Abstract
Aiming at evaluating new candidate species for the cut flower market of Greece and beyond, in this study, the vase life of three Greek tulip plant species, Tulipa cretica ‘Hilde’ (CRH, local endemic of Crete, Greece), T. clusiana ‘Chrysantha’ (CLC, naturalized in Chios Island, Greece), and T. australis (AUS, native in the Mediterranean and Greece), was investigated in comparison to the commercial tulip hybrid Île-de-France (IDF). To this end, pre-cooled at 4 °C bulbs of the abovementioned Greek tulip plant species were bought from Dutch nurseries and grown in pots placed in unheated greenhouses located at two different climatic conditions in Northern Greece. The plants were uprooted when the flowers reached a slightly open stage. Half of the flowering stems were immediately placed into bottles with deionized water, while the rest were placed in a preservative solution containing citric acid 5% and sulfuric acid 1% and then remained under laboratory conditions until the entire tepal wilted (end of vase life). The measurements performed concerned: (a) flower stem length and flower maximum diameter, (b) fresh weight (FW) of initial stems, leaves, flowers, and bulbs and at the end of vase life, (c) flower color parameters (L, a, b, c, and H) in all treated flowers, (d) leaf chlorophyll content (SPAD values), and (e) initial and final water volume after removing the flowering stems. The aforementioned measurements showed that CRH cut flowers may exhibit consistent floral opening patterns and were associated with a long mean vase life of 5.7 days, which can be further prolonged to 6.5 days by carefully selecting a cultivation location with proper climatic conditions. The vase life of CLC cut flowers was significantly affected by the climatic parameters (temperature) of the area where the plants were cultivated. The immersion of cut flowering stems in a preservative solution with citric and sulfuric acids did not yield a notable increase in the longevity of cut flowers during the postharvest period. Moreover, this treatment did not have any significant impact on leaf chlorophyll content or flower color at the end of the flowers’ vase life. The data of this work show that cut flowers from the native species T. cretica and T. clusiana have satisfactory vase life, especially when plants were grown in favorable climate condition; the latter is an important criterion for their entry into the cut flower market.
1. Introduction
Floriculture is probably the most competitive sector of agriculture since many different plant species are produced and marketed [1,2]. To stand the global competitiveness and meet consumers’ demand for cut flowers, the ornamental industry frequently introduces new flower crops with special features [1,2]. Typically, the native flora of different regions serves as a reservoir from which new floricultural species originate, and annually, hundreds of new wild plant species are evaluated worldwide for their potential to compete in the ornamental plant market. Noteworthy efforts to assess the sustainable exploitation potential for the ornamental sector of neglected and underutilized local endemics originating from selected Mediterranean regions have taken place recently, resulting in the identification of both opportunities and extant gaps [3]. Native tulips originating from the Balkans and Central Asia are a typical example of wild plant species from which many commercial tulip varieties were developed [4,5]. Although the utilization of endemic and native species offers many benefits, such as new interesting ornamental features in combination with reduced crop requirements due to natural adaptation to climatic conditions and inherent resistance to pests and diseases [3,6,7], cut flowers from wild-growing plants cannot be placed directly into the flower market [8]. The high level of variability in yield, the unbalanced architecture of the flowering parts (shoot length, foliage shape, number, and size of the flowering buds), and irregular time of flowering accompanied by their short postharvest life may undermine and reduce their aesthetic value and concomitant marketability [1,9]. Preharvest and postharvest factors are strongly affecting the abovementioned features of cut flowers [10,11]. Genetic/varietal factors, plant age, climatic conditions during the growing period, plant health, cultivation practices, and the harvesting stage are among the most important preharvest factors [12], which are responsible for creating a product with good quality (large flower with desired coloring, high water and carbohydrates content). In addition, bulb size and bulb weight of bulbous plants play a significant role both on geophytes flower quality and their postharvest vase life [13,14,15]. On the other hand, postharvest treatments mainly aim to maintain the cut flowers’ quality characteristics for as long as possible in order to extend their vase life. For this purpose, appropriate storage conditions and handling are applied to reduce transpiration, maintain a high concentration of carbohydrates in plant tissues, limit microbial infection, and inhibit ethylene biosynthesis and action.
To achieve the abovementioned objectives, flower preservative solutions, with both surfactant and antimicrobial effects, are usually used to prolong the postharvest life of cut flowers, particularly when flowers cannot be kept at controlled temperature and relative humidity conditions to decrease their respiration and dehydration [16]. Specific preservative solutions containing substances with antimicrobial activity, such as sodium hypochlorite [17], silver nitrate [18], physan 20, 8-hydroxyquinoline [19], silver thiosulfate [18], and chlorine dioxide [20], can prevent the growth of microbes inside the flowering stem, which are responsible for occlusions of shoots vessels. Thus, the addition of these substances in the preservation solution facilitates the absorption of water and increases the vase life of the flowers. The extended postharvest life of flowers guarantees customer contentment and fosters sustained product purchase, supporting the entire flower production network and trade chain.
This study aimed to assess the vase life of three Greek tulip species, Tulipa cretica ‘Hilde’, T. clusiana ‘Chrysantha’, and T. australis, with shorter flower stem length, smaller flower size and leaf area, as well as greater adaptability to the Mediterranean climate in comparison to a commercially cultivated hybrid variety Île-de-France. The investigation sought to determine their suitability for potential introduction in the cut flower market of Greece in a sustainable way, with vase life serving as a crucial evaluation criterion.
2. Materials and Methods
2.1. Cultivation Conditions and Sampling
In February of 2022, three-month pre-cooled (at 4 °C) bulbs from three Greek native tulip plant species, i.e., T. cretica ‘Hilde’ (local endemic of Crete, Greece), T. clusiana ‘Chrysantha’ (naturalized in Chios Island, Greece), T. australis (native in the Mediterranean region including Greece), and the tulip hybrid Île-de-France (Figure 1), were planted in 3.6 L pots. Plants were grown in unheated greenhouses covered with plastic film, which were installed in Northern Greece in the Institute of Plant Breeding and Genetic Resources (Hellenic Agricultural Organization Demeter) located in Thermi and in commercial greenhouses (Thermokipia Athina) located in Epanomi (Figure 1). The experiments were conducted in the framework of the research project TULIPS.GR. The substrate where plants were grown was a mixture of peat and sand (4:1, v:v). Plants were drip-irrigated regularly on a weekly basis with adequate amounts of water, similar to commercial practice. All plants in both locations received integrated fertilization with a complete mix of nutrients, similar to popular commercial fertilization regimes. A total of 658 pots with flowering plants from the different species (Table 1) were transported to the Laboratory of Floriculture and Landscape Architecture of the University of Thessaly, Greece, during March and April 2022 for postharvest evaluation.

Figure 1.
Greek tulip plant species investigated in this study with views of their experimental cultivation. (A) Tulipa cretica ‘Hilde’ (local endemic of Crete, Greece); (B) T. clusiana ‘Chrysantha’ (naturalized in Chios Island, Greece); (C) T. australis (native in the Mediterranean region including Greece); (D) Tulip hybrid Île-de-France; (E) Experimental cultivation in unheated research greenhouse (Thermi, Northern Greece); (F) Experimental cultivation in a commercial greenhouse (Epanomi, Northern Greece).

Table 1.
Number of plants from each cultivation location harvested at different days and placed in deionized water with and without the preservative for the four studied tulip species (Tulipa cretica ‘Hilde’ (CRH), Tulipa australis (AUS), Tulipa clusiana ‘Chrysantha’ (CLC), and tulip hybrid Île-de-France (IDF)).
2.2. Sample Preparation
All plants were uprooted when flowers were at a slightly open stage (Figure 2, Stage 1). The bulbs were separated, and half of the flowering stems from each species were placed directly into 0.5 L plastic bottles containing a pre-weighed amount of deionized water, while the other half of flowering stems were placed in the bottles after pulsing for 10 s within a preservative solution containing citric acid 5%, sulfuric acid 1%, and water 94%. The flowering stems were placed in a room with natural lighting. The room temperature and relative humidity were recorded in real time.

