Usage of Machine Learning Algorithms for Establishing an Effective Protocol for the In Vitro Micropropagation Ability of Black Chokeberry (Aronia melanocarpa (Michx.) Elliott)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Preparation of Explant, and Explant Sterilization
2.2. Culture Conditions
2.3. Analysis of Variance
2.4. Modeling Using Machine Learning (ML) Algorithms
3. Results
3.1. Analysis of Variance for In Vitro Features
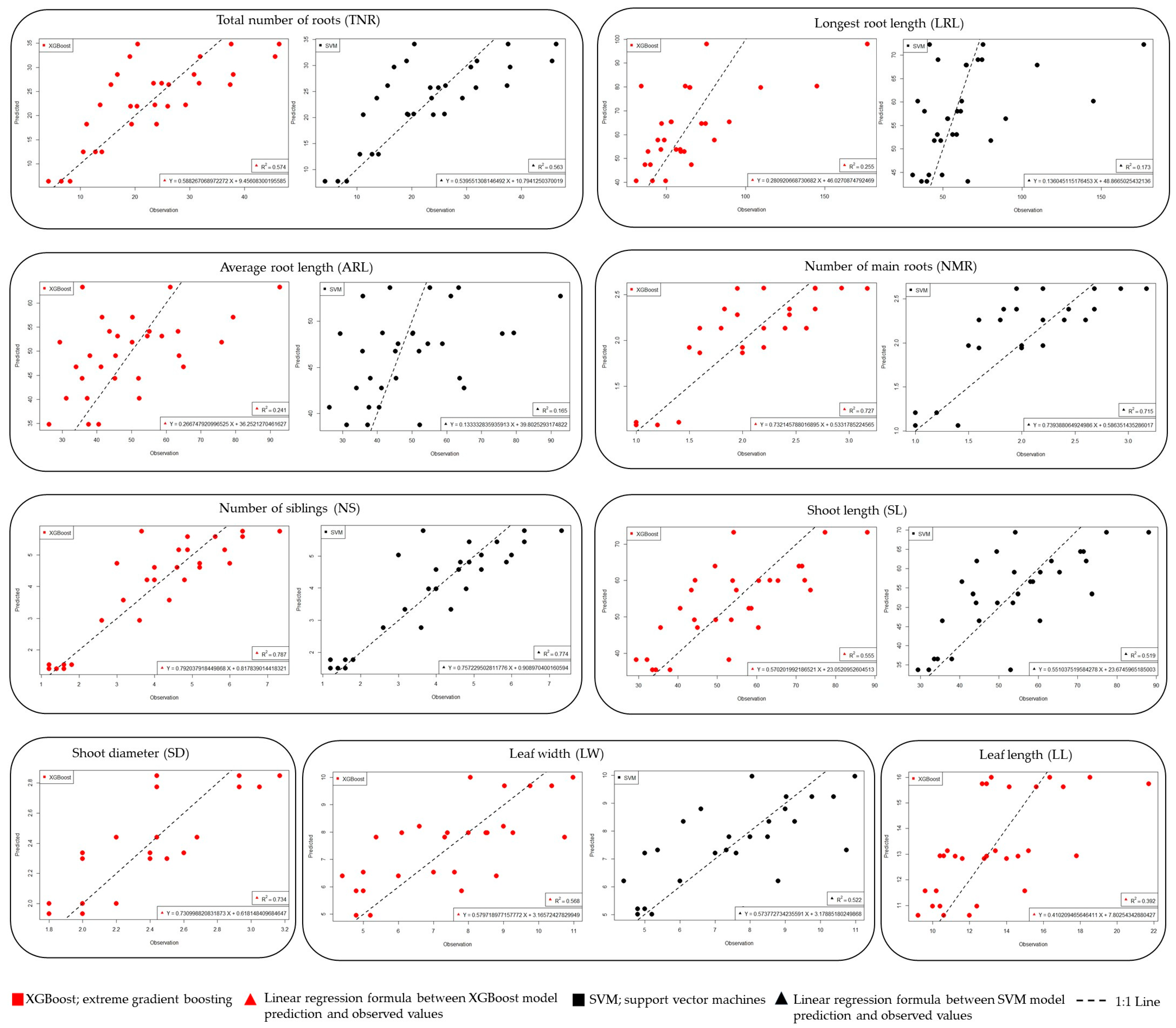
3.2. Machine Learning (ML) Analysis
4. Discussion
4.1. Evaluation of the In Vitro Micropropagation Ability of Black Chokeberry
4.2. Usage of Machine Learning Algorithms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ochmian, I.D.; Grajkowski, J.; Smolik, M. Comparison of some morphological features, quality and chemical content of four cultivars of chokeberry fruits (Aronia melanocarpa). Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 253–260. [Google Scholar] [CrossRef]
- Yilmaz, A.; Güler, E.; Soydemir, H.E.; Demirel, S.; Mollahaliloğlu, S.; Çiftçi, V.; Karadeniz, T. Miracle Plant: Black Chokeberry (Aronia melanocarpa). MAS J. Appl. Sci. 2021, 6, 83–94. [Google Scholar] [CrossRef]
- Šnebergrová, J.; Čížková, H.; Neradova, E.; Kapci, B.; Rajchl, A.; Voldřich, M. Variability of characteristic components of aronia. Czech J. Food Sci. 2014, 32, 25–30. [Google Scholar] [CrossRef]
- Jeppsson, N.; Johansson, R. Changes in fruit quality in black chokeberry (Aronia melanocarpa) during maturation. J. Hortic. Sci. Biotechnol. 2000, 75, 340–345. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Winterhalter, P. Preparation of dimeric procyanidins B1, B2, B5, and B7 from a polymeric procyanidin fraction of black chokeberry (Aronia melanocarpa). J. Agric. Food Chem. 2010, 58, 5147–5153. [Google Scholar] [CrossRef]
- Konić–Ristić, A.; Srdić–Rajić, T.; Kardum, N.Đ.; Glibetić, M. Biological activity of Aronia melanocarpa antioxidants pre–screening in an intervention study design. J. Serbian Chem. Soc. 2013, 78, 429–443. [Google Scholar] [CrossRef]
- Benvenuti, S.; Pellati, F.; Melegari, M.A.; Bertelli, D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Rubus, Ribes, and Aronia. J. Food Sci. 2004, 69, 164–169. [Google Scholar] [CrossRef]
- Hardin, J.W. The enigmatic chokeberries (Aronia, Rosaceae). Bull. Torrey Bot. Club 1973, 100, 178–184. [Google Scholar] [CrossRef]
- Ara, V. Fachthemen–Schwarzfruchtige Aronia: Gesund––und bald in aller Munde? Fluss. Obs. 2002, 69, 653–657. [Google Scholar]
- Scott, R.W.; Skirvin, R.M. Black chokeberry (Aronia melanocarpa Michx.): A semi–edible fruit with no pests. J. Am. Pomol. Soc. 2007, 61, 135. [Google Scholar]
- Gonzalez–Molina, E.; Moreno, D.A.; Garcia–Viguera, C. Aronia–enriched lemon juice: A new highly antioxidant beverage. J. Agric. Food Chem. 2008, 56, 11327–11333. [Google Scholar] [CrossRef] [PubMed]
- Stralsjö, L.; Åhlin, H.; Witthöft, C.M.; Jastrebova, J. Folate determination in Swedish berries by radioprotein–binding assay (RPBA) and high performance liquid chromatography (HPLC). Eur. Food Res. Technol. 2003, 216, 264–269. [Google Scholar] [CrossRef]
