Alterations in Nonvolatile Components of Tea (Camellia sinensis) Induced by Insect Feeding under Field Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Field Setting
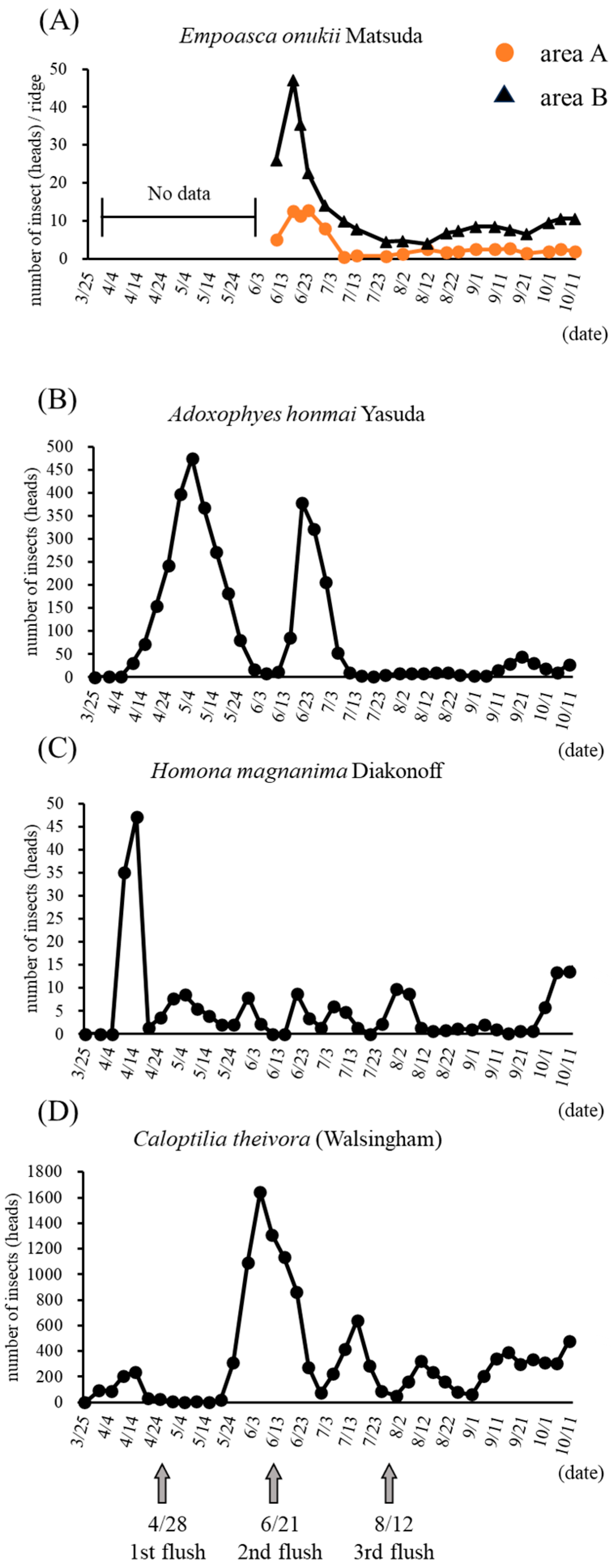
2.3. Monitoring the Number of Insects
2.4. Tea Sample Preparation
2.5. Quantification Analysis
2.6. Ultra-Performance Liquid Chromatography–Mass Spectrometry (UPLC–MS) Analysis
2.7. Data Analysis
2.8. Molecular Networking Analysis
2.9. Color Difference
2.10. Statistical Analysis
3. Results
3.1. Number of Pest Insects
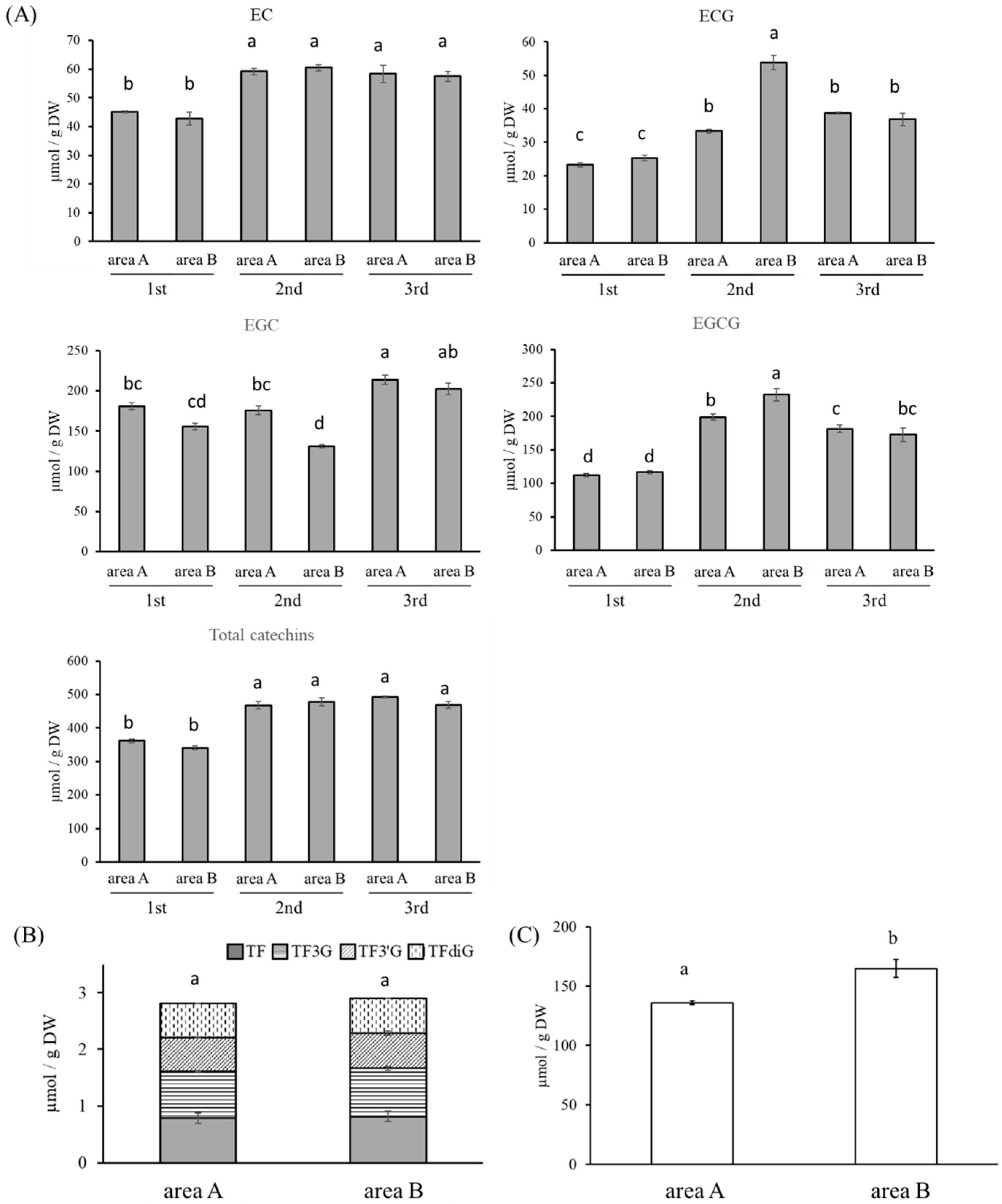
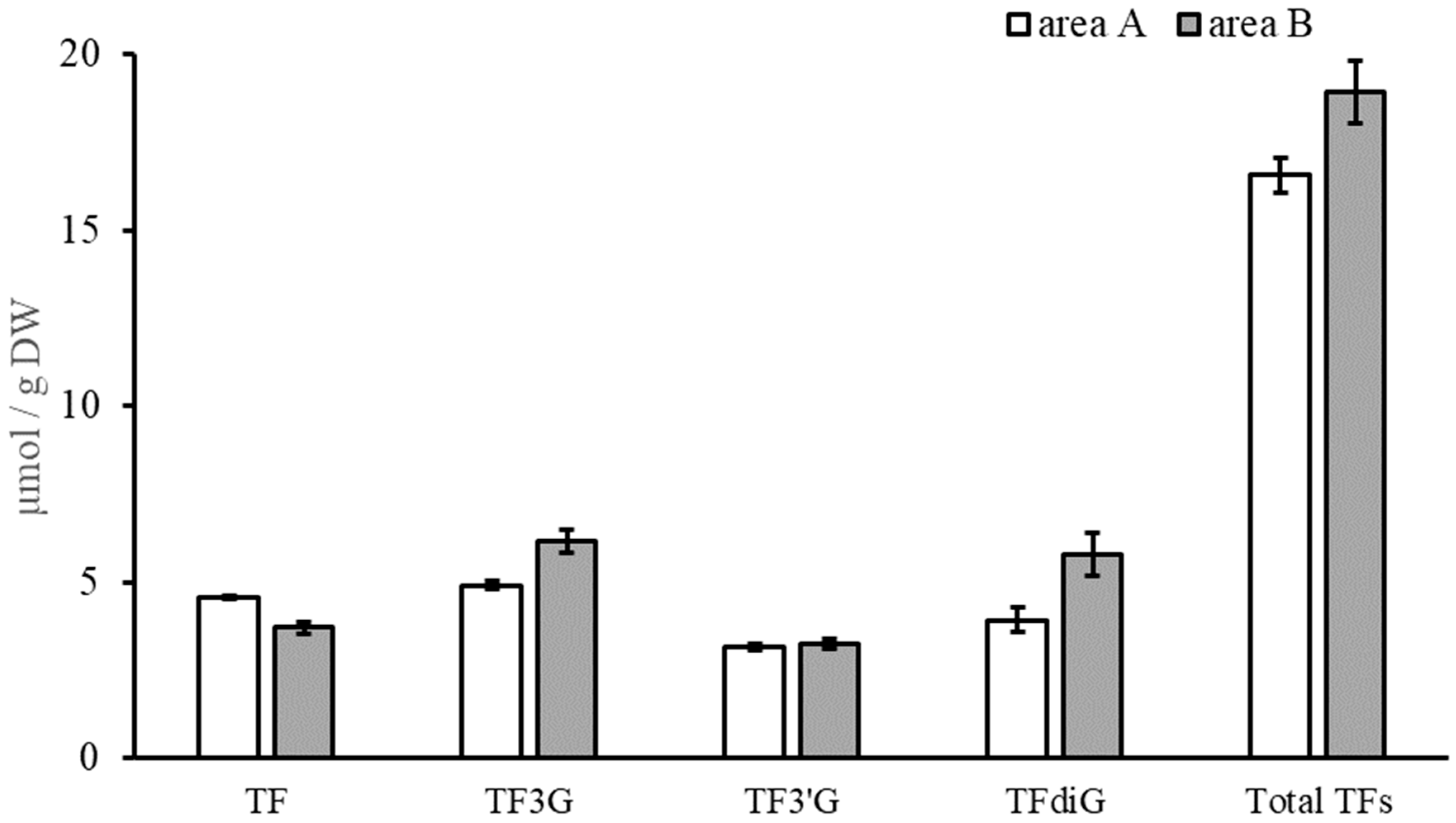
3.2. Quantification of Catechins, TFs, and Caffeine
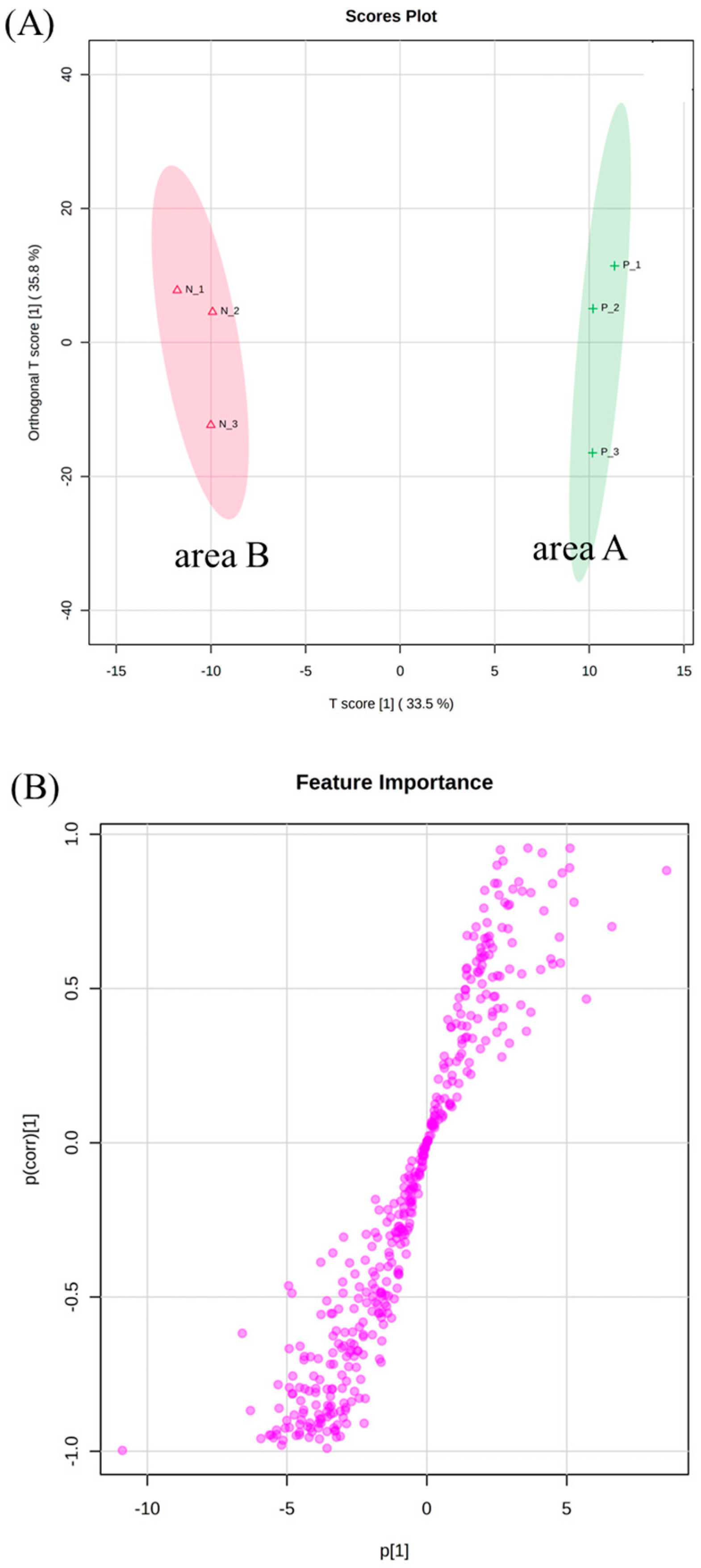
3.3. OPLS-DA of Second-Flush Fresh Leaves
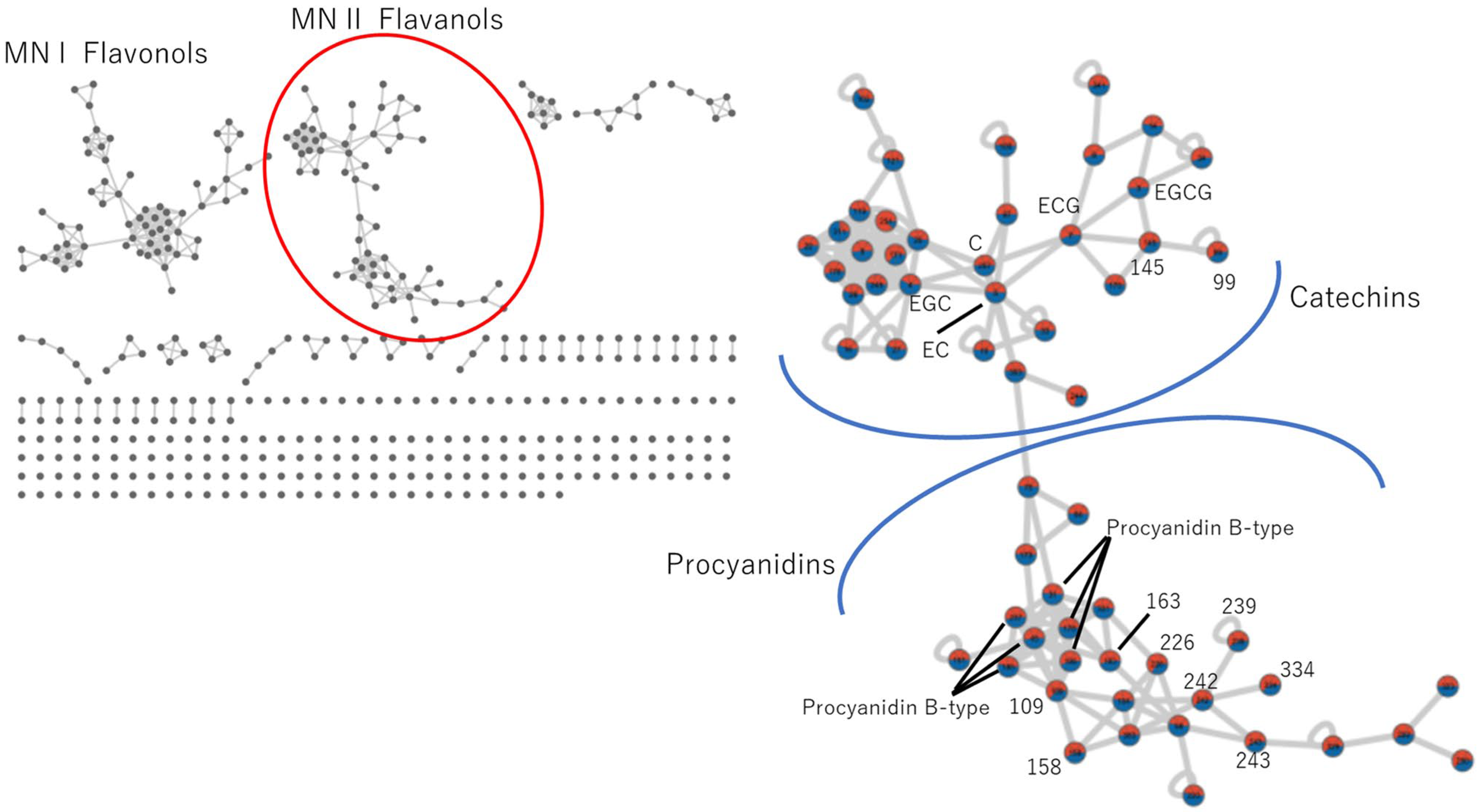
3.4. Molecular Networking (MN) Analysis
3.4.1. Catechins and Procyanidin
3.4.2. Hydrolyzable Tannin
3.4.3. Volatile Compound Glycoside
