Nutrients Use Efficiency in Coupled and Decoupled Aquaponic Systems
Abstract
:1. Introduction
2. Materials and Methods
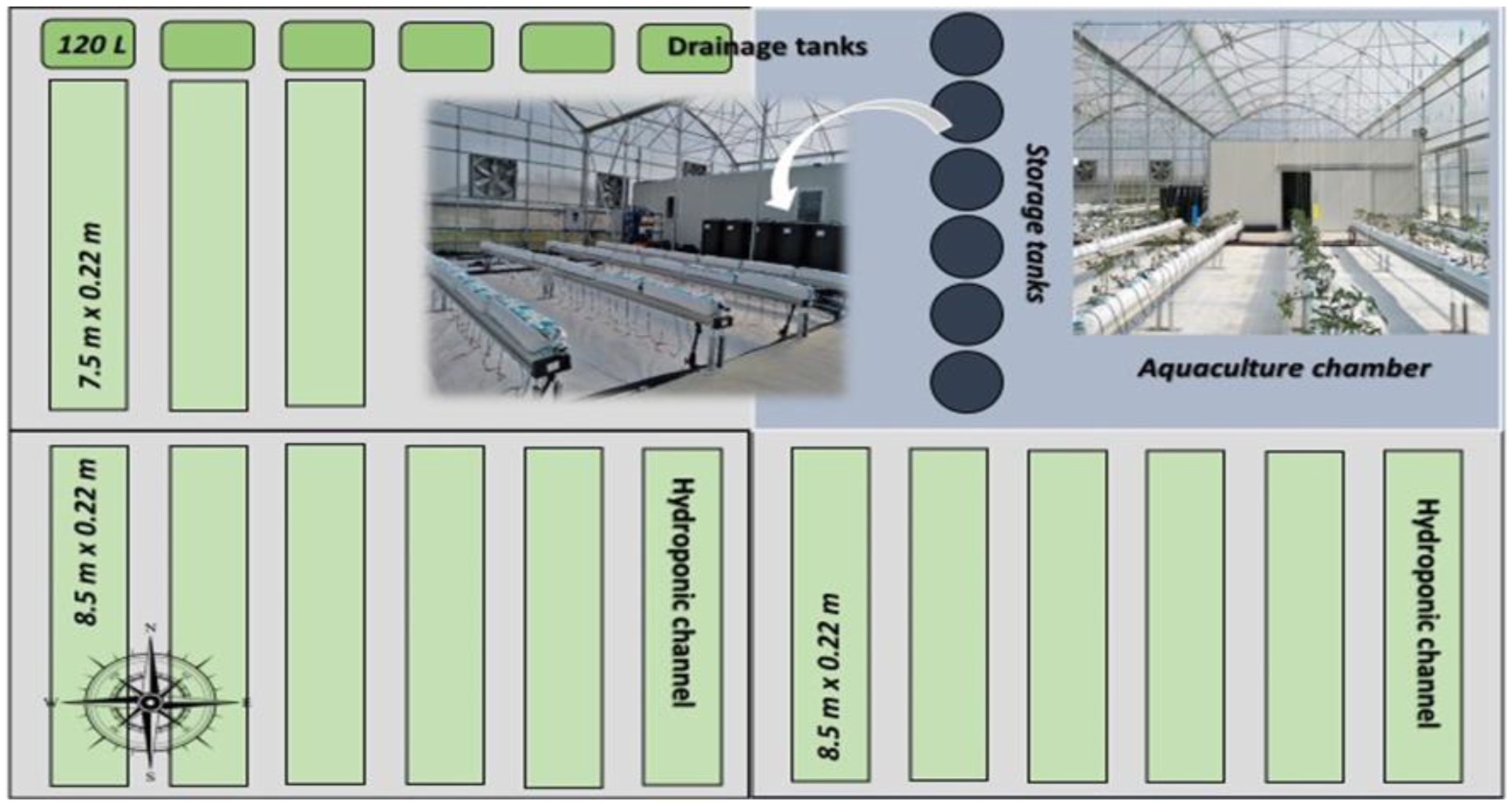
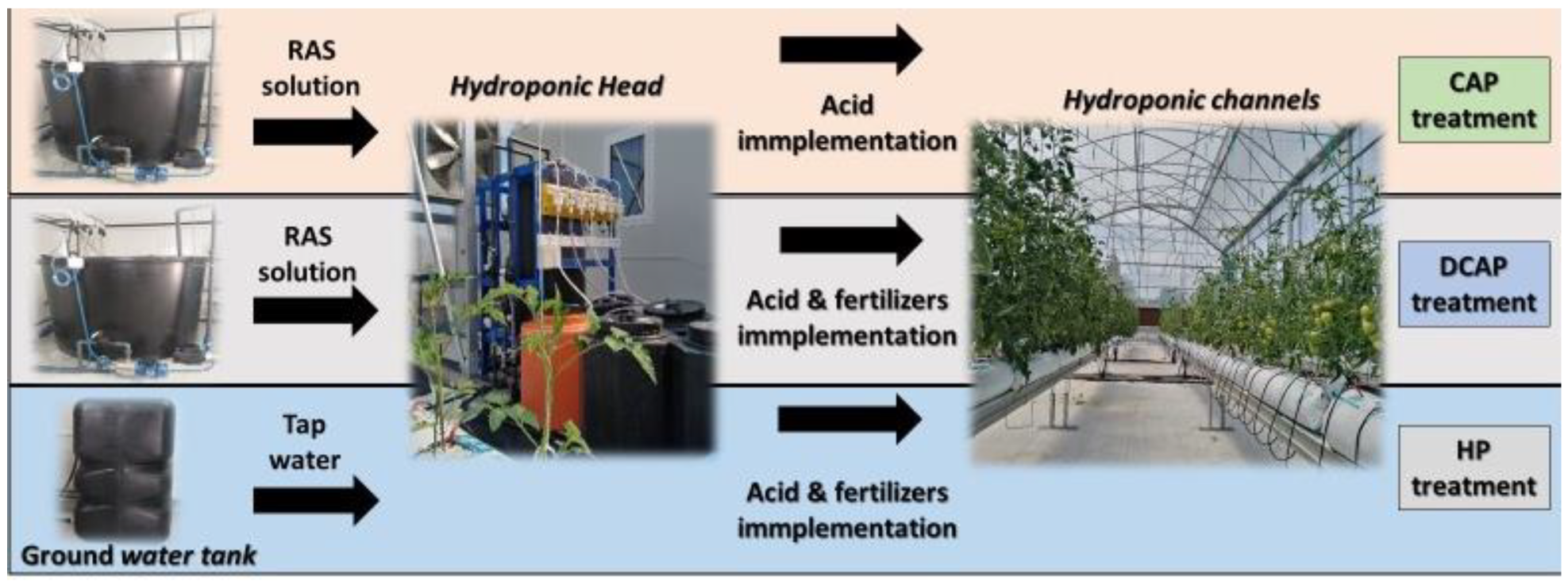
2.1. Experimental Setup
2.2. Experimental Set Up
2.3. Measurements and Analyses
2.4. Calculations
2.5. Statistical Analysis
3. Results
3.1. Water Quality Parameters
3.2. Nutrient Fluctuation in the System
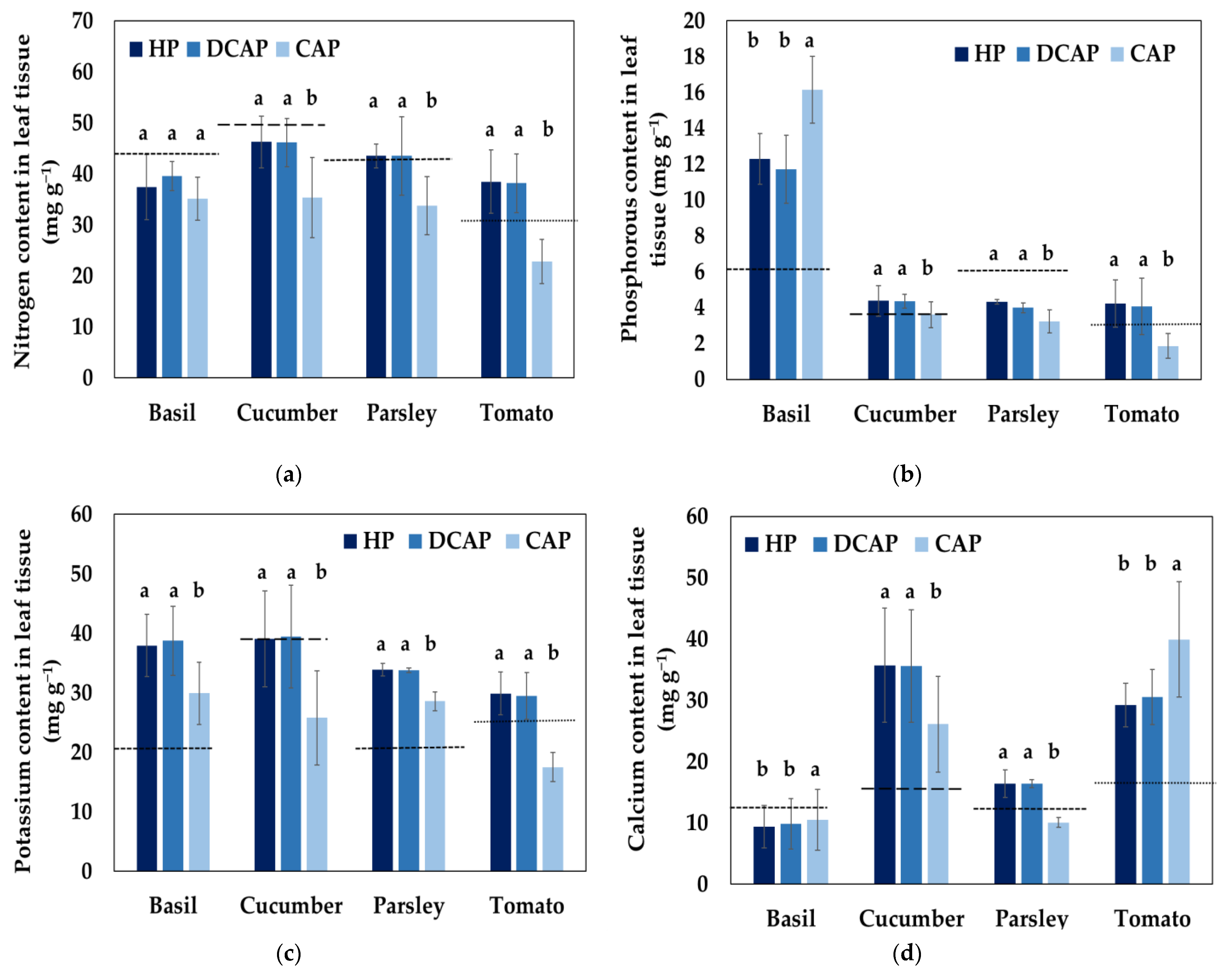
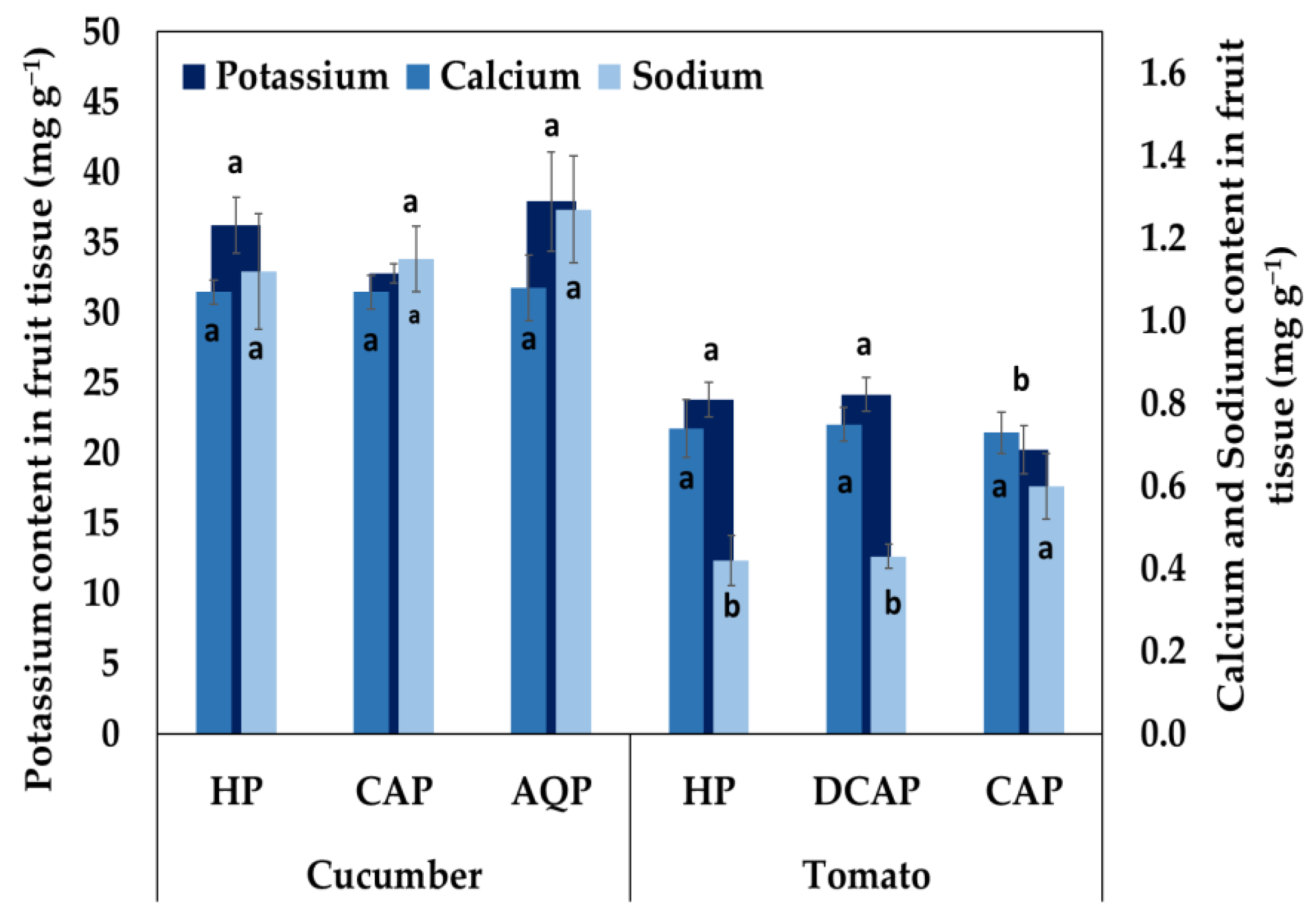
3.3. Nutrients in Leaf and Fruit Tissue
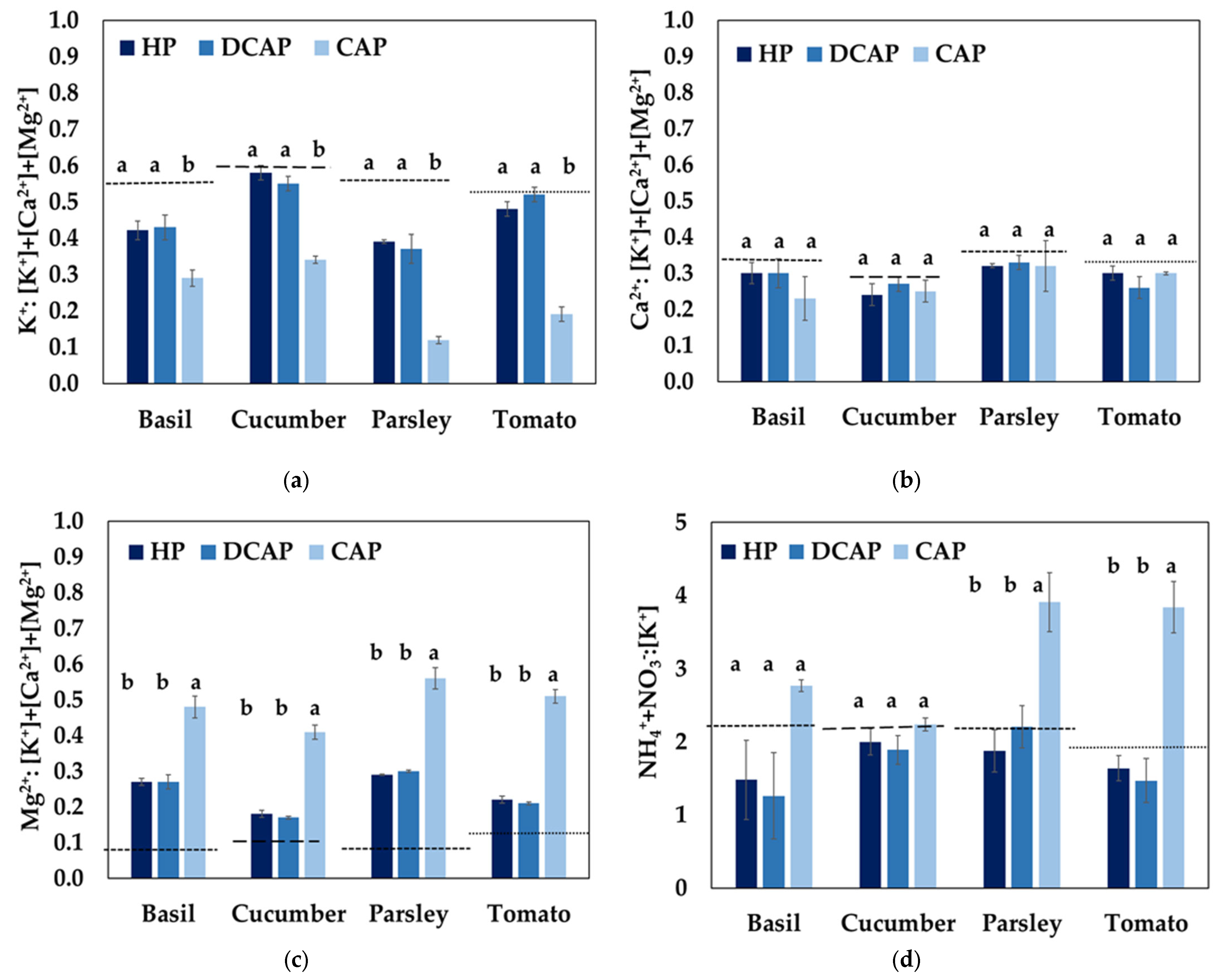
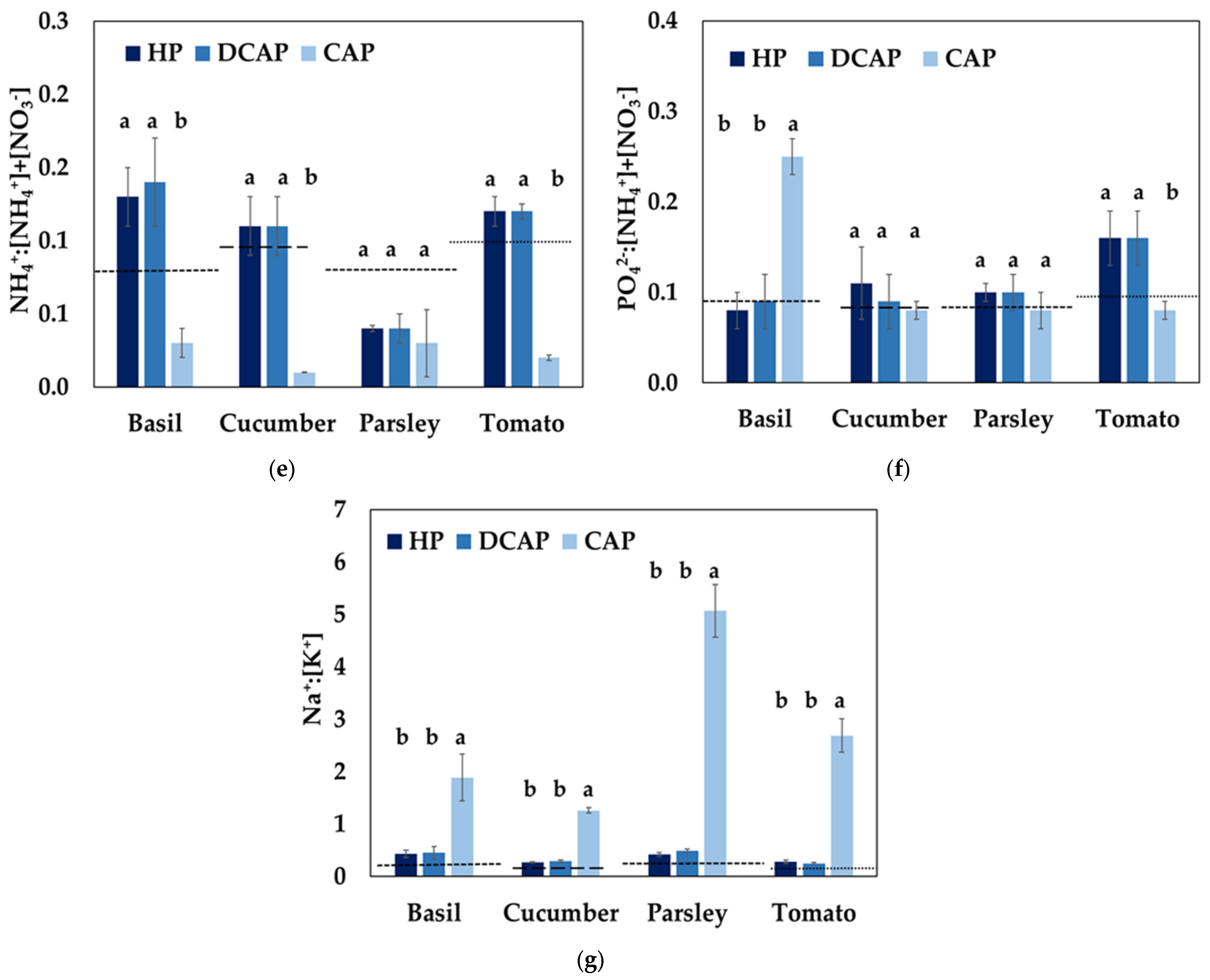
3.4. Nutrient Use Efficiency
4. Discussion
4.1. Nutrient Quality Assessment
4.2. Systems Evaluation Based on the Nutrient Use Efficiency
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Palm, H.W.; Knaus, U.; Appelbaum, S.; Goddek, S.; Strauch, S.M.; Vermeulen, T.; Kotzen, B. Towards commercial aquaponics: A review of systems, designs, scales and nomenclature. Aquac. Int. 2018, 26, 813–842. [Google Scholar] [CrossRef]
- Naegel, L.C. Combined production of fish and plants in recirculating water. Aquaculture 1977, 10, 17–24. [Google Scholar] [CrossRef]
- Lewis, W.M.; Yopp, J.H.; Schramm, H.L., Jr.; Brandenburg, A.M. Use of hydroponics to maintain quality of recirculated water in a fish culture system. Trans. Am. Fish. Soc. 1978, 107, 92–99. [Google Scholar] [CrossRef]
- Goddek, S.; Joyce, A.; Kotzen, B.; Burnell, G.M. Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Springer Nature: Berlin/Heidelberg, Germany, 2019; p. 619. [Google Scholar]
- Kloas, W.; Groß, R.; Baganz, D.; Graupner, J.; Monsees, H.; Schmidt, U.; Rennert, B. A new concept for aquaponic systems to improve sustainability, increase productivity, and reduce environmental impacts. Aquac. Environ. Interact. 2015, 7, 179–192. [Google Scholar] [CrossRef]
- Rahman, S. Effluent Water Characterization of Intensive Tilapia Culture Units and Its Application in an Integrated Lettuce Aquaponic Production Facility. Ph.D. Thesis, Auburn University, Auburn, AL, USA, 2010. [Google Scholar]
- Rakocy, J.E.; Masser, M.P.; Losordo, T.M. Recirculating aquaculture tank production systems: Aquaponics–integrating fish and plant culture. SRAC Publ. 2006, 454, 1–16. [Google Scholar]
- Suhl, J.; Baganz, D.; Kloas, W.; Dannehl, D.; Jobs, S.; Scheibe, G.; Schmidt, U. The potential of double recirculating aquaponic systems for intensive tomato production. In Proceedings of the III International Symposium on Horticulture in Europe-SHE2016 1242, Crete, Greece, 17–21 October 2016; pp. 679–686. [Google Scholar]
- Monsees, H.; Suhl, J.; Paul, M.; Kloas, W.; Dannehl, D.; Würtz, S. Lettuce (Lactuca sativa, variety Salanova) production in decoupled aquaponic systems: Same yield and similar quality as in conventional hydroponic systems but drastically reduced greenhouse gas emissions by saving inorganic fertilizer. PLoS ONE 2019, 14, e0218368. [Google Scholar] [CrossRef]
- Andriani, Y.; Dhahiyat, Y.; Zahidah, Z.; Zidni, I. The effect of stocking density ratio of fish on water plant productivity in aquaponics culture system. Nusant. Biosci. 2017, 9, 31–35. [Google Scholar] [CrossRef]
