Abstract
In apricots and other stonefruit, chilling injury (CI) symptoms like mealiness, rubberiness, and gel formation are associated with cell wall properties. Apricots were stored at 0 °C for 5 weeks and ripened at 20 °C to induce CI and compared with fruit ripened at 20 °C from harvest at similar firmness. In those apricots without CI, degradation of middle-lamella pectin during softening weakened cell-cell adhesion and intercellular junctions. Pectin was still present in middle lamella regions but pectin that filled the intercellular spaces at harvest had disappeared. Fruit with combinations of CI symptoms showed different pectin solubilities, molecular weight distribution, and differences in pectin staining compared with fruit that were severely chilling-injured, exhibiting all symptoms. The perception of mealiness correlated with the presence of pectin in the cell lumen, and rubberiness with the presence of pectin in tricellular corners. We concluded that in chilling-injured apricots, the normal softening process is not being resumed after fruit have been taken out of cold storage. Cell wall degradation is disrupted, affecting the normal weakening of cell walls during softening. Hence, cell walls were less likely to break open during chewing, and when cells did break, any juice released might be bound by pectin present in the cell walls and cell lumen, leaving a sensation of rubberiness and mealiness.
Keywords:
apricot; cell wall; chilling injury; immunolabelling; mealiness; pectin; softening; rubberiness 1. Introduction
Like most stonefruit, apricots (Prunus armeniaca L.) have a short postharvest life that can be extended through cold storage. Cold temperature, however, can result in the development of chilling injury (CI) symptoms such as gel and mealiness, which may not become apparent until after fruit have been removed from cold storage and have ripened at room temperature, although some symptoms may already develop during cold storage [1]. Apricot cultivar ‘CluthaGold’, for example, softens rapidly and becomes rubbery (leathery) in cold storage at 0 °C, and develops mealiness and gel during subsequent ripening at 20 °C [1]. Cultivar, maturity at which apricot fruit were harvested, and storage temperature have a significant effect on how fruit perform after cold storage. Apricots harvested at a more mature stage were more prone to mealiness and gel formation after cold storage, whereas those that were less mature developed more rubbery symptoms. Storage temperatures between 4 °C and 7 °C resulted in more mealy fruit than those stored at 0 °C or lower [2,3]. The development of CI limits consumer acceptance of apricots and also other stonefruit like nectarine and peach. Storage of ‘Shushanggan’ and ‘Xiaobai’ apricots at near-freezing temperature (−1 °C to −1.9 °C) delayed the postharvest softening process and reduced CI remarkably [4,5].
There are differences among stonefruit species and even cultivars of the same species in the changes occurring in the cell wall during ripening-related softening or development of CI. This is particularly evident in the extent of solubilisation and depolymerisation of the polyuronide backbone of pectin in peach and nectarine [6,7,8]. Cell wall changes during apricot softening often involve a reduction in molecular weight (MW) of CDTA-soluble pectin (CSP) when fruit harvested just before eating-ripeness (20 N) were coldstored for 42 days [9], but whether these fruit exhibited CI symptoms was not investigated. Changes in MW profiles of pectins were also reported for the Japanese apricot (Prunus mume), but only during ripening at room temperature [10]. A previous study [1] showed that in ‘CluthaGold’ apricots, the correlation between the amount of water-soluble pectin (WSP) and fruit firmness was similar for mealy and non-mealy fruit. However, when cultivars ‘CluthaGold’ and ‘Larclyd’ ripened after cold storage, mealiness and gel formation was accompanied not only by an increase in yields of WSP but also in CSP, whereas in apricots ripened at 20 °C straight after harvest, yields of WSP increased but CSP slightly decreased. It was concluded that solubilisation of pectin was a major feature of softening in apricots and that differences in CSP amounts may reflect differences in the strength of cell adhesion leading to CI [1]. A study on ‘Shushanggan’ apricots [4] showed that storage at near freezing temperature suppressed the loss of side chains in CDTA-soluble pectin, and strongly inhibited the solubilisation of Na2CO3-soluble pectin by the suppression of cell wall modifying enzymes and related gene expression. Storage of ‘Xiaobai’ apricots at near-freezing-temperature also significantly inhibited the activity of cell wall modifying enzymes and delayed the solubilisation of WSP [5].
In a previous paper [1], we looked at the effects of harvest maturity and cold storage conditions on sensory attributes and fruit properties during ripening of ‘Larclyd’ and ‘CluthaGold’ apricots, in order to establish whether there are any mathematical correlations between postharvest treatment and biological properties to predict the development and extent of CI. In this study, cell wall changes in apricots with chilling injury (CI) symptoms after cold storage followed by ripening at 20 °C were compared with cell wall changes in fruit ripened without CI at 20 °C straight after harvest, in order to identify changes in structure, pectin localisation, uronic acid content, and molecular weight distribution of pectin extracted from fruit cell walls during softening. Cell wall-related patterns were identified in fruit that had only gel formation, rubberiness (leatheriness), or gel formation and rubberiness, or all three CI symptoms (gel formation, rubberiness, and mealiness). Achieving an understanding of the relationship between fruit ripening, CI, and softening-related cell wall degradation and depolymerisation in apricots will assist in developing strategies for improving fruit quality, particularly regarding texture and reduction of chilling injuries.
2. Materials and Methods
2.1. Plant Material and Experimental Design
‘CluthaGold’ apricots were harvested at commercial maturity from mature multi-leader trees on a grower property at Earnscleugh, Central Otago, New Zealand. Fruit from eight trees (80 per tree) were selected, weighed, and placed in trays with plixes and liners to limit water loss during storage. Fruit from each tree were kept separate and not mixed. Equal numbers of trays with fruit from each tree were stored at 0 °C and at 20 °C (relative humidity 85%). For immediate harvest assessments, 16 fruit (2 per tree) were used. These fruit had an average flesh firmness of 59 N and soluble solids content of 12.2%. From fruit left to ripen at 20 °C, phenotyping assessments were made every second day using 16 fruit (2 per tree) per assessment day, until fruit were over-soft at firmnesses of less than 10 N [11]. Fruit in 0 °C cold storage were removed after 5 weeks, held at 20 °C for 4 h to warm and assessed (2 fruit per tree, 16 fruit in total). The rest of the fruit were left to ripen at 20 °C and assessed every second day (2 fruit per tree, 16 fruit per assessment day) until over-soft.
2.2. Fruit Phenotyping and Sensory Assessments
At each assessment day, the following measurements were made on every fruit: fresh weight, flesh firmness, and sensory assessments such as rubbery score and gel formation score as described in [1,12].
