Monitoring of Seasonal Under-Vine CO2 Effluxes in a Vineyard under Different Fertilization Practices
Abstract
:1. Introduction
2. Materials and Methods
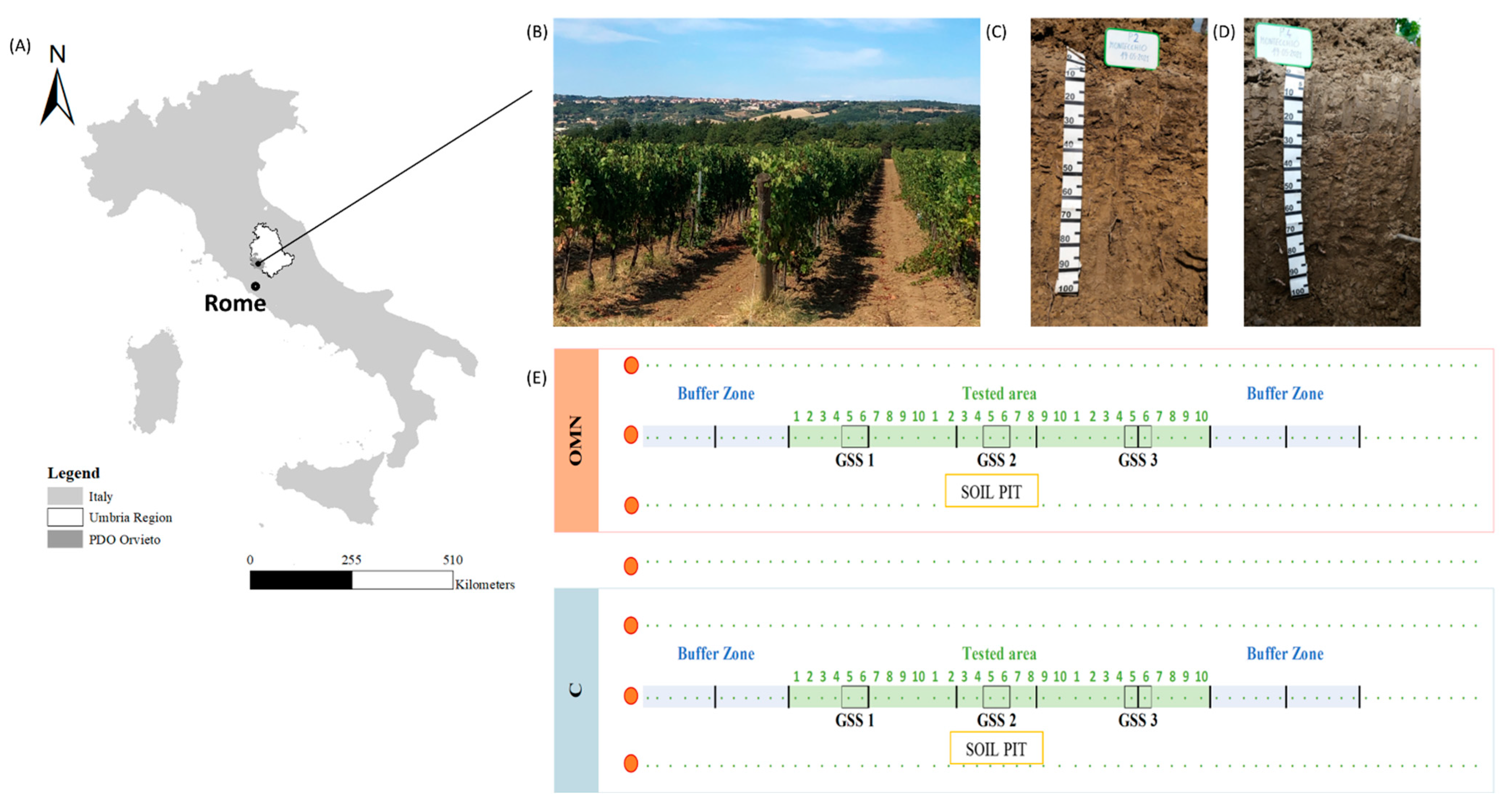
2.1. Study Area Characterization
2.2. Experimental Design
2.3. Soil Surveying
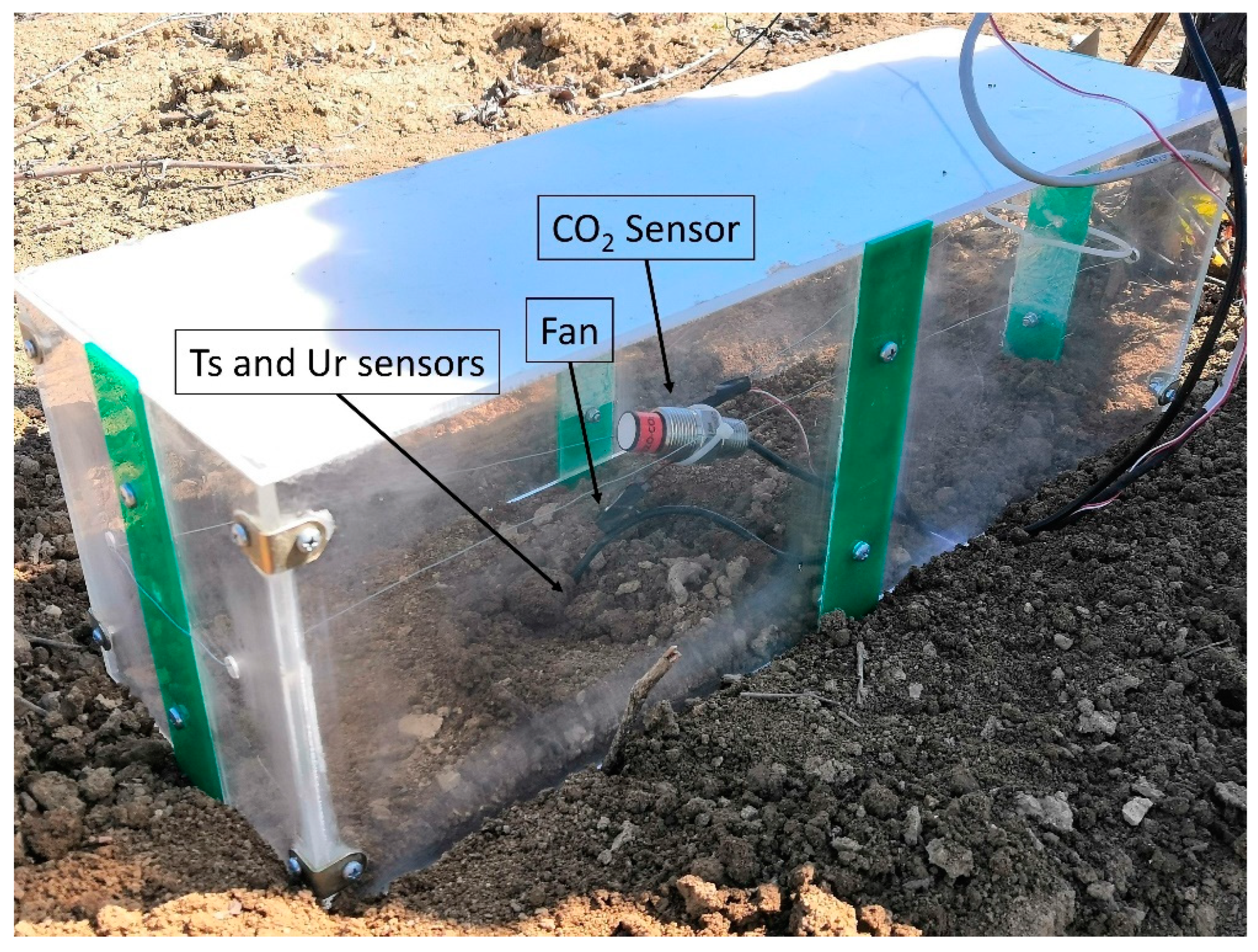
2.4. Soil Respiration Determination by Chamber Prototype
2.4.1. Prototypes for Continuous TSR Assessment
2.4.2. Sensoristics
2.4.3. Installation of SRCps, Data Acquisition, and Processing
2.4.4. Statistical Analysis
3. Results
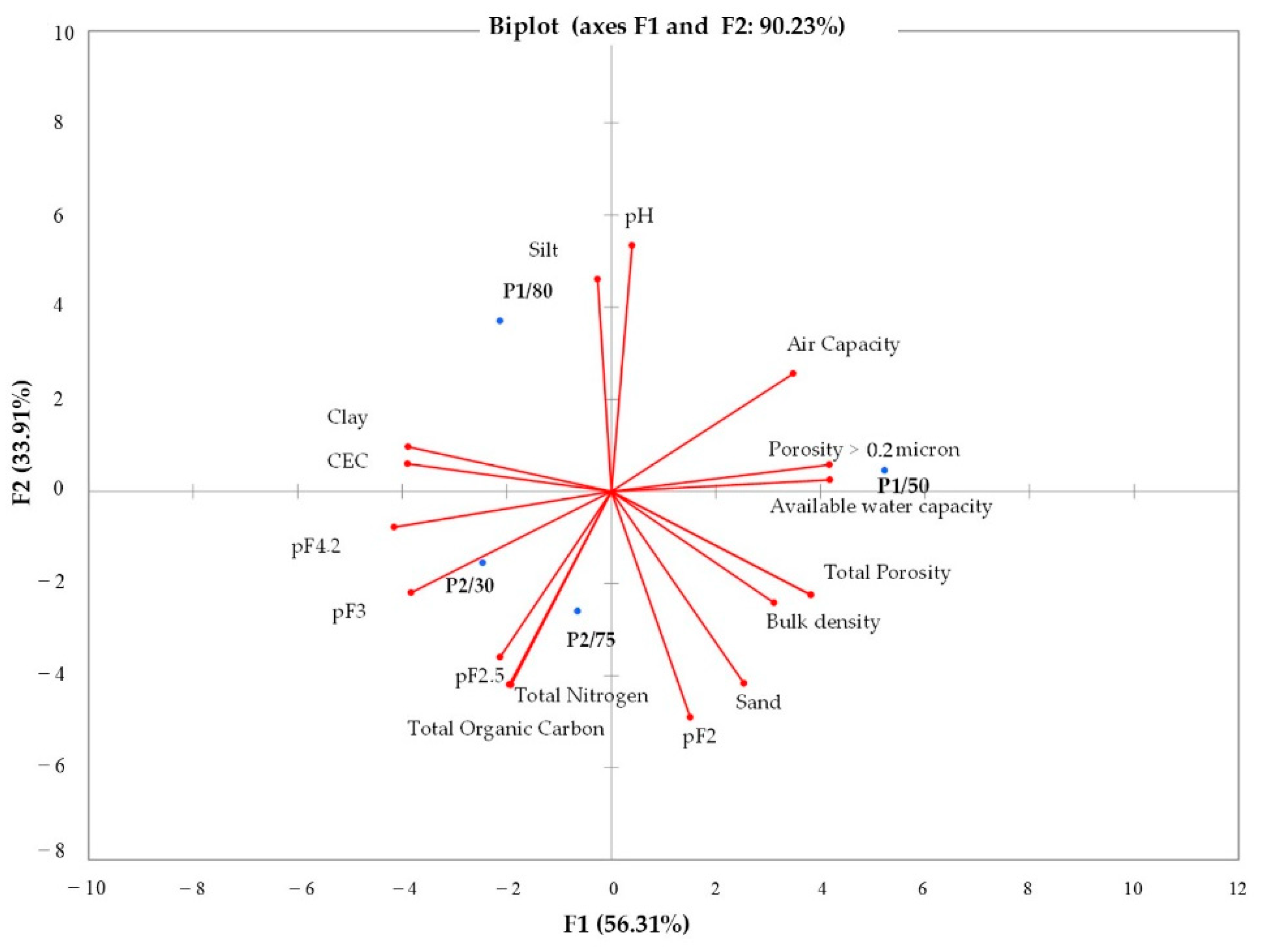
3.1. Soil Characterization
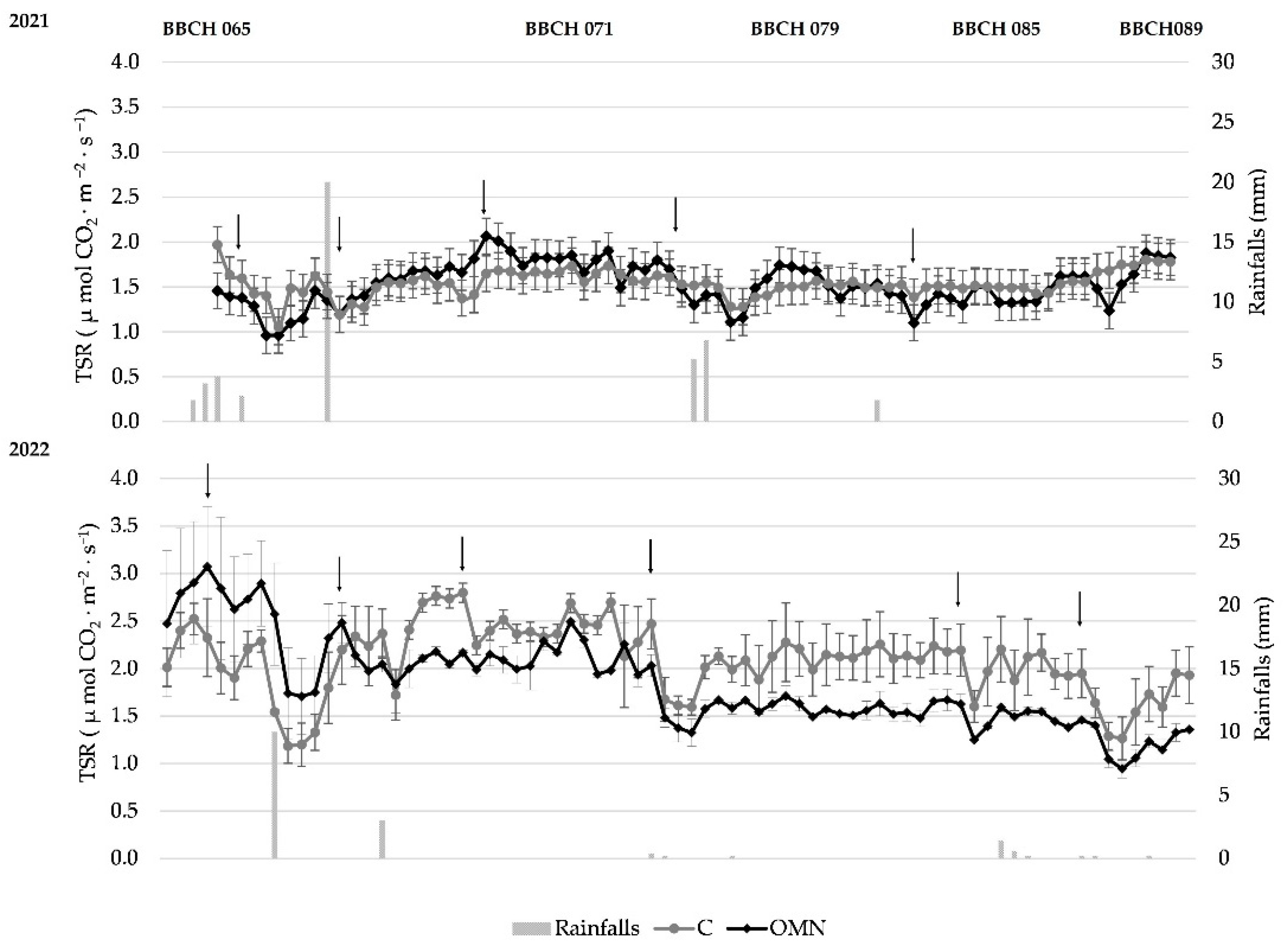
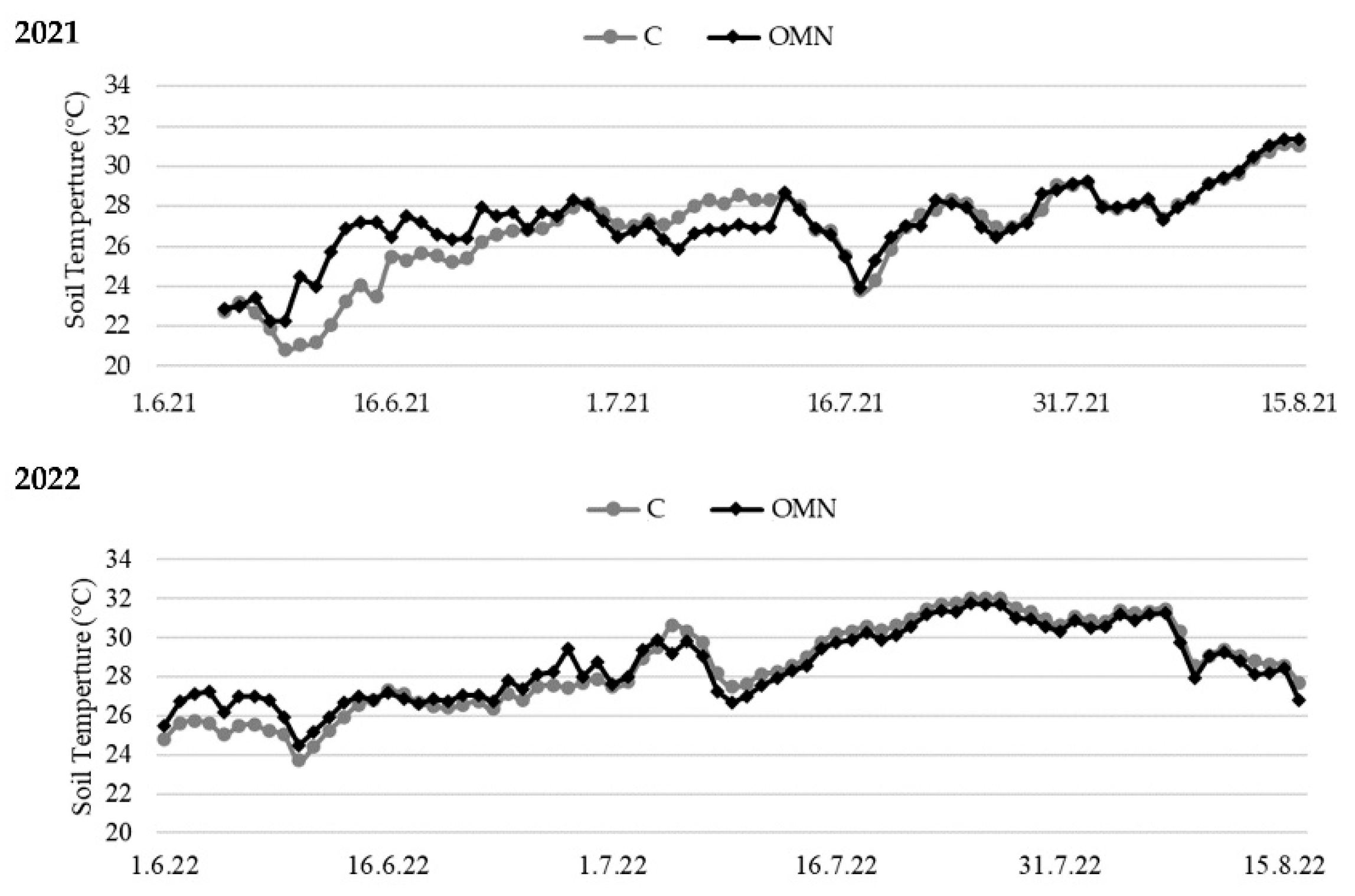
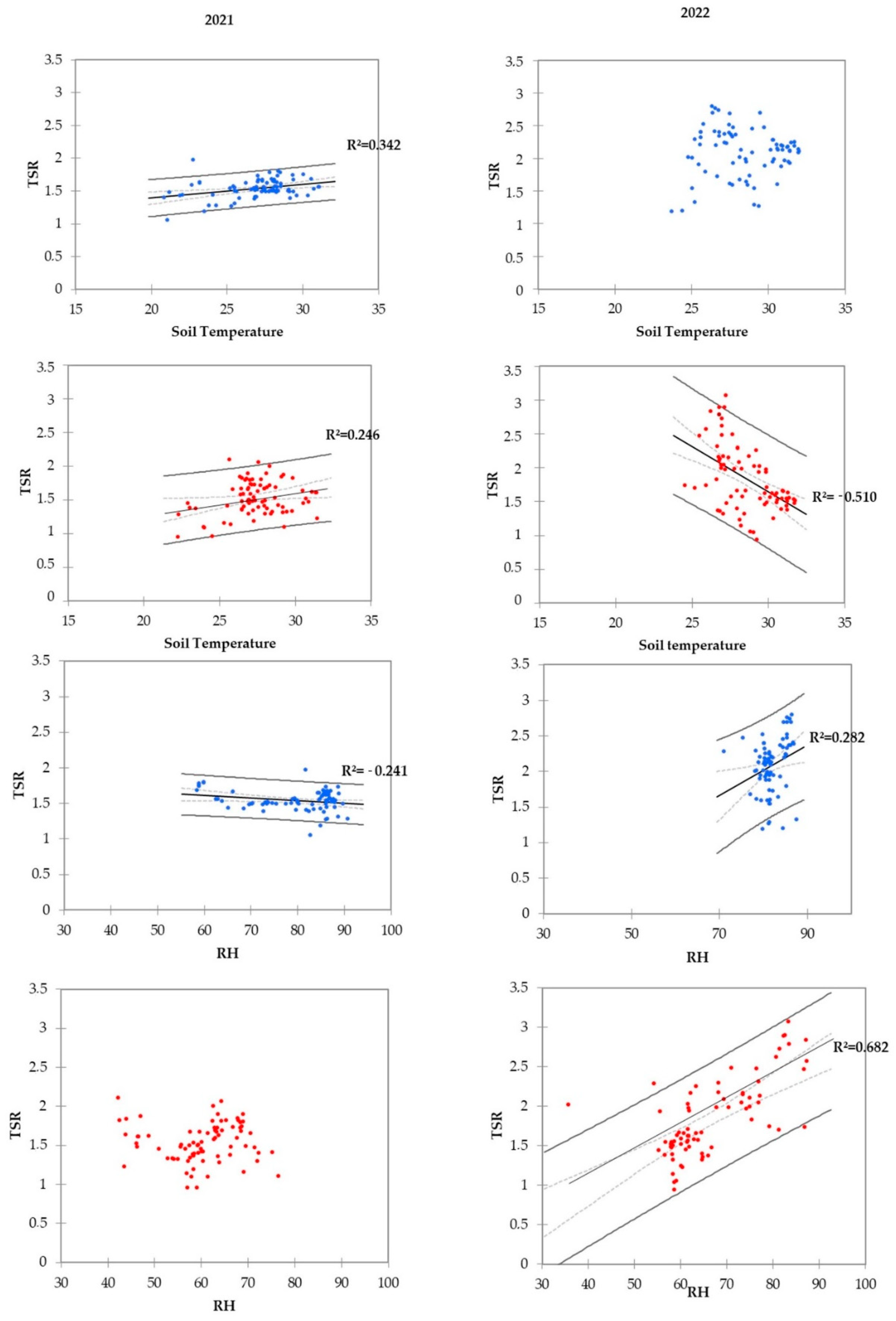
3.2. CO2, Ts, and Ur Measurements of the 2021 and 2022 Seasons
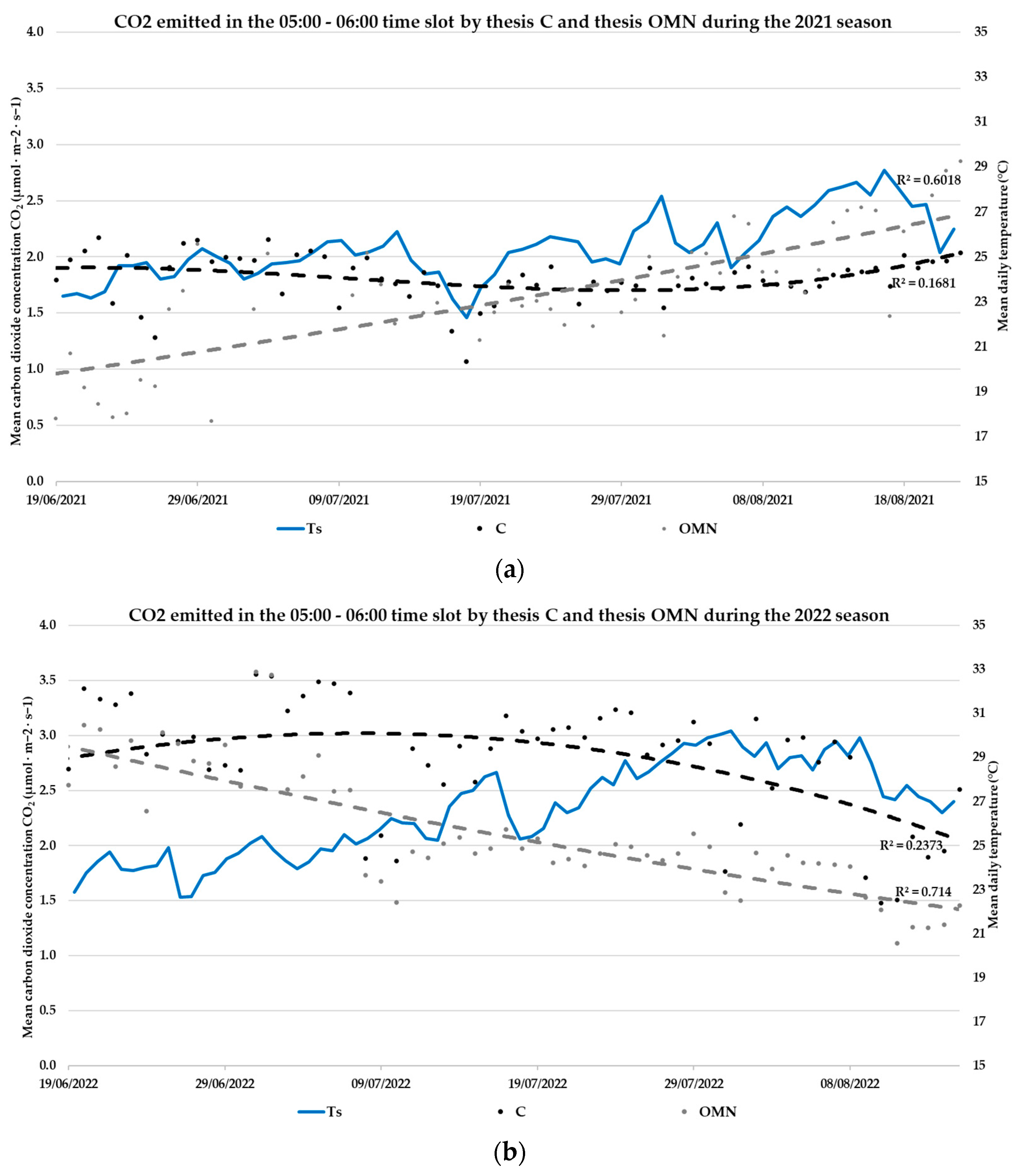
3.3. Daily CO2 Fluxes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- EIP-AGRI Workshop ‘Towards Carbon Neutral Agriculture’: Final Report Workshop Report 24–25 March 2021; European Commission: Brussels, Belgium, 2021.
- Hannah, R.; Max, R.; Pablo, R. CO2 and Greenhouse Gas Emissions; Our World in Data: Oxford, UK, 2020. [Google Scholar]
- Vermunt, D.A.; Negro, S.O.; Van Laerhoven, F.S.J.; Verweij, P.A.; Hekkert, M.P. Sustainability transitions in the agri-food sector: How ecology affects transition dynamics. Environ. Innov. Soc. Transit. 2020, 36, 236–249. [Google Scholar] [CrossRef]
- Munjonji, L.; Ayisi, K.K.; Mafeo, T.P.; Maphanga, T.; Mabitsela, K.E. Seasonal variation in soil CO2 emission and leaf gas exchange of well-managed commercial Citrus sinensis (L.) orchards. Plant Soil 2021, 465, 65–81. [Google Scholar] [CrossRef]