Figure 2.
Postharvest stages of Tulipa australis flowers as defined during postharvest evaluation: (1) slightly open; (2) half-opened; (3) fully opened; (4) slightly wilted at the tepal edge; (5) whole tepal wilted; (6) whole tepal severely wilted with abscission.
2.3. Measurements
After the separation from the bulb, the flower stem length and maximum diameter were recorded.
In addition, the study also involved the measurement of certain parameters every two days. These included (a) flower color parameters such as L, a, b, c, and H, which were measured in three different tepals in each one of all treated flowers using a PCE-CSM-1 colorimeter (PCE Instruments), and (b) leaf greenness, as indicated by SPAD values, was measured on the abaxial surface of three fully expanded leaves, utilizing the CCM-200plus Chlorophyll Content Meter, following the methodology outlined by Khan et al. [21].
Flower quality was evaluated every second day by four different observers (floriculture laboratory staff) using a scale ranging from 1 to 6 according to tepal senescence [22]. Figure 2 shows the flower quality corresponding to the evaluation scale from 1 to 6. Similar senescence stages for tepals based on the degree of wilting and abscission were followed for the cut flowers of all tulip species treated in this experiment.
In this study, the vase life of cut flowers was considered as the number of days between harvest day and the time when the entire tepal of the flower has withered (Figure 2, stage 5), leading to the loss of its commercial quality. To estimate the period during which 50% of cut flowers of CRH, CLC, AUS, and IDF reached stage 5, the plants of each treatment (Table 1) were divided into four groups. Each of these groups contained 4 to 17 plants depending on the available number of plants. The flowering stems were gradually removed from the bottles when flowers reached the 5th stage (Figure 2). After removing the flowering stems from the bottle, the FW of the flowering stems and leaves were measured separately. The preservation period (number of days) and the weight of the preservative solution that remained in each bottle were recorded.
2.4. Statistical Analysis
The obtained featured values, as the average, were statistically verified by means of variance analysis method (ANOVA). The difference among the means was compared by Multiple Range Tests, Kruskal–Wallis, and Friendman Tests at the 95.0% confidence level by using STATGRAPHICS computer package. Differences among treatments were considered significant only when p ≤ 0.05.
3. Results and Discussion
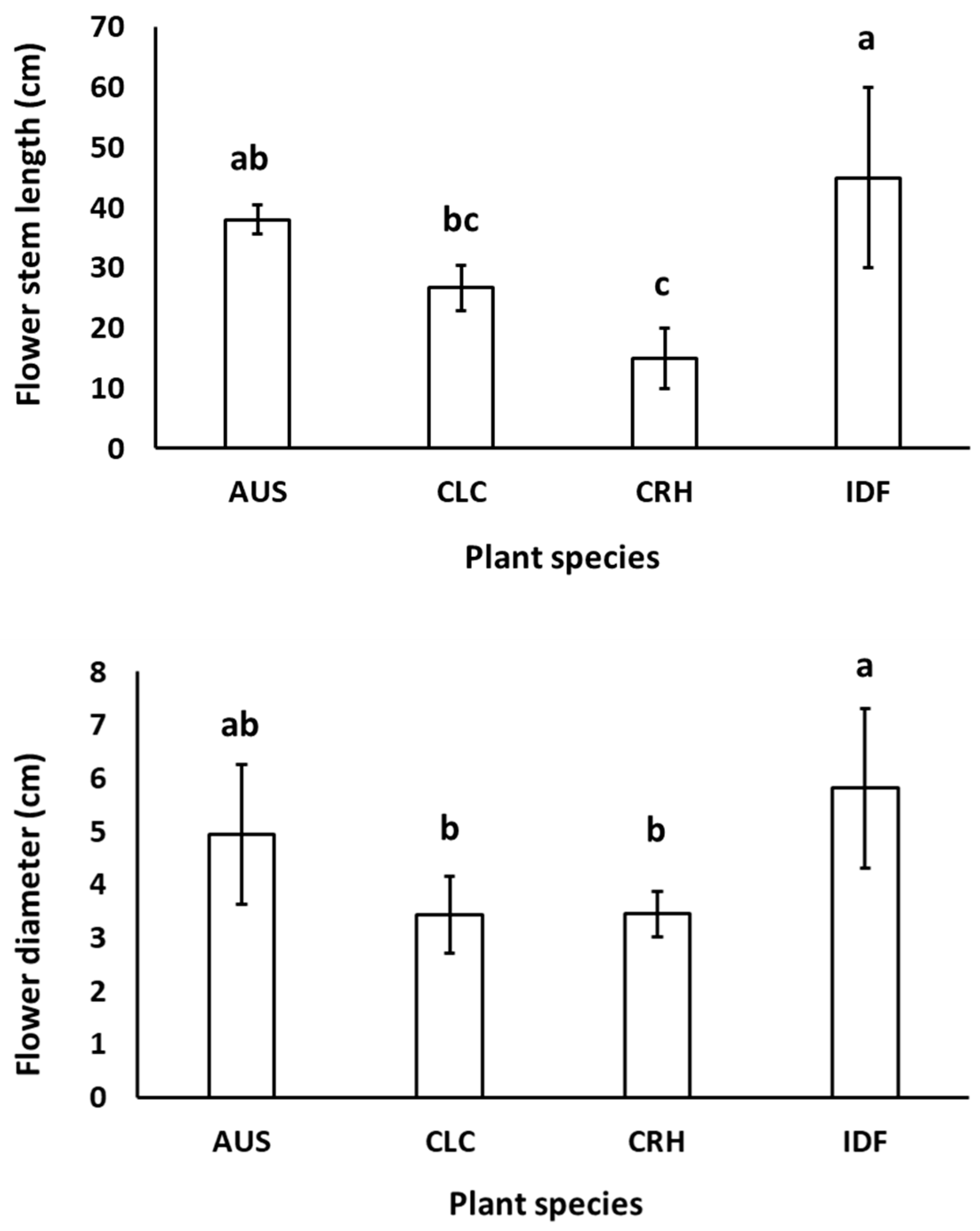
The native tulip plant species CRH, CLC, AUS, as well as the tulip hybrid Île-de-France grown in both Epanomi and Thermi, showed flower stem height and flower diameter similar to those reported from botanical and hybrid tulip bulb producers (https://www.farmergracy.co.uk/collections/botanical-tulips and https://www.gardenia.net/plant/tulipa-ile-de-france-triumph-tulip, respectively, accessed on 2 August 2023) as shown in Figure 3.
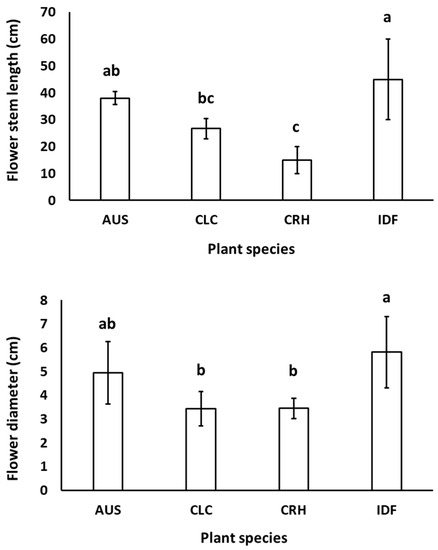
Figure 3.
Mean flower stem length and flower diameter of Tulipa australis (AUS), Tulipa clusiana ‘Chrysantha’ (CLC), Tulipa cretica ‘Hilde’ (CRH), and tulip hybrid Île-de-France (IDF) harvested from plants grown in Epanomi and Thermi. Different lowercase letters indicate statistically significant differences (p ≤ 0.05). The vertical bars indicate standard deviation (SD).