- Engin, S.P.; Mert, C. The effects of harvesting time on the physicochemical components of aronia berry. Turk. J. Agric. For. 2020, 44, 361–370. [Google Scholar] [CrossRef]
- Shahin, L.; Phaal, S.S.; Vaidya, B.N.; Brown, J.E.; Joshee, N. Aronia (Chokeberry): An underutilized, highly nutraceutical plant. J. Med. Act. Plants 2019, 8, 46–63. [Google Scholar]
- Demir, B.; Sayinci, B.; Sümbül, A.; Yaman, M.; Yildiz, E.; Çetin, N.; Karakaya, O.; Ercişli, S. Bioactive compounds and physical attributes of genotypes through multivariate approaches. Folia Hortic. 2020, 32, 189–202. [Google Scholar] [CrossRef]
- Demir, B.; Sayıncı, B.; Yaman, M.; Sümbül, A.; Yıldız, E.; Karakaya, O.; Bobuş Alkaya, G.; Ercişli, S. Biochemical composition and shape–dimensional traits of rosehip genotypes. Folia Hortic. 2021, 33, 293–308. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef]
- Andrzejewska, J.; Sadowska, K.; Kloska, L.; Rogowski, L. The effect of plant age and harvest time on the content of chosen components and antioxidative potential of black chokeberry fruit. Acta Sci. Pol. Hortorum Cultus 2015, 14, 105–114. [Google Scholar]
- Yaman, M. Evaluation of genetic diversity by morphological, biochemical and molecular markers in sour cherry genotypes. Mol. Biol. Rep. 2022, 49, 5293–5301. [Google Scholar] [CrossRef]
- Thorpe, T.A. History of plant tissue culture. Mol. Biotechnol. 2007, 37, 169–180. [Google Scholar] [CrossRef]
- Haliloğlu, K.; Türkoğlu, A.; Balpınar, Ö.; Öztürk, H.; Özkan, G.; Poczai, P. Mammalian sex hormones effects on in vitro organogenesis of common bean (Phaseolus vulgaris L.). Sci. Rep. 2023, 13, 3337. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.R.; Abido, M.S. The role of biotechnology in the conservation of biodiversity. J. Exp. Biol. 2014, 2, 352–363. [Google Scholar]
- Uzun, S.; Yukselgungor, D. Micropropagation of some onobrychis species through in vitro shoot regeneration. Acta Sci. Pol. Hortorum Cultus 2020, 19, 45–52. [Google Scholar] [CrossRef]
- Uzun, S.; Ilbaş, A.İ.; Ipek, A.; Arslan, N.; Barpete, S. Efficient in vitro plant regeneration from immature embryos of endemic Iris sari and I. schachtii. Turk. J. Agric. For. 2014, 38, 348–353. [Google Scholar] [CrossRef]
- Sarasan, V.; Kite, G.C.; Sileshi, G.W.; Stevenson, P.C. Applications of phytochemical and in vitro techniques for reducing over–harvesting of medicinal and pesticidal plants and generating income for the rural poor. Plant Cell Rep. 2011, 30, 1163–1172. [Google Scholar] [CrossRef]
- Bulunuz Palaz, E.; Ugur, R.; Yaman, M. Micropropagation Protocols of New Prunus Hybrids with Significant Rootstock Potential in Fruit Breeding and Cultivation. Erwerbs-Obstbau 2023, 65, 1359–1364. [Google Scholar] [CrossRef]
- Twaij, B.M.; Jazar, Z.H.; Hasan, M.N. Trends in the use of tissue culture, applications and future aspects. Int. J. Plant Biol. 2020, 11, 8385. [Google Scholar] [CrossRef]