3.5. Influence on Black Tea Processing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, G.Y.; Zhao, C.N.; Xu, X.Y.; Gan, R.Y.; Cao, S.Y.; Liu, Q.; Shang, A.; Mao, Q.Q.; Li, H. Bin Phytochemical Composition and Antioxidant Capacity of 30 Chinese Teas. Antioxidants 2019, 8, 180. [Google Scholar] [CrossRef] [PubMed]
- Lassed, S.; Deus, C.M.; Djebbari, R.; Zama, D.; Oliveira, P.J.; Rizvanov, A.A.; Dahdouh, A.; Benayache, F.; Benayache, S. Protective Effect of Green Tea (Camellia sinensis (L.) Kuntze) against Prostate Cancer: From in Vitro Data to Algerian Patients. Evid.-Based Complement. Altern. Med. 2017, 2017, 1691568. [Google Scholar] [CrossRef] [PubMed]
- Cerezo-Guisado, M.I.; Zur, R.; Lorenzo, M.J.; Risco, A.; Martín-Serrano, M.A.; Alvarez-Barrientos, A.; Cuenda, A.; Centeno, F. Implication of Akt, ERK1/2 and Alternative P38MAPK Signalling Pathways in Human Colon Cancer Cell Apoptosis Induced by Green Tea EGCG. Food Chem. Toxicol. 2015, 84, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Williamson, M.P.; McCormick, T.G.; Nance, C.L.; Shearer, W.T. Epigallocatechin Gallate, the Main Polyphenol in Green Tea, Binds to the T-Cell Receptor, CD4: Potential for HIV-1 Therapy. J. Allergy Clin. Immunol. 