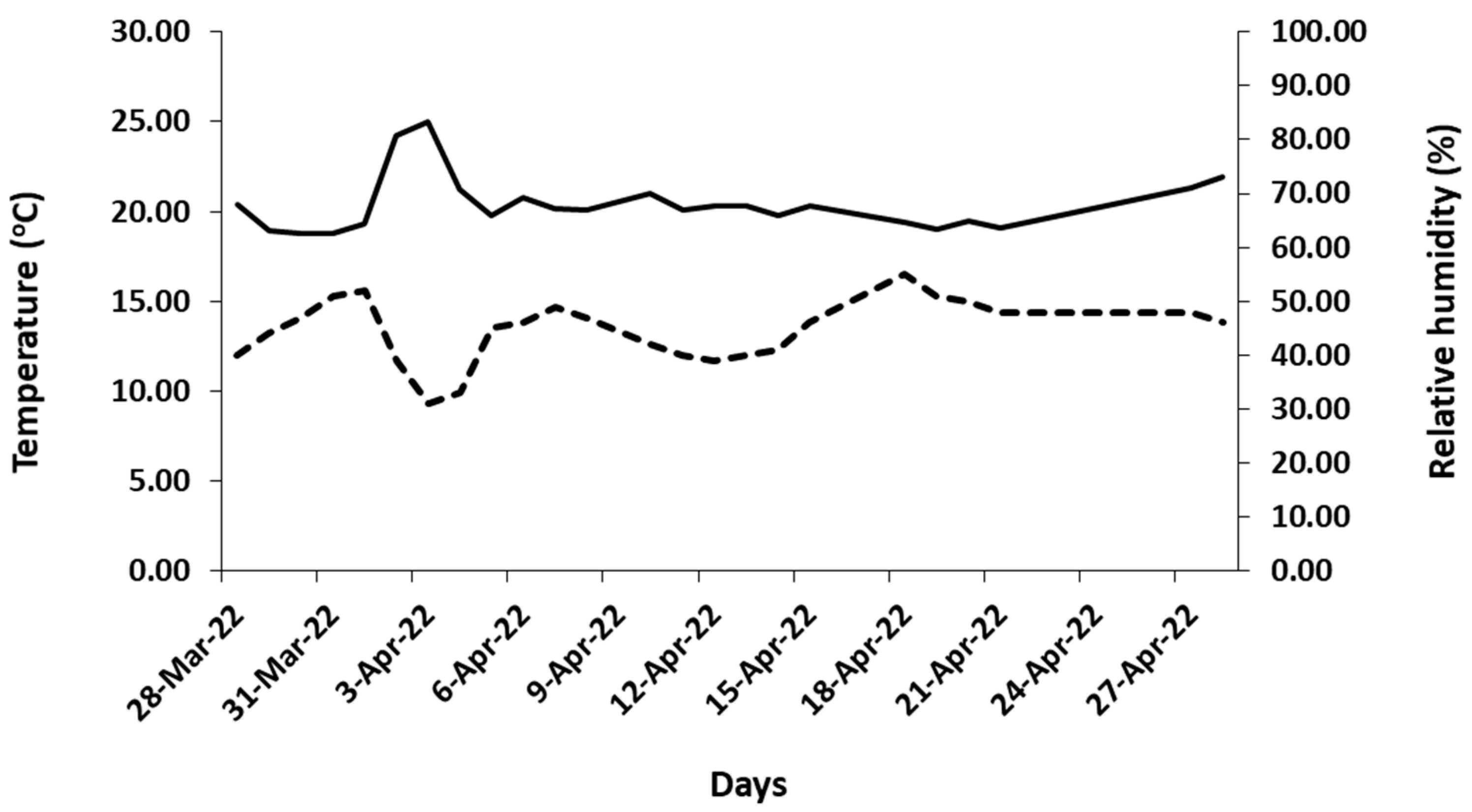
The temperature and humidity inside the laboratory where the experiments were conducted varied from 18.8 to 25 °C and from 31 to 55%, respectively (Figure 4). The average air temperature and relative humidity were 20.4 (±1.5) and 44.6 (±5.9), respectively. The abovementioned values were similar to those measured from several researchers inside the Mediterranean residents during March and April [23,24,25]. The photosynthetic active radiation (PAR) above the cut flowers ranged from 4.6 to 5.31 μmol/m2/s, and the photoperiod was the natural one for the period when the experiments were conducted (11 h and 30 min to 12 h and 30 min, respectively).
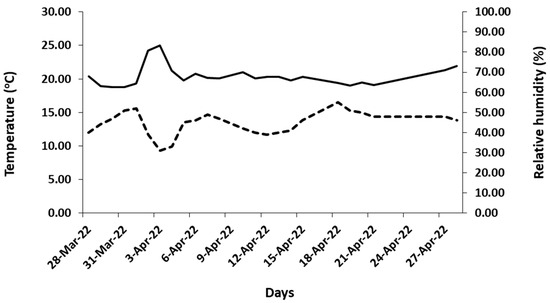
Figure 4.
Indoor air temperature (°C) (—) and relative humidity (%) (- -), where the harvested flowering stems of studied tulips were placed to experimentally assess their vase life.
Under similar to the above-described climate conditions, the vase life of the cut flowers of the commercial cultivar IDF, which is a member of Triumph tulips group and cultivated both for field and greenhouse cut tulip production in many different climatic zones, ranged from 3.5 to 8 days depending on the bulb maturity stage [22,26,27]. The measurements conducted on the average vase life of the cut flowers revealed statistically significant differences among the four tulip species, irrespective of the growth location and the use of preservatives. Specifically, the average vase life (days) of cut flowers of IDF measured in this experiment was in agreement with those measured in the abovementioned studies [22,26,27]. The cut flowers of IDF reached the 5th stage (Figure 2) after 4.0 days (±1.7) from harvest (Figure 5). The flowers of the native species T. cretica ‘Hilde’ (CRH) presented the highest average vase life (5.7 days ±1.5), followed by T. australis (AUS), which had slightly lower preservation period (5.1 days ±2.82). In addition, the T. cretica flowers had almost half the SD value compared to that of T. australis flowers, probably due to lower genetic homogeneity. These characteristics can become important in selecting a plant species as a candidate ornamental plant for cut flower production [28,29,30], since they promote uniformity of the floral opening and, thus, increased marketability. Additional studies have evaluated T. cretica, showing high potential for the ornamental sector concerning the suitability for targeted floricultural sub-sectors (e.g., pot/patio plants, home gardening, landscaping, and xeroscaping) coupled with an adequate level for sustainable exploitation feasibility [3]. The third examined species, namely T. clusiana ‘Chrysantha’ (CLC), had shorter vase life (3.5 days ±1.19) compared to all other tulip species used in this study. This postharvest life of CLC was under the minimum acceptable standard for vase life of cut tulips, which is perceived to be 5 to 6 days [31]. From the evaluation of the worldwide electronic trade of Greek botanical tulips, the ex-situ conservation of different Greek species and the sustainable exploitation challenges of Greek tulips have revealed that both well-established value chains and research gaps exist in the market of tulips, raising significant concerns regarding the effectiveness of ex-situ conservation [32]. Nevertheless, the current study contributes to the creation of a sustainable value chain for the Greek tulips. For T. cretica, in particular, its previously defined readiness timescale for sustainable exploitation in the long term [3] could be upgraded based on the results of the current work as achievable in the short term after bridging of previously extant research gaps regarding its cultivation and postharvest vase life.