- Quilleré, I.; Roux, L.; Marie, D.; Roux, Y.; Gosse, F.; Morot-Gaudry, J.F. An artificial productive ecosystem based on a fish/bacteria/plant association. 2. Performance. Agric. Ecosyst. Environ. 1995, 53, 19–30. [Google Scholar] [CrossRef]
- Roosta, H.R. Effects of foliar spray of K on mint, radish, parsley and coriander plants in aquaponic system. J. Plant Nutr. 2014, 37, 2236–2254. [Google Scholar] [CrossRef]
- Castillo-Castellanos, D.; Zavala-Leal, I.; Ruiz-Velazco, J.M.J.; Radilla-García, A.; Nieto-Navarro, J.T.; Romero-Bañuelos, C.A.; González-Hernández, J. Implementation of an experimental nutrient film technique-type aquaponic system. Aquac. Int. 2016, 24, 637–646. [Google Scholar] [CrossRef]
- Sutton, R.J.; Lewis, W.M. Further observations on a fish production system that incorporates hydroponically grown plants. Progress. Fish-Cult. 1982, 44, 55–59. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, Z.; Zhang, J.; Xie, H.; Liang, S.; Wang, J.; Yan, R. Attempts to improve nitrogen utilization efficiency of aquaponics through nitrifies addition and filler gradation. Environ. Sci. Pollut. Res. 2016, 23, 6671–6679. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cabanás, V.M.; Pérez-Urrestarazu, L.; Juárez, A.; Kaufman, N.T.; Gross, J.A. Comparative analysis of horizontal and vertical decoupled aquaponic systems for basil production and effect of light supplementation by LED. Agronomy 2020, 10, 1414. [Google Scholar] [CrossRef]
- Rakocy, J.E. Integration of vegetable hydroponics with fish culture: A review. Tech. Mod. Aquac. 1993, 112–136. [Google Scholar] [CrossRef]
- Wortman, S.E. Crop physiological response to nutrient solution electrical conductivity and pH in an ebb-and-flow hydroponic system. Sci. Hortic. 2015, 194, 34–42. [Google Scholar] [CrossRef]
- Diem, T.N.T.; Konnerup, D.; Brix, H. Effects of recirculation rates on water quality and Oreochromis niloticus growth in aquaponic systems. Aquac. Eng. 2017, 78, 95–104. [Google Scholar] [CrossRef]
- Al Tawaha, A.R.; Wahab, P.E.M.; Jaafar, H.B.; Zuan, A.T.K.; Hassan, M.Z. Effects of fish stocking density on water quality, growth performance of tilapia and yield of butterhead Lettuce grown in decoupled recirculation aquaponic systems. J. Ecol. Eng. 2021, 22, 8–19. [Google Scholar] [CrossRef]
- Knaus, U.; Palm, H.W. Effects of fish biology on ebb and flow aquaponical cultured herbs in northern Germany (Mecklenburg Western Pomerania). Aquaculture 2017, 466, 51–63. [Google Scholar] [CrossRef]
- Colt, J. Water quality requirements for reuse systems. Aquac. Eng. 2006, 34, 143–156. [Google Scholar] [CrossRef]
- Monsees, H.; Keitel, J.; Paul, M.; Kloas, W.; Wuertz, S. Potential of aquacultural sludge treatment for aquaponics: Evaluation of nutrient mobilization under aerobic and anaerobic conditions. Aquac. Environ. Interact. 2017, 9, 9–18. [Google Scholar] [CrossRef]
- Goddek, S.; Delaide, B.; Mankasingh, U.; Ragnarsdottir, K.V.; Jijakli, H.; Thorarinsdottir, R. Challenges of sustainable and commercial aquaponics. Sustainability 2015, 7, 4199–4224. [Google Scholar] [CrossRef]
- Graber, A.; Junge, R. Aquaponic Systems: Nutrient recycling from fish wastewater by vegetable production. Desalination 2009, 246, 147–156. [Google Scholar] [CrossRef]
