Abstract
Citrus processing side-streams are largely represented by waste orange peels (WOP), and there are several techniques developed for polyphenol extraction from WOP; yet, there are a significant lack of methodologies based on non-conventional, green solvents. On this basis, this study was performed to assess a deep eutectic solvent (DES) synthesized with glycerol and sodium butyrate, for its capacity to extract WOP polyphenols. Optimization of the process was carried out using a response surface methodology, which revealed that a maximum total polyphenol yield of 73.36 mg gallic acid equivalent (GAE) g−1 dry mass (DM) could be achieved with a solvent system of DES/water (80% w/w), a residence time of 120 min, and a temperature of 90 °C. Using these settings, the polyphenol extraction from WOP with the DES/water solvent system was found to have outstanding performance compared to aqueous or hydroethanolic extraction, while the extracts generated possessed significantly enhanced antioxidant properties. The chromatographic analyses of the extracts demonstrated that the DES/water extract was particularly enriched in hesperidin (21.81 mg g−1 dry mass), a bioflavonoid with promising pharmaceutical potential. This is a first report on the use of this particular DES for WOP polyphenol extraction, which may be used to produce hesperidin-enriched extracts, by implementing the methodology developed.
1. Introduction
There is, to date, the admission that linear economy models are no longer effective enough to sustain the ever-increasing world population, whereas insufficient strategies of bioresource harnessing lead to raw material overexploitation and depletion, as well as to an unprecedented aggravation of the environment, with detrimental consequences to land conservation, food security, and eventually to human well-being. The unavoidable shift to bioeconomy-based concepts of agricultural production and food processing has created overwhelming opportunities for the valorization of biomass, which, through biorefining technologies, may provide a wide range of products, from low-value soil adjuvants to high value-added specialty chemicals. In this regard, food industry residues are now appreciated as materials with an exceptional potential for recovering a spectrum of molecules with an assortment of properties, such as antioxidants, proteins, pigments, flavorings, etc. [1].
Amongst other precious phytoconstituents that may be retrieved from various agri-food wastes and by-products (stems, roots, peels, leaves, seeds, etc.), polyphenolic compounds hold a prominent position as constituents with peculiar bioactive properties. This vast group of secondary metabolites encompasses several subclasses, including simple phenolics (e.g., hydroxycinnamates such as caffeic and ferulic acids and the derivatives thereof), and more structurally complex flavonoids (e.g., flavanones, flavones, etc.). A high number of these phytochemicals have been shown to possess significant biological properties, embracing cardioprotective and chemoprotective effects, and also anti-inflammatory, antioxidant, and antimicrobial activities [2,3]. Therefore, a large volume of examinations have focused on valorization technologies with the objective of exploiting polyphenol-rich rejected materials [4,5].
Citrus are globally the most prevalent fruit crop, with orange having the highest share of about 60% of the total citrus production. Oranges are mainly destined for juicing, which yields approximately 50% of juice on a fresh weight basis. Thus, there is almost 50% residual mass, consisting primarily of pulp, peel, and seeds. The rejected material also includes defective fruits, which are discarded [6]. Global orange production was estimated in 2019 to reach approximately 46 million metric tons, 37% of which were used for the manufacturing of various commodities. Considering the above, it is clear that orange processing residues are a major source of organic waste material. In this regard, frameworks encompassing the reuse/valorization of orange processing solid wastes for polyphenol recovery become imminent, especially in the light of several studies, which have demonstrated the activities of citrus flavonoids. Commodities of flavonoid-rich citrus peel extracts are commercially available as health supplements and nutraceuticals [7].
Polyphenols can be efficiently extracted from various plant tissues with solid–liquid extraction, using solvents of appropriate polarity. On the other hand, in accordance with the principles of green chemistry, the replacement of petroleum-based, conventional solvents with alternative, green ones is of paramount importance in establishing sustainable downstream processes in a biorefinery framework [8,9,10]. On this ground, there has been an outstanding number of investigations over the past few years, dealing with the use of neoteric liquids, known as deep eutectic solvents (DESs), specifically designed for polyphenol extraction [4,11].
In most cases reported in the literature, DESs are synthesized using a hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD) [12,13]. The synthesis of these solvents is facile and straightforward, using food-grade substances, such as organic acids (i.e., citric acid, lactic acid), glycerol, choline chloride, salts of organic acids (i.e., sodium citrate), etc., and they are characterized by an absence of toxicity, particularly low vapor pressure, composition tunability, recyclability, and foods/pharmaceuticals/cosmetics compatibility. These unique features make DESs highly suitable for developing sustainable processes for the extraction of numerous plant food wastes [14]. Furthermore, several DESs have been reported to exhibit exceptional solvation properties for a variety of structurally diversified polyphenols, while polyphenol extraction yield with DESs may frequently outperform that achieved with common, conventional solvents [15]. However, research on WOP polyphenol extraction with DES is rather quite limited [16,17,18,19,20].
Considering the above-mentioned, this investigation was carried out to examine the use of a previously reported DES, composed of glycerol and sodium butyrate [21], in the development of an extraction process, which aimed to produce flavonoid-enriched orange peel extracts. Process optimization was performed with a response surface methodology, accompanied by the examination of the polyphenolic profile and the antioxidant activity of the extracts generated, on a comparative basis. As far as the authors are aware, this is the first study on such a process with regard to the valorization of waste orange peels.