2006, 118, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, S.; Sarethy, I.P.; Dang, S.; Gabrani, R. Green Tea Extract: Possible Mechanism and Antibacterial Activity on Skin Pathogens. Food Chem. 2012, 135, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Han, Y.; Wang, J.; Wang, Y.; Chen, X.; Shao, Y.; Cheng, Y.; Zhou, Z.; Lu, X.; Wu, Z. A review on anti-cancer effect of green tea catechins. J. Func. Foods. 2020, 74, 104172. [Google Scholar] [CrossRef]
- Ng, H.S.; Teoh, A.N.; Lim, J.C.W.; Tan, J.S.; Wan, P.K.; Yim, H.S.; Show, P.L.; Lan, J.C.W. Thermo-sensitive aqueous biphasic extraction of polyphenols from Camellia sinensis var. assamica leaves. J. Taiwan Inst. Chem. Eng. 2017, 79, 151–157. [Google Scholar] [CrossRef]
- Lai, W.F.; Baig, M.M.F.A.; Wong, W.T.; Zhu, B.T. Epigallocatechin-3-gallate in functional food development: From concept to reality. Trends Food Sci. Technol. 2020, 102, 271–279. [Google Scholar] [CrossRef]
- Wakamatsu, M.; Yamanouchi, H.; Sahara, H.; Iwanaga, T.; Kuroda, R.; Yamamoto, A.; Minami, Y.; Sekijima, M.; Yamada, K.; Kajiya, K. Catechin and Caffeine Contents in Green Tea at Different Harvest Periods and Their Metabolism in Miniature Swine. Food Sci. Nutr. 2019, 7, 2769–2778. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, B.J.; Chung, J.O.; Kim, H.N.; Kim, E.H.; Jung, S.; Lee, H.; Lee, S.J.; Hong, Y.S. Metabolomic Unveiling of a Diverse Range of Green Tea (Camellia sinensis) Metabolites Dependent on Geography. Food Chem. 2015, 174, 452–459. [Google Scholar] [CrossRef]
- Dong, F.; Yang, Z.; Baldermann, S.; Sato, Y.; Asai, T.; Watanabe, N. Herbivore-Induced Volatiles from Tea (Camellia sinensis) Plants and Their Involvement in Intraplant Communication and Changes in Endogenous Nonvolatile Metabolites. J. Agric. Food Chem. 2011, 59, 13131–13135. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Yu, Z.; Liu, X.; Zeng, L.; Cheng, S.; Li, J.; Tang, J.; Yang, Z. Effect of Major Tea Insect Attack on Formation of Quality-Related Nonvolatile Specialized Metabolites in Tea (Camellia sinensis) Leaves. J. Agric. Food Chem. 2019, 67, 6716–6724. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Zhang, C.; Lv, Z.; Shen, C. Pre- and Post-Harvest Exposure to Stress Influence Quality-Related Metabolites in Fresh Tea Leaves (Camellia sinensis). Sci. Hortic. 2021, 281, 109984. [Google Scholar] [CrossRef]
- Han, B.Y.; Chen, Z.M. Composition of the Volatiles from Intact and Mechanically Pierced Tea Aphid-Tea Shoot Complexes and Their Attraction to Natural Enemies of the Tea Aphid. J. Agric. Food Chem. 2002, 50, 2571–2575. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, D.C.; Lincoln, D.E. Volatile emissions from an odorous plant in response to herbivory and methyl jasmonate exposure. J. Chem. Ecol. 2006, 32, 725–743. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Watanabe, N.; Yang, Z. Understanding the Biosyntheses and Stress Response Mechanisms of Aroma Compounds in Tea (Camellia sinensis) to Safely and Effectively Improve Tea Aroma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Liu, X.; Zhou, Y.; Wang, X.; Zeng, L.; Fu, X.; Li, J.; Tang, J.; Dong, F.; Yang, Z. Formation and Emission of Linalool in Tea (Camellia sinensis) Leaves Infested by Tea Green Leafhopper (Empoasca (Matsumurasca) onukii Matsuda). Food Chem. 2017, 237, 356–363. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Peter Constabel, C. Tannins in Plant-Herbivore Interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
- Scott, E.R.; Li, X.; Wei, J.P.; Kfoury, N.; Morimoto, J.; Guo, M.M.; Agyei, A.; Robbat, A.; Ahmed, S.; Cash, S.B.; et al. Changes in Tea Plant Secondary Metabolite Profiles as a Function of Leafhopper Density and Damage. Front. Plant Sci. 2020, 11, 636. [Google Scholar] [CrossRef]
- Takino, Y.; Imagawa, H.; Horikawa, H.; Tanaka, A. Studies on the Mechanism of the Oxidation of Tea Leaf Catechins. Agric. Biol. Chem. 1964, 28, 64–71. [Google Scholar] [CrossRef]
- Gifu Ken Byogaityu Bozyo Center The Number of Pests (Graph). Available online: https://www.pref.gifu.lg.jp/page/2979.html (accessed on 2 March 2023).