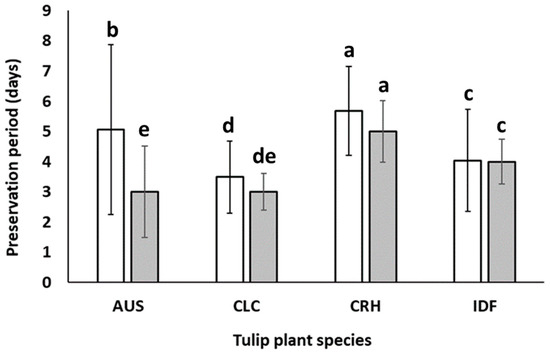
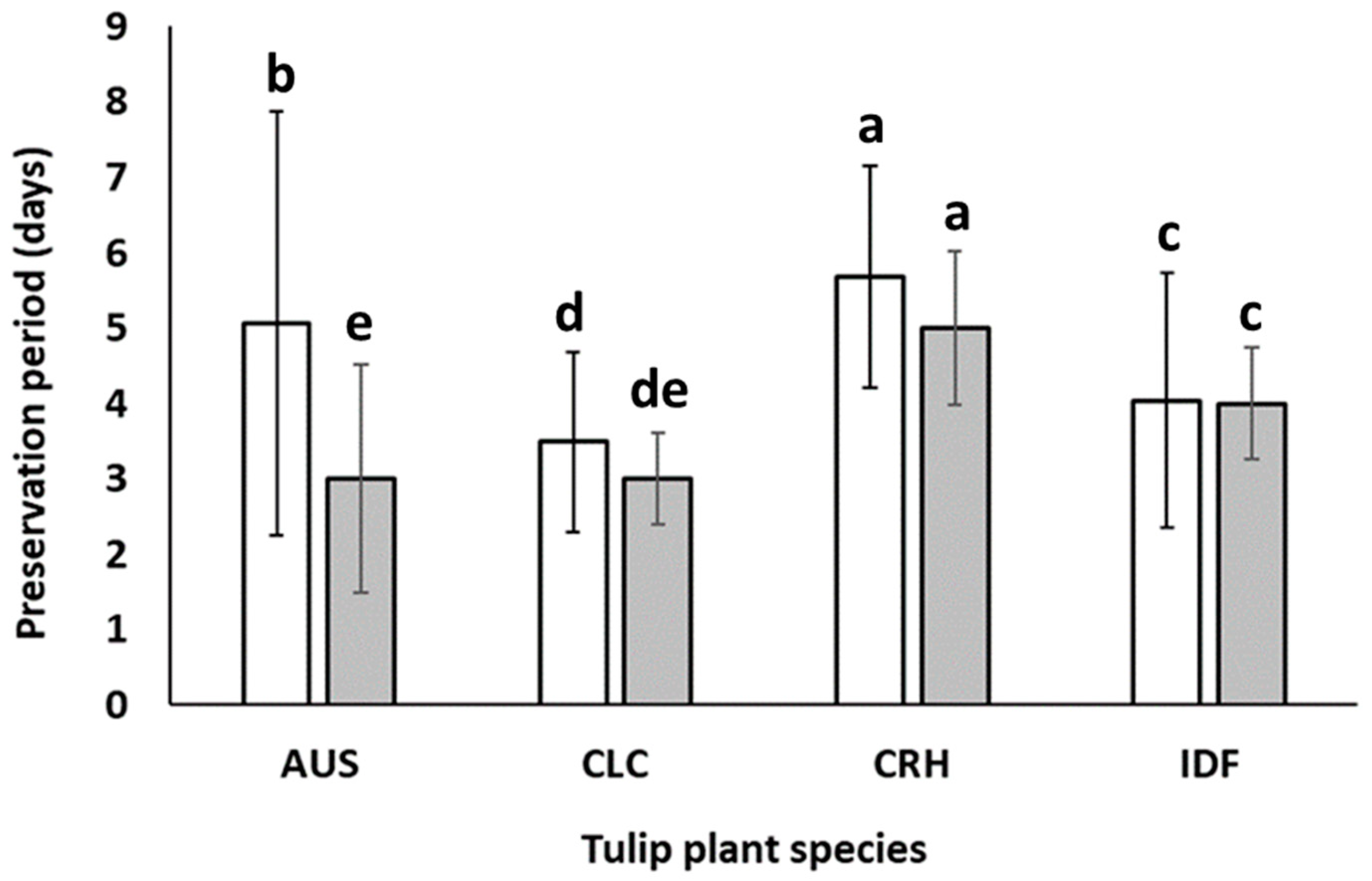
Figure 5.
Vase life (□) and the period (■) that 50% of the flowers of Tulipa australis (AUS), Tulipa clusiana ‘Chrysantha’ (CLC), Tulipa cretica ‘Hilde’ (CRH), and tulip hybrid Île-de-France (IDF) reached stage 5. Measurements concern flowers harvested from plants grown both in Epanomi and Thermi and remained in solution with and without the preservative. Different lowercase letters indicate statistically significant differences (p ≤ 0.05). The vertical bars indicate standard deviation (SD).
To further enhance the assessment of the aesthetic value of the studied tulip species, we estimated the timeframe in which 50% of the cut flowers of CRH, CLC, AUS, and IDF reached stage 5 (as depicted in Figure 5). According to the obtained results, 50% of cut flowers of CRH reached stage 5 in five days after harvesting, which is almost equal to their vase life (5.6 days being the period that the average number of all CRH flowers reached the same senescence stage). In other words, all treated cut flowers of CRH appear to lose their aesthetic value almost at the same time, which strengthens the hypothesis for strong genetic homogeneity among the cultivated individuals of this species, as well as enhances the ability of its commercial exploitation for the production of cut flowers in Greece. The flowers of CLC also showed a similar pattern, while the senescence of AUS flower stems continued for another two days after 50% of the flowers of this species had reached stage 5 (Figure 5). As observed in the aforementioned graph, it is evident that all cut flowers of the commercial cultivar IDF underwent a simultaneous decline in their aesthetic value.
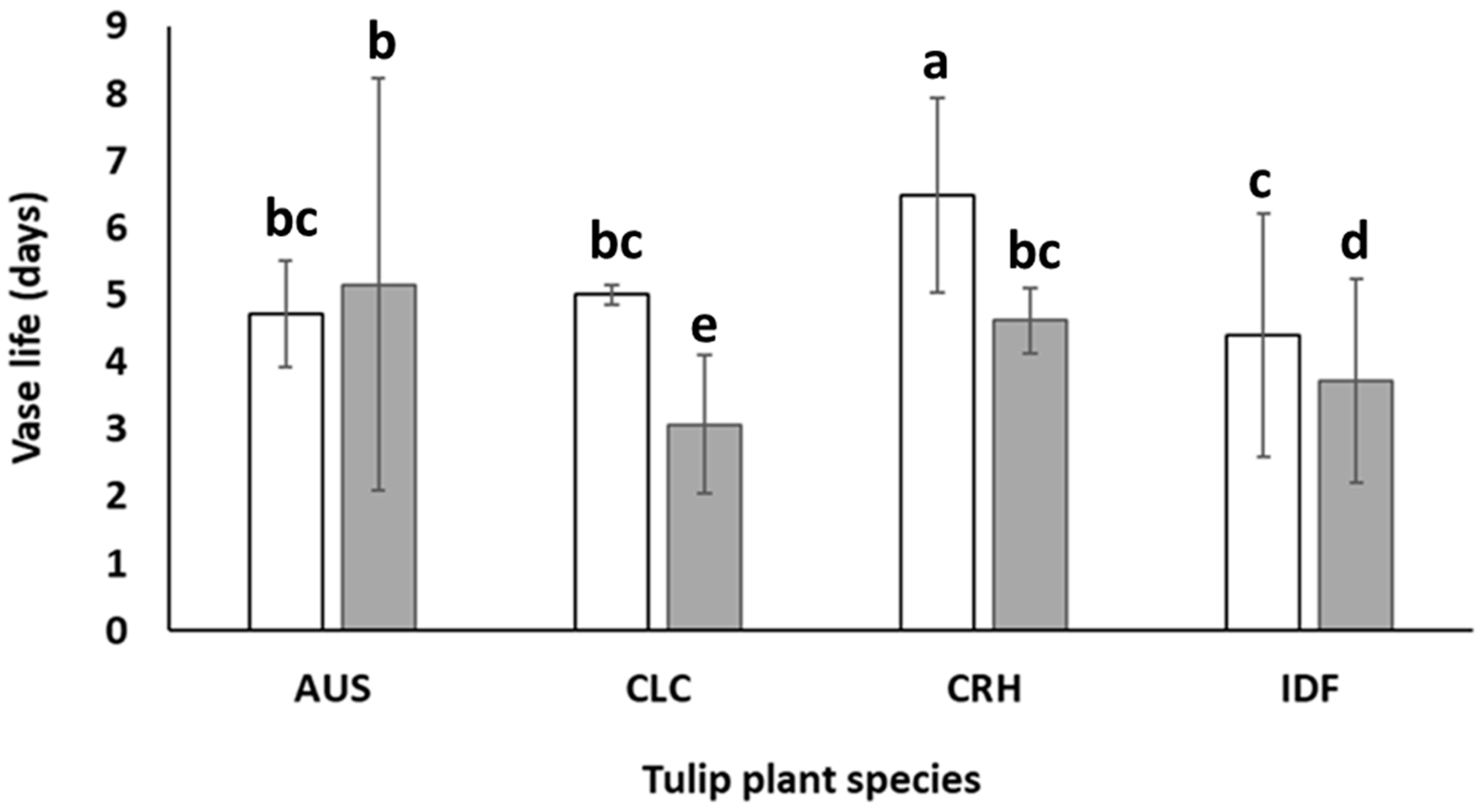
The climatic conditions where plants were grown significantly affected the postharvest life of the cut flowers examined for each studied species. In specific, apart from AUS (in which no statistically significant differences were observed), the vase life of cut flowers from plants from each species grown in Epanomi was longer than those grown in Thermi, as shown in Figure 6. This is probably due to the higher temperatures of the area of Thermi area compared to those of Epanomi during the growth period of the plants. The outdoor temperature difference ranged from 0.5 °C during January to 1.1 °C during April. It has been reported in several studies that preharvest high temperatures promote an elevated respiration rate and decrease carbohydrate reserves, and thus ultimately limiting the quality of flowers and their vase life [33,34,35]. However, the possible interaction of temperature with other factors, such as the sufficiency or deficiency in nutrients, complicates the understanding of temperature effects on vase life [35].