- Monthony, A.S.; Page, S.R.; Hesami, M.; Jones, A.M.P. The past, present and future of Cannabis sativa tissue culture. Plants 2021, 10, 185. [Google Scholar] [CrossRef]
- Litwinczuk, W. Propagation of black chokeberry (Aronia melanocarpa Elliot) through in vitro culture. Electron. J. Pol. Agric. Univ. Ser. Hortic. 2002, 5, 1–7. [Google Scholar]
- Rusea, I.; Popescu, A.; Isac, V.; Șuțan, A.; Hoza, D. High efficiency shoot multiplication from in vitro cultured meristems of Aronia melanocarpa cv. Nero. Sci. Pap. Ser. B Hortic. 2019, 63, 65–74. [Google Scholar]
- Şuţan, N.A.; Isac, V.; Duminică, C.; Popescu, A. Studies on the in vitro micropropagation ability of Aronia melanocarpa (Michx.) Elliot. Curr. Trends Nat. Sci. 2017, 6, 85–92. [Google Scholar]
- Kukharchik, N.V.; Kastritskaya, M.S.; Malinovskaya, A.M. Process guide of production of Improved in iv vitro planting material of chokeberry (Arónia melanocárpa). Fruit Grow. 2014, 26, 233–240. [Google Scholar]
- Aasim, M.; Katirci, R.; Baloch, F.S.; Mustafa, Z.; Bakhsh, A.; Nadeem, M.A.; Ali, S.A.; Hatipoğlu, R.; Çiftçi, V.; Habyarimana, E.; et al. Innovation in the breeding of common bean through a combined approach of in vitro regeneration and machine learning algorithms. Front. Genet. 2022, 13, 897696. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Jones, A.M.P. Application of artificial intelligence models and optimization algorithms in plant cell and tissue culture. Appl. Microbiol. Biotechnol. 2020, 104, 9449–9485. [Google Scholar] [CrossRef]
- Silva, J.C.F.; Teixeira, R.M.; Silva, F.F.; Brommonschenkel, S.H.; Fontes, E.P. Machine learning approaches and their current application in plant molecular biology: A systematic review. Plant Sci. 2019, 284, 37–47. [Google Scholar] [CrossRef]
- Butler, K.T.; Davies, D.W.; Cartwright, H.; Isayev, O.; Walsh, A. Machine learning for molecular and materials science. Nature 2018, 559, 547–555. [Google Scholar] [CrossRef]
- Olden, J.D.; Lawler, J.J.; Poff, N.L. Machine learning methods without tears: A primer for ecologists. Q. Rev. Biol. 2008, 83, 171–193. [Google Scholar] [CrossRef]
- Pepe, M.; Hesami, M.; Small, F.; Jones, A.M.P. Comparative analysis of machine learning and evolutionary optimization algorithms for precision micropropagation of Cannabis sativa: Prediction and validation of in vitro shoot growth and development based on the optimization of light and carbohydrate sources. Front. Plant Sci. 2021, 12, 757869. [Google Scholar]
- Pepe, M.; Hesami, M.; Jones, A.M.P. Machine learning–mediated development and optimization of disinfection protocol and scarification method for improved in vitro germination of cannabis seeds. Plants 2021, 10, 2397. [Google Scholar] [CrossRef]
- Lozano–Milo, E.; Landin, M.; Gallego, P.P.; García–Pérez, P. Machine Learning Deciphers Genotype and Ammonium as Key Factors for the Micropropagation of Bryophyllum sp. Medicinal Plants. Horticulturae 2022, 8, 987. [Google Scholar] [CrossRef]