In brief, flesh firmness was assessed on opposite cheeks of the fruit using a 7.9 mm probe using a GUSS FTA penetrometer (GUSS Manufacturing Ltd., Strand, South Africa) after a small circle of skin was removed. Penetration speed, trigger force, and penetration distance were 10 mm s−1, 0.5 Newton (N), and 8 mm, respectively.
Rubberiness was assessed on a scale from 0 (no rubberiness) to 3 (severe rubberiness) and was judged visually by noting if the GUSS probe penetrated into the flesh or bounced off on one (score of 2) or two (score of 3) sides. If the probe penetrated the flesh only at the very end of its trajectory, it received a score of 1. Rubberiness is a textural change and not visible to the eye.
Gel formation was visually assessed on a scale from 0 (no gel) to 3 (severe gel). The score was based on the proportion of flesh having a glassy appearance: 1 being <10% glassy, 2 being 10–50% glassy, and 3 being >50% glassy (Figure S1).
At each phenotyping (16 fruit), the six fruit closest to the mean flesh firmness were assessed for mealiness and juiciness by three trained panellists independently tasting a slice from each fruit. Mealiness and juiciness were scored on a scale from 0 (no mealiness or juiciness) to 3 (very mealy or very juicy). Mealiness was considered to be a combination of lack of juice and a lumpy or woolly texture. Mealiness is a textural change and not visible to the eye.
2.3. Cell Wall Isolation and Extraction
Fruit samples to examine changes in cell wall chemistry and structure during softening at 20 °C and after cold storage at 0 °C were selected based on firmness and degree of CI as indicated in Table 1. Fruit similar in storage method, firmness, and chilling injury symptom extent (at least 10 fruit for each sampling point across the 8 replicate trees) were pooled, peeled, and flesh (middle mesocarp) chopped, frozen in liquid nitrogen and stored at −80 °C.

Table 1.
Summary of ‘CluthaGold’ apricots that were selected for structural and pectin-related changes in the cell wall during ripening at 20 °C straight after harvest or after cold storage at 0 °C for 5 weeks followed by ripening at 20 °C. Table shows values ±SD.
Frozen tissue (50 g) was ground into a fine powder in liquid nitrogen using a mortar and pestle, and cell wall pectic fractions prepared [1]. In brief, after extraction of ground tissue with ice-cold methanol- chloroform- water- formic acid to inactivate enzymes and extract low molecular weight (MW) metabolites [13], the pellet was sequentially extracted with water, calcium chelator trans- 1,2-cyclohexane diaminetetraacetic acid (CDTA) and sodium carbonate (Na2CO3) to give the water-soluble pectin (WSP), chelator-soluble pectin (CSP), Na2CO3-soluble pectin (NSP), and the cell wall residue (CWR), respectively. Extracts were extensively dialysed and freeze-dried. Uronic acids (UA) were measured as described previously [14] using galacturonic acid as a standard.
2.4. Size Exclusion Chromatography
WSP, CSP, and NSP were separated by size exclusion chromatography on a column of Superose™ 6 10/300 (GE Healthcare, Chicago, IL, USA). Polysaccharides were dissolved in water (1 mg mL−1) and 1 mL loaded onto the column (eluent 50 mM Na acetate pH 5.0, flow rate 0.25 mL min−1). Collection of fractions (0.25 mL) commenced 20 min after injection. Elution was monitored using the uronic acid assay as described above. Note that higher fraction numbers mean lower molecular weight.
2.5. Light Microscopy and Immunolabelling
Individual fruit (1–4 for each CI combination) were selected for microscopy examination to provide examples of different combinations of absence or presence of gel formation and mealiness and different juiciness scores for a range of flesh firmnesses. For those fruit samples, immediately after phenotyping, the skin was removed, and sections of affected apricot flesh cut vertically from outer to inner cortex in the same location within the fruit to ensure tissue similarity [15]. For structural observations, 1 µm sections were stained in a 0.05% solution of Toluidine blue in benzoate buffer pH 4.4 (0.125% w/v benzoic acid, 0.145% w/v sodium benzoate in water), then air-dried and mounted in Shurmount (Triangle Biomedical Sciences, Durham, NC, USA). For immunolabelling, two antibodies to pectin epitopes were used, JIM5 and JIM7 (PlantProbes, Leeds, UK). Sections (200 nm) were wetted with phosphate-buffered saline with 0.1% Tween 80 (PBS-T) for 10 min, then incubated with 0.1% bovine serum albumin c (BSA-c) (Aurion, Wageningen, Netherlands) in PBS-T to block non-specific labelling (15 min), followed by incubation with primary antibody (dilution 1:20 v/v in 0.1% BSA-c in PBS-T) overnight at 4 °C in a moist chamber. Slides were then washed in PBS-T and incubated for 2 h in the dark at room temperature with goat anti-rat IgG AlexaFluor® 488 (Molecular Probes, Eugene, OR, USA) diluted 1: 600 (v/v) in PBS. Sections were washed in ultrapure water, dried at room temperature, and coverslip-mounted onto the slide using anti-fade agent Citifluor (Citifluor, London, UK) [16]. Sections were viewed using an Olympus Vanox AHBT3 microscope (Olympus Optical Co Ltd., Tokyo, Japan) using an interference blue filter set for observation of the fluorescence of the antibody labelled material. Images were collected with a Photometrics CoolSnap digital camera (Roper Scientific Ltd., Tucson, AZ, USA). Images were further processed using Adobe Photoshop Version 6.0.
2.6. Statistical Analysis
Statistical analyses were carried out using Student’s unpaired t-test to compare the means at p < 0.05 of samples with and without chilling injuries at similar firmness.
3. Results
3.1. Pectin Metabolism Is Different between Apricots with and without Chilling Injury (CI) Symptoms of Mealiness, Gel Formation, and Rubberiness
Uronic acid (UA) contents of extracts and molecular weight profiles of pectin extracted from apricots that had ripened normally at room temperature (20 °C) straight from harvest and from apricots that developed chilling injuries at subsequent ripening at 20 °C after cold storage at 0 °C were evaluated (Table 1).
In normally ripening fruit at 20 °C as well as in chilling-injured fruit, the UA content of WSP in the cell wall roughly tripled from harvest to the over-soft stage at 5 N (Figure 1A), therefore showing considerable pectin solubilisation from the cell wall. In the other cell wall extracts, the UA content either decreased overall or remained similar during ripening in fruit at similar firmness, chilling-injured or not (Figure 1B–D). Except in the WSP, the UA content was generally higher in chilling-injured fruit over the softening period than in fruit that ripened at 20 °C straight from harvest.