- Vergote, N.; Vermeulen, J. Recirculation aquaculture system (RAS) with tilapia in a hydroponic system with tomatoes. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 927, Lisbon, Portugal, 22–27 August 2010; pp. 67–74. [Google Scholar]
- Villaverde, S.; Garcia-Encina, P.A.; Fdz-Polanco, F. Influence of pH over nitrifying biofilm activity in submerged biofilters. Water Res. 1997, 31, 1180–1186. [Google Scholar] [CrossRef]
- Tyson, R.V.; Simonne, E.H.; Treadwell, D.D.; White, J.M.; Simonne, A. Reconciling pH for ammonia biofiltration and cucumber yield in a recirculating aquaponic system with perlite biofilters. HortScience 2008, 43, 719–724. [Google Scholar] [CrossRef]
- Webster, C.D.; Lim, C. (Eds.) Tilapia: Biology, Culture, and Nutrition; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Pickens, J. Integrating Effluent from Recirculating Aquaculture Systems with Greenhouse Cucumber and Tomato Production. Ph.D. Thesis, Auburn University, Auburn, AL, USA, 2015. [Google Scholar]
- Suhl, J.; Dannehl, D.; Baganz, D.; Schmidt, U.; Kloas, W. An innovative suction filter device reduces nitrogen loss in double recirculating aquaponic systems. Aquac. Eng. 2018, 82, 63–72. [Google Scholar] [CrossRef]
- Yep, B.; Zheng, Y. Aquaponic trends and challenges—A review. J. Clean. Prod. 2019, 228, 1586–1599. [Google Scholar] [CrossRef]
- Schmautz, Z.; Loeu, F.; Liebisch, F.; Graber, A.; Mathis, A.; Griessler Bulc, T.; Junge, R. Tomato productivity and quality in aquaponics: Comparison of three hydroponic methods. Water 2016, 8, 533. [Google Scholar] [CrossRef]
- Nichols, M.A.; Savidov, N.A. Aquaponics: A nutrient and water efficient production system. In Proceedings of the II International Symposium on Soilless Culture and Hydroponics 947, Puebla, Mexico, 15–19 May 2011; pp. 129–132. [Google Scholar]
- Monsees, H.; Kloas, W.; Wuertz, S. Decoupled systems on trial: Eliminating bottlenecks to improve aquaponic processes. PLoS ONE 2017, 12, e0183056. [Google Scholar] [CrossRef] [PubMed]
- Delaide, B.; Teerlinck, S.; Decombel, A.; Bleyaert, P. Effect of wastewater from a pikeperch (Sander lucioperca L.) recirculated aquaculture system on hydroponic tomato production and quality. Agric. Water Manag. 2019, 226, 105814. [Google Scholar] [CrossRef]
- Rodgers, D.; Won, E.; Timmons, M.B.; Mattson, N. Complementary Nutrients in Decoupled Aquaponics Enhance Basil Performance. Horticulturae 2022, 8, 111. [Google Scholar] [CrossRef]
- Savidov, N.; Brooks, A.B. Evaluation and Development of Aquaponics Production and Product Market Capabilities in Alberta; Crop Diversification Centre South, Alberta Agriculture, Food and Rural Development: Brooks, AB, Canada, 2004. [Google Scholar]
- Roosta, H.R.; Hamidpour, M. Effects of foliar application of some macro-and micro-nutrients on tomato plants in aquaponic and hydroponic systems. Sci. Hortic. 2011, 129, 396–402. [Google Scholar] [CrossRef]
- Pattillo, D.A.; Foshee, W.G.; Blythe, E.K.; Pickens, J.; Wells, D.; Monday, T.A.; Hanson, T.R. Performance of aquaculture effluent for tomato production in outdoor raised beds. HortTechnology 2020, 30, 624–631. [Google Scholar] [CrossRef]