- Montanaro, G.; Doupis, G.; Kourgialas, N.; Markakis, E.; Kavroulakis, N.; Psarras, G.; Koubouris, G.; Dichio, B.; Nuzzo, V. Management options influence seasonal CO2 soil emissions in Mediterranean olive ecosystems. Eur. J. Agron. 2023, 146, 126815. [Google Scholar] [CrossRef]
- Yan, Z.; Miles, D.; Huan-jie, C.; Li-bing, S.; Hui, C. The effects of aerated irrigation on soil respiration, oxygen, and porosity. J. Integr. Agric. 2019, 18, 2854–2868. [Google Scholar]
- Buchmann, N. Biotic and abiotic factors controlling soil respiration rates in Picea abies stands. Soil Biol. Biochem. 2000, 32, 1625–1635. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.; Wan, S. Predominant role of water in regulating soil and microbial respiration and their responses to climate change in a semiarid grassland. Glob. Chang. Biol. 2009, 15, 184–195. [Google Scholar] [CrossRef]
- Chen, W.; Jia, X.; Zha, T.; Wu, B.; Zhang, Y.; Li, C.; Chen, G. Soil respiration in a mixed urban forest in China in relation to soil temperature and water content. Eur. J. Soil Biol. 2013, 54, 63–68. [Google Scholar] [CrossRef]
- Brunori, E.; Farina, R.; Biasi, R. Sustainable viticulture: The carbon-sink function of the vineyard agro-ecosystem. Agric. Ecosyst. Environ. 2016, 223, 10–21. [Google Scholar] [CrossRef]
- Carlisle, E.; Smart, D.; Williams, L.E.; Summers, M. California Vineyard Greenhouse Gas Emissions: Assessment of the Available Literature and Determination of Research Needs; California Sustainable Winegrowing Alliance: San Francisco, CA, USA, 2010. [Google Scholar]
- Comas, L.H.; Bauerle, T.L.; Eissenstat, D.M. Biological and environmental factors controlling root dynamics and function: Effects of root ageing and soil moisture. Aust. J. Grape Wine Res. 2010, 16, 131–137. [Google Scholar] [CrossRef]