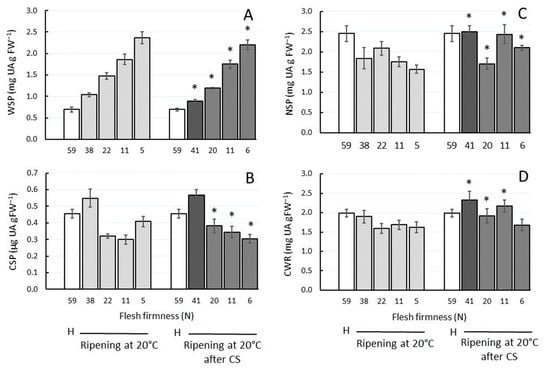
Figure 1.
Uronic acid (UA) content of cell wall extracts of ‘CluthaGold’ apricot fruit during ripening at 20 °C and during cold storage (CS) at 0 °C for 5 weeks (dark grey bars, flesh firmness 41 N) and subsequent ripening at 20 °C (light grey bars) on fresh weight (FW) basis. H = harvest. (A) water-soluble pectin (WSP), (B) CDTA-soluble pectin (CSP), (C) Na2CO3-soluble pectin (NSP), (D) cell wall residue (CWR). Note the different scales. Data are means of three analyses with three technical replicates each ± SD. (*) represents statistical significance (p value < 0.05) between ripening at 20 °C and ripening at 20 °C after CS at similar firmness (38 N vs. 41 N; 22 N vs. 20 N; 11 N vs. 11 N = eating-soft; 5 N vs. 6 N = over-soft) within the same cell wall extract.
The MW distribution of WSP in 20 °C ripened fruit gradually increased in size from harvest until fruit were eating-soft (11 N) and decreased again when fruit became over-soft (5 N) to a size distribution similar to that at harvest (Figure 2A). The MW profiles of WSP from chilling-injured fruit, however, did not show such an increase while softening (Figure 2B). This shows that the solubilisation of increasingly large pectin molecules occurs during normal ripening, and that this is prevented during CI development. Additionally, the slightly rubbery fruit at 41 N that had just been taken out of cold storage showed a smaller pectin population between fractions 25 and 35 that was not present in harvest fruit or in fruit that ripened without cold storage to a similar firmness of 38 N B and Figure S2A). At the over-soft stage (6 N), MW profiles were similar between fruit with and without chilling injuries (Figure 2B and Figure S2C).
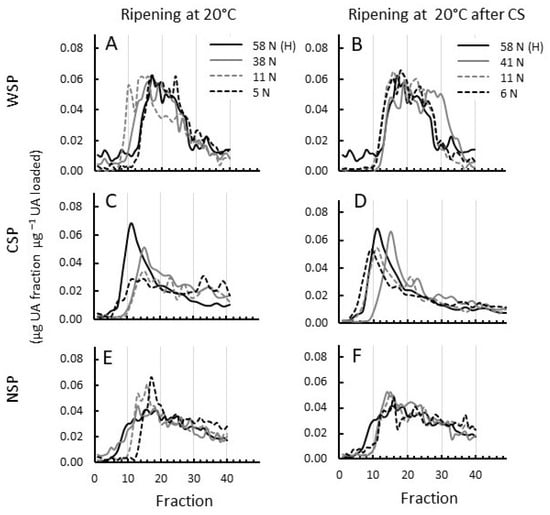
Figure 2.
Molecular weight distribution of cell wall pectin fractions extracted from apricots ripened at 20 °C with and without prior cold storage. (A,B) water-soluble pectin (WSP), (C,D) CDTA-soluble pectin (CSP), and (E,F) Na2CO3-soluble pectin (NSP) for ‘CluthaGold’ apricots during ripening at 20 °C straight from harvest (A,C,E) and during cold storage (CS) at 0 °C for 5 weeks and subsequent ripening at 20 °C (B,D,F). Extracts were eluted through a Superose™ 6 10/300 column (eluent 50 mM Na acetate pH 5.0, flow rate 0.25 mL min−1). Collection of fractions (0.25 mL) commenced 20 min after injection. UA content of each fraction was determined using galacturonic acid as standard. Results are presented in μg UA per fraction per μg total UA content loaded. A typical elution profile of 2–3 separations per extract is shown.
The MW distribution of CSP, representing pectin largely derived from the middle lamellae, dropped considerably while fruit ripened to 38 and 41 N, respectively, regardless of cold storage or ripening at 20 °C straight from harvest. In normally ripening fruit, the MW distribution continues to decrease (Figure 2C and Figure S2B,C), whereas in coldstored fruit, the MW distribution of CSP is increasing again while CI symptoms like gel, mealiness, and rubberiness further develop (Figure 2D and Figure S2E,F).
The MW distribution of NSP decreased in both normally ripening fruit and in those developing CI symptoms and was quite similar over the ripening period (Figure 2E,F).
Figure S2 shows a direct comparison between MW profiles of pectins from fruit ripened with and without CI at similar firmness.
3.2. Changes in Extent of Cell Separation and Cell Shape in Fruit Ripened at 20 °C and after Cold Storage
In firm ‘CluthaGold’ fruit at harvest, mesocarp cells had irregular shapes, with distinctly stained and undulating cell walls, considerable cell-cell separation, and relatively large intercellular spaces at tricellular junctions. There was also staining in the region between separated cell walls (Figure 3A). After softening to 5 N at 20 °C, staining of the cell walls with toluidine blue was less intense, indicating loss of cell wall material during softening. Whereas cell wall swelling was comparable to that at the harvest stage, the extent of cell-cell separation seemed reduced, perhaps due to increased osmotic pressure due to sugar accumulation within the cells (Figure 3B). After ripening subsequent to cold storage, cells in maximum chilling-injured fruit at eating firmness (5–15 N) had developed more irregular shapes with some apparent collapse, and the extent of both intercellular spaces and cell-cell separation had increased, although some cells did remain in close contact (Figure 3C). Cell wall staining appeared comparable to that of fruit ripened at 20 °C and there seemed to be no appreciable cell wall swelling (Figure 3A,C). Cells from tissue with gel symptoms had a similar appearance as cells at the harvest stage although they were eating soft (Figure 3A,D), whereas cells from tissue with gel and mealy CI symptoms were of the appearance of cells from tissue without CI with a slight increase in cell separation and an increase in intercellular space (Figure 3B,E). Mesocarp cells from firm, rubbery, gel fruit after 5 weeks cold storage showed strong and sharp cell wall staining. Notable is the pectin staining of intercellular spaces (Figure 3F).