2. Materials and Methods
2.1. Reagents and Chemicals
Sodium butyrate was purchased from Glentham (Corsham, U.K.). Folin–Ciocalteu reagent, absolute ethanol, and gallic acid monohydrate were from Panreac (Barcelona, Spain). 2,2-Diphenyl-1-picrylhydrazyl (DPPH•) radical was from Alfa Aesar (Karlsruhe, Germany). Sodium acetate anhydrous, glycerol anhydrous (99%), and sodium carbonate anhydrous were from Penta (Prague, Czechia). Iron(III) chloride hexahydrate (FeCl3•6H2O) was purchased from Merck (Darmstadt, Germany). 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) was purchased from Fluka (Steinheim, Germany). L-Ascorbic acid was from Carlo Erba (Milano, Italy). Neochlorogenic acid, luteolin 7-O-rutinoside, caffeic acid, ferulic acid, chlorogenic acid, narirutin, and hesperidin were from Sigma-Aldrich (Steinheim, Germany). Solvents used for chromatographic determinations were high-performance liquid chromatography (HPLC) grade. The DES used in the extraction process development was composed of glycerol (hydrogen bond donor) and sodium butyrate (hydrogen bond acceptor), and it was synthesized using a glycerol/sodium butyrate molar ratio of 6 as described elsewhere [21]. Briefly, appropriate amounts of both compounds were weighted and placed in a glass vial, and heated mildly until the formation of a transparent liquid was observed. This liquid, termed “DES”, was used without any further treatment.
2.2. Collection, Handling, and Drying of Waste Orange Peels (WOP)
A realistic WOP sample of approximately 9 kg, representative of food-processing residue, was obtained by collecting orange juicing residues, composed, essentially, of orange peels, from various catering facilities (Larissa, Central Greece), which carry out orange juicing on a daily basis to produce fresh orange juice. The collection was accomplished within 3 consecutive days, and then residues were transported to the laboratory within 2 h. Following the receipt of the residues, the material was screened for foreign matter and infected peels, and oven-dried at 80 °C, for 24 h, using a Binder BD56 oven (Bohemia, NY, USA). Conditions of drying, as well as further handling and storage, have been previously described in detail [22].
2.3. Protocol and Procedure of Waste Orange Peel Extraction
A liquid-to-solid ratio of 20 mL g−1 and a 500 rpm stirring speed were used for all extractions performed. A volume of 20 mL of solvent was placed in a 25-mL Duran™ bottle and heated at the desired temperature using an oil bath on a temperature-controlled hotplate (Witeg, Wertheim, Germany). To ensure that the predetermined temperature was acquired by the solvent, a period of 10 min was allowed to elapse before adding the pulverized WOP (1.00 g). Then, extractions were carried out for selected increments of residence time. In the case of response surface optimization, the extraction period was dictated by the experimental design. After the extractions, all mixtures were centrifuged at 10,000× g to remove cell debris and obtain a clear extract.
2.4. Experimental Design and Response Surface Optimization
Numerous previous investigations emphasized the importance of three key parameters (independent variables), namely time, temperature, and DES/water ratio in the extraction of polyphenols [13]. Based on this finding, the optimization of the extraction process was accomplished by implementing a response surface methodology, with a Box–Behnken experimental design. To assess the influence of the independent variables on the yield of total polyphenols (the dependent variable), there was a selection of ranges for each independent variable, which were codified in three levels, as shown in Table 1.

Table 1.
The independent variables used for the experimental design and the codification of their actual levels.
The codification to three levels, −1, 0, and 1, was carried out as described in detail elsewhere [23]. Likewise, the ranges for DES/water proportion, residence time, and temperature were selected considering both earlier results [20] and preliminary experiments. Both an analysis of variance (ANOVA) and lack-of-fit tests were used to appraise the model derived, and to determine overall model significance, as well as the significance of each term of the model. The final model was given in the form of a mathematical equation, which contained only the significant terms, whereas the non-significant terms (p > 0.05) were not considered.
2.5. Total Polyphenol, Total Flavonoid, and Antioxidant Activity Determination
A previously established protocol was followed to determine the yield in total polyphenols [24], based on the Folin–Ciocalteu methodology. All samples were appropriately diluted with methanol prior to determinations. The results were given as mg gallic acid equivalents (GAE) per g of dry mass (DM). Total flavonoids were determined as analytically described elsewhere [25], using the aluminum chloride/sodium acetate reagent, and expressed as mg rutin equivalents [RtE] per g DM. The methodologies used for the determination of both the antiradical activity (AAR) and ferric-reducing power (PR) were fully described in an earlier study [26]. In short, for the AAR determination, the stable radical probe DPPH• was used, and results were reported as μmol DPPH per g DM. For the PR determination, TPTZ was used as the chromophore complexing agent for Fe2+, and results were given as μmol ascorbic acid equivalents (AAE) per g DM.
2.6. Chromatographic Determinations
Samples were appropriately diluted with HPLC-grade methanol before the analysis. The flavones nobiletin, didymin, dimethylnobiletin, and sinensetin were tentatively identified by liquid chromatography–mass spectrometry, carried out in positive ionization mode, using the chromatographic and mass spectra acquisition settings reported earlier [27], while mass spectra and ultraviolet (UV)–visible (vis) data dereplication was performed as previously described [28]. The quantification of these flavones was carried out using luteolin 7-O-glucoside as an external standard, which was employed to construct a calibration curve (R2 = 0.9980). Quantifications of neochlorogenic acid, chlorogenic acid, caffeic acid, ferulic acid, narirutin, and hesperidin were also carried out with external standards, and calibration curves were constructed with original standards prepared in HPLC-grade methanol, immediately before the determinations, using a concentration range of 0–50 μg mL−1. The chromatographic device and specific details pertaining to analyses were reported in a previously published examination [29].
2.7. Statistical Processing and Analyses
The design of the experiment and Response Surface Methodology (RSM), along with all associated statistical determinations (i.e., the lack of goodness of fit and an ANOVA) were accomplished with JMP™ Pro 16 (SAS, Cary, NC, USA). Regressions (at least 95% significance level) were performed with SigmaPlot™ 15.0 (Systat Software Inc., San Jose, CA, USA). A Shapiro–Wilk test was employed to examine the normality of the experimental data, which were found to be normally distributed. Thus, IBM SPSS Statistics™ 29 (SPSS Inc., Chicago, IL, USA) was used to examine statistically significant differences, based on the Kruskal–Wallis test. Single-factor experiments and all extractions included in the experimental design were carried out at least twice. Chromatographic and spectrophotometric determinations were performed in triplicate. Values were given as average ± standard deviation (SD).