- Ito, A.; Yanase, E. Study into the Chemical Changes of Tea Leaf Polyphenols during Japanese Black Tea Processing. Food Res. Int. 2022, 160, 111731. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular Framework for Processing, Visualizing, and Analyzing Mass Spectrometry-Based Molecular Profile Data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
- Nothias, L.F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-Based Molecular Networking in the GNPS Analysis Environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Mohimani, H.; Gurevich, A.; Shlemov, A.; Mikheenko, A.; Korobeynikov, A.; Cao, L.; Shcherbin, E.; Nothias, L.F.; Dorrestein, P.C.; Pevzner, P.A. Dereplication of Microbial Metabolites through Database Search of Mass Spectra. Nat. Commun. 2018, 9, 4035. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Schmid, R.; Petras, D.; Nothias, L.F.; Wang, M.; Aron, A.T.; Jagels, A.; Tsugawa, H.; Rainer, J.; Garcia-Aloy, M.; Dührkop, K.; et al. Ion Identity Molecular Networking for Mass Spectrometry-Based Metabolomics in the GNPS Environment. Nat. Commun. 2021, 12, 3832. [Google Scholar] [CrossRef]
- Hashimoto, F.; Nonaka, G.; Nishioka, I. Tannins and Related Compounds. XC.: 8-C-Ascorbyl (-)-Epigallocatechin 3-O-Gallate and Novel Dimeric Flavan -3-Ols, Oolonghomobisflavans A and B, from Oolong Tea. (3). Chem. Pharm. Bull. 1989, 37, 3255–3263. [Google Scholar] [CrossRef]
- Nonaka, G.; Kawahara, O.; Nishioka, I. Tannins and Related Compounds. XV. A New Class of Dimeric Flavan-3-Ol Gallates, Theasinensins A and B, and Proanthocyanidin Gallates from Green Tea Leaf. (1). Chem. Pharm. Bull. 1983, 31, 3906–3914. [Google Scholar] [CrossRef]
- Zhang, L.; Ho, C.T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and Biological Activities of Processed Camellia Sinensis Teas: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Matsuo, Y. Production Mechanisms of Black Tea Polyphenols. Chem. Pharm. Bull. 2020, 68, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Dai, W.; Lu, M.; Lv, H.; Guo, L.; Zhang, Y.; Zhu, Y.; Peng, Q.; Lin, Z. Study of the Dynamic Changes in the Non-Volatile Chemical Constituents of Black Tea during Fermentation Processing by a Non-Targeted Metabolomics Approach. Food Res. Int. 2016, 79, 106–113. [Google Scholar] [CrossRef]
- Samanta, T.; Cheeni, V.; Das, S.; Roy, A.B.; Ghosh, B.C.; Mitra, A. Assessing Biochemical Changes during Standardization of Fermentation Time and Temperature for Manufacturing Quality Black Tea. J. Food Sci. Technol. 2015, 52, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Q.X.; Song, B. Pesticidal Activity and Mode of Action of Monoterpenes. J. Agric. Food Chem. 2022, 70, 4556–4571. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Qi, D.; Yang, T.; Lv, H.; Guo, L.; Zhang, Y.; Zhu, Y.; Peng, Q.; Xie, D.; Tan, J.; et al. Nontargeted Analysis Using Ultraperformance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry Uncovers the Effects of Harvest Season on the Metabolites and Taste Quality of Tea (Camellia sinensis L.). J. Agric. Food Chem. 2015, 63, 9869–9878. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Redfern, S.P.; Kirkup, D.; Porter, E.A.; Kite, G.C.; Terry, L.A.; Berry, M.J.; Simmonds, M.S.J. Variation of Theanine, Phenolic, and Methylxanthine Compounds in 21 Cultivars of Camellia Sinensis Harvested in Different Seasons. Food Chem. 2017, 220, 517–526. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, L.; Liu, L.; Yang, Q.; Lu, Z.; Nie, Z.; Wang, Y.; Xia, T. Purification and Characterization of a Novel Galloyltransferase Involved in Catechin Galloylation in the Tea Plant (Camellia sinensis). J. Biol. Chem. 2012, 287, 44406–44417. [Google Scholar] [CrossRef]