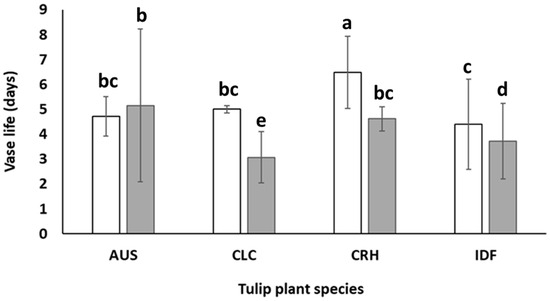
Figure 6.
Vase life of cut flowers of Tulipa australis (AUS), Tulipa clusiana ‘Chrysantha’ (CLC), Tulipa cretica ‘Hilde’ (CRH), and tulip hybrid Île-de-France (IDF) harvested from plants grown in Epanomi (□) and Thermi (■). Different lowercase letters indicate statistically significant differences (p ≤ 0.05). The vertical bars indicate standard deviation (SD).
According to Figure 6, the cultivation of IDF, CRH, and CLC tulips exclusively in the greenhouse located in Epanomi could increase the average vase life of cut flowers at 0.3, 0.8, and 1.5 days, respectively, compared to the values presented in Figure 5. In this way, cut flowers from CLC and CRH tulips could reach a commercial vase life of 5 and 6.4 days, respectively. However, it is important to mention that IDF cut flowers produced from plants grown in Epanomi’s greenhouse increased their vase life only by 0.3 days. The abovementioned results denote that there is a great potential in increasing the cut flowers’ vase life of Greek tulips when a proper selection of the cultivation area is made.
No statistically significant differences in vase life were observed among cut flowers harvested from plants grown in the same location (Epanomi or Thermi) and remained in deionized water or previously dipped in preservatives (Table 2). These results are in agreement with previous studies, where it has been pointed out that tulips do not respond well to the general range of postharvest floral preservatives [31,36,37,38]. Cut flowers are often held in holding or vase solutions, the composition of which may vary according to the flower species [39]. Each holding solution must contain at least two components, i.e., sugar and germicides. The preservative used in this experiment that contained citric and sulfuric acids to lower the pH of the flowering stem sap as well as to control and reduce microbial proliferation is a requirement for extending quality and longevity of cut flowers [40,41,42,43]. The addition of sugar to the preservative solution was not considered necessary since sugar in tulip preservatives is not recommended [44].

Table 2.
Mean vase life (days ± standard deviation) of cut flowers harvested from plants cultivated in the greenhouses of Epanomi and Thermi. The cut flowers remained in bottles of deionized water, with or without prior immersion in the preservative.
Physiological wilting, which results from the inability of cut flowers to absorb water after harvesting (water stress), is a major factor contributing to cut flower senescence and, thus, dermination of their postharvest life. In cut flowers, water uptake diminishes over time [45]. The limited ability of cut flowers to absorb water is mainly due to the proliferation of microorganisms in the vase preservative solution, which gradually leads to flower stem vascular occlusion [46,47,48,49]. For this reason, measuring the amount and rate of water absorption by the cut flowers is an important indication of the possibility of their long-term preservation in the vase [50]. The results of this work showed that cut flowers from the tulip hybrid IDF absorbed three to eight times more water than those of the Greek species studied herein (Table 3). In contrast, water absorption by the cut flowers of each Greek tulip species was similar regardless of the location where the plants were grown and was not affected significantly by the use of preservatives.

Table 3.
Average water uptake (mL) throughout the vase life and per day during the same period performed by cut flowers of Tulipa australis (AUS), Tulipa clusiana ‘Chrysantha’ (CLC), Tulipa cretica ‘Hilde’ (CRH), and tulip hybrid Île-de-France (IDF) cultivated in Epanomi (E) and Thermi (T), with or without pulsing within the preservative.
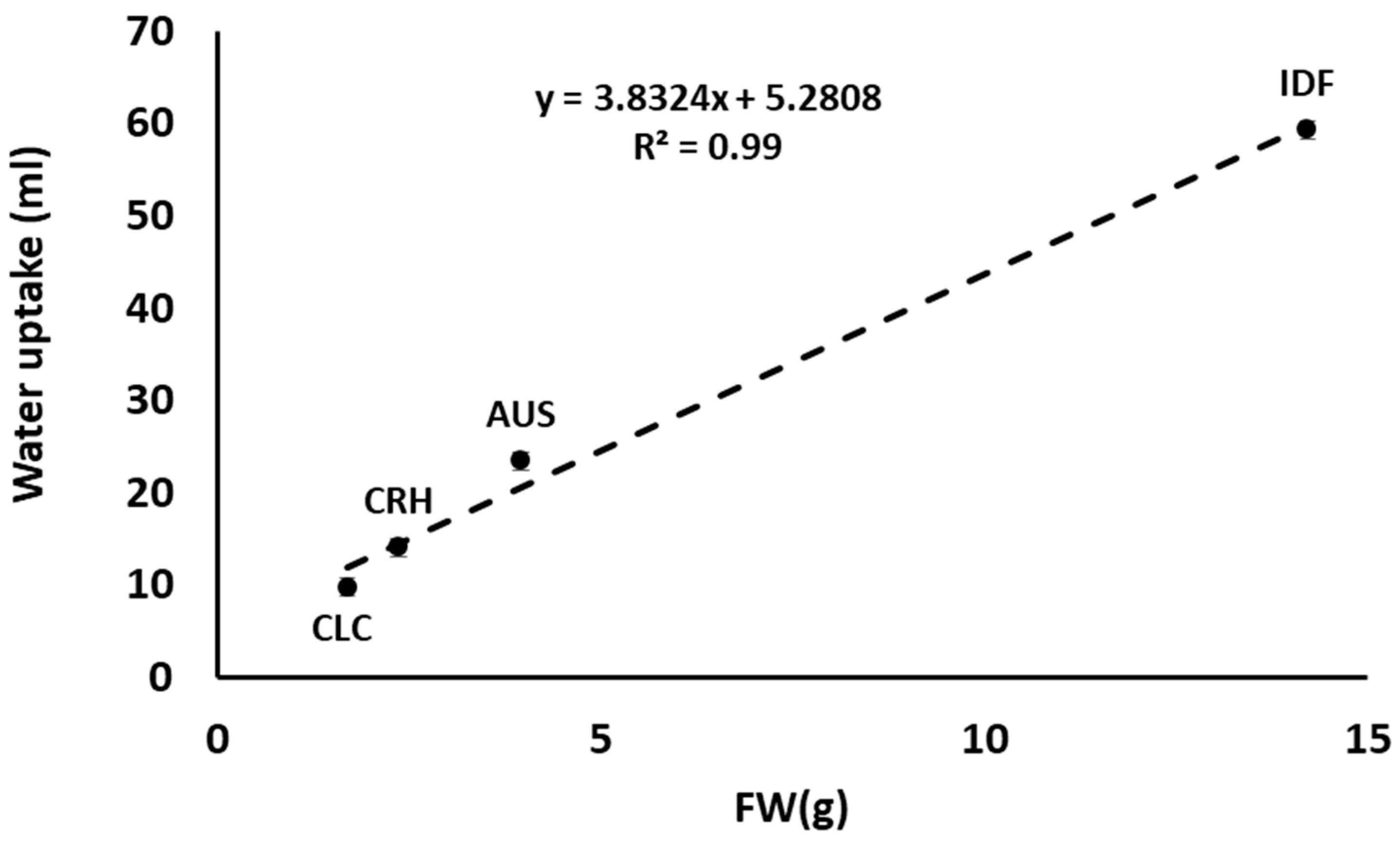
This pattern remained almost the same when the measurements were normalized by estimating the average amount of water absorbed by cut flowers per day of their vase life. Even though the cut flowers of Greek tulip species (AUS, CLC, and CRH) did not show a statistically significant difference regarding water absorption, CLC and CRH tulips showed the longest vase life and tended to absorb the least amount of water (Table 3). However, cut flower water balance depends on the water uptake and transpiration rate. Impaired water uptake may be due to bacterial growth and/or physiological processes (production of polyphenolic compounds) and, therefore, an increased resistance of water flow through xylem vessels. The transpiration rate (apart from climatic conditions) is reported to be affected significantly by plant species [51,52] since it depends on morphological characteristics, such as the number and size of stomata across the epidermis. The much higher water uptake of IDF’s cut flowers compared to those of the Greek plant species (AUS, CLS, and CRH) should probably be attributed to the higher leaf mass and longer flowering stems of IDF, resulting in an average FW of 14.2 g (±4.8) compared to the AUS, CLS, and CRH leaves FW (3.9 g ±1.1, 1.7 g ±0.4, and 2.3 g ±0.88, respectively). The latter was also confirmed from the linear correlation with high R2 = 0.99 between the FW of different species’ leaves and the absorbed water measured (Figure 7).
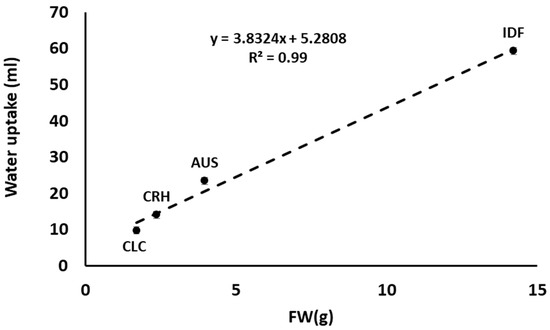
Figure 7.