3. Results and Discussion
3.1. Single-Factor Experiments
The initial stage for the examination of the efficiency of the DES to recover polyphenols was to clarify the role of water proportion. This feature of the solvent system is critical to extraction performance, as revealed by several previous investigations [30], because mixing DES with water may affect its physicochemical properties, such as polarity and viscosity, which pertain to polyphenol extractability. Therefore, the appropriate tuning of DES behavior would entail an increased extraction performance [13]. In this direction, and considering the data provided in an earlier study [20], the DES/water proportion (termed C) was tested from 50 to 80% w/w (Figure 1).
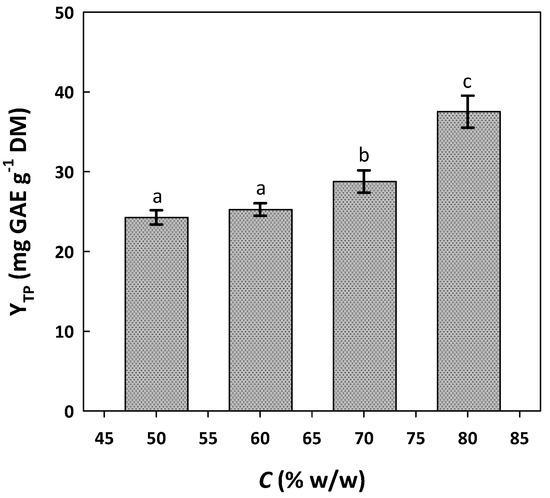
Figure 1.
Effect of DES/water proportion (C) on the yield of total polyphenol extraction (YTP) from WOP. Extractions were accomplished at 70 °C, for 120 min, under stirring at 500 rpm. Columns designated with different letters (a, b, c) display statistically different levels (p < 0.05).
Up to 60%, the solvent system displayed no significant difference, but when C was increased to 70%, the yield in total polyphenol extraction (YTP) was also significantly increased (p < 0.05). The same phenomenon was observed when the C was switched to 80%. A further increase in DES proportion was precluded, because it would lead to the solvent being particularly viscous, making extract handling problematic. Thus, a C of 80% was adopted for subsequent testing. Using this solvent system, residence time screening indicated that a significant increase in YTP could be achieved up to 120 min (p < 0.05) (Figure 2). By contrast, YTP declined significantly upon the extension of the duration beyond this point and up to 240 min (p < 0.05) (Figure 2). This outcome evidenced that the optimal residence time might lie between 120 and 180 min.
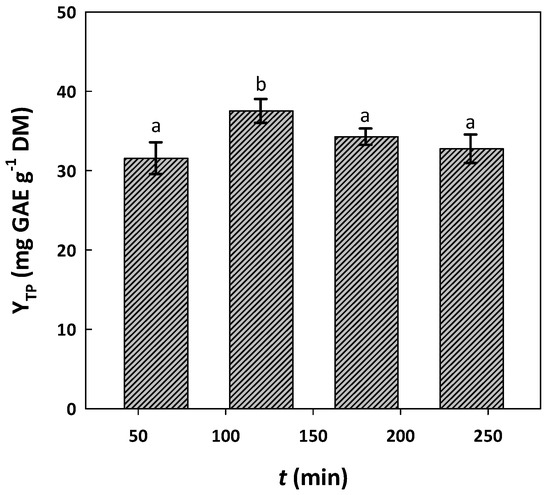
Figure 2.
Effect of residence time (t) on the yield of total polyphenol extraction (YTP) from WOP. Extractions were accomplished at 70 °C, for 120 min, under stirring at 500 rpm, with the solvent system DES/water (80% w/w). Columns designated with different letters (a, b) display statistically different levels (p < 0.05).
Likewise, the effect of temperature on YTP was tested by maintaining a residence time of 120 min. The level of 90 °C was chosen as the upper limit tested, in line with previous studies, to avoid possible thermally induced polyphenol decomposition [28]. As can be seen in Figure 3, the increase in temperature from 50 to 70 °C was accompanied by a significant increase in YTP. The effect from 70 to 90 °C was similar, pointing to a positive influence of temperature on polyphenol extraction.
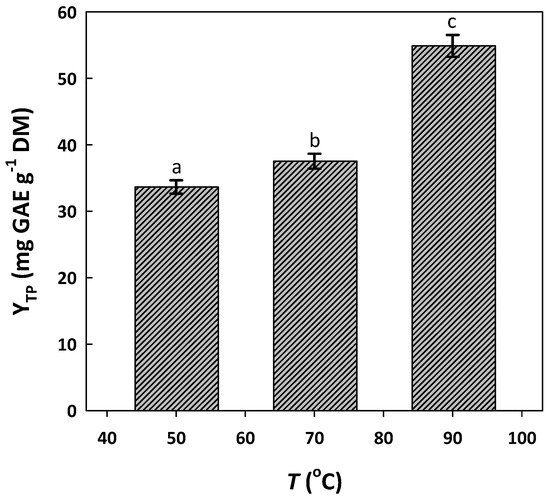
Figure 3.
Effect of extraction temperature (T) on the yield of total polyphenol extraction (YTP) from WOP. Extractions were performed for 120 min, under stirring at 500 rpm. Columns assigned with different letters (a, b, c) represent statistically different levels (p < 0.05).
3.2. Process Optimization
On the ground of the results obtained from the single-factor investigation, there was a delimitation of the variable ranges; then, an experimental design was set up to scrutinize the effect of the three process variables, DES/water proportion (C), residence time (t), and temperature (T), by deploying an RSM. Thus, it was made possible to evaluate the effect of the individual variables, but also to detect cross (synergistic) functions. Using an analysis of variance (ANOVA) test, the closeness of the predicted and measured (actual) values (Table 2 and Figure 4B) was the basis for checking the validity of the models, also considering the significance of the lack-of-fit.

Table 2.