- Sadat–Hosseini, M.; Arab, M.M.; Soltani, M.; Eftekhari, M.; Soleimani, A. Applicability of soft computing techniques for in vitro micropropagation media simulation and optimization: A comparative study on Salvia macrosiphon Boiss. Ind. Crops Prod. 2023, 199, 116750. [Google Scholar] [CrossRef]
- Demirel, F.; Eren, B.; Yilmaz, A.; Türkoğlu, A.; Haliloğlu, K.; Niedbała, G.; Bujak, H.; Jamshidi, B.; Pour–Aboughadareh, A.; Bocianowski, J.; et al. Prediction of Grain Yield in Wheat by CHAID and MARS Algorithms Analyses. Agronomy 2023, 13, 1438. [Google Scholar] [CrossRef]
- Faraz, A.; Tırınk, C.; Önder, H.; Şen, U.; Ishaq, H.M.; Tauqir, N.A.; Wheed, A.; Nabeel, M.S. Usage of the XGBoost and MARS algorithms for predicting body weight in Kajli sheep breed. Trop. Anim. Health Prod. 2023, 55, 276. [Google Scholar] [CrossRef] [PubMed]
- Gökmen, F.; Uygur, V.; Sukuşu, E. Extreme Gradient Boosting Regression Model for Soil Available Boron. Eura. Soil Sci. 2023, 56, 738–746. [Google Scholar] [CrossRef]
- Hassan, S.M.; Jasinski, M.; Leonowicz, Z.; Jasinska, E.; Maji, A.K. Plant disease identification using shallow convolutional neural network. Agronomy 2021, 11, 2388. [Google Scholar] [CrossRef]
- Dong, J.; Zeng, W.; Wu, L.; Huang, J.; Gaiser, T.; Srivastava, A.K. Enhancing short–term forecasting of daily precipitation using numerical weather prediction bias correcting with XGBoost in different regions of China. Eng. Appl. Artif. Intell. 2023, 117, 105579. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plantar. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- McCown, B.H.; Sellmer, J.C. General media and vessels suitable for woody plant culture. In Cell and Tissue Culture in Forestry: General Principles and Biotechnology; Springer: Dordrecht, The Netherlands, 1987; pp. 4–16. [Google Scholar]
- Noble, W.S. What is a support vector machine? Nat. Biotechnol. 2006, 24, 1565–1567. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd ACM Sigkdd International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Appelhans, T.; Mwangomo, E.; Hardy, D.R.; Hemp, A.; Nauss, T. Evaluating machine learning approaches for the interpolation of monthly air temperature at Mt. Kilimanjaro, Tanzania. Spat. Stat. 2015, 14, 91–113. [Google Scholar] [CrossRef]
- Rasmussen, C.E. Gaussian processes in machine learning. In Summer School on Machine Learning; Springer: Berlin/Heidelberg, Germany, 2003; pp. 63–71. [Google Scholar]
- Eren, B.; Türkoğlu, A.; Haliloğlu, K.; Demirel, F.; Nowosad, K.; Özkan, G.; Niedbała, G.; Pour–Aboughadareh, A.; Bujak, H.; Bocianowski, J. Investigation of the Influence of Polyamines on Mature Embryo Culture and DNA Methylation of Wheat (Triticum aestivum L.) Using the Machine Learning Algorithm Method. Plants 2023, 12, 3261. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.; Wing, J.; Weston, S.; Williams, A.; Keefer, C.; Engelhardt, A.; Cooper, T.; Mayer, Z.; Kenkel, B.; Benesty, M. Caret: Classification and Regression Training: R Package; Cran: Vienna, Austria, 2019. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org/ (accessed on 1 December 2022).