Correlation between the fresh weight (FW) of leaves and flowering stems from Tulipa australis (AUS), Tulipa clusiana ‘Chrysantha’ (CLC), Tulipa cretica ‘Hilde’ (CRH), and tulip hybrid Île-de-France (IDF) associated with the amount of water (mL) absorbed during vase life.
However, it is well established that not only the negative water balance causes wilting symptoms on leaves and flowers, but also biocides used as preservatives can affect the wilting of leaves [47,53], their photosynthesis and membrane permeability, as well as cut flower’s quality physiology and flower opening [46,47,54]. When citric acid is used as a preservative, it may reduce chlorophyll fluorescence and increase ion leakage during the last days of vase life as membrane permeability decreases [42]. However, the effect of citric acid on the physiological responses of cut flowers has not been adequately studied as additive in flower preservative solutions. The results of this work showed that the use of citric acid as a preservative for the cut flowers of AUS, CLC, CRH, and IDF, which were cultivated in the same location (Epanomi or Thermi), did not cause significant alteration in leaf greenness (SPAD values) (Table 4). The above is in agreement with the results of other researchers [55] regarding the effect of citric acid as an additive in cut flower preservatives since a concentration of 100 to 200 ppm citric acid can improve the chlorophyll content of tulip cut flowers to overcome the loss of photosynthetic activity caused by citric acid absorption [56]. However, the addition of citric acid in the preservative solution did not appear to affect anthocyanin leakage [57] and, consequently, the color of flowers. In specific, in experiments concerning the preservation of cut tulip flowers [58], the addition of 100 ppm citric acid to the preservation solution, instead of 50 ppm used in the present work, resulted in an increased flower vase life, solution uptake, chlorophyll content, flower diameter, and their fresh and dry weight, whereas at the same time, delayed senescence initiation and stem bending.

Table 4.
Mean leaves SPAD values and L,a,b,c, and H color parameter values of cut flowers harvested from Tulipa australis (AUS), Tulipa clusiana ‘Chrysantha’ (CLC), Tulipa cretica ‘Hilde’ (CRH), and tulip hybrid Île-de-France (IDF) plants grown in Epanomi (E) and Thermi (T) location, with or without pulsing within the preservative.
In addition, in almost all treatments, no variation of the color parameters (L, a,b,c, and H) was observed. Only in the case of AUS cultivated both in Epanomi and Thermi, b and c flowers’ color parameters showed higher values when no preservative was used (Table 4). Higher values of b indicate more intense yellow coloration of the flower, while higher values of parameter c indicate higher brightness. According to the results shown in Table 4, the use of citric acid reduced the coloring and brightness of the color in flowers harvested from AUS plants grown in Epanomi and Thermi.
4. Conclusions
Measurements regarding the average vase life of the cut flowers showed that there were statistically significant differences between the three Greek tulip species, T. cretica ‘Hilde’, T. clusiana ‘Chrysantha’, T. australis, and the commercialized tulip hybrid Île-de-France, regardless of the cultivation location and the use of preservatives. Cut flowers from T. cretica were characterized from a long vase life of 5.7 days, which can be extended to 6.5 days if a location with proper climate conditions is selected for plant cultivation. In addition, they are characterized by uniformity of the flower opening. These are basic criteria for selecting a potential plant species as a candidate for cut flower production, and therefore, T. cretica could serve as a potential candidate ornamental plant for cut flower production. In addition, cut flowers from T. clusiana showed similar characteristics to T. cretica, but their vase life was probably affected to a greater extent by the climate parameters of the area where the plants were grown. The vase life of the flowers harvested from the tulip hybrid Île-de-France, cultivated in Epanomi (the location with favorable conditions for the growth of T. cretica and T. clusiana), showed the same or shorter vase life compared to the other Greek species studied. However, the cut flowers of the tulip hybrid Île-de-France absorbed almost three times more the amount of water from the vase that T. cretica and T. clusiana cut flowers absorbed in the comparative experiment performed herein due to their larger flower stems and leaves.
Pulsing cut flowering stems within a preservative containing 5% citric acid and 1% sulfuric acid did not significantly increase the postharvest lifetime of the cut flowers in any of the herein studied tulips and did not affect the green color of leaves and the color of flowers at the end of their vase life. However, according to the results of relative experiments, the increase of citric acid content to 10% could improve flower characteristics measured in this experiment. The above data furnished herein show that the native species T. cretica and T. clusiana have sufficient vase life, which is an important criterion for their entry into the cut flower market. These data may be used to pave the road for the facilitation of the sustainable exploitation of the Greek tulip species with high ornamental value.
