Abstract
Caprification is the process of hanging caprifig fruits on edible fig trees to transfer the pollen inside the caprifig to the edible fig via the wasp (Blastophaga psenes) living in the caprifig. It needs to be repeated several times for the proper fruit set of edible figs. The present study was conducted to determine the change in the number of Blastophaga psenes, the duration of Blastophaga’s exit, and pollen viability in case the caprifigs to be used in the caprification process are stored until use. The number of Blastophaga and in vitro pollen viability were tested at day 0 (harvest day) and after 4, 8, 12, 16 and 20 days of storage at three different temperatures (0, 4, and 8 °C). Afterwards, the effect of pollination frequency on the edible fig fruit set and quality was determined by using Blastophaga psenes as a vector in the pollination of stored caprifig fruits, pollinating five times with 4-day intervals and three times with 8-day intervals. Approximately a 50% reduction in the number of B. psenes was detected after 4 (180.22), 12 (174.11) and 16 (192.66) days of caprifigs storage at 0, 4, and 8 °C, respectively. The pollen germination percentage of the caprifigs increased with storage and was higher in those stored at 8 °C (43.96%) and 4 °C (41.70%). The highest fruit set was obtained when the caprifigs stored at 4 °C (76.41%) and 8 °C (71.38%) five times with 4-day intervals were used for pollination. The pollination practice repeated five times with 4-day intervals resulted in a lower proportion of extra-large fruits with a weight of >100 g, a higher proportion of fruits with no or slight ostiole damage and early ripening of fruits. These results suggest that B. psenes and pollen viability can be preserved by storing caprifigs at 4 or 8 °C and that fruit set and fruit characteristics would be positively affected with the use of stored caprifigs in the pollination practice repeated five times with 4-day intervals.
1. Introduction
One of the most economically important tree species in the Mediterranean region is the fig tree. Turkey is the world’s leading producer of figs, with a diverse range of cultivated and wild fig species, including caprifigs [1]. According to cropping/pollination characteristics, the edible figs ‘Smyrna’ and ‘San Pedro’ require pollination to produce a commercial crop [2,3]. Only female flowers were produced by edible figs on the female tree, which had a functional long-style. The other type, known as ‘caprifig’ (male fig), provides pollen for the main crop of edible figs. The syconium of male fig plants, caprifigs, has both functional male and non-functional female flowers (gall flowers) [4]. Caprifig pollen is carried by a single wasp species (Blastophaga psenes L.) [5,6]. Caprification is the process of hanging the caprifig fruits on the female trees and allowing the wasp to reach female fruits. Because the syconia of female fig trees gradually become receptive, it is critical to identify two or three caprifig cultivars in order to extend the caprification period [7,8]. Alternatively, fruits of caprifig cultivars can be stored at 4 °C for 14 days and used for caprification [9]. For a successful caprification, caprifig fruits need to maintain the number of wasps, pollen viability, and germination rate [10,11,12]. The changes in the number of wasps in stored caprifig fruits has been reported only by Anjam et al. [13]. Some estimates of caprifig’s pollen viability and germination percentages at caprifig harvest day have been reported [12,14,15,16]. However, there has been no published report about how many days caprifig fruits can be stored at 4 °C or while using an alternative storage temperature without losing pollen viability and germination. Several studies have shown that storing pollen at low temperatures is effective for their long-term preservation, including almond [17], cherimoya [18], mango [19], cherry [20], apple [21], pecan [22], gấc melon [23], plum [24], and rose [25]. However, because the fig’s pollen ripens in the male fig fruits (caprifig), no studies have been found to determine the effect of pollen storage within caprifigs on the viability and germination of the pollen.