- Metivier, P.S.; Yeung, E.C.; Patel, K.R.; Thorpe, T.A. In vitro rooting of microshoots of Cotinus coggygria Mill, a woody ornamental plant. In Vitro Cell Dev. Biol. Plant 2007, 43, 119–123. [Google Scholar] [CrossRef]
- Ali, A.; Ahmad, T.; Abbasi, N.A.; Hafiz, I.A. Effect of different concentrations of auxins on in vitro rooting of olive cultivar ‘Moraiolo’. Pak. J. Bot. 2009, 41, 1223–1231. [Google Scholar]
- Singh, C.K.; Raj, S.R.; Jaiswal, P.S.; Patil, V.R.; Punwar, B.S.; Chavda, J.C.; Subhash, N. Effect of plant growth regulators on in vitro plant regeneration of sandalwood (Santalum album L.) via organogenesis. Agrofor. Syst. 2016, 90, 281–288. [Google Scholar] [CrossRef]
- Hunt, M.A.; Trueman, S.J.; Rasmussen, A. Indole–3–butyric acid accelerates adventitious root formation and impedes shoot growth of Pinus elliottii var. elliottii × P. caribaea var. hondurensis cuttings. New For. 2011, 41, 349–360. [Google Scholar] [CrossRef]
- Fallahpour, M.; Miri, S.M.; Bouzari, N. Propagation Of ‘Gisela 5′Rootstock As Affected By Mineral Composition Of Media And Plant Growth Regulators. J. Hortic. Res. 2015, 23, 57–64. [Google Scholar] [CrossRef]
- Sisko, M. In vitro propagation of Gisela 5 (Prunus cerasus × P. canescens). Agric. Slov. 2011, 8, 31–34. [Google Scholar]
- Hui–Mei, W.; Yuan–Gang, Z.; Feng–Li, D.; Xiao–Ju, Z. Assessment of factors affecting in vitro shoot regeneration from axillary bud explant of Camptotheca acuminata. J. For. Res. 2005, 16, 52–54. [Google Scholar] [CrossRef]
- Haradzi, N.A.; Khor, S.P.; Subramaniam, S.; Chew, B.L. Regeneration and micropropagation of Meyer lemon (Citrus × meyeri) supported by polymorphism analysis via molecular markers. Sci. Hortic. 2021, 286, 110225. [Google Scholar] [CrossRef]
- El–Agamy, S.Z.; Mostafa, R.A.; Shaaban, M.M.; El–Mahdy, M.T. In vitro propagation of Manfalouty and Nab El–gamal pomegranate cultivars. Res. J. Agric. Biol. Sci. 2009, 5, 1169–1175. [Google Scholar]
- Lozzi, A.; Abdelwahd, R.; Mentag, R.; Abousalim, A. Development of a new culture medium and efficient protocol for in vitro micropropagation of Ceratonia siliqua L. In Vitro Cell Dev. Biol. Plant 2019, 55, 615–624. [Google Scholar] [CrossRef]
- Glass, A.D.; Britto, D.T.; Kaiser, B.N.; Kinghorn, J.R.; Kronzucker, H.J.; Kumar, A.; Okamoto, M.; Rawat, S.; Siddiqi, M.Y.; Unkles, S.E.; et al. The regulation of nitrate and ammonium transport systems in plants. J. Exp. Bot. 2002, 53, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Mansseri–Lamrioui, A.; Louerguioui, A.; Bonaly, J.; Yakoub–Bougdal, S.; Allili, N.; Gana–Kebbouche, S. Proliferation and rooting of wild cherry: The influence of cytokinin and auxin types and their concentration. Afr. J. Biotechnol. 2011, 10, 8613–8624. [Google Scholar]
- Reddy, A.S. Calcium: Silver bullet in signaling. Plant Sci. 2001, 160, 381–404. [Google Scholar] [CrossRef]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef]