- Freibauer, A.; Rounsevell, M.D.A.; Smith, P.; Verhagen, J. Carbon sequestration in the agricultural soils of Europe. Geoderma 2004, 122, 1–23. [Google Scholar] [CrossRef]
- Ma, T.; Zhu, G.; Ma, J.; Kun, Z.; Wang, S.; Tuo, H.; Shasha, S. Soil respiration in an irrigated oasis agroecosystem: Linking environmental controls with plant activities on hourly, daily and monthly timescales. Plant Soil 2020, 447, 347–364. [Google Scholar] [CrossRef]
- Tedesco, S.; Fevereiro, P.; Kragler, F.; Pina, A. Plant grafting and graft incompatibility: A review from the grapevine perspective. Sci. Hortic. 2022, 299, 111019. [Google Scholar] [CrossRef]
- Moyano, F.E.; Manzoni, S.; Chenu, C. Responses of soil heterotrophic respiration to moisture availability: An exploration of processes and models. Soil Biol. Biochem. 2013, 59, 72–85. [Google Scholar] [CrossRef]
- Phillips, S.C.; Varner, R.K.; Frolking, S.; Munger, J.W.; Bubier, J.L.; Wofsy, S.C.; Crill, P.M. Interannual, seasonal, and diel variation in soil respiration relative to ecosystem respiration at a wetland to upland slope at Harvard Forest. J. Geophys. Res. Biogeosci. 2010, 115, G02019. [Google Scholar] [CrossRef]
- De Dato, G.; Lagomarsino, A.; Lellei-Kovacs, E.; Liberati, D.; Abou Jaoudé, R.; Marabottini, R.; Stazi, S.R.; Guidolotti, G.; Kovacs-Land, E.; Kroel-Dulay, G.; et al. The response of soil CO2 efflux to water limitation is not merely a climatic issue: The role of substrate availability. Forests 2017, 8, 241. [Google Scholar] [CrossRef]
- Payen, F.T.; Sykes, A.; Aitkenhead, M.; Alexander, P.; Moran, D.; MacLeod, M. Soil organic carbon sequestration rates in vineyard agroecosystems under different soil management practices: A meta-analysis. J. Clean. Prod. 2021, 290, 125736. [Google Scholar] [CrossRef]