Author Contributions
Conceptualization, C.L., N.K. and G.T.; methodology, C.L. and G.T.; validation, C.L., M.Z., N.K., I.S., E.K., S.P., E.V., I.P., M.A.S. and G.T.; formal analysis, C.L. and M.Z.; investigation, C.L., M.Z., I.S., E.K., M.A.S., S.P., E.V. and G.T.; resources, C.L. and M.Z.; data curation, C.L., M.Z., I.S. and M.A.S.; writing—original draft preparation, C.L., M.Z., E.K., I.S. and N.K.; writing—review and editing, C.L., M.Z., E.K., I.S., G.T. and N.K.; visualization, C.L., M.Z. and I.S.; software, C.L. and M.Z.; supervision, C.L., N.K. and G.T.; project administration, C.L., N.K. and G.T.; funding acquisition, C.L., N.K. and G.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been co-financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH-CREATE-INNOVATE (project code: T2EDK-05115; acronym: TULIPS.GR), entitled “Value chain for Greek native tulips: development of documented propagation material and integrated conservation for sustainable exploitation”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding authors on request.
Acknowledgments
We are thankful to the staff of the Balkan Botanic Garden of Kroussia, Institute of Plant Breeding and Genetic Resources, Hellenic Agricultural Organization Demeter for the hosting of ex situ conservation of the accessions of TULIPS.GR.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Weiss, D. Introduction of New Cut Flowers: Domestication of New Species and Introduction of New Traits Not Found in Commercial Varieties. In Breeding for Ornamentals: Classical and Molecular Approaches; Vainstein, A., Ed.; Springer: Dordrecht, The Netherlands, 2002. [Google Scholar] [CrossRef]
- Hentig, W. The development of ”new ornamental plants” in Europe. Acta Hortic. 1995, 397, 9–30. [Google Scholar] [CrossRef]
- Krigas, N.; Tsoktouridis, G.; Anestis, I.; Khabbach, A.; Libiad, M.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Lamchouri, F.; Tsiripidis, I.; Tsiafouli, M.A.; et al. Exploring the potential of neglected local endemic plants of three Mediterranean regions in the ornamental sector: Value chain feasibility and readiness timescale for their sustainable exploitation. Sustainability 2021, 13, 2539. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Govaerts, R.; David, J.C.; Hall, T.; Borland, K.; Roberts, P.S.; Tuomisto, A.; Buerki, S.; Chase, M.W.; Fay, M.F. Tiptoe through the tulips: Cultural history, molecular phylogenetics and classification of Tulipa (Liliaceae). Bot. J. Linn. Soc. 2013, 172, 280–328. [Google Scholar] [CrossRef]
- Stefanaki, A.; Walter, T.; Andel, T. Tracing the introduction history of the tulip that went wild (Tulipa sylvestris) in sixteenth-century Europe. Sci. Rep. 2022, 12, 9786. [Google Scholar] [CrossRef]
- Beruto, M. Introduction of new ornamental plants and production technologies: Case studies. Acta Hortic. 2013, 1000, 23–34. [Google Scholar] [CrossRef]
- Dragovic, M.J.O. Selection and domestication of endemic species from Macaronesia with ornamental value. Acta Hortic. 2015, 1097, 193–198. [Google Scholar] [CrossRef]
- Armitage, A.M. New ornamental crop introduction: A model of cooperation between industry and academia. Acta Hortic. 2003, 624, 25–27. [Google Scholar] [CrossRef]
- Sedgley, M. Banksia: New proteaceous cut flower crop. Hortic. Rev. 1998, 22, 1–26. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Lim, J.H. Do eco-friendly floral preservative solutions prolong vase life better than chemical solutions? Horticulturae 2021, 7, 415. [Google Scholar] [CrossRef]
- Oene, S.; Mattiuz, C.; Brito, T.; Pan, R. Post-harvest preservation of roses cv. Ipanema. Commun. Plant Sci. 2019, 9, 70–80. [Google Scholar] [CrossRef]
- Çelikel, F.G.; Karaçalý, Y. Effect of preharvest factors on flower quality and longevity of cut carnations (Dianthus caryophyllus L.). Acta Hortic. 1995, 405, 156–163. [Google Scholar] [CrossRef]
- Kim, H.H.; Ohkawa, K.; Nitta, E. Effect of bulb weight on the growth and flowering of Leucocoryne coquimbensis F. Phill. Acta Hortic. 1998, 454, 341–346. [Google Scholar] [CrossRef]
- Kapczyńska, A. Effect of bulb size on growth, flowering and bulb formation in lachenalia cultivars. Hortic. Sci. 2014, 41, 89–94. [Google Scholar] [CrossRef]
- Almeida, D.; Barbosa, J.; Grossi, J.; Finger, F.; Heidemann, J. Influence of vernalization and bulb size on the production of lily cut flowers and lily bulbs. Semin. Cienc. Agrar. 2017, 38, 2399. [Google Scholar] [CrossRef]
- Kader, A.A. Postharvest Technology of Horticultural Crops, 3rd ed.; Agriculture and Natural Resources, Publication 3311; University of California: Davis, CA, USA, 2007; p. 535. [Google Scholar]
- Macnish, A.J.; Jiang, C.; Reid, M.S. Treatment with thidiazuron improves opening and vase life of iris flowers. Postharvest Biol. Technol. 2010, 56, 77–84. [Google Scholar] [CrossRef]
- Figueroa, I.; Colinas, M.T.; Mejia, J.; Ramirez, F. Postharvest physiological changes in roses of different vase life. Int. J. Agric. Nat. Resour. 2005, 32, 167–176. Available online: https://www.rcia.uc.cl/index.php/ijanr/article/view/1301 (accessed on 25 June 2023). [CrossRef]
- Aros, A.; Silva, C.; Char, C.; Prat, L.; Escalona, V. Role of flower preservative solutions during postharvest of Hydrangea macrophylla cv. Bela. Cien. Inv. Agr. 2016, 43, 418–428. [Google Scholar] [CrossRef]
- Macnish, A.J.; Leonard, R.T.; Nell, T.A. Treatment with chlorine dioxide extends the vase life of selected cut flowers. Postharvest Biol. Technol. 2008, 50, 197–207. [Google Scholar] [CrossRef]
- Khan, W.; Prithviraj, B.; Smith, D.L. Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant Physiol. 2003, 160, 485–492. [Google Scholar] [CrossRef]
- Iwaya-Inoue, M.; Takata, M. Trehalose plus chloramphenicol prolong the vase life of tulip flowers. HortScience 2001, 36, 946–950. [Google Scholar] [CrossRef]
- Sarbu, I.; Pacurar, C. Experimental and numerical research to assess indoor environment quality and schoolwork performance in university classrooms. Build Environ. 2015, 93, 141–154. [Google Scholar] [CrossRef]
- De Masi, F.R.; Ruggiero, S.; Vanoli, P.G. Hygro-thermal performance of an opaque ventilated façade with recycled materials during wintertime. Energy Build. 2021, 245, 110994. [Google Scholar] [CrossRef]
- Hofmann, M.; Geyer, C.; Kornad, O. Dependencies of the indoor climate on the course of the seasons and derivation of regressions from long-term measurements. Indoor Air 2022, 32, e13058. [Google Scholar] [CrossRef]
- Derbyshire, G. Cultivation Aspects of Hydroponic Cut Tulip (Tulipa gesneriana) Production in South Africa. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2013. Available online: https://scholar.sun.ac.za/server/api/core/bitstreams/88c6dea3-2078-4160-8b70-4cd73d3f4f62/content (accessed on 25 June 2023).