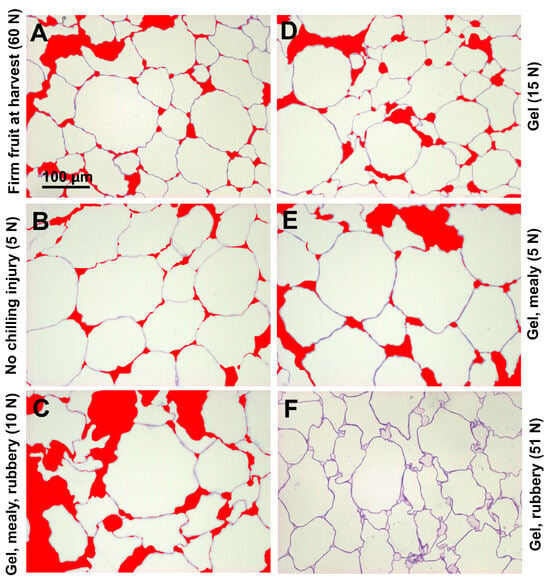
Figure 3.
Structural observations of apricot mesocarp cells of ‘CluthaGold’ with and without chilling injury symptoms and combinations thereof. Apricot mesocarp cells at harvest (A); after ripening at 20 °C straight from harvest to an over-soft stage with flesh firmness of 5 N (B); after cold storage at 0 °C and ripening at 20 °C to an eating-soft stage with gel, mealy, and rubbery symptoms (C); eating-soft firmness of 15 N with gel symptoms but not mealy or rubbery (D); over-soft fruit with gel and mealy symptoms but not rubbery (E); and fruit straight out of cold storage with gel and rubbery symptoms while still very firm (F). All images are reproduced to the same magnification. Note that the firmnesses indicated in the panels are from individual fruit whereas the firmnesses in Table 1 are an average from bulk samples for cell wall analyses. Cell-cell separation and intercellular spaces are marked in red except (F) to not obscure the stained intercellular spaces. Figure S3 shows the original images of mesocarp cells of ‘CluthaGold’.
3.3. Intercellular Spaces and Cell Lumen of Eating-Soft Chilling-Injured Apricots Were Filled with Pectin, Similar to Fruit at Harvest
The monoclonal antibodies JIM5 (specific for non- or low methyl-esterified pectin) and JIM7 (specific for highly esterified pectin) both gave intense labelling and unlike toluidine blue staining, the intensity did not change during softening (Figure 4). In the at-harvest samples, both antibodies labelled middle lamella regions as well as tricellular junctions strongly. Labelling also showed that intercellular spaces were filled with pectin with varying degrees of esterification recognized by both antibodies (Figure 4A,B). Intercellular spaces in fruit are usually gas-filled but as fruit tissue softens, the intercellular space can become flooded with fluid either from within the cells or with apoplastic fluid, including pectin that has been solubilised from the cell wall. In cell walls of fruit that ripened at 20 °C after harvest, this pectin staining within the intercellular spaces eventually disappeared. Both antibodies still labelled cell walls strongly at middle lamellae regions and possibly beyond, exposing areas of cell separation (Figure 4C,D). In soft fruit with CI symptoms of mealiness, rubberiness, and gel formation, both antibodies labelled the cell walls as well as pectin material between separating cell walls and in intercellular spaces. There was also grainy pectin labelling in the cell lumen (Figure 4E,F). Although cell wall labelling with JIM5 showed a similar intensity and sharpness to material that ripened to eating firmness, labelling with JIM7 appeared more intense. This was possibly caused by labelling the cell wall to a greater extent, perhaps indicating some cell wall swelling. Whereas in fruit without CI, pectin-filled intercellular spaces disappeared during ripening (Figure 4C,D), they remained in fruit with CI (Figure 4E,F).
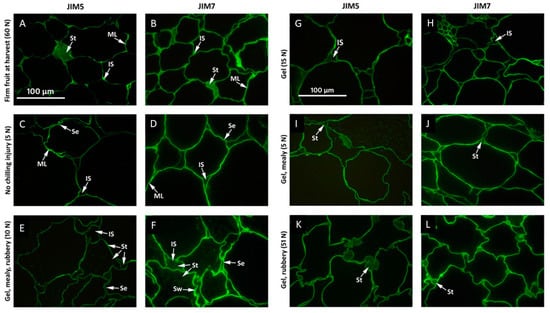
Figure 4.
Immunofluorescence labelling of ‘CluthaGold’ apricot mesocarp cells with and without chilling injury symptoms and combinations thereof using monoclonal antibodies JIM5 and JIM7, specific for low-esterified or high-esterified pectin, respectively. Immunofluorescence labelling with antibody JIM5 (A,C,E,G,I,K) or JIM7 (B,D,F,H,J,L) in ‘CluthaGold’ apricot mesocarp cell walls from fruit at harvest (A,B); ripened at 20 °C straight from harvest to an over-soft flesh firmness of 5 N (C,D); ripened at 20 °C to an eating-soft flesh firmness of 10 N after cold storage at 0 °C with high gel, mealy, and rubberiness scores (E,F); ripened at 20 °C after cold storage at 0 °C for 5 weeks to an eating soft stage of 15 N with high gel score but no mealiness or rubberiness (G,H); ripened at 20 °C after cold storage at 0 °C for 5 weeks to an over-soft stage of 5 N with high gel and mealy scores but no rubberiness (I,J); fruit straight out of cold storage with gel and rubbery symptoms while still very firm (K,L). Flesh firmness in Newton (N) and chilling injury symptoms for each fruit is indicated in each panel. All images are reproduced to the same magnification. Abbreviations: IS, intercellular spaces; ML, middle lamella; Se, cell-cell separation; St, pectin staining of intercellular spaces, Sw, cell lumen and between separating cell walls. Note that the firmness indicated in panels is from an individual fruit whereas firmnesses in Table 1 are an average from bulk samples for cell wall analyses.
3.4. Fruit with Combinations of CI Symptoms Had Different Pectin Localisation, Content, and Molecular Weight Distribution
To identify patterns in cell wall changes for fruit that have particular CI combinations, comparisons were made between fruit that had only gel formation, or gel formation and rubberiness, or all three CI symptoms (gel formation, rubberiness, and mealiness). For this comparison, the results of structural, immunological, and biochemical investigations were drawn together. Fruit that ripened without cold storage, and apart from a very low rubbery score had no CI symptoms, were included. As there are differences in pectin changes during softening, only fruit that had approximately the same firmness (eating-ripe between 5 and 15 N) were compared (Table 1).