- Pou, A.; Balda, P.; Cifre, J.; Ochogavia, J.M.; Ayestaran, B.; Guadalupe, Z.; Llompart, M.; Bota, J.; Martínez, L. Influence of non-irrigation and seasonality on wine colour, phenolic composition and sensory quality of a grapevine (Vitis vinifera cv. Callet) in a Mediterranean climate. OENO One 2023, 57, 217–233. [Google Scholar] [CrossRef]
- Iovieno, P.; Morra, L.; Leone, A.; Pagano, L.; Alfani, A. Effect of organic and mineral fertilizers on soil respiration and enzyme activities of two Mediterranean horticultural soils. Biol. Fertil. Soils 2009, 45, 555–561. [Google Scholar] [CrossRef]
- Sun, Q.; Granco, G.; Groves, L.; Voong, J.; Van Zyl, S. Viticultural Manipulation and New Technologies to Address Environmental Challenges Caused by Climate Change. Climate 2023, 11, 83. [Google Scholar] [CrossRef]
- Bastida, F.; Kandeler, E.; Moreno, J.L.; Ros, M.; García, C.; Hernández, T. Application of fresh and composted organic wastes modifies structure, size and activity of soil microbial community under semiarid climate. Appl. Soil Ecol. 2008, 40, 318–329. [Google Scholar] [CrossRef]
- Jie, Z.; Yanzhi, J.; Yanjie, G.; Xing, Y.; Yannan, L.; Jian, H.; Yang, L.; Chen, W.; Wenzan, W.; Yusha, L.; et al. Responses of soil respiration and microbial community structure to fertilizer and irrigation regimes over 2 years in temperate vineyards in North China. Sci. Total Environ. 2022, 840, 156469. [Google Scholar]
- Delle Vedove, G.; Alberti, G.; Zuliani, M.; Peressotti, A. Automated monitoring of soil respiration: An improved automatic chamber system. Ital. J. Agron. 2007, 2, 377–382. [Google Scholar] [CrossRef]