- Savidov, N.A.; Hutchings, E.; Rakocy, J.E. Fish and plant production in a recirculating aquaponic system: A new approach to sustainable agriculture in Canada. In Proceedings of the International Conference and Exhibition on Soilless Culture: ICESC 2005 742, Singapore, 5–8 September 2005; pp. 209–221. [Google Scholar]
- Cooper, A. The ABC of NFT. Nutrient Film Technique; Grower Books: New York, NY, USA, 1979. [Google Scholar]
- Katsoulas, N.; Savvas, D.; Kitta, E.; Bartzanas, T.; Kittas, C. Extension and evaluation of a model for automatic drainage solution management in tomato crops grown in semi-closed hydroponic systems. Comput. Electron. Agric. 2015, 113, 61–71. [Google Scholar] [CrossRef]
- Savvas, D. Soilless Crops: Hydroponics, Substrates; Agrotypos Press: Athens, Greece, 2011. [Google Scholar]
- Vainshtein, E.E.; Lebedev, V.I. New determinations of Li, Na, K, Rb, Cs, Ca, and Sr in standards W-1 and G-1 by flame photometry. Geochemistry 1961, 10, 386–388. [Google Scholar]
- APHA. Standard Methods of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005; pp. 2–61. ISBN 0875530478. [Google Scholar]
- Kjeldahl, C. A new method for the determination of nitrogen in organic matter. Z. Anal. Chem. 1883, 22, 366. [Google Scholar] [CrossRef]
- Jones, J.B. Hydroponic Cropping; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Silva, J.A.; Uchida, R.S. Plant Nutrient Management in Hawaii’s Soils: Approaches for Tropical and Subtropical Agriculture; University of Hawaii: Honolulu, HI, USA, 2000. [Google Scholar]
- Owen, W.G.; Cockson, P.; Henry, J.; Whipker, B.; Currey, C.J. Basil (Ocimum basilicum). e-Gro Nutr. Monit. 2018, 1, 7. [Google Scholar]
- Sonneveld, C.; Voogt, W. Plant nutrition in future greenhouse production. In Plant Nutrition of Greenhouse Crops; Springer: Dordrecht, The Netherlands, 2009; pp. 393–403. [Google Scholar]
- Samarakoon, U.C.; Weerasinghe, P.A.; Weerakkody, W.A.P. Effect of electrical conductivity (EC) of the nutrient solution on nutrient uptake, growth and yield of leaf lettuce (Lactuca sativa L.) in stationary culture. Trop. Agric. Res. 2006, 18, 13. [Google Scholar]
- Blidariu, F.; Radulov, I.; Lalescu, D.; Drasovean, A.; Grozea, A. Evaluation of nitrate level in green lettuce conventional grown under natural conditions and aquaponic system. Anim. Sci. Biotechnol. 2013, 46, 244–250. [Google Scholar]
- Bittsanszky, A.; Uzinger, N.; Gyulai, G.; Mathis, A.; Junge, R.; Villarroel, M.; Kőmíves, T. Nutrient supply of plants in aquaponic systems. Ecocycles 2016, 2, 17–20. [Google Scholar] [CrossRef]
- Tsoumalakou, E.; Mente, E.; Kormas, K.A.; Katsoulas, N.; Vlahos, N.; Kapsis, P.; Levizou, E. Precise monitoring of lettuce functional responses to minimal nutrient supplementation identifies aquaponic system’s nutrient limitations and their time-course. Agriculture 2022, 12, 1278. [Google Scholar] [CrossRef]
- Resh, H.M. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Popma, T.; Masser, M. Tilapia Life History and Biology; Publication No. 283; Southern Regional Aquaculture Center: Stoneville, MS, USA, 1999. [Google Scholar]
- Masser, M.P.; Rakocy, J.; Losordo, T.M. Recirculating aquaculture tank production systems. In Management of Recirculating Systems; SRAC Publication 452; Southern Regional Aquaculture Center: Stoneville, MS, USA, 1999. [Google Scholar]