The design points used to set up the experimental design, and the actual (measured) and predicted values of the response (YTP).
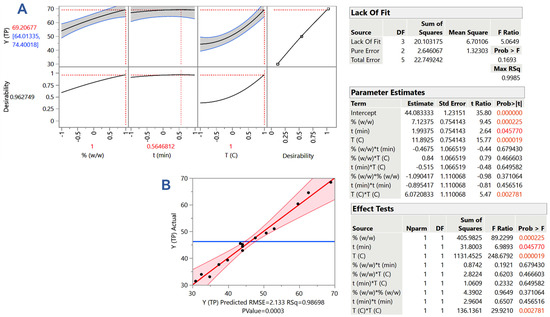
Figure 4.
Plot (A) presents the desirability faction, the maximum predicted YTP, and the optimum C, T, and t. Plot (B) shows the correlation between the measured (actual) and predicted values of the response (YTP), derived from the response surface methodology. Critical statistical parameters (square correlation coefficient (R2), p-value for the model) are also provided. The inset tables display statistics related to the response surface methodology. Values assigned with colors are statistically significant (red: p < 0.05; orange, p < 0.001).
It can be seen in Figure 4 (inset table “Lack Of Fit”) that the lack-of-fit was significant, evidencing the credibility of the model derived. Furthermore, considering the parameter estimates (Figure 4, inset table “Parameter Estimates”), it was shown that all three variables tested had a statistically significant effect on the response (YTP), but that no significant synergistic effects were observed. Therefore, the mathematical equation describing the model was composed of only the significant terms, as follows:
YTP = 44.08 + 7.12X1 + 1.99X2 + 11.89X3 + 6.07X32
This quadratic model was visually depicted in the form of 3D plots (Figure 5), to provide an at-a-glance impression of the effect of simultaneous variation in the three variables on the response. In addition, the tool of desirability function (Figure 4A) permitted the estimation of the optimal predicted settings for the process, which were C = 80% (w/w), t = 154 min, and T = 90 °C. Under these conditions, the maximum predicted response (YTP) was determined to be 69.21 ± 5.20 mg GAE g−1 DM.
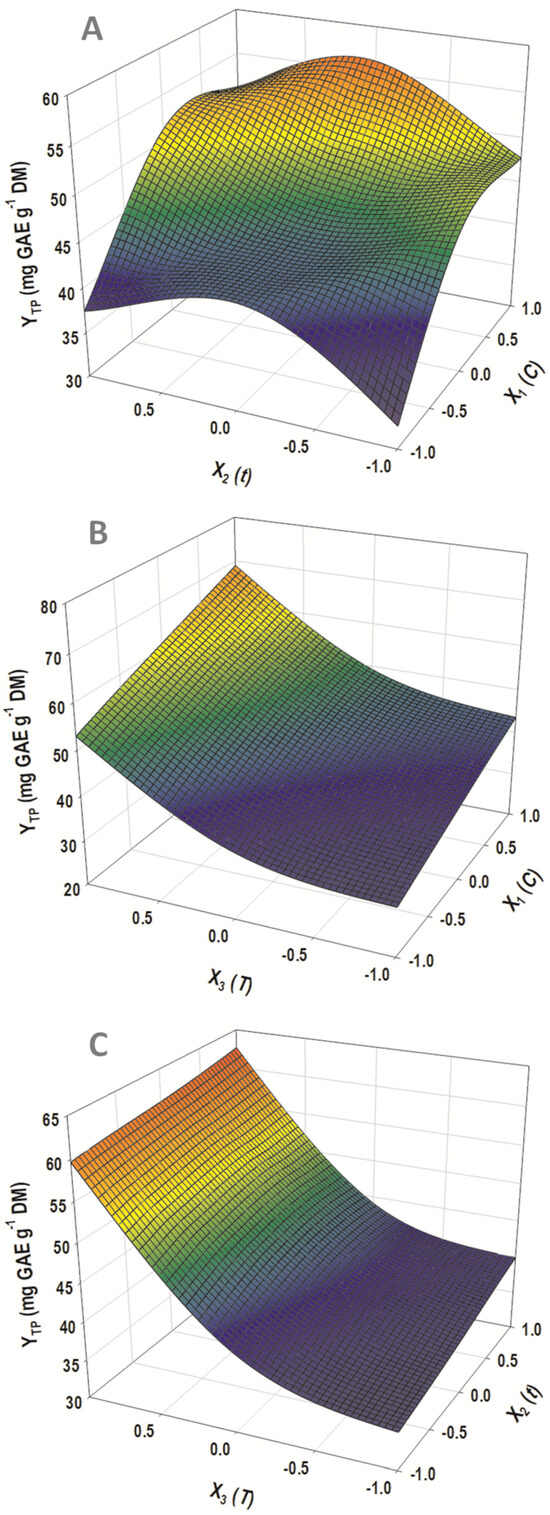
Figure 5.
Three-dimensional diagram depicting the yield in total polyphenols (YTP) as a function of DES composition (C) and residence time (t) (A), DES composition (C) and temperature (T) (B), and temperature (T) and residence time (t) (C).
Since the R2, which is an indication of the total variability around the mean provided by the model, was 0.99 and the p value for lack-of-fit, for a significance level of at least 95%, was not significant, then it would be argued that equation (1) had an excellent fitting to the actual (experimental) data, and that the model derived may be a very good predictor. To ascertain the applicability of the model, the optimal conditions were used to carry out three individual extractions and determine YTP. The outcome of this experiment was YTP = 73.36 ± 1.40 mg GAE g−1 DM, which had no statistical difference from the optimum predicted value. This finding evidenced the reliability of the model established.
All three variables considered had a significant and positive effect on the response (YTP), but temperature also exerted a significant quadratic effect. This was manifested by the non-linear increase in YTP, as a response to increasing temperature (Figure 5B,C). As discussed earlier (Section 3.1), the role of suitably regulating the water content of a DES-based solvent is of utmost importance to the extraction performance.