- Aranda–Peres, A.N.; Peres, L.E.P.; Higashi, E.N.; Martinelli, A.P. Adjustment of mineral elements in the culture medium for the micropropagation of three Vriesea bromeliads from the Brazilian Atlantic Forest: The importance of calcium. HortScience 2009, 44, 106–112. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Katagiri, T.; Shinozaki, K.; Qi, Z.; Tatsumi, H.; Furuichi, T.; Kishigami, A.; Sokabe, M.; Kojima, I.; Sato, S.; et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl. Acad. Sci. USA 2007, 104, 3639–3644. [Google Scholar] [CrossRef]
- Niazian, M. Application of genetics and biotechnology for improving medicinal plants. Planta 2019, 249, 953–973. [Google Scholar] [CrossRef]
- Hesami, M.; Condori–Apfata, J.A.; Valderrama Valencia, M.; Mohammadi, M. Application of artificial neural network for modeling and studying in vitro genotype–independent shoot regeneration in wheat. Appl. Sci. 2020, 10, 5370. [Google Scholar] [CrossRef]
- Niazian, M.; Niedbała, G. Machine learning for plant breeding and biotechnology. Agriculture 2020, 10, 436. [Google Scholar] [CrossRef]
- Aasim, M.; Akin, F.; Ali, S.A.; Taskin, M.B.; Colak, M.S.; Khawar, K.M. Artificial neural network modeling for deciphering the in vitro induced salt stress tolerance in chickpea (Cicer arietinum L). Physiol. Mol. Biol. Plants 2023, 29, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Naderi, R.; Tohidfar, M. Modeling and optimizing in vitro sterilization of chrysanthemum via multilayer perceptron–non–dominated sorting genetic algorithm–II (MLP–NSGAII). Front. Sci. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Niazian, M.; Noori, S.A.S.; Galuszka, P.; Tohidfar, M.; Mortazavian, S.M.M. Genetic stability of regenerated plants via indirect somatic embryogenesis and indirect shoot regeneration of Carum copticum L. Ind. Crops Prod. 2017, 97, 330–337. [Google Scholar] [CrossRef]
- Mirza, K.; Aasim, M.; Katırcı, R.; Karataş, M.; Ali, S.A. Machine learning and artificial neural networks–based approach to model and optimize ethyl methanesulfonate and sodium azide induced in vitro regeneration and morphogenic traits of water hyssops (Bacopa monnieri L.). J. Plant Growth Regul. 2023, 42, 3471–3485. [Google Scholar] [CrossRef]
- Arab, M.M.; Yadollahi, A.; Shojaeiyan, A.; Ahmadi, H. Artificial neural network genetic algorithm as powerful tool to predict and optimize in vitro proliferation mineral medium for G× N15 rootstock. Front. Plant Sci. 2016, 7, 1526. [Google Scholar] [CrossRef]
- Kirtis, A.; Aasim, M.; Katırcı, R. Application of artificial neural network and machine learning algorithms for modeling the in vitro regeneration of chickpea (Cicer arietinum L.). Plant Cell Tissue Organ Cult. 2022, 150, 141–152. [Google Scholar] [CrossRef]
- Aasim, M.; Ali, S.A.; Bekiş, P.; Nadeem, M.A. Light–emitting diodes induced in vitro regeneration of Alternanthera reineckii mini and validation via machine learning algorithms. In Vitro Cell. Dev. Biol. Plant 2022, 58, 816–825. [Google Scholar] [CrossRef]

| Medium | Dose (mg L−1) | TNR 1 | LRL 2 | ARL 3 | NMR 4 | NS 5 | SL 6 | SD 7 | LW 8 | LL 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| MS | 0.0 | 6.33 ± 2.01 | 53.73 ± 6.46 b10 | 53.12 ± 6.49 abc | 1.13 ± 0.23 | 1.40 ± 0.20 | 38.18 ± 12.85 | 2.00 ± 0.00 c | 5.87 ± 1.67 | 11.60 ± 2.96 |