- Rakocy, J.E.; Bailey, D.S.; Shultz, R.C.; Thoman, E.S. Update on tilapia and vegetable production in the UVI aquaponic system. In New Dimensions on Farmed Tilapia: Proceedings of the Sixth International Symposium on Tilapia in Aquaculture, Manila, Philippines, 12–16 September 2004; Creative Unlimited: Somerset, NJ, USA, 2004; pp. 12–16. [Google Scholar]
- Guadagnin, S.G.; Rath, S.; Reyes, F.G.R. Evaluation of the nitrate content in leaf vegetables produced through different agricultural systems. Food Addit. Contam. 2005, 22, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Adler, P.R.; Summerfelt, S.T.; Glenn, D.M.; Takeda, F. Mechanistic approach to phytoremediation of water. Ecol. Eng. 2003, 20, 251–264. [Google Scholar] [CrossRef]
- Briat, J.F.; Curie, C.; Gaymard, F. Iron utilization and metabolism in plants. Curr. Opin. Plant Biol. 2007, 10, 276–282. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Cani, A.C.P.; Azevedo, R.V.D.; Pereira, R.N.; Oliveira, M.A.D.; Chaves, M.A.; Braga, L.G.T. Phytodepuration of the effluents in a closed system of fish production. Rev. Bras. Saúde Prod. Anim. 2013, 14, 371–381. [Google Scholar] [CrossRef]
- Cerozi, B.D.S.; Fitzsimmons, K. Phosphorus dynamics modeling and mass balance in an aquaponics system. Agric. Syst. 2017, 153, 94–100. [Google Scholar] [CrossRef]
- Malvi, U.R. Interaction of micronutrients with major nutrients with special reference to potassium. Karnataka J. Agric. Sci. 2011, 24, 106–109. [Google Scholar]
- Huett, D.O. Production and quality of sim carnations grown hydroponically in rockwool substrate with nutrient solutions containing different levels of calcium, potassium and ammonium-nitrogen. Aust. J. Exp. Agric. 1994, 34, 691–697. [Google Scholar] [CrossRef]
- Sonneveld, C. Growing cucumbers and tomatoes in rockwool. In Proceedings of the Fifth International Congress on Soilless Culture, Wageningen, The Netherlands, 18–24 May 1980; pp. 253–262. [Google Scholar]
- Bhatnagar, A.; Devi, P. Water quality guidelines for the management of pond fish culture. Int. J. Environ. Sci. 2013, 3, 1980–2009. [Google Scholar]
- Yang, T.; Kim, H.J. Comparisons of nitrogen and phosphorus mass balance for tomato-, basil-, and lettuce-based aquaponic and hydroponic systems. J. Clean. Prod. 2020, 274, 122619. [Google Scholar] [CrossRef]
- Nicoletto, C.; Maucieri, C.; Schmautz, Z.; Borin, M.; Sambo, P.; Junge, R. Baby leaf NFT production and water management in aquaponic system. In Proceedings of the International Symposium on Greener Cities for More Efficient Ecosystem Services in a Climate Changing World 1215, Bologna, Italy, 12–15 September 2017; pp. 159–164. [Google Scholar]
- Schachtman, D.; Liu, W. Molecular pieces to the puzzle of the interaction between potassium and sodium uptake in plants. Trends Plant Sci. 1999, 4, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Geisenhoff, L.O.; Jordan, R.A.; Santos, R.C.; Oliveira, F.C.D.; Gomes, E.P. Effect of different substrates in aquaponic lettuce production associated with intensive tilapia farming with water recirculation systems. Eng. Agríc. 2016, 36, 291–299. [Google Scholar] [CrossRef]
- Adams, P. Nutrition of greenhouse vegetables in NFT and hydroponic systems. In Proceedings of the International Symposium on New Cultivation Systems in Greenhouse 361, Cagliari, Italy, 26–30 April 1993; pp. 245–257. [Google Scholar]