The optimum 80% (w/w) determined in this study was very close to the one previously reported for glycerol/sodium propionate DES [26] and matches exactly the outcome of other studies on DES-based polyphenol extraction [31,32]. However, the comparison with bibliographic data is often problematic, given that several researchers report DES/water proportion as w/w, w/v and v/v. In general, the addition of a specific amount of water in glycerol-based DES is necessary to appropriately regulate viscosity and polarity [33], but water proportion cannot exceed a certain point, because this would cause DES disintegration, abrogating its peculiar solvation properties [34].
In an extraction process, time and temperature are interdependent variables affecting extraction yield and extract composition. However, in most instances related to polyphenol extraction with DES, optimal temperatures did not exceed 80 °C [15,35]. In a recent study on polyphenol extraction from WOP, the preferred temperature was significantly lower, set at 45 °C [17], while in other investigations the optimum temperatures found for polyphenol recovery from WOP with DES were 50 °C [18] and 60 °C [19]. Raising the temperature may greatly facilitate polyphenol extractability, because there is a significant decrease in solvent viscosity, which results in more diffusion and polyphenol solubilization; however, polyphenol instability issues may preclude temperature increases beyond a certain point [15]. Indeed, for polyphenol recovery with a glycerol/sodium acetate DES, extensive polyphenol degradation was observed in the extract obtained at 135 °C [36], an effect attributed to the slightly alkaline pH of that DES. Given that the glycerol/sodium butyrate DES used in this study was also slightly alkaline (pH of the 80% mixture = 8.24), then the upper limit of 90 °C selected could be considered a fair compromise to achieve maximum extraction yield without risking polyphenol loss.
Likewise, choosing an optimum residence time is of paramount importance, to avoid unnecessary time-consuming processes which may also have detrimental consequences on the polyphenolic constituents of the extracts [15]. In this regard, the determination of the optimum t clearly defines the treatment duration, since prolonging the residence time, beyond a certain point, is not accompanied by a significant increase in the extraction yield. Such a phenomenon has been demonstrated for the DES used in this study (glycerol/sodium butyrate) in an earlier examination, where polyphenol extraction from Origanum dictamnus was optimal at approximately 50 min, and thereafter the extraction yield remained practically unchanged [21].
3.3. Extraction Yield and Metabolite Composition
The effectiveness of the treatment developed can be highlighted by comparison with commonly used, conventional green solvents, such as water and 60% (v/v) aqueous ethanol. On this basis, extractions were performed under optimized conditions and the following two representative indices were used: the yield in total polyphenols (Figure 6A) and in total flavonoids (Figure 6B).
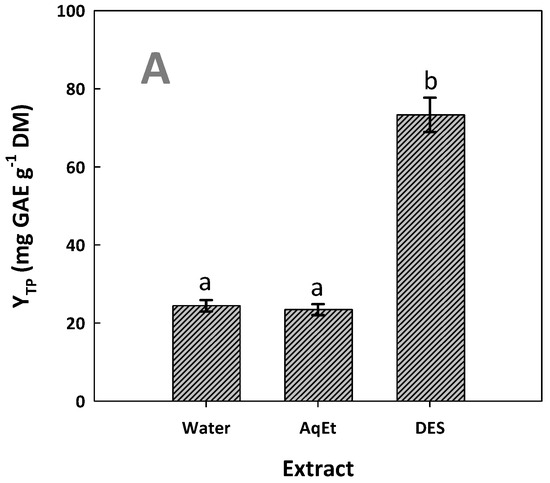
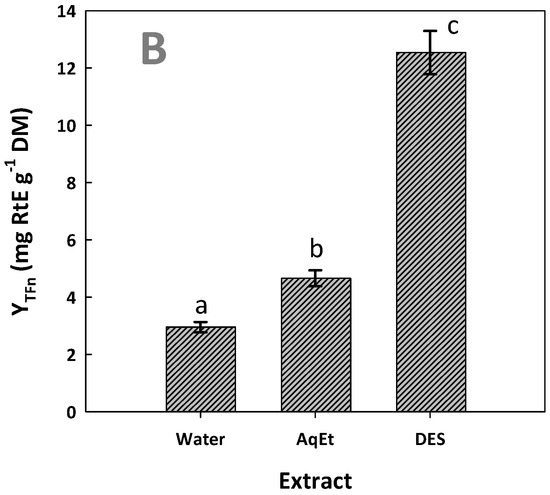
Figure 6.
Total polyphenol (A) and total flavonoid yields (B) achieved after performing extractions with the DES/water solvent system (assigned as DES) and water and 60% aqueous ethanol (assigned as AqEt), under optimized conditions (t = 154 min, and T = 90 °C). Values denoted with different letters (a, b, c) display statistically significant differences (p < 0.05).
Based on the results for both YTP and YTFn, the extract generated with DES was far more enriched, compared to both the aqueous and hydroethanolic extracts (p < 0.05). With reference to YTP, extraction with the DES was shown to have three times higher efficiency than the aqueous and the hydroethanolic extraction, while, based on YTFn, the DES extraction afforded a 4.2 and 2.7 times higher flavonoid yield when compared to the aqueous and the hydroethanolic extraction, respectively. These findings highlighted the ability of the DES tested to recover WOP polyphenols. The maximum yield of 73.36 ± 1.40 mg GAE g−1 DM achieved was exceptionally high and, to the best of the authors’ knowledge, along with 75.77 mg GAE g−1 DM obtained with choline chloride/fructose and proline/malic acid deep eutectic solvent extraction [16], is the highest yield reported in the literature. Levels of 44.09 mg GAE g−1 DM have also been reported, using a glycerol-based organosolv treatment of WOP [28], but the usual yields reported may vary from 7 to over 26 mg GAE g−1 DM, obtained with techniques including cyclodextrin-aided extraction [29], ultrasound-assisted extraction [37], and microwave-assisted extraction [38,39].