| 0.5 | 21.86 ± 3.63 | 80.27 ± 10.01 ab | 51.87 ± 7.44 abc | 1.90 ± 0.36 | 4.20 ± 0.53 | 52.39 ± 10.24 | 2.30 ± 0.26 b | 7.97 ± 0.55 | 12.80 ± 1.20 | |
| 1.0 | 26.42 ± 3.87 | 52.93 ± 12.62 b | 44.33 ± 8.14 bcd | 2.13 ± 0.50 | 4.60 ± 0.60 | 49.13 ± 4.71 | 2.00 ± 0.20 c | 8.20 ± 1.38 | 13.13 ± 2.21 | |
| 1.5 | 18.20 ± 6.48 | 47.35 ± 16.02 b | 40.20 ± 10.82 cd | 1.87 ± 0.23 | 2.93 ± 0.57 | 47.00 ± 12.52 | 2.00 ± 0.00 c | 6.53 ± 1.36 | 10.60 ± 1.40 | |
| 2.0 | 28.53 ± 3.25 | 65.33 ± 13.45 ab | 46.73 ± 4.81 bcd | 2.13 ± 0.30 | 4.73 ± 0.64 | 60.00 ± 14.20 | 2.33 ± 0.31 b | 6.40 ± 2.22 | 12.93 ± 4.21 | |
| Means | 20.27 ± 8.82 B | 59.92 ± 15.90 B | 47.25 ± 8.23 B | 1.83 ± 0.47 B | 3.57 ± 1.37 B | 49.34 ± 12.13 B | 2.13 ± 0.23 B | 6.99 ± 1.61 B | 12.21 ± 2.44 B | |
| WPM | 0.0 | 12.47 ± 1.72 | 40.53 ± 9.42 b | 34.78 ± 7.57 d | 1.07 ± 0.11 | 1.53 ± 0.30 | 35.33 ± 2.34 | 1.93 ± 0.11 c | 4.93 ± 0.23 | 10.93 ± 1.28 |
| 0.5 | 26.68 ± 4.43 | 97.93 ± 7.00 a | 63.28 ± 10.57 a | 2.32 ± 0.43 | 5.12 ± 0.64 | 63.91 ± 12.50 | 2.81 ± 0.18 a | 9.72 ± 0.67 | 15.62 ± 1.46 | |
| 1.0 | 32.23 ± 7.26 | 64.58 ± 15.40 ab | 54.09 ± 9.92 abc | 2.60 ± 0.61 | 5.61 ± 0.73 | 59.94 ± 5.75 | 2.44 ± 0.24 b | 10.00 ± 1.69 | 16.02 ± 2.69 | |
| 1.5 | 22.20 ± 7.91 | 57.77 ± 16.54 ab | 49.04 ± 13.20 abcd | 2.28 ± 0.28 | 3.58 ± 0.70 | 57.34 ± 15.27 | 2.44 ± 0.00 b | 7.97 ± 1.66 | 12.93 ± 1.71 | |
| 2.0 | 34.81 ± 7.33 | 79.71 ± 13.27 ab | 57.02 ± 10.65 ab | 2.60 ± 0.61 | 5.78 ± 0.55 | 73.20 ± 17.33 | 2.85 ± 0.37 a | 7.81 ± 2.71 | 15.78 ± 2.03 | |
| Means | 25.68 ± 9.73 A | 68.10 ± 23.26 A | 51.64 ± 13.34 A | 2.17 ± 0.67 A | 4.33 ± 1.75 A | 57.95 ± 16.49 A | 2.49 ± 0.40 A | 8.08 ± 2.33 A | 14.26 ± 2.84 A | |
| Mean Dose | 0.0 | 9.40 ± 3.75 d | 47.13 ± 10.22 d | 43.95 ± 11.86 c | 1.10 ± 0.16 c | 1.47 ± 0.24 d | 36.76 ± 8.41 c | 1.97 ± 0.08 c | 5.40 ± 1.18 c | 11.27 ± 2.07 b |
| 0.5 | 24.27 ± 4.47 b | 89.10 ± 12.37 a | 57.57 ± 10.29 a | 2.11 ± 0.42 b | 4.66 ± 0.73 b | 58.15 ± 12.01 b | 2.55 ± 0.38 a | 8.84 ± 1.10 a | 14.21 ± 1.95 a | |
| 1.0 | 29.33 ± 6.10 a | 58.76 ± 14.11 c | 49.21 ± 9.71 bc | 2.36 ± 0.56 a | 5.11 ± 0.81 a | 54.54 ± 7.56 b | 2.22 ± 0.31 b | 9.10 ± 1.69 a | 14.58 ± 2.71 a | |
| 1.5 | 20.20 ± 6.83 c | 52.56 ± 16.97 cd | 44.62 ± 11.82 c | 2.07 ± 0.32 b | 3.26 ± 0.67 c | 52.17 ± 13.71 b | 2.22 ± 0.24 b | 7.25 ± 1.56 b | 11.77 ± 1.89 b | |
| 2.0 | 31.67 ± 6.13 a | 72.52 ± 14.31 b | 51.87 ± 9.29 b | 2.37 ± 0.39 a | 5.25 ± 0.92 a | 66.60 ± 15.91 a | 2.59 ± 0.41 a | 7.10 ± 2.35 b | 14.36 ± 3.80 a | |
| MSM | 219.31 *** | 501.70 * | 144.55 * | 0.87 *** | 4.23 *** | 555.42 *** | 1.01 *** | 8.97 ** | 31.31 *** | |
| MSD | 464.44 *** | 1717.94 *** | 188.19 *** | 1.65 *** | 15.29 *** | 714.46 *** | 0.41 *** | 13.49 *** | 15.06 *** | |
| MS(MxD) | 1.42 ns | 225.87 * | 243.45 *** | 0.08 ns | 0.22 ns | 63.24 ns | 0.09 * | 1.97 ns | 3.52 ns | |
| Traits | ML 1 Criteria | SVM 2 | RF 3 | XGBoost 4 | KNN 5 | GP 6 |
|---|---|---|---|---|---|---|
| TNR | R2 | 0.575 | 0.523 | 0.589 | 0.498 | 0.569 |
| MSE | 7.079 | 7.504 | 6.965 | 7.692 | 7.131 | |
| MAPE | 26.431 | 34.981 | 25.686 | 37.587 | 29.218 | |