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agric. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- Mourantian, A.; Aslanidou, M.; Mente, E.; Katsoulas, N.; Levizou, E. Basil functional and growth responses when cultivated via different aquaponic and hydroponics systems. PeerJ 2023, 11, e15664. [Google Scholar] [CrossRef]
- Wongkiew, S.; Hu, Z.; Chandran, K.; Lee, J.W.; Khanal, S.K. Nitrogen transformations in aquaponic systems: A review. Aquac. Eng. 2017, 76, 9–19. [Google Scholar] [CrossRef]
- Delaide, B. A Study on the Mineral Elements Available in Aquaponics, Their Impact on Lettuce Productivity and the Potential Improvement of Their Availability. Ph.D. Thesis, University of Liege, Liege, Belgium, 2017. [Google Scholar]





| Basil and Parsley | Cucumber (Vegetative Stage) | Cucumber (Fruity Stage) | Tomato (3rd Truss) | Tomato (5th Truss) | Tomato (10th Truss) | |
|---|---|---|---|---|---|---|
| Macronutrients (mg L−1) | ||||||
| Nitrate (NO3−) | 682 | 915 | 853 | 887 | 763 | 769 |
| Ammonium (NH4+) | 18 | 25 | 25 | 27 | 22 | 22 |
| Phosphate (PO4−) | 97 | 119 | 109 | 142 | 142 | 142 |
| Potassium (K+) | 200 | 242 | 281 | 274 | 293 | 313 |
| Calcium (Ca2+) | 150 | 166 | 136 | 204 | 188 | 180 |
| Magnesium (Mg2+) | 80 | 39 | 34 | 58 | 53 | 51 |
| Sulfur (SO4−2) | 288 | 125 | 134 | 346 | 394 | 384 |
| Micronutrients (μmol L−1) | ||||||
| Iron (Fe) | 5 | 15 | 15 | 15 | 15 | 15 |
| Boron (B) | 20 | 25 | 25 | 35 | 30 | 30 |
| Copper (Cu) | 1 | 0.8 | 0.8 | 0.8 | 0.8 | 0.7 |
| Zinc (Zn) | 5 | 5 | 5 | 5 | 5 | 5 |
| Manganese (Mn) | 5 | 10 | 10 | 10 | 10 | 10 |
| Molybdenum (Mo) | 5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Basil | Cucumber | Parsley | Tomato | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Nutrient Solution | Drainage Solution | Nutrient Solution | Drainage Solution | Nutrient Solution | Drainage Solution | Nutrient Solution | Drainage Solution | ||
| pH | HP | 6.1 ± 0.1 Aa | 6.1 ± 0.2 Ba | 6.0 ± 0.0 Aa | 5.9 ± 0.2 Ba | 5.7 ± 0.1 Ab | 6.4 ± 0.2 Ba | 5.8 ± 0.0 Aa | 5.2 ± 0.3 Bb |
| DCAP | 6.05 ± 0.9 Aa | 6.2 ± 0.1 Ba | 6.0 ± 0.1 Aa | 6 ± 0.2 Ba | 5.6 ± 0.1 Ab | 6.3 ± 0.2 Ba | 5.9 ± 0.0 Aa | 5.5 ± 0.2 Bb | |
| CAP | 6.2 ± 0.8 Ab | 7.1 ± 0.2 Aa | 6.1 ± 0.1 Ab | 7.0 ± 0.2 Aa | 5.9 ± 0.1 Ab | 7.6 ± 0.3 Aa | 5.8 ± 0.1 Ab | 7.5 ± 0.4 Aa | |
| RAS | 8.1 ± 0.7 | 7.8 ± 0.2 | 7.8 ± 0.0 | 8.1 ± 0.1 | |||||
| EC | HP | 2.2 ± 0.0 Aa | 2.5 ± 0.1 Aa | 2.3 ± 0.1 Ab | 2.8 ± 0.1 Aa | 2.1 ± 0.0 Ab | 2.4 ± 0.1 Aa | 3.0 ± 0.1 Ab | 4.1 ± 0.5 Aa |
| DCAP | 2.2 ± 0.1 Aa | 2.6 ± 0.1 Aa | 2.4 ± 0.0 Ab | 2.7 ± 0.1 Aa | 2.0 ± 0.0 Ab | 2.4 ± 0.1 Aa | 2.9 ± 0.1 Ab | 3.9 ± 0.4 Aa | |
| CAP | 1.3 ± 0.1 Ba | 1.3 ± 0.1 Bb | 1.2 ± 0.1 Ba | 1.1 ± 0.1 Bb | 0.9 ± 0.1 Bb | 1.3 ± 0.0 Ba | 1.1 ± 0.0 Ba | 1.1 ± 0.1 Ba | |
| RAS | 0.98 ± 0.0 | 0.88 ± 0.0 | 0.83 ± 0.1 | 0.94 ± 0.0 | |||||
| Basil | Cucumber | Parsley | Tomato | |||||
|---|---|---|---|---|---|---|---|---|
| Concentrations in the RAS | Nutrient Availability | Concentrations in the RAS | Nutrient Availability | Concentrations in the RAS | Nutrient Availability | Concentrations in the RAS | Nutrient Availability | |
| NO3− | 130.1 | 19.1 | 111.3 | 12.0 | 92.4 | 13.6 | 113.7 | 14.1 |