Furthermore, to portray the analytical polyphenolic composition and to spot possible important differences, the extracts were also analyzed by HPLC, leading to ten compounds being tentatively identified (Figure 7). Thus, the distinction of the efficacy of the solvents tested was based on the quantification of these compounds.
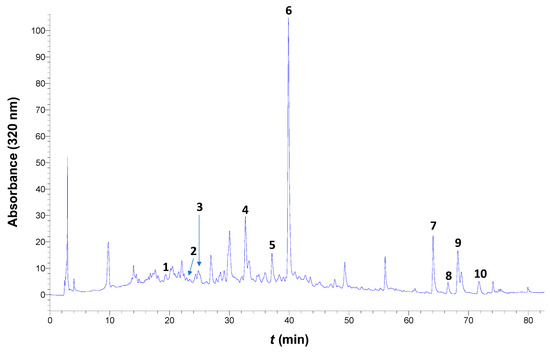
Figure 7.
Representative HPLC traces at 320 nm of the WOP extract generated with the DES, under optimized conditions. Peak assignment: 1, neochlorogenic acid; 2, chlorogenic acid; 3, caffeic acid; 4, ferulic acid; 5, narirutin; 6, hesperidin, 7, didymin; 8, sinensetin; 9, nobiletin; 10, dimethylnobiletin.
It can be seen in Table 3 that water and aqueous ethanol were, in general, more effective in recovering neochlorogenic acid, chlorogenic acid, and caffeic acid, whereas significantly higher amounts of ferulic acid were recovered using the DES. By contrast, the recovery of neochlorogenic acid, chlorogenic acid, and caffeic acid using the DES was poor.

Table 3.
Polyphenolic composition of the extracts produced after performing extractions with the DES/water solvent system (assigned as DES), water and 60% aqueous ethanol (assigned as AqEt), under optimized conditions (t = 154 min, and T = 90 °C).
To the contrary, extraction with DES was far more effective for the recovery of the flavanone fraction, including narirutin and hesperidin. It is to be emphasized that extraction with DES afforded almost fifteen and two times higher yields in total flavanones when compared to the aqueous and hydroethanolic extraction, respectively. Furthermore, it should be highlighted that hesperidin was the predominant polyphenol in the DES extract and accounted for about 88% of the total polyphenols determined. In addition, the hesperidin amount recovered (21.81 mg g−1 DM) may be considered to be exceptionally high, in the light of previous examinations, which reported values from 0.2 to just over 8 mg g−1 [39,40,41]. However, orange peel extraction with a sequential subcritical water process was shown to yield 22.99 mg hesperidin g−1 DM [42], while alkaline hot water extraction was reported to give 26.81 mg hesperidin g−1 DM [43]. With respect to flavones, the hydroethanolic solvent and the DES were virtually of equivalent effectiveness, since there was only a 4% difference in the total flavones extracted. Overall, the yield attained with the DES was 26.5 mg g−1, being 1.75 and 8.63 times higher than those obtained with aqueous ethanol and water, respectively. This finding pointed to the supremacy of the DES over common conventional solvents.
3.4. Antioxidant Characteristics
The antioxidant tests were carried out to ascertain whether the increased polyphenol concentration in the extracts generated with DES would be accompanied by enhanced antioxidant activity. The results obtained confirmed that the DES extract was indeed far more active than both the aqueous and the hydroethanolic extracts. In particular, the DES extract displayed an AAR, which was significantly higher (p < 0.05), and it was almost three times more active than the extract produced with aqueous ethanol (Figure 8A).
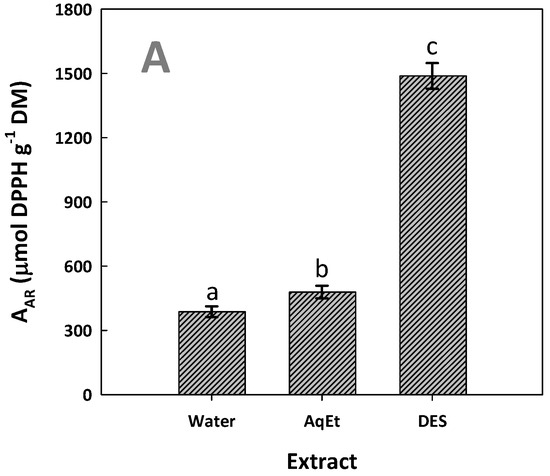
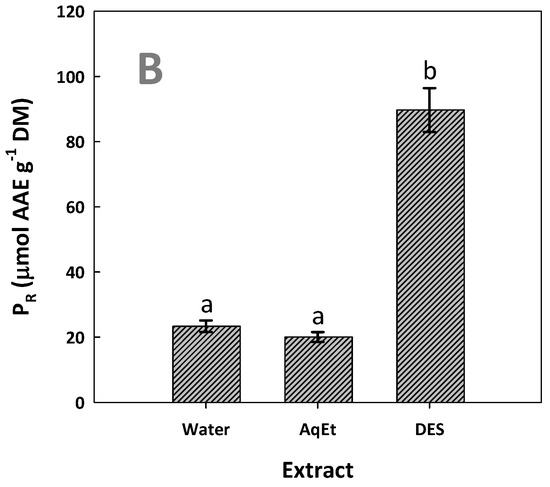
Figure 8.
The antiradical activity (AAR) (A) and ferric-reducing power (PR) (B) of the WOP extracts tested. The extracts were produced with DES/water solvent system (assigned as DES) and water and 60% aqueous ethanol (assigned as AqEt), under optimized conditions (t = 154 min, and T = 90 °C). Values denoted with different letters (a, b, c) are statistically significant (p < 0.05).
Likewise, the DES extract exhibited significantly higher PR, which was 3.8 times higher than that determined for the aqueous extract (Figure 8B). This outcome did confirm that the DES extract, being more enriched in polyphenols, had the strongest antioxidant activity. This observation is in line with previous studies, where WOP extracts with higher polyphenolic concentrations also showed stronger antioxidant activity [28]. Furthermore, it has been found that the antioxidant activity of WOP extracts, determined by both DPPH and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) methodologies, were positively correlated with the total polyphenol concentration, but not with the total flavonoid concentration [43]. Similar results concerning the correlation between total polyphenol concentration and antioxidant activity, estimated by different tests, were reported by other authors too [44].