| MAD | 5.184 | 6.196 | 5.279 | 6.216 | 5.523 | |
| LRL | R2 | 0.138 | 0.245 | 0.281 | 0.100 | 0.231 |
| MSE | 29.085 | 27.231 | 26.564 | 30.371 | 27.472 | |
| MAPE | 23.360 | 31.523 | 30.833 | 35.882 | 30.017 | |
| MAD | 16.760 | 19.317 | 19.212 | 21.129 | 18.747 | |
| ARL | R2 | 0.135 | 0.207 | 0.267 | 0.038 | 0.206 |
| MSE | 14.210 | 13.608 | 13.080 | 14.990 | 13.616 | |
| MAPE | 20.653 | 23.443 | 22.129 | 27.245 | 23.129 | |
| MAD | 10.392 | 10.871 | 10.359 | 12.327 | 10.824 | |
| NMR | R2 | 0.714 | 0.673 | 0.736 | 0.606 | 0.711 |
| MSE | 0.317 | 0.339 | 0.304 | 0.372 | 0.318 | |
| MAPE | 13.231 | 16.740 | 13.069 | 18.023 | 14.256 | |
| MAD | 0.245 | 0.300 | 0.253 | 0.316 | 0.267 | |
| NS | R2 | 0.780 | 0.721 | 0.795 | 0.642 | 0.786 |
| MSE | 0.783 | 0.880 | 0.755 | 0.997 | 0.771 | |
| MAPE | 14.825 | 24.614 | 15.012 | 30.063 | 15.716 | |
| MAD | 0.546 | 0.739 | 0.572 | 0.846 | 0.588 | |
| SL | R2 | 0.534 | 0.506 | 0.571 | 0.436 | 0.544 |
| MSE | 9.989 | 10.285 | 9.591 | 10.990 | 9.882 | |
| MAPE | 14.652 | 18.140 | 15.435 | 19.743 | 16.592 | |
| MAD | 7.752 | 9.114 | 7.936 | 9.717 | 8.491 | |
| SD | R2 | 0.648 | 0.664 | 0.743 | 0.439 | 0.713 |
| MSE | 0.217 | 0.213 | 0.186 | 0.275 | 0.196 | |
| MAPE | 6.712 | 7.294 | 5.637 | 9.775 | 6.334 | |
| MAD | 0.159 | 0.171 | 0.133 | 0.222 | 0.150 | |
| LW | R2 | 0.535 | 0.518 | 0.583 | 0.349 | 0.527 |
| MSE | 1.373 | 1.398 | 1.300 | 1.625 | 1.384 | |
| MAPE | 13.881 | 17.827 | 14.984 | 21.524 | 16.974 | |
| MAD | 0.990 | 1.213 | 1.051 | 1.414 | 1.168 | |
| LL | R2 | 0.188 | 0.366 | 0.380 | 0.274 | 0.368 |
| MSE | 2.633 | 2.326 | 2.241 | 2.491 | 2.301 | |
| MAPE | 13.526 | 13.883 | 13.237 | 15.554 | 14.085 | |
| MAD | 1.896 | 1.859 | 1.777 | 2.030 | 1.874 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirel, F.; Uğur, R.; Popescu, G.C.; Demirel, S.; Popescu, M. Usage of Machine Learning Algorithms for Establishing an Effective Protocol for the In Vitro Micropropagation Ability of Black Chokeberry (Aronia melanocarpa (Michx.) Elliott). Horticulturae 2023, 9, 1112. https://doi.org/10.3390/horticulturae9101112
Demirel F, Uğur R, Popescu GC, Demirel S, Popescu M. Usage of Machine Learning Algorithms for Establishing an Effective Protocol for the In Vitro Micropropagation Ability of Black Chokeberry (Aronia melanocarpa (Michx.) Elliott). Horticulturae. 2023; 9(10):1112. https://doi.org/10.3390/horticulturae9101112
Chicago/Turabian StyleDemirel, Fatih, Remzi Uğur, Gheorghe Cristian Popescu, Serap Demirel, and Monica Popescu. 2023. "Usage of Machine Learning Algorithms for Establishing an Effective Protocol for the In Vitro Micropropagation Ability of Black Chokeberry (Aronia melanocarpa (Michx.) Elliott)" Horticulturae 9, no. 10: 1112. https://doi.org/10.3390/horticulturae9101112
APA StyleDemirel, F., Uğur, R., Popescu, G. C., Demirel, S., & Popescu, M. (2023). Usage of Machine Learning Algorithms for Establishing an Effective Protocol for the In Vitro Micropropagation Ability of Black Chokeberry (Aronia melanocarpa (Michx.) Elliott). Horticulturae, 9(10), 1112. https://doi.org/10.3390/horticulturae9101112