After staining with toluidine blue, tissue of fruit with only gel formation (Figure 3D), or with both gel formation and mealiness (Figure 3E), looked very similar to tissue from fruit that had all three CI symptoms (Figure 3C), although irregular cell shapes, cell-cell separation, and large intercellular spaces seemed not quite as extensive in fruit with CI combinations. Fruit that developed gel formation and rubberiness already when still quite firm (51 N) during cold storage showed quite different cell characteristics (Figure 3F). Cells had irregular shapes similar to eating-soft maximum chilling-injured samples (Figure 3C), but cell-cell separation was not extensive and resembled that of fruit at harvest (Figure 3A). There was distinct staining of intercellular spaces and regions between separated cell walls which was seen at a much lower intensity in fruit at harvest.
Immunolocalisation using JIM5 and JIM7 antibodies showed that in fruit with only gel symptoms, no pectin staining could be detected in the cell lumen or intercellular spaces at tricellular junctions with either antibody (Figure 4G,H), but staining could be observed in fruit that showed gel and mealiness, especially in cell corners (Figure 4I,J) and was most intense in fruit with all three CI symptoms (Figure 4E,F). In fruit with only gel symptoms, labelling of cell walls with JIM7 appeared less intense than in fruit with gel and mealiness (Figure 4J) or with all CI symptoms (Figure 4F), and represented more the intensity of JIM7 labelling of an eating-soft fruit without CI (Figure 4D). Firm fruit (51 N) with gel formation and rubberiness that developed during cold storage showed characteristics of fruit at harvest as well as eating-soft fruit using either antibody (Figure 4K,L). Pectin-filled tricellular corners and little cell-cell separation resembled fruit at harvest (Figure 4A,B), whereas the irregular cell shapes and lack of undulating cell walls were similar to fruit with CI symptoms.
The UA content of WSP of fruit with all three CI symptoms was similar to control fruit without CI, whereas in fruit that had rubberiness and gel, or only gel formation, the UA content was significantly less (Figure 5A). The MW profiles showed that WSP of fruit with all three chilling injuries as well as rubbery fruit with gel formation was more degraded compared with fruit with only gel or fruit without CI (Figure 5B).
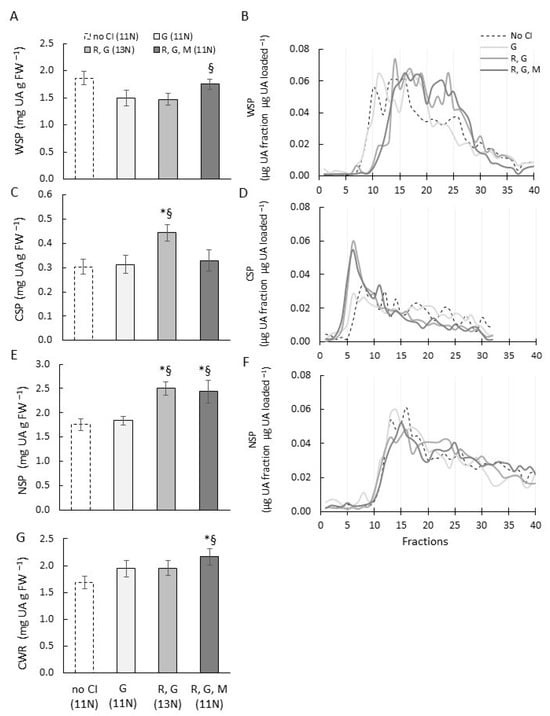
Figure 5.
Pectin (uronic acid) content and molecular weight distribution of pectin fractions extracted from cell walls of ‘CluthaGold’ apricots with combinations of CI symptoms and without CI. Comparison of uronic acid (UA) content (A,C,E,G) and molecular weight distribution (B,D,F) of cell wall fractions of ‘CluthaGold’ apricot fruit with mealiness, gel, and/or rubberiness after cold storage and subsequent ripening at 20 °C, and control fruit ripened at 20 °C without chilling injury (CI). (A) Water-soluble pectin (WSP); (B) chelator-soluble pectin (CSP); (C) Na2CO3-soluble pectin (NSP); (D) insoluble cell wall residue (CWR). X axis indicates chilling injury symptoms (G, gel; M, mealy; R, rubbery; no CI, no chilling injury) and associated firmnesses of bulk samples. UA data are means of three replicate samples measured in triplicate (n = 3 ± SD). (*) indicates statistical significance between fruit with chilling injury and control fruit without chilling injury, and (§) between fruit with chilling injury symptoms (p value < 0.05). For molecular weight profiles, extracts were separated on a Superose™ 6 10/300 column (eluent 50 mM Na acetate pH 5.0, flow rate 0.25 mL min−1). Collection of fractions (0.25 mL) commenced 20 min after injection. UA content of each fraction was determined using galacturonic acid as standard and results presented as μg UA per fraction per μg total UA content loaded. A typical elution profile of 2–3 separations is shown.
The CSP in fruit that were rubbery and had gel contained significantly more UA than fruit that had no CI, or only gel, or all three chilling injuries. The UA content of CSP in these fruit was similar to each other (Figure 5C). Fruit with gel and rubberiness and fruit with all three CI symptoms showed high molecular weight populations (eluting approximately between fractions 3 to 7) which were not evident to this extent in fruit without CI and in fruit that had only gel formation (Figure 5D).
The UA content in NSP of fruit that had gel formation and were rubbery, as well as fruit that had all three CI symptoms, was significantly higher than the UA content of fruit with only gel formation and control fruit (Figure 5E). NSP had very similar MW elution profiles across fruit with CI combinations and without CI (Figure 5F). The UA content in the insoluble cell wall residue of fruit that had all three chilling injuries was significantly higher than in fruit with gel, or with gel and rubberiness symptoms, or in control fruit without chilling injuries (Figure 5G).
4. Discussion
Sensory juiciness in apples and nectarines is associated with the breaking open of cell walls to release the cellular juice [17,18]. In fruit that ripen to a soft-melting texture such as peach, this is achieved by changes to pectin and hemicellulose solubility and structure, which results in loosening and weakening of the cell wall, as well as changes in cell adhesion mediated by changes in middle lamellae pectin [6]. Cell wall changes in ‘CluthaGold’ apricots without CI followed a pattern that was consistent with those in other fruit that ripen to a soft-melting texture. More, and increasingly larger, pectin molecules were becoming water-soluble and were hence removed from the cell wall network, aiding fruit softening, as this left the cell wall progressively weaker (Figure 1A and Figure 2A). This is also indicated by less intense staining of eating-soft cell walls with toluidine blue compared with cell walls at harvest (Figure 3A,B and Figure S3A,B). The presence of intercellular spaces and some cell-to-cell separation also matches previous observations of the tissue structure of normally ripened apricots [19]. In apricots that developed gel, mealiness, and rubberiness during softening, UA content in NSP and in the CWR was higher compared with fruit that ripened without CI (Figure 5E,G). WSP did not increase in size as much as in fruit that softened without cold storage, leaving pectin with higher MWs in the cell wall. As a consequence, restructuring and weakening of the cell wall for juice release when chewed might not be as pronounced as in normally ripened fruit. Hence, rubberiness could be linked to pectin that is bound tightly to the cell wall (NSP, pectin in CWR) (Figure 5G), rendering the cell wall and intercellular connections stronger and giving the sensation of rubberiness.