- Bashir, M.; Khan, M.A.; Muhammad, Q.; Basra, S.M.A. Evaluation of commercial tulip accessions for flowering potential in climatic conditions of Faisalabad. Int. J. Agric. Biol. 2018, 20, 25–32. [Google Scholar]
- Ichimura, K.; Kishimoto, M.; Norishikori, R.; Kawabata, Y.; Yamada, K. Soluble carbohydrates and variation in vase life of cut rose cultivars ‘Delilah’ and ‘Sonia’. J. Hortic. Sci. Biotechnol. 2005, 80, 280–286. [Google Scholar] [CrossRef]
- Krause, M.R.; Santos, M.; Moreira, K.F.; Tolentino, M.M.; Mapeli, A.M. Extension of the vase life of Lilium pumilum cut flowers by pulsing solution containing sucrose, citric acid and silver thiosulfate. Ornamental. Hortic. 2021, 27, 344–350. [Google Scholar] [CrossRef]
- Sun, J.; Guo, H.; Tao, J. Effects of harvest stage, storage, and preservation technology on postharvest ornamental value of cut Peony (Paeonia lactiflora) flowers. Agronomy 2022, 12, 230. [Google Scholar] [CrossRef]
- Salunkhe, D.K.; Bhat, N.R.; Desai, B.B. Postharvest of Flowers and Ornamental Plants; Springer: Berlin, Germany, 1990. [Google Scholar]
- Krigas, N.; Lykas, C.; Ipsilantis, I.; Matsi, T.; Weststrand, S.; Havström, M.; Tsoktouridis, G. Greek tulips: Worldwide electronic trade over the internet, global ex situ conservation and current sustainable exploitation challenges. Plants 2021, 10, 580. [Google Scholar] [CrossRef]
- Halevy, A.H.; Mayak, S. Senescence and postharvest physiology of cut flowers, part 1. Hortic. Rev. 1997, 1, 204–236. [Google Scholar] [CrossRef]
- Fanourakis, D.; Pieruschka, R.; Savvides, A.; Macnish, A.J.; Sarlikioti, V.; Woltering, E.J. Sources of vase life variation in cut roses: A review. Postharvest Biol. Technol. 2013, 78, 1–15. [Google Scholar] [CrossRef]
- Sahniwal, S.S.; Abbey, L. Cut flower vase life—Influential factors, metabolism and organic formulation. Hortic. Int. J. 2019, 3, 275–281. [Google Scholar] [CrossRef]
- De Hertogh, A.A.; Le Nard, M. The Physiology of Flower Bulbs. A Comprehensive Treatise on the Physiology and Utilization of Ornamental Flowering Bulbous and Tuberous Plants; Elsevier: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Lukaszewska, A.J. Distribution of sugars in tulip flower parts as affected by ethrel and GA3 in the holding solution. Acta Hortic. 1995, 405, 351–355. [Google Scholar] [CrossRef]
- Dole, J.M.; Wilkins, H.F. Floriculture: Principles and Species, 2nd ed.; Pearson Prentice-Hall: New Jersey, NJ, USA, 2004. [Google Scholar]
- Asrar, A.W.A. Effects of some preservative solutions on vase life and keeping quality of snapdragon (Antirrhinum majus L.) cut flowers. J. Saudi Soc. Agric. Sci. 2012, 11, 29–35. [Google Scholar] [CrossRef]
- Ichimura, K.; Yumoto, H.S. Extension of the vase life of cut roses by treatment with sucrose before and during simulated transport. Bull. Natl. Inst. Flor. Sci. 2007, 7, 17–27. Available online: https://www.naro.go.jp/publicity_report/publication/archive/files/naro-se/NIFS07-03.pdf (accessed on 6 March 2023).
- Elhindi, K.M. Evaluation of several holding solutions for prolonging vase-life and keeping quality of cut sweet pea flowers (Lathyrus odoratus L.). Saudi J. Biol. Sci. 2012, 19, 195–202. [Google Scholar] [CrossRef]
- Jowkar, Μ.Μ.; Kafi, Μ.; Khalighi, A.; Hasanzadeh, Ν. Reconsideration in using citric acid as vase solution preservative for cut rose flowers. Curr. Res. J. Biol. 2012, 4, 427–436. Available online: https://maxwellsci.com/print/crjbs/v4-427-436.pdf (accessed on 13 April 2023).
- Ahmad, I.; Dole, J.M. Homemade floral preservatives affect postharvest performance of selected specialty cut flowers. HortTechnology 2014, 24, 384–393. [Google Scholar] [CrossRef]
- Han, S.S. Sugar and Acidity in Preservative Solutions for Field-Grown Cut Flowers. UMass Extension Greenhouse Crops and Floriculture Program. 2023. Available online: https://ag.umass.edu/greenhouse-floriculture/fact-sheets/sugar-acidity-in-preservative-solutions-for-field-grown-cut#links (accessed on 10 May 2023).
- Halevy, A.H. Treatments to improve water balance of cut flowers. Acta Hortic. 1976, 64, 223–230. [Google Scholar] [CrossRef]
- Van Doorn, W.G.; De Witte, Y.; Perik, R.R.J. Effect of antimicrobial compounds on the number of bacteria in stems of cut rose flowers. J. Appl. Bacteriol. 1990, 68, 117–122. [Google Scholar] [CrossRef]
- Bleeksma, H.C.; Van Doorn, W.G. Embolism in rose stems as a result of vascular occlusion by bacteria. Postharvest Biol. Technol. 2003, 29, 334–340. [Google Scholar] [CrossRef]
- He, S.; Joyce, D.C.; Irving, D.E.; Faragher, J.D. Stem end blockage in cut Grevillea ‘Crimson Yul-lo’ inflorescences. Postharvest Biol. Technol. 2006, 41, 78–84. [Google Scholar] [CrossRef]
- Liu, J.; He, S.; Zhang, Z.; Cao, J.; Lv, P.; He, S.; Cheng, G.; Joyce, D.C. Nano-silver pulse treatments inhibit stem-end bacteria on cut gerbera cv. Ruikou flowers. Postharvest Biol. Technol. 2009, 54, 59–62. [Google Scholar] [CrossRef]
- In, B.C.; Chang, M.K.; Son, K.C. Effect of vase water temperature and preservative on water relation and flower opening characteristics in cut roses. Korean J. Hort. Sci. Technol. 2009, 27, 116–122. Available online: https://www.researchgate.net/profile/Byung-Chun-In/publication/264065593_Effect_of_Vase_Water_Temperature_and_Preservative_on_Water_Relation_and_Flower_Opening_Characteristics_in_Cut_Roses/links/54929fe60cf2302e1d073771/Effect-of-Vase-Water-Temperature-and-Preservative-on-Water-Relation-and-Flower-Opening-Characteristics-in-Cut-Roses.pdf (accessed on 12 June 2023).
- Woltering, E.J.; Paillart, M.J.M. Effect of cold storage on stomatal functionality, water relations and flower performance in cut roses. Postharvest Biol. Technol. 2018, 136, 66–73. [Google Scholar] [CrossRef]
- Ahmadi-Majd, M.; Rezaei Nejad, A.; Mousavi-Fard, S.; Fanourakis, D. Deionized water as vase solution prolongs flower bud opening and vase life in cut carnation and rose through sustaining an improved water balance. Eur. J. Hortic. Sci. 2021, 86, 682–693. [Google Scholar] [CrossRef]
- Torre, S.; Fjeld, T. Water loss and postharvest characteristics of cut roses grown at high or moderate relative air humidity. Sci. Hortic. 2001, 89, 217–226. [Google Scholar] [CrossRef]
- Florack, D.E.A.; Stiekema, W.J.; Bosch, D. Toxicity of peptides to bacteria present in the vase water of cut roses. Postharvest Biol. Technol. 1996, 8, 285–291. [Google Scholar] [CrossRef]
- Khan, F.U.; Khan, F.A.; Hayat, N.; Bhat, S.A. Influence of certain chemicals on vase life of cut tulip. Indian J. Plant Physiol. 2007, 12, 127–132. [Google Scholar]
- Rahimian-Boogar, A.; Salehi, H.; Mir, N. Influence of citric acid and hydrogen peroxide on postharvest quality of tuberose (Polianthes tuberosa L. ‘Pearl’) cut flowers. J. Hortic. Res. 2016, 24, 13–19. [Google Scholar] [CrossRef]
- Zamani, S.; Hadavi, E.; Kazemi, M.; Hekmati, J. Effect of some chemical treatments on keeping quality and vase life of chrysanthemum cut flowers. World Appl. Sci. J. 2011, 12, 1962–1966. [Google Scholar]
- Ullah, M.; Bashir, M.; Gul, H.; Shahzad, A.; Shahzad, M. Use of citric acid and iron sulfate in promoting post-harvest longevity of cut tulips (Tulipa gesneriana L. cv. Marylin) in vase solutions. Contemp. Agric. 2022, 71, 57–64. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).