The number of ‘Bursa Siyahı’ edible fig orchards has increased in recent years in order to improve Turkey’s export potential [26]. However, because the cultivar requires pollination for fruit set, there can be fluctuations in fruit yield, primarily due to pollination problems (caprification) [27]. Factors such as caprifig type, pollination intensity and frequency influence pollination efficiency [7,28,29,30]. The effect of pollination frequency on the fruit set, ripening time and fruit quality of edible fig cultivars commonly grown in the region has been reported in Tunusia [28,29] and Iran [7]. Edible fig’s fruit quality is affected by factors such as fruit diameter, fruit skin thickness, large fruit flesh, small fruit cavity, soluble solids content, titratable acidity, flesh and skin color. Medium-sized fruits with small ostiole damage and fruit cavity are regarded as good-quality fruit [30]. It is important to note that a large ostiole in the fig is an undesirable characteristic, as pests and pathogens enter the fruit [31]. Pink and red flesh color are preferred by the consumer. Moreover, early or late ripening of edible figs is important in terms of staying in the market longer and increasing export potential [32]. However, there has been only one published report [33] on the effect of pollination frequency on the fruit quality of the ‘Bursa Siyahı’ cultivar, one of the high-quality fresh fig products preferred in the world market with increasing export potential. Besides determining pollen viability and germination characteristics of caprifig fruits under in vitro conditions based on storage potential, it is also necessary to test them under in vivo conditions [12,15,16]. The present study aimed to develop a protocol by determining the in vitro wasp-pollen quality characteristics of caprifig fruits stored at different temperatures for pollination. In addition, it was aimed to determine the optimal frequency of caprification for fruit set and quality in ‘Bursa Siyahı’ figs.
2. Materials and Methods
2.1. Plant Materials and Experimental Design
The study was carried out at the Agricultural Application and Research Center of Bursa Uludag University between 2020 and 2022. According to the meteorological data, the area has a maximum temperature of 43.8 °C, a minimum temperature of −19.2 °C, and an annual rainfall of 700 mm. The experimental orchard has typical sandy soils with a pH of 7.1–7.5. Agricultural techniques, such as pruning, irrigation and fertilization were carried out according to standard procedures in the area. In the pollination experiment, the ‘Karabulut’ cultivar, which was 4 years old and planted at 5 × 5 m intervals, was used as the male fig (caprifig), whereas the ‘Bursa Siyahı’ cultivar, which was 20 years old and planted at 10 × 10 m intervals, was used as the female fig.
2.2. Caprifig Storage Conditions and Pollen Collection
The caprifig fruits of the ‘Karabulut’ cultivar were harvested when Blastophaga psenes wasps emerged from the profichi (spring crop of caprifig) fruit and the pollen matured in the early morning hours. The samples were stored at 0, 4 and 8 ± 0.5 °C at 85–90% humidity conditions for 20 days and analyzed every 4 days. The caprifig fruits belonging to the control (0 days) group were not stored at 0, 4, or 8 °C; they were analyzed on the harvest day. Fifteen caprifig fruits taken every 4 days from different storage temperatures were brought to room temperature and sliced open longitudinally. Then, the male flowers on the fruit were lightly tapped with a glass rod to ensure that the anthers remained on the mesh attached to the petri dishes, and the pollen passed through the mesh to the petri dishes [34].
2.3. Evaluation of In Vitro Blastophaga and Pollen Quality
2.3.1. Number of Blastophaga psenes and Duration of Blastophaga’s Exit
To calculate the number of B. psenes every 4 days in caprifig fruits stored at different temperatures, one fruit was put in a jar (8 × 10 cm) with four replications, and the number of wasps that emerged from the caprifig fruit over a few days was counted. For the control group (0 days), these procedures were carried out in the morning before the wasp’s emergence occurred on the harvest day. The mouth of the jars was closed with mesh to prevent the escape of wasps, and the jars were kept in the climate rooms at 26 °C in 16 h light:8 h dark [13]. The first exiting wasp was transferred to bottles containing 70% alcohol, the first and last exit day was recorded, and the duration of Blastophaga’s exit was determined.
2.3.2. Pollen Viability and Pollen Germination
Viability and germination tests were carried out immediately on the harvest day when the pollen was obtained. Pollen viability was determined using 2,3,5-Triphenyltetrazolium chloride (TTC) staining. The pollen was uniformly distributed in TTC solution, dripped onto the slide, and incubated in sunlight for two hours at room temperature. A light microscope was then used to analyze the pollen grains (×40; Leica DC 500). Pollen viability was determined using four replicates of approximately 150 grains; in the analysis, bright red color indicated viability and colorless indicated non-viability [35].
The Petri dish technique was utilized to determine in vitro pollen germination [35]. The pollen grains were planted on the culture media and incubated in the dark for 24 h at 25 °C. The culture media contained 1% agar, 5% sucrose and 5 ppm H3BO3 at pH 5.0 [14]. The pollen germination was tested in two random fields of two Petri dishes per treatment. It was considered germinated when the pollen tube length was equal to or larger than the diameter of the pollen grain.
2.4. Pollination Treatments
About ten days before caprification, the syconia in