Leatheriness in peaches is very similar to rubberiness in apricots after cold storage, but occurred when fruits were firmer (>30 N). Enzyme activities associated with cell wall degradation and ethylene production were lower, whilst the insoluble pectin content was higher in leathery peach fruit than in juicy or mealy fruit [20]. Peaches harvested at a less mature stage were more prone to leatheriness, an observation also noted for apricots [12]. Leathery peaches had very deformed mesocarp cells and significant staining of the intercellular matrix, presumably pectin [21], which were similar to those seen in the rubbery apricots in this study (Figure 3F). However, the greater cell wall thickening in leathery peaches compared with juicy or mealy fruit was not seen in rubbery apricots. Leathery peaches showed less depolymerisation within the CSP fraction than mealy peaches [22]. For apricots, however, there was no difference in depolymerisation of CSP between rubbery fruit on removal from cold storage (41 N) and juicy fruit at a similar firmness (Figure 2C,D and Figure S2A), but differences were evident for mean molecular weight of WSP (Figure 2A,B and Figure S2D).
Adhesion between cells might be expected to influence the way fruit tissue breaks when chewed by determining whether (1) the fruit tissue breaks into small fragments containing undamaged cells leaving a dry sensation in the mouth, or (2) individual cells rupture when crushed, leaving a pulp of connecting cell wall material and a moist sensation in the mouth [18]. Chemistry and localisation of pectin in the cell wall, especially in middle lamellae and intercellular junctions, might therefore determine tissue breakage and juice release. It is likely that in apricots without CI, the weakened cell wall, as well as degradation and disappearance of pectin in intercellular junctions, aided in tissue breakage and juice release, resulting in a fruit that was perceived as juicy. However, in apricots showing mealiness, gel, and rubberiness, these changes did not take place. Although cell separation was more pronounced, middle lamella-related CSP did not disappear from cell junctions, and degradation of this pectin was also not noticeable (Figure 4 and Figure 5). It is likely that cells either do not break open in these chilling-injured fruit because cell walls remain too strong, or that cells break open but the amount of pectin present in cell wall and cell lumen will bind juices released, resulting in a fruit that is perceived as dry. This was also suggested for nectarine and peach [23]. Peach mealiness, or woolliness, was found to be caused by cell fluids forming calcium-pectate gel complexes in the middle lamella, as a result of de-esterification accompanied by a lack of depolymerisation [24].
Solubilisation and depolymerisation patterns of pectins differed between apricot and peach during ripening. Apricot showed a slow increase in the proportion of short length CSPs in apricots with prolonged 0 °C storage [9]. In peach, depolymerisation was not seen until fruit were at a firmness of approximately 20 N [6]. There was little change in depolymerisation of NSP until ‘Clutha Gold’ apricots reached a firmness of approximately 10 N (Figure 2E), whereas peach showed no change in MW [6]. WSP in apricot increased in molecular weight until the over-soft stage while ripening at 20 °C (Figure 2A), whereas in peach a peak of high MW WSPs remained until fruit became ripe, at which time there was rapid depolymerisation [7]. These changes are consistent with the different textures of ripe peaches (high amounts of melting and juice) and apricots (moderate melting and low juice).
Symptoms of mealiness and gel formation were absent or very minor for ‘Clutha Gold’ apricots immediately after they were removed from cold storage at 0 °C after 5 weeks, even though they could subsequently only ripen further with the development of CI (Table 1). However, there were significant differences between the cell wall characteristics of these apricots compared with fruit that ripened at 20 °C straight from harvest to similar firmness (41 and 38 N, respectively). The lower UA content of the WSP extract (Figure 1A), the accompanying higher UA content in the insoluble cell wall residue (Figure 1B), the absent increase in molecular weight of WSP characteristics for apricots ripening at 20 °C to similar firmness (Figure 2B), as well as the UA content of NSP remaining similar to that of fruit at harvest instead of decreasing (Figure 1C), all indicate that ‘normal’ softening events were already altered during softening in cold storage and were not resumed after taking fruit out of cold storage. In tomato, although not a stonefruit, it has been shown that most cell wall enzymes do not recover expression after cold storage when returned to shelf temperature of 20 °C [25].
Mealy fruit could be differentiated from non-mealy fruit for peach and plum by examining the MW profiles of the various pectin fractions [22,26]. In both fruits, there was reduced depolymerisation of CSP (peach) or WSP (plums) for mealy fruit compared with non-mealy fruit. Also in our study, different molecular weight patterns were observed between fruit with and without chilling injuries. ‘Clutha Gold’ apricots that were mealy, rubbery, and had gel showed no increase in MW distribution of WSP (Figure 2B) and no decrease in MW distribution of CSP except straight after removal from cold storage (Figure 2D and Figure S2D), compared with fruit that ripened normally without CI. There were also differences in pectin MW distribution in apricots with combinations of CI symptoms. Apricots that showed only gel formation had WSP of larger MW distribution (Figure 5B) and CSP that looked more degraded than fruit with any other combination of CI symptoms (Figure 5D).
Mealiness (wooliness) in nectarine and peach has been associated with the inhibition of ethylene and aroma-related volatiles [27,28,29]. This contrasts with many other fruits such as kiwifruit, lemons, and Japanese plums in which the development of chilling injury after removal from cold storage has been accompanied by an increase in ethylene production and respiration [30,31,32]. It has been suggested that the different patterns are due to the type of chilling injury, being pitting and water-soaking symptoms in kiwifruit and lemons, whereas mealiness is an internal injury in nectarines and peaches [27]. However, the chilling injury symptoms in Japanese plum were internal, yet ethylene production increased after removal from cold storage [32]. Another experiment on peaches found an initial burst of ethylene occurred within 24 h of removal from cold storage after which ethylene production dropped dramatically [33]. The development of mealiness in peaches and nectarines has also been associated with the downregulation of genes associated with the ethylene and the cell wall disassembly pathways and associated enzymes for peaches, compared with cold-stored fruit that received intermittent warming during storage, which subsequently did not show mealiness symptoms [24,28].