- Egidio, L.; Maria, A.; Vitale, N.; Cristos, X.; Giuseppe, C. Variability of total soil respiration in a Mediterranean vineyard. Soil Res. 2015, 53, 531–541. [Google Scholar]
- Philippe, R.; Gordon, L.H. Measurement of Soil Respiration in situ: Chamber Techniques. Micrometeorol. Agric. Syst. 2005, 47, 247–286. [Google Scholar]
- Phillips, C.L.; Nickerson, N. Soil Respiration. In Reference Module in Earth Systems and Environmental Sciences; Elias, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Masnaghetti, A. I Cru di Enogea, Orvieto: Vigneti e Zone; Alessandro Masnaghetti Editore: Monza, Italy, 2015. [Google Scholar]
- Biasi, R.; Brunori, E.; Ferrara, C.; Salvati, L. Assessing impacts of climate change on phenology and quality traits of Vitis vinifera L.: The contribution of local knowledge. Plants 2019, 8, 121. [Google Scholar] [CrossRef]
- Horwath, W. Carbon cycling and formation of soil organic matter. In Soil Microbiology, Ecology and Biochemistry, 3rd ed.; Eldor, A.P., Ed.; Academic Press: Cambridge, MA, USA, 2007; pp. 303–339. [Google Scholar]
- Goupil, K.; Nkongolo, K. Assessing soil respiration as an indicator of soil microbial activity in reclaimed metal contaminated lands. Am. J. Environ. Sci. 2014, 10, 403–411. [Google Scholar] [CrossRef]
- Soil Survey Laboratory Staff. Soil Survey Laboratory Manual, Soil Survey Investigation Report No. 42; Government Printing Office: Washington, DC, USA, 1996. [Google Scholar]
- Rubio, C.M. A Laboratory Procedure to Determine the Thermal Properties of Silt Loam Soils Based on ASTM D 5334. Appl. Ecol. Environ. Sci. 2013, 1, 45–48. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 8th ed.; Government Printing Office: Washington, DC, USA, 1998; p. 541. [Google Scholar]