| NH4+ | 0.1 | 0.7 | 0.1 | 0.6 | 0.1 | 0.8 | 0.1 | 0.5 |
| PO4− | 13.7 | 14.1 | 11.7 | 9.8 | 9.3 | 9.6 | 8.3 | 5.8 |
| K+ | 19.1 | 9.5 | 19.3 | 7.9 | 11.2 | 5.6 | 17.7 | 6.0 |
| Ca2+ | 39.3 | 26.2 | 36.7 | 22.1 | 35.4 | 23.6 | 37.9 | 19.9 |
| Mg2+ | 42.5 | 53.1 | 41.4 | 106.5 | 37.5 | 46.8 | 42.5 | 78.2 |
| SO42− | 60.4 | 20.9 | 33.8 | 27.0 | 14.7 | 5.1 | 48.2 | 12.9 |
| Fe2+ | 0.1 | 8.8 | 0.1 | 8.2 | 0.1 | 8.1 | 0.1 | 9.5 |
| Na+ | 46.5 | - | 38.8 | - | 41.6 | - | 43.8 | - |
| Basil | ||||||
|---|---|---|---|---|---|---|
| Nutrient Element | Treatment | Ν | Nutrient Solution (NS; mg L−1) | Drainage Solution (DS; mg L−1) | Uptaken Concentration (Cu; mg L−1) | Removal Rate (RM; mg g−1) |
| NO3− | HP | 18 | 509.4 ± 131.9 a | 590.7 ± 144.6 a | 519.6 | 489.3 |
| DCAP | 18 | 511.4 ± 96.4 a | 557.77 ± 144.0 a | 521.6 | 488.0 | |
| CAP | 18 | 215.6 ± 55.4 b | 188.3 ± 55.1 b | 219.9 | 236.9 | |
| NH4+ | HP | 18 | 14.1 ± 6.1 a | 5.4 ± 2.4 a | 24.0 | 19.6 |
| DCAP | 18 | 14.9 ± 4.0 a | 5.0 ± 1.0 a | 25.3 | 22.9 | |
| CAP | 18 | 2.6 ± 1.0 b | 1.1 ± 0.7 b | 3.9 | 3.5 | |
| PO42− | HP | 18 | 80.5 ± 16.8 a | 73.4 ± 17.2 a | 101.8 | 96.3 |
| DCAP | 18 | 79.1 ± 12.5 a | 67.8 ± 13.5 a | 99.1 | 94.0 | |
| CAP | 18 | 82.8 ± 14.5 a | 67.1 ± 24.5 a | 106.5 | 100.4 | |
| K+ | HP | 36 | 206.4 ± 43.7 a | 196.2 ± 38.1 a | 219.4 | 224.4 |
| DCAP | 36 | 204.6 ± 34.6 a | 208.3 ± 40.8 a | 217.6 | 223.2 | |
| CAP | 36 | 41.1 ± 7.3 b | 32.4 ± 6.7 b | 52.2 | 47.3 | |
| Ca2+ | HP | 36 | 125.4 ± 30.8 a | 154.8 ± 34.9 a | 121.7 | 114.8 |
| DCAP | 36 | 127.3 ± 18.7 a | 163.5 ± 34.6 a | 123.7 | 117.5 | |
| CAP | 36 | 28.0 ± 11.7 b | 31.0 ± 8.0 b | 21.7 | 26.9 | |
| Na+ | HP | 36 | 49.9 ± 15.7 a | 72.2 ± 19.2 a | 28.3 | 39.3 |
| DCAP | 36 | 53.0 ± 13.7 a | 76.6 ± 25.5 a | 42.6 | 43.6 | |
| CAP | 36 | 48.9 ± 15.4 a | 62.0 ± 15.3 a | 36.4 | 42.5 | |
| Parsley | ||||||
| NO3− | HP | 24 | 495.5 ± 41.8 a | 521.3 ± 49.3 a | 466.3 | 161.0 |
| DCAP | 24 | 496.6 ± 48.4 a | 500.7 ± 76.1 a | 467.2 | 167.1 | |
| CAP | 24 | 90.6 ± 12.5 b | 81.9 ± 6.4 b | 111.9 | 87.2 | |
| NH4+ | HP | 24 | 2.6 ± 0.3 a | 1.0 ± 0.3 a | 4.0 | 1.4 |
| DCAP | 24 | 2.7 ± 0.4 a | 1.2 ± 0.3 a | 4.1 | 1.8 | |
| CAP | 24 | 0.7 ± 0.2 b | 0.6 ± 0.2 b | 1.3 | 0.7 | |
| PO42− | HP | 24 | 70.5 ± 8.1 a | 65.4 ± 4.8 a | 93.5 | 27.6 |
| DCAP | 24 | 65.2 ± 5.2 a | 54.2 ± 9.2 a | 85.6 | 20.9 | |
| CAP | 24 | 12.8 ± 1.8 b | 12.4 ± 1.6 b | 14.0 | 2.0 | |
| K+ | HP | 27 | 156.8 ± 2.6 a | 185.3 ± 6.4 a | 146.9 | 50.8 |
| DCAP | 27 | 136.7 ± 2.4 b | 167.0 ± 9.0 b | 128.5 | 34.7 | |
| CAP | 27 | 16.7 ± 0.3 b | 33.4 ± 3.3 c | 32.8 | 5.7 | |
| Ca2+ | HP | 27 | 138.2 ± 3.9 a | 149.3 ± 5.8 a | 126.9 | 48.4 |
| DCAP | 27 | 142.9 ± 4.0 a | 168.3 ± 10.8 a | 132.0 | 41.4 | |
| CAP | 27 | 38.7 ± 0.4 b | 49.5 ± 4.3 b | 27.9 | 27.9 | |
| Na+ | HP | 27 | 39.5 ± 1.2 a | 55.5 ± 5.2 a | 29.0 | 10.4 |
| DCAP | 27 | 40.4 ± 0.8 a | 58.6 ± 5.0 a | 32.1 | 9.1 | |
| CAP | 27 | 39.7 ± 1.5 a | 54.3 ± 4.0 a | 29.7 | 26.2 | |
| Cucumber | ||||||
|---|---|---|---|---|---|---|
| Nutrient Element | Treatment | Ν | Nutrient Solution (NS; mg L−1) | Drainage Solution (DS; mg L−1) | Uptake Concentration (Cu; mg L−1) | Removal Rate (RM; mg g−1) |
| NO3− | HP | 27 | 670.1 ± 93.4 a | 753.5 ± 215.8 a | 645.0 | 403.3 |