Hesperidin is a flavonoid that expresses antioxidant activity [45] and, owing to its high concentration, could be a key antioxidant in the extracts tested. The strong antioxidant potency of hesperidin has also been confirmed by chemical tests [46] and in vitro cell studies [47]. On this basis, it could be claimed that the significantly higher antioxidant activity determined for extracts produced with the DES might reflect the high hesperidin concentration. Furthermore, aside from the antioxidant potency, hesperidin is a bioflavonoid that has been under scrutiny for potential biological effects, and its involvement in several diseases, such as neurological disorders [47] and cancer [48], has been well documented. Thus, the generation of highly hesperidin-enriched extracts employing green methodologies might have an important prospect in biomedical applications.
4. Conclusions
The investigation presented herein showed that a neoteric deep eutectic solvent, composed of glycerol and sodium butyrate, may be highly effective in the recovery of polyphenolic substances from waste orange peels. The appropriate combination with water, along with the use of optimized settings of extraction time and temperature, enabled the development of a process which had exceptional extraction performance, providing a total polyphenol yield of 73.36 mg GAE g−1 DM. The extract produced was also shown to express enhanced antioxidant activity. The chromatographic analysis revealed that the extract obtained under optimized conditions was also particularly enriched in hesperidin (21.81 mg g−1 DM), which represented almost 88% of the polyphenols determined. The prospect seen from the results of this study may be applicable in biorefinery concepts, as it is based on a green solvent and pertains to the valorization of an agri-food waste. Such strategies may enable the establishment of sustainable frames for biowaste exploitation, and a further contribution to bioeconomy policies.
Author Contributions
Conceptualization, D.P.M.; formal analysis, D.K., and D.P.; investigation, D.K., and D.P.; data curation, D.K., and D.P.; writing—original draft preparation, D.P.M. and D.K.; writing—review and editing, D.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jin, Q.; Yang, L.; Poe, N.; Huang, H. Integrated processing of plant-derived waste to produce value-added products based on the biorefinery concept. Trends Food Sci. Technol. 2018, 74, 119–131. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Torres-Valenzuela, L.S.; Ballesteros-Gómez, A.; Rubio, S. Green solvents for the extraction of high added-value compounds from agri-food waste. Food Eng. Rev. 2020, 12, 83–100. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Boateng, N.A.S.; Ma, H. Latest developments in polyphenol recovery and purification from plant by-products: A review. Trends Food Sci. Technol. 2020, 99, 375–388. [Google Scholar] [CrossRef]
- Dassoff, E.S.; Guo, J.X.; Liu, Y.; Wang, S.C.; Li, Y.O. Potential development of non-synthetic food additives from orange processing by-products—A review. Food Qual. Saf. 2021, 5, fyaa035. [Google Scholar] [CrossRef]
- Suri, S.; Singh, A.; Nema, P.K. Recent advances in valorization of citrus fruits processing waste: A way forward towards environmental sustainability. Food Sci. Biotech. 2021, 30, 1601–1626. [Google Scholar] [CrossRef]
- Li, Z.; Smith, K.H.; Stevens, G.W. The use of environmentally sustainable bio-derived solvents in solvent extraction applications—A review. Chin. J. Chem. Eng. 2016, 24, 215–220. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Ravi, H.K. Towards petroleum-free with plant-based chemistry. Cur. Opin. Green Sustain. Chem. 2021, 28, 100450. [Google Scholar] [CrossRef]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging green techniques for the extraction of antioxidants from agri-food by-products as promising ingredients for the food industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef]
- Ivanović, M.; Islamčević Razboršek, M.; Kolar, M. Innovative extraction techniques using deep eutectic solvents and analytical methods for the isolation and characterization of natural bioactive compounds from plant material. Plants 2020, 9, 1428. [Google Scholar] [CrossRef] [PubMed]
- Kalhor, P.; Ghandi, K. Deep eutectic solvents for pretreatment, extraction, and catalysis of biomass and food waste. Molecules 2019, 24, 4012. [Google Scholar] [CrossRef]
- Skarpalezos, D.; Detsi, A. Deep eutectic solvents as extraction media for valuable flavonoids from natural sources. Appl. Sci. 2019, 9, 4169. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep eutectic solvents for the extraction of bioactive compounds from natural sources and agricultural by-products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- Lu, W.; Liu, S. Choline chloride–based deep eutectic solvents (Ch-DESs) as promising green solvents for phenolic compounds extraction from bioresources: State-of-the-art, prospects, and challenges. Biomass Convers. Bioref. 2020, 12, 2949–2962. [Google Scholar] [CrossRef]
- Panić, M.; Andlar, M.; Tišma, M.; Rezić, T.; Šibalić, D.; Bubalo, M.C.; Redovniković, I.R. Natural deep eutectic solvent as a unique solvent for valorisation of orange peel waste by the integrated biorefinery approach. Waste Manag. 2021, 120, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Urios, C.; Vinas-Ospino, A.; Puchades-Colera, P.; Blesa, J.; López-Malo, D.; Frígola, A.; Esteve, M.J. Choline chloride-based natural deep eutectic solvents for the extraction and stability of phenolic compounds, ascorbic acid, and antioxidant capacity from Citrus sinensis peel. LWT 2023, 177, 114595. [Google Scholar] [CrossRef]