- ISO 11260:2018; Soil Quality—Determination of Effective Cation Exchange Capacity and Base Saturation Level Using Barium Chloride Solution. International Organization for Standardization: Geneva, Switzerland, 1994.
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis, Part—Chemical Methods; Soil Science Society of America: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- ISO 10694:1995(E); Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). International Organization for Standardization: Geneva, Switzerland, 1995.
- Pleysier, J.L.; Juo, A.S.R. A Single-Extraction Method Using Silver-Thiourea for Measuring Exchangeable Cations and Effective CEC in Soils with Variable Charges. Soil Sci. 1980, 129, 205–211. [Google Scholar] [CrossRef]
- Salter, P.J.; Berry, G.; Williams, J.B. The influence of texture on the moisture characteristics of soils: III, Quantitative relationships between particle size, composition, and available-water capacity. Eur. J. Soil Sci. 1966, 17, 93–98. [Google Scholar] [CrossRef]
- Hu, X.; Hirota, M.; Wuyunna; Kawada, K.; Li, H.; Meng, S.; Tamura, K.; Kamijo, T. Responses in gross primary production of Stipa krylovii and Allium polyrhizum to a temporal rainfall in a temperate grassland of Inner Mongolia, China. J. Arid Land 2019, 11, 824–836. [Google Scholar] [CrossRef]
- Davidson, E.A.; Savage, K.; Verchot, L.; Navarro, R. Minimizing artifacts and biases in chamber-based measurements of soil respiration. Agric. For. Meteorol. 2002, 113, 21–37. [Google Scholar] [CrossRef]
- Adafruit-SHT-10 Mesh-Protected Weather-Proof Temperature/Humidity Sensor. Available online: https://www.adafruit.com/product/1298 (accessed on 7 December 2020).