| DCAP | 27 | 660.4 ± 133.7 a | 742.9 ± 121.5 a | 636.4 | 425.3 | |
| CAP | 27 | 188.4 ± 62.7 b | 170.1 ± 95.2 b | 200.7 | 162.0 | |
| NH4+ | HP | 27 | 23.1 ± 1.5 a | 7.9 ± 1.7 a | 39.2 | 22.6 |
| DCAP | 27 | 24.7 ± 1.6 a | 8.0 ± 2.3 a | 42.2 | 25.3 | |
| CAP | 27 | 0.9 ± 0.5 b | 0.8 ± 0.4 b | 1.6 | 0.6 | |
| PO42− | HP | 27 | 110.5 ± 8.5 a | 108.8 ± 25.0 a | 108.7 | 72.4 |
| DCAP | 27 | 103.3 ± 8.7 a | 99.3 ± 21.0 a | 101.6 | 65.1 | |
| CAP | 27 | 28.7 ± 10.3 b | 23.4 ± 27.7 b | 29.0 | 25.9 | |
| K+ | HP | 36 | 239.8 ± 6.7 a | 236.3 ± 16.4 a | 233.6 | 148.7 |
| DCAP | 36 | 221.3 ± 5.0 a | 226.2 ± 7.7 a | 217.5 | 153.7 | |
| CAP | 36 | 47.0 ± 10.1 b | 35.7 ± 11.1 b | 54.7 | 43.5 | |
| Ca2+ | HP | 36 | 107.9 ± 5.3 a | 142.7 ± 6.2 a | 90.5 | 55.2 |
| DCAP | 36 | 121.3 ± 4.8 a | 161.5 ± 9.1 a | 101.2 | 76.3 | |
| CAP | 36 | 42.2 ± 5.3 b | 42.1 ± 4.5 b | 37.1 | 34.3 | |
| Na+ | HP | 36 | 42.4 ± 0.8 a | 70.1 ± 3.9 a | 27.1 | 16.5 |
| DCAP | 36 | 42.4 ± 1.2 a | 72.3 ± 3.8 a | 27.0 | 18.2 | |
| CAP | 36 | 40.7 ± 1.0 a | 52.6 ± 2.0 b | 25.0 | 26.3 | |
| Tomato | ||||||
| NO3− | HP | 27 | 695.4 ± 6.6 a | 802.8 ± 13.2 a | 715.7 | 421.8 |
| DCAP | 27 | 691.8 ± 17.7 a | 806.3 ± 21.2 a | 712.5 | 457.2 | |
| CAP | 27 | 135.5 ± 2.7 b | 70.5 ± 2.8 b | 172.5 | 225.4 | |
| NH4+ | HP | 45 | 22.9 ± 1.1 a | 9.3 ± 0.6 a | 31.0 | 20.6 |
| DCAP | 45 | 22.5 ± 1.0 a | 8.4 ± 0.5 a | 30.5 | 21.8 | |
| CAP | 45 | 1.1 ± 0.1 b | 0.3 ± 0.0 b | 2.1 | 1.9 | |
| PO42− | HP | 45 | 163.0 ± 6.0 a | 183.7 ± 6.8 a | 166.6 | 105.1 |
| DCAP | 45 | 164.3 ± 7.4 a | 182.1 ± 7.8 a | 167.8 | 117.2 | |
| CAP | 45 | 17.9 ± 0.9 b | 6.3 ± 0.8 b | 23.3 | 30.7 | |
| K+ | HP | 36 | 256.0 ± 4.3 a | 319.6 ± 20.3 a | 235.4 | 153.5 |
| DCAP | 36 | 259.0 ± 3.0 a | 305.3 ± 18.4 a | 237.7 | 173.0 | |
| CAP | 36 | 27.7 ± 0.7 b | 12.0 ± 2.2 b | 37.2 | 46.1 | |
| Ca2+ | HP | 36 | 180.7 ± 3.5 a | 264.6 ± 26.3 a | 141.5 | 93.8 |
| DCAP | 36 | 176.8 ± 3.2 a | 244.6 ± 50.4 a | 138.7 | 110.5 | |
| CAP | 36 | 41.6 ± 0.5 b | 47.9 ± 2.7 b | 37.2 | 48.5 | |
| Na+ | HP | 36 | 42.3 ± 1.1 a | 75.5 ± 7.6 a | 28.3 | 16.9 |
| DCAP | 36 | 43.2 ± 0.8 a | 76.6 ± 9.1 a | 29.8 | 20.5 | |
| CAP | 36 | 41.6 ± 0.9 a | 58.7 ± 3.6 b | 27.1 | 38.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslanidou, M.; Elvanidi, A.; Mourantian, A.; Levizou, E.; Mente, E.; Katsoulas, N. Nutrients Use Efficiency in Coupled and Decoupled Aquaponic Systems. Horticulturae 2023, 9, 1077. https://doi.org/10.3390/horticulturae9101077
Aslanidou M, Elvanidi A, Mourantian A, Levizou E, Mente E, Katsoulas N. Nutrients Use Efficiency in Coupled and Decoupled Aquaponic Systems. Horticulturae. 2023; 9(10):1077. https://doi.org/10.3390/horticulturae9101077
Chicago/Turabian StyleAslanidou, Maria, Angeliki Elvanidi, Anastasia Mourantian, Efi Levizou, Eleni Mente, and Nikolaos Katsoulas. 2023. "Nutrients Use Efficiency in Coupled and Decoupled Aquaponic Systems" Horticulturae 9, no. 10: 1077. https://doi.org/10.3390/horticulturae9101077
APA StyleAslanidou, M., Elvanidi, A., Mourantian, A., Levizou, E., Mente, E., & Katsoulas, N. (2023). Nutrients Use Efficiency in Coupled and Decoupled Aquaponic Systems. Horticulturae, 9(10), 1077. https://doi.org/10.3390/horticulturae9101077