- Bansal, S.; Choudhary, S.; Sharma, M.; Kumar, S.S.; Lohan, S.; Bhardwaj, V.; Syan, N.; Jyoti, S. Tea: A Native Source of Antimicrobial Agents. Food Res. Int. 2013, 53, 568–584. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins—A Final Frontier in Flavonoid Research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Jaros, A.; Lee, G.; Mozola, C.; Weir, Q.; Salminen, J.P. Hydrolyzable Tannins as “Quantitative Defenses”: Limited Impact against Lymantria Dispar Caterpillars on Hybrid Poplar. J. Insect Physiol. 2009, 55, 297–304. [Google Scholar] [CrossRef]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent Studies of the Volatile Compounds in Tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
| ID | RT | Parent Mass m/z | MS2 Fragmentation Ion | Dereplication Results | Exact Mass m/z | Δppm |
|---|---|---|---|---|---|---|
| 7 | 12.23 | 443.0981 | 291, 273, 153, 139, 123 | Epicatechin gallate | 443.0973 | 1.87 |
| 22 | 12.23 | 273.0759 | 147, 123 | N/A | ||
| 99 | 13.72 | 449.0848 | 303, 279 | N/A | ||
| 145 | 13.77 | 427.1021 | 275, 257, 153, 139, 107 | N/A | ||
| 171 | 13.79 | 257.0815 | 139, 131, 107 | N/A | ||
| 176 | 14.03 | 289.0703 | 205, 179, 163, 151, 139 | N/A | ||
| 244 | 7.76 | 286.1283 | 271, 201, 163, 135, 127, 123 | N/A | ||
| 109 | 9.90 | 731.1571 | 579, 443, 427, 409, 289 | N/A | ||
| 158 | 10.85 | 715.1650 | 579, 443, 393, 273 | N/A | ||
| 163 | 7.39 | 747.1565 | 595, 459, 427, 409, 289 | N/A | ||
| 195 | 13.12 | 579.1499 | 427, 409, 291, 287 | Procyanidin B2 | 579.1497 | 0.34 |
| 226 | 2.37 | 611.1401 | 443, 425, 307 | N/A | ||
| 239 | 9.69 | 921.1475 | 751 | N/A | ||
| 242 | 9.54 | 899.1673 | 729, 579, 457, 443, 425, 409 | N/A | ||
| 243 | 7.20 | 915.1590 | 745, 595, 459, 457, 425 | N/A | ||
| 334 | 12.09 | 867.1735 | 697, 547, 443, 425, 393, | N/A | ||
| 80 | 15.97 | 355.1733 | 203 | p-Menth-1-ene-4,7-diol 4-glucoside a | 355.1727 | 1.72 |
| 154 | 24.56 | 471.2206 | 335, 333 | Linalyl beta-vicianoside a | 471.2201 | 1.08 |
| 61 | 5.48 | 185.0414 | 153, 126, 125, 107 | Methyl gallate | 185.0444 | −16.23 |
| 1 | 7.97 | 195.0855 | 138, 110 | Caffeine | 195.0877 | −10.91 |
| 131 | 11.92 | 659.0836 | 489 | N/A |
| ID | RT | m/z | Predicted Annotation |
|---|---|---|---|
| 226 | 2.37 | 611.1401 | prodelphidin |
| 109 | 9.90 | 731.1571 | C-CG dimer |
| 163 | 7.39 | 747.1565 | C-GCG dimer |
| 242 | 9.54 | 899.1673 | GCG-CG dimer |
| 239 | 9.69 | 921.1475 | Na adduct of ID242 |
| 243 | 7.20 | 915.1590 | GCG-GCG dimer |
| 158 | 10.85 | 715.1650 | afzelechin-CG dimer |
| 334 | 12.09 | 867.1735 | afzelechin gallate-CG dimer |
| Sample | L* | a* | b* | ΔE |
|---|---|---|---|---|
| area A | 78.48 ± 0.24 | 11.47 ± 0.14 | 76.57 ± 0.49 | ― |
| area B | 70.62 ± 0.21 | 17.36 ± 0.12 | 80.24 ± 0.38 | 10.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, A.; Kamiya, J.; Katsuno, N.; Yanase, E. Alterations in Nonvolatile Components of Tea (Camellia sinensis) Induced by Insect Feeding under Field Conditions. Horticulturae 2023, 9, 1078. https://doi.org/10.3390/horticulturae9101078
Ito A, Kamiya J, Katsuno N, Yanase E. Alterations in Nonvolatile Components of Tea (Camellia sinensis) Induced by Insect Feeding under Field Conditions. Horticulturae. 2023; 9(10):1078. https://doi.org/10.3390/horticulturae9101078
Chicago/Turabian StyleIto, Ayumi, Jin Kamiya, Nakako Katsuno, and Emiko Yanase. 2023. "Alterations in Nonvolatile Components of Tea (Camellia sinensis) Induced by Insect Feeding under Field Conditions" Horticulturae 9, no. 10: 1078. https://doi.org/10.3390/horticulturae9101078
APA StyleIto, A., Kamiya, J., Katsuno, N., & Yanase, E. (2023). Alterations in Nonvolatile Components of Tea (Camellia sinensis) Induced by Insect Feeding under Field Conditions. Horticulturae, 9(10), 1078. https://doi.org/10.3390/horticulturae9101078