- AtlasScientific—EZO-CO2™ Embedded Carbon Dioxide Sensor Title of Site. Available online: https://atlas-scientific.com/probes/co2-sensor/ (accessed on 7 December 2020).
- Santos, J.A.; Yang, C.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.T.; Correia, C.; Moriondo, M.; Bindi, M.; Leolini, L.; et al. Short-term adaptation of European viticulture to climate change: An overview from the H2020 Clim4Vitis action. IVES Tech. Rev. Vine Wine 2021. [Google Scholar] [CrossRef]
- Pingel, M.; Reineke, A.; Leyer, I. Disentangling the mixed effects of soil management on microbial diversity and soil functions: A case study in vineyards. Sci. Rep. 2023, 13, 3568. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ferreiro, J.; Fu, S. Biological indices for soil quality evaluation: Perspectives and limitations. Land Degrad. Dev. 2016, 27, 14–25. [Google Scholar] [CrossRef]
- Costa, J.M.; Egipto, R.; Aguiar, F.C.; Marques, P.; Nogales, A.; Madeira, M. The role of soil temperature in mediterranean vineyards in a climate change context. Front. Plant Sci. 2023, 14, 1145137. [Google Scholar] [CrossRef]
- Doğan, B.; Gülser, C. Assessment of Soil Quality for Vineyard Fields: A Case Study in Menderes District of Izmir, Turkey. Eurasian J. Soil Sci. 2019, 8, 176–183. [Google Scholar] [CrossRef]
- Kurtural, S.K. Desired Soil Properties for Vineyard Site Preparation. Universtiy of Kentucky, College of Agriculture. Coop. Ext. Serv. 2011, 31. [Google Scholar]
- Lazcano, C.; Decock, C.; Wilson, S.G. Defining and Managing for Healthy Vineyard Soils, Intersections with the Concept of Terroir. Front. Environ. Sci. 2020, 8, 68. [Google Scholar] [CrossRef]
- Agota, H.; Eszter, T.; Györgyi, G.; Dencső, M.; Imre, P. Soil CO2 and N2O Emission Drivers in a Vineyard (Vitis vinifera) under Different Soil Management Systems and Amendments. Sustainability 2018, 10, 1811. [Google Scholar]
- Quan, Z.; Richard, P.P.; Stefano, M.; Russell, L.S.; Andrew, C.O.; Adrien, F.; Edoardo, D.; Rodrigo, V.; Kimberly, N. Changes in photosynthesis and soil moisture drive the seasonal soil respiration-temperature hysteresis relationship. Agric Meteorol. 2018, 259, 184–195. [Google Scholar]
- Annette, B.; Meriem, S.; Aurore, K.; Pierre, R. Soil microbial community responses to heat wave components: Drought and high temperature. Clim. Res. 2015, 66, 243–264. [Google Scholar]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Federica, G.; Patrick, M.; Nicola, B.; Lorenzo, L.; Flavio, F.; Diego, T. Influence of compost addition on soil properties, root growth and vine performances of Vitis vinifera cv Cabernet sauvignon. Sci. Hortic. 2017, 225, 88–95. [Google Scholar]
- Wang, B.; Zha, T.S.; Jia, X.; Wu, B.; Zhang, Y.Q.; Qin, S.G. Soil moisture modifies the response of soil respiration to temperature in a desert shrub ecosystem. Biogeosciences 2014, 11, 259–268. [Google Scholar] [CrossRef]
- Soil Quality Indicators. USDA Natural Resources Conservation Service. Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Official Soil Series Descriptions. Available online: https://www.nrcs.usda.gov/sites/default/files/2022-10/indicator_sheet_guide_sheet.pdf (accessed on 18 February 2023).
- Lal, R. Challenges and opportunities in soil organic matter research. Eur. J. Soil Sci. 2009, 60, 158–169. [Google Scholar] [CrossRef]
- Pete, S.; Changming, F.; Julián, J.M.; John, M. Impact of Global Warming on Soil Organic Carbon. Adv. Agron. 2008, 97, 1–43. [Google Scholar]
- Marion, N.; Mark, J.H. Warming increases soil respiration in a carbon-rich soil without changing microbial respiratory potential. Biogeosciences 2020, 17, 4405–4420. [Google Scholar]
- Ferreira, C.S.; Seifollahi-Aghmiuni, S.; Destouni, G.; Ghajarnia, N.; Kalantari, Z. Soil degradation in the European Mediterranean region: Processes, status and consequences. Sci. Total Environ. 2022, 805, 150106. [Google Scholar] [CrossRef]
- Miko, U.F.K. The temperature dependence of organic-matter decomposition-still a topic of debate. Soil. Biol. Biochem. 2006, 38, 2510–2518. [Google Scholar]
- Eric, D.; Ivan, J. Temperature Sensitivity of Soil Carbon Decomposition and Feedbacks to Climate Change. Nature 2006, 440, 165–173. [Google Scholar]
- Miguel, C.; Margarida, V.; José, E.; Ricardo, E.; Carlos, L.; Hipolito, M.; Manuela, C.M. Modern viticulture in southern Europe: Vulnerabilities and strategies for adaptation to water scarcity. Agric. Water Manag. 2015, 164, 5–18. [Google Scholar]
- Kerri, S.; Danielle, P.; Eli, C.; Robert, S.; David, S. A Vineyard Agroecosystem: Disturbance and Precipitation Affect Soil Respiration under Mediterranean Conditions. Soil Sci. Soc. Am. J. 2010, 74, 231–239. [Google Scholar]


| cm H2O | KPa | pF | bar |
|---|---|---|---|
| 0 | 0 | 0 | 0 |