- Xu, M.; Ran, L.; Chen, N.; Fan, X.; Ren, D.; Yi, L. Polarity-dependent extraction of flavonoids from citrus peel waste using a tailor-made deep eutectic solvent. Food Chem. 2019, 297, 124970. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Separ. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Viñas-Ospino, A.; Panić, M.; Bagović, M.; Radošević, K.; Esteve, M.; Redovniković, I.R. Green approach to extract bioactive compounds from orange peel employing hydrophilic and hydrophobic deep eutectic solvents. Sustain. Chem. Pharm. 2023, 31, 100942. [Google Scholar] [CrossRef]
- Slim, Z.; Jancheva, M.; Grigorakis, S.; Makris, D.P. Polyphenol extraction from Origanum dictamnus using low-transition temperature mixtures composed of glycerol and organic salts: Effect of organic anion carbon chain length. Chem. Eng. Com. 2018, 205, 1494–1506. [Google Scholar] [CrossRef]
- Kalompatsios, D.; Athanasiadis, V.; Palaiogiannis, D.; Lalas, S.I.; Makris, D.P. Valorization of waste orange peels: Aqueous antioxidant polyphenol extraction as affected by organic acid addition. Beverages 2022, 8, 71. [Google Scholar] [CrossRef]
- Mota, I.; Rodrigues Pinto, P.C.; Novo, C.; Sousa, G.; Guerreiro, O.; Guerra, A.R.; Duarte, M.F.; Rodrigues, A.E. Extraction of polyphenolic compounds from Eucalyptus globulus bark: Process optimization and screening for biological activity. Ind. Eng. Chem. Res. 2012, 51, 6991–7000. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Patsea, M.; Stefou, I.; Grigorakis, S.; Makris, D.P. Screening of natural sodium acetate-based low-transition temperature mixtures (LTTMs) for enhanced extraction of antioxidants and pigments from red vinification solid wastes. Environ. Proc. 2017, 4, 123–135. [Google Scholar] [CrossRef]
- Stefou, I.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Development of sodium propionate-based deep eutectic solvents for polyphenol extraction from onion solid wastes. Clean Technol. Environ. Pol. 2019, 21, 1563–1574. [Google Scholar] [CrossRef]
- Anagnostopoulou, M.A.; Kefalas, P.; Kokkalou, E.; Assimopoulou, A.N.; Papageorgiou, V.P. Analysis of antioxidant compounds in sweet orange peel by HPLC–diode array detection–electrospray ionization mass spectrometry. Biomed. Chrom. 2005, 19, 138–148. [Google Scholar] [CrossRef]
- Abdoun, R.; Grigorakis, S.; Kellil, A.; Loupassaki, S.; Makris, D.P. Process optimization and stability of waste orange peel polyphenols in extracts obtained with organosolv thermal treatment using glycerol-based solvents. ChemEngineering 2022, 6, 35. [Google Scholar] [CrossRef]
- Lakka, A.; Lalas, S.; Makris, D.P. Hydroxypropyl-β-cyclodextrin as a green co-solvent in the aqueous extraction of polyphenols from waste orange peels. Beverages 2020, 6, 50. [Google Scholar] [CrossRef]
- Vilková, M.; Płotka-Wasylka, J.; Andruch, V. The role of water in deep eutectic solvent-base extraction. J. Mol. Liquids 2020, 304, 112747. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, J.-Z.; Wang, L.-T.; Kang, Y.-F.; Meng, Y.; Jiao, J.; Fu, Y.-J. Sustainable deep eutectic solvents preparation and their efficiency in extraction and enrichment of main bioactive flavonoids from sea buckthorn leaves. J. Clean. Prod. 2018, 184, 826–835. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.-G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chrom. A 2013, 1285, 22–30. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Bozinou, E.; Palaiogiannis, D.; Athanasiadis, V.; Chatzilazarou, A.; Lalas, S.I.; Makris, D.P. Glycerol-based deep eutectic solvents for simultaneous organosolv treatment/extraction: High-performance recovery of antioxidant polyphenols from onion solid wastes. Sustainability 2022, 14, 15715. [Google Scholar] [CrossRef]
- Dalmau, E.; Rosselló, C.; Eim, V.; Ratti, C.; Simal, S. Ultrasound-assisted aqueous extraction of biocompounds from orange byproduct: Experimental kinetics and modeling. Antioxidants 2020, 9, 352. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Mihoubi Boudhrioua, N. Phytochemical characteristics of citrus peel and effect of conventional and nonconventional processing on phenolic compounds: A review. Food Rev. Inter. 2017, 33, 587–619. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Boudhrioua, N.M.; Ghoul, M. Effect of different operating conditions on the extraction of phenolic compounds in orange peel. Food Bioprod. Proc. 2015, 96, 161–170. [Google Scholar] [CrossRef]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.-S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Screening the effect of four ultrasound-assisted extraction parameters on hesperidin and phenolic acid content of aqueous citrus pomace extracts. Food Biosci. 2018, 21, 20–26. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Baseggio, A.M.; Mayanga-Torres, P.; Junior, M.R.M.; Rostagno, M.; Martínez, J.; Forster-Carneiro, T. Subcritical water extraction of flavanones from defatted orange peel. J. Supercrit. Fluids 2018, 138, 7–16. [Google Scholar] [CrossRef]
- Chen, X.-M.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.T.; Rodríguez Mellado, J.M. Spectrophotometric and electrochemical assessment of the antioxidant capacity of aqueous and ethanolic extracts of Citrus flavedos. Oxygen 2022, 2, 99–108. [Google Scholar] [CrossRef]
- Choi, S.-S.; Lee, S.-H.; Lee, K.-A. A comparative study of hesperetin, hesperidin and hesperidin glucoside: Antioxidant, anti-inflammatory, and antibacterial activities in vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Termentzi, A.; Gabrieli, C.; Niopas, I.; Georgarakis, M.; Kokkalou, E. The phytochemical analysis and antioxidant activity assessment of orange peel (Citrus sinensis) cultivated in Greece–Crete indicates a new commercial source of hesperidin. Biomed. Chrom. 2009, 23, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and anti-inflammatory effects of citrus flavonoid hesperetin: Special focus on neurological disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Babiker, A.Y.; Anwar, S. Hesperidin, a bioflavonoid in cancer therapy: A review for a mechanism of action through the modulation of cell signaling pathways. Molecules 2023, 28, 5152. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).