–224. [Google Scholar] [CrossRef]
- Shao, X.; Han, Y.; Qi, C.; Tian, Y.; Fan, S. Effects of different concentrations of selenium fertilizer on tomato quality. Vegetables 2017, 8, 25–28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Su, Y.; Yang, R.; Xie, Z.; Gong, H. Effect of Foliar Application of Silicon and Selenium on the Growth, Yield and Fruit Quality of Tomato in the Field. Horticulturae 2023, 9, 1126. https://doi.org/10.3390/horticulturae9101126
Hu W, Su Y, Yang R, Xie Z, Gong H. Effect of Foliar Application of Silicon and Selenium on the Growth, Yield and Fruit Quality of Tomato in the Field. Horticulturae. 2023; 9(10):1126. https://doi.org/10.3390/horticulturae9101126
Chicago/Turabian StyleHu, Wanxing, Yan Su, Rui Yang, Zhilong Xie, and Haijun Gong. 2023. "Effect of Foliar Application of Silicon and Selenium on the Growth, Yield and Fruit Quality of Tomato in the Field" Horticulturae 9, no. 10: 1126. https://doi.org/10.3390/horticulturae9101126
APA StyleHu, W., Su, Y., Yang, R., Xie, Z., & Gong, H. (2023). Effect of Foliar Application of Silicon and Selenium on the Growth, Yield and Fruit Quality of Tomato in the Field. Horticulturae, 9(10), 1126. https://doi.org/10.3390/horticulturae9101126