| 100 | 10 | 2 | 0.1 |
| 333 | 33.3 | 2.5 | 0.333 |
| 1000 | 100 | 3 | 1 |
| 15,000 | 1500 | 4.2 | 15 |
| Profile | Profile P1 | Profile P2 | ||||
|---|---|---|---|---|---|---|
| Horizon denomination | Ap2 | Bk | BC | Ap1 | Bw1 | Bw2 |
| Horizon depth | 10–35 cm | 35–55 cm | 55–100 cm | 0–25 cm | 25–60 cm | 60–80 cm |
| Sand (%) | 32 | 37 | 24 | 34 | 31 | 38 |
| Silt (%) | 35 | 35 | 39 | 28 | 35 | 27 |
| Clay (%) | 33 | 28 | 37 | 38 | 34 | 35 |
| Textural class | CL | CL | CL | CL | CL | CL |
| Profile | Profile P1 | Profile P2 | ||||
|---|---|---|---|---|---|---|
| Horizon denomination | Ap2 | Bk | BC | Ap1 | Bw1 | Bw2 |
| Horizon depth | 10–35 cm | 35–55 cm | 55–100 cm | 0–25 cm | 25–60 cm | 60–80 cm |
| pH | 8.4 | 8.4 | 8.5 | 8.3 | 8.3 | 8.3 |
| Organic matter (%) | 0.66 | 0.39 | 0.33 | 1.25 | 0.92 | 0.66 |
| CSC (meq/100 g) | 24.19 | 20.35 | 28.48 | 29.98 | 26.04 | 27.25 |
| Total N (%) | 0.046 | 0.03 | 0.026 | 0.079 | 0.062 | 0.046 |
| Horizon/Sample | Saturation | Moisture Content by Volume % pF | Available Water Capacity | Air Capacity | Total Porosity % | 60% | Porosity > 0.2 Microns % | Apparent Density in gr/cm3 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pF2 | pF2.5 | pF3 | pF4.2 | ||||||||
| P1/50 | 45.5 | 38.5 | 35.5 | 32 | 20 | 18.5 | 6.9 | 45.5 | 27.3 | 25.4 | 1.6 |
| P1/80 | 44.8 | 36.3 | 36.3 | 33.7 | 23.9 | 14.2 | 6.7 | 44.8 | 26.9 | 21 | 1.5 |
| P2/30 | 45 | 38.6 | 36.9 | 34.6 | 24.5 | 14.1 | 6.4 | 45 | 27 | 20.5 | 1.5 |
| P2/75 | 45.2 | 38.6 | 38.6 | 34.4 | 23.8 | 14.7 | 6.6 | 45.2 | 27.1 | 21.3 | 1.6 |
| Soil Variables | Saturation | pF2 | pF2.5 | pF3 | pF4.2 | Available Water Capacity (AWC) (%) | Air Capacity (AC) (%) | Total Porosity (TP) (%) | Porosity > 0.2 Microns (%) | Bulk Density (BD) (gr/cm3) | Sand (%) | Silt (%) | Clay (%) | pH | Soil Organic Content (SOC) (%) | CEC (meq/100 g) | Total Nitrogen (TN) (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saturation | 1 | ||||||||||||||||
| pF2 | 0.699 | 1 | |||||||||||||||
| pF2.5 | −0.172 | 0.299 | 1 | ||||||||||||||
| pF3 | −0.659 | 0.027 | 0.764 | 1 | |||||||||||||
| pF4.2 | −0.839 | −0.236 | 0.621 | 0.962 | 1 | ||||||||||||
| Available Water Capacity (AWC) (%) | 0.884 | 0.334 | −0.582 | −0.931 | −0.995 | 1 | |||||||||||
| Air Capacity (AC) (%) | 0.563 | −0.198 | −0.566 | −0.928 | −0.872 | 0.820 | 1 | ||||||||||
| Total Porosity (TP) (%) | 1.000 | 0.699 | −0.172 | −0.659 | −0.839 | 0.884 | 0.563 | 1 | |||||||||
| Porosity > 0.2 microns (%) | 0.858 | 0.271 | −0.599 | −0.952 | −0.999 | 0.997 | 0.858 | 0.858 | 1 | ||||||||
| Bulk Density (BD) (gr/cm3) | 0.870 | 0.560 | 0.198 | −0.464 | −0.646 | 0.674 | 0.555 | 0.870 | 0.665 | 1 | |||||||
| Sand | 0.873 | 0.870 | 0.320 | −0.225 | −0.477 | 0.547 | 0.198 | 0.873 | 0.508 | 0.894 | 1 | ||||||
| Silt | −0.421 | −0.677 | −0.821 | −0.320 | −0.084 | 0.022 | 0.191 | −0.421 | 0.053 | −0.688 | −0.800 | 1 | |||||
| Clay | −0.908 | −0.569 | 0.533 | 0.790 | 0.904 | −0.941 | −0.579 | −0.908 | −0.915 | −0.596 | −0.627 | 0.034 | 1 | ||||
| pH | −0.321 | −0.890 | −0.629 | −0.479 | −0.229 | 0.128 | 0.585 | −0.321 | 0.193 | −0.302 | −0.674 | 0.761 | 0.135 | 1 | |||
| Soil Organic Content (SOC) (%) | −0.109 | 0.631 | 0.515 | 0.712 | 0.550 | −0.461 | −0.880 | −0.109 | −0.523 | −0.213 | 0.229 | −0.406 | 0.146 | −0.867 | 1 | ||
| CEC (meq/100 g) | −0.881 | −0.512 | 0.593 | 0.822 | 0.917 | −0.948 | −0.606 | −0.881 | −0.926 | −0.556 | −0.570 | −0.036 | 0.997 | 0.070 | 0.191 | 1 | |
| Total Nitrogen (TN) (%) | −0.102 | 0.636 | 0.507 | 0.705 | 0.542 | −0.453 | −0.876 | −0.102 | −0.515 | −0.211 | 0.232 | −0.403 | 0.136 | −0.868 | 1.000 | 0.181 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirigliano, P.; Cresti, A.; Rengo, A.; D’Arcangelo, M.E.M.; Brunori, E. Monitoring of Seasonal Under-Vine CO2 Effluxes in a Vineyard under Different Fertilization Practices. Horticulturae 2023, 9, 1107. https://doi.org/10.3390/horticulturae9101107
Cirigliano P, Cresti A, Rengo A, D’Arcangelo MEM, Brunori E. Monitoring of Seasonal Under-Vine CO2 Effluxes in a Vineyard under Different Fertilization Practices. Horticulturae. 2023; 9(10):1107. https://doi.org/10.3390/horticulturae9101107
Chicago/Turabian StyleCirigliano, Pasquale, Andrea Cresti, Andrea Rengo, Mauro Eugenio Maria D’Arcangelo, and Elena Brunori. 2023. "Monitoring of Seasonal Under-Vine CO2 Effluxes in a Vineyard under Different Fertilization Practices" Horticulturae 9, no. 10: 1107. https://doi.org/10.3390/horticulturae9101107
APA StyleCirigliano, P., Cresti, A., Rengo, A., D’Arcangelo, M. E. M., & Brunori, E. (2023). Monitoring of Seasonal Under-Vine CO2 Effluxes in a Vineyard under Different Fertilization Practices. Horticulturae, 9(10), 1107. https://doi.org/10.3390/horticulturae9101107