Modified storage regimes such as near-freezing temperature are reducing CI in apricots considerably by affecting ethylene production [4,5,34] which subsequently leads to the inhibition of cell wall modifying enzymes and cell wall-related genes. The loss of side chains was the main modification in CDTA-soluble pectin in ‘Shushanggan’ apricots during normal storage and made the main contribution to the softening of apricots, while the loss of side chains was suppressed by near-freezing temperature storage [4]. During storage of ‘Xiaobai’ apricots at this ultra-low temperature, reduced membrane lipid peroxidation was found to be effectively mitigating the increase in malondialdehyde content and membrane permeability of apricots and maintaining the stability of cell membranes [5]. Near-freezing temperature storage also contributed to the inhibition of cell wall enzymes, thus delaying the damage of cell structure and function caused by cell wall polysaccharides depolymerisation in this apricot cultivar. In nectarines and peaches, ethylene has a role in the prevention of CI [27], whereas ethylene increases CI in plums [32]. Apricots treated with putrescine or spermidine prior to near-freezing storage had less ethylene production, were firmer, and had less CI and longer shelf life than control fruit after removal from cold storage [35]. Both maturity at harvest and cultivar also affected the development of CI after cold storage [1,36]. ‘Larclyd’ apricots exhibit extremely low ethylene production rates during ripening compared with ‘CluthaGold’ (Stanley, pers. com.), a characteristic that has been associated with longer storage potential and may suggest it has suppressed climacteric characteristics similar to those found in some plum cultivars [36]. ‘CluthaGold’ softened more rapidly at 0 °C and developed more mealiness than ‘Larclyd’ [1], which also suggests that cultivars that have higher ethylene production will be more prone to CI. It would be interesting to know how quickly ethylene production develops after removal from cold storage and whether the conversion of 1-aminocyclopropane-1-carboxylic acid to ethylene is suppressed in some apricot cultivars. Since ethylene is essential for the correct sequence of cell wall degradation that is required for the normal ripening process [24,27], it seems likely that ethylene production may be suppressed, at least for a period of time, after removal from cold storage in some circumstances.
Modified storage regimes, such as near-freezing temperature which affects ethylene production [4,5], calcium treatment [37], polyamine application [34], or intermittent warming [28], may provide short-term strategies for lengthening the storage life of apricots, whilst breeding new cultivars that have the ability to enable ethylene production to be up-regulated through exogenous ethylene supply could be a longer-term solution.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/horticulturae9101115/s1: Figure S1. Apricot with gel formation after cold storage. As more than 50% of the fruit has a glassy appearance, the gel score would be 3 (with 0 being no gel and 3 being >50% gel). Figure S2. Comparison of molecular weight profiles of pectin fractions extracted from CluthaGold apricots at similar firmness while softening at 20 °C straight from harvest or at 20 °C after removal from cold storage. Figure S3. Unmarked images of the mesocarp cells of ‘CluthaGold’ apricots at the same time points as presented in Figure 3.
Author Contributions
Conceptualisation, C.J.S.; methodology, C.J.S., C.S., I.C.H. and R.S.; formal analysis, investigation, data curation, C.J.S., C.S., I.C.H. and R.S.; supervision, I.C.H. and R.S.; writing—original draft preparation, C.J.S. and R.S.; writing—review and editing, C.J.S., I.C.H. and R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Business Innovation and Employment (Contract number C06X0806), Plant & Food Research, and Summerfruit New Zealand.
Data Availability Statement
Data are contained within this manuscript and as Supplementary Material Figures S2 and S3.
Acknowledgments
Thanks to Roneel Prakash for technical assistance and to Mark Wohlers who advised on statistical analyses. Our special gratitude goes to David Brummell for his advice, discussions, and encouragement during the writing of this manuscript. We are also indebted to David Brummell and Ross Atkinson who both critically reviewed the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stanley, J.; Prakash, R.; Marshall, R.; Schröder, R. Effect of harvest maturity and cold storage on correlations between fruit properties during ripening of apricot (Prunus armeniaca). Postharvest Biol. Technol. 2013, 82, 39–50. [Google Scholar] [CrossRef]
- Manolopoulou, H.; Mallidis, C. Storage and processing of apricots. Acta Hortic. 1999, 488, 567–576. [Google Scholar] [CrossRef]
- Stanley, J.; Marshall, R.; Ogwaro, J.; Feng, R.; Wohlers, M.; Woolf, A.B. Postharvest storage temperatures impact significantly on apricot fruit quality. Acta Hortic. 2010, 880, 525–532. [Google Scholar] [CrossRef]
- Fan, X.; Jiang, W.; Gong, H.; Yang, Y.; Zhang, A.; Liu, H.; Caob, J.; Guod, F.; Cuie, K. Cell wall polysaccharides degradation and ultrastructure modification of apricot during storage at a near freezing temperature. Food Chem. 2019, 300, 125194. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Y.; Zhang, Z.; He, H.; Shi, L.; Zhu, X.; Cui, K. Near-freezing temperature storage improves shelf-life and suppresses chilling injury in postharvest apricot fruit (Prunus armeniaca L.) by regulating cell wall metabolism. Food Chem. 2022, 387, 132921. [Google Scholar] [CrossRef]
- Brummell, D.A.; Dal Cin, V.; Crisosto, C.H.; Labavitch, J.M. Cell wall metabolism during maturation, ripening and senescence of peach fruit. J. Exp. Bot. 2004, 55, 2029–2039. [Google Scholar] [CrossRef]
- Muramatsu, N.; Tanaka, K.; Asakura, T.; Haji, T. Changes in cell wall polysaccharides and physical properties of peach (Prunus persica Batsch) fruit during ripening. J. Jpn. Soc. Hortic. Sci. 2004, 73, 534–540. [Google Scholar] [CrossRef]
- Fruk, G.; Cmelika, Z.; Jemrica, T.; Hribarb, J.; Vidrihb, R. Pectin role in woolliness development in peaches and nectarines: A review. Sci. Hortic. 2014, 180, 1–5. [Google Scholar] [CrossRef]
- Liu, H.; Chen, F.S.; Yang, H.S.; Yao, Y.Z.; Gong, X.Z.; Xin, Y.; Ding, C.H. Effect of calcium treatment on nanostructure of chelate-soluble pectin and physicochemical and textural properties of apricot fruits. Food Res. Int. 2009, 42, 1131–1140. [Google Scholar] [CrossRef]
- Tsuchida, Y.; Yakushiji, H.; Oe, T.; Negoro, K.; Gato, N.; Kotani, T.; Onishi, Y.; Kobata, T.; Tamura, M. Differences in cell-wall polysaccharide degradation during softening process in two cultivars of Japanese apricot fruits. J. Jpn. Soc. Hortic. Sci. 2014, 83, 81–89. [Google Scholar] [CrossRef]
- Stanley, J.; Feng, J.; Olsson, S. Crop load and harvest maturity effects on consumer preferences for apricots. J. Sci. Food Agric. 2014, 95, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J. Control of Pre-Harvest and Postharvest Factors to Improve Fruit Quality of Apricot. Ph.D. Thesis, School of Natural Sciences, Griffith University, Brisbane, Australia, 2015. [Google Scholar]
- Schröder, R.; Nicolas, P.; Vincent, S.J.F.; Fischer, M.; Reymond, S.; Redgwell, R.J. Purification and characterisation of a galactoglucomannan from kiwifruit (Actinidia deliciosa). Carbohydr. Res. 2001, 331, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M.; Falourd, X.; Quemener, B.; Devaux, M.F.; Audergon, J.M. Histological and cell wall polysaccharide chemical variability among apricot varieties. LWT Food Sci. Technol. 2014, 58, 486–496. [Google Scholar] [CrossRef]
- Sutherland, P.; Hallett, I.; Jones, M. Probing cell wall structure and development by the use of antibodies: A personal perspective. N. Z. J. For. Sci. 2009, 39, 197–205. [Google Scholar]
- Harker, F.R.; Hallett, I.C. Physiological-changes associated with development of mealiness of apple fruit during cool storage. HortScience 1992, 27, 1291–1294. [Google Scholar] [CrossRef]
- Harker, F.R.; Sutherland, P.W. Physiological changes associated with fruit ripening and the development of mealy texture during storage of nectarines. Postharvest Biol. Technol. 1993, 2, 269–277. [Google Scholar] [CrossRef]
- Kovacs, E.; Meresz, P.; Kristof, Z.; Nemeth-Szerdahelyi, E. Ripening and microstructure of apricot (Prunus armeniaca L.). Acta Aliment. 2008, 37, 23–39. [Google Scholar] [CrossRef]
- Ju, Z.G.; Duan, J.S.; Ju, Z.Q. Leatheriness and mealiness of peaches in relation to fruit maturity and storage temperature. J. Hortic. Sci. 2000, 75, 86–91. [Google Scholar] [CrossRef]
- Luza, J.G.; Vangorsel, R.; Polito, V.S.; Kader, A.A. Chilling injury in peaches—A cytochemical and ultrastructural cell-wall study. J. Am. Soc. Hortic. Sci. 1992, 117, 114–118. [Google Scholar] [CrossRef]
- Brummell, D.A.; Dal Cin, V.; Lurie, S.; Crisosto, C.H.; Labavitch, J.M. Cell wall metabolism during the development of chilling injury in cold-stored peach fruit: Association of mealiness with arrested disassembly of cell wall pectins. J. Exp. Bot. 2004, 55, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Harker, R.F.; Maindonald, J.H. Ripening of nectarine fruit. Plant Physiol. 1994, 106, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Lurie, S.; Crisosto, C.H. Chilling injury in peach and nectarine. Postharvest Biol. Technol. 2005, 37, 195–208. [Google Scholar] [CrossRef]
- Hunter, D.A.; Napier, N.J.; Erridge, Z.A.; Saei, A.; Chen, R.K.Y.; McKenzie, M.J.; O’Donoghue, E.M.; Hunt, M.; Favre, L.; Lill, R.E.; et al. Transcriptome responses of ripe cherry tomato fruit exposed to chilling and rewarming identify reversible and irreversible gene expression changes. Front. Plant Sci. 2021, 12, 685416. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Vicente, A.R.; Crisosto, C.H.; Labavitch, J.M. Cell wall modifications in chilling-injured plum fruit (Prunus salicina). Postharvest Biol. Technol. 2008, 48, 77–83. [Google Scholar] [CrossRef]
- Zhou, H.W.; Dong, L.; Ben-Arie, R.; Lurie, S. The role of ethylene in the prevention of chilling injury in nectarines. J. Plant Physiol. 2001, 158, 55–61. [Google Scholar] [CrossRef]
- Zhou, H.W.; Lurie, S.; Ben-Arie, R.; Dong, L.; Burd, S.; Weksler, A.; Lers, A. Intermittent warming of peaches reduces chilling injury by enhancing ethylene production and enzymes mediated by ethylene. J. Hortic. Sci. Biotechnol. 2001, 76, 620–628. [Google Scholar]
- Zhang, B.; Xi, W.; Wei, W.; Shen, J.; Ferguson, I.; Chen, K. Changes in aroma-related volatiles and gene expression during low temperature storage and subsequent shelf-life of peach fruit. Postharvest Biol. Technol. 2011, 60, 7–16. [Google Scholar] [CrossRef]
- Leguizamon, G.; Luchsinger, L.; Razeto, B. Water loss and ethylene as non-destructive symptoms of chilling injury in ‘Eureka’ lemons. Acta Hortic. 2001, 553, 297–298. [Google Scholar] [CrossRef]
- Feng, J.; Maguire, K.; MacKay, B.R. Factors affecting ethylene production of Hayward kiwifruit. Acta Hortic. 2003, 610, 203–209. [Google Scholar] [CrossRef]
- Khan, A.S.; Ahmed, M.J.; Singh, Z. Increased ethylene biosynthesis elevates incidence of chilling injury in cold-stored ‘Amber Jewel’ Japanese plum (Prunus salicina Lindl.) during fruit ripening. Int. J. Food Sci. Technol. 2011, 46, 642–650. [Google Scholar] [CrossRef]
- Fernández-Trujillo, J.P.; Cano, A.; Artés, F. Physiological changes in peaches related to chilling injury and ripening. Postharvest Biol. Technol. 1998, 13, 109–119. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, H.; Fan, X.; Jiao, W.; Cao, J.; Jiang, W. Near freezing point temperature storage inhibits chilling injury and enhances the shelf life quality of apricots following long-time cold storage. J. Food Process. Preserv. 2019, 43, e13958. [Google Scholar] [CrossRef]
- Koushesh saba, M.; Arzani, K.; Barzegar, M. Postharvest polyamine application alleviates chilling injury and affects apricot storage ability. J. Agric. Food Chem. 2012, 60, 8947–8953. [Google Scholar] [CrossRef] [PubMed]
- Abdi, N.; McGlasson, W.B.; Holford, P.; Williams, M.; Mizrahi, Y. Responses of climacteric and suppressed-climacteric plums to treatment with propylene and 1-methylcyclopropene. Postharvest Biol. Technol. 1998, 14, 29–39. [Google Scholar] [CrossRef]
- Liu, H.; Chen, F.; Lai, S.; Tao, J.; Yang, H.; Jiao, Z. Effects of calcium treatment and low temperature storage on cell wall polysaccharide nanostructures and quality of postharvest apricot (Prunus armeniaca). Food Chem. 2017, 225, 87–97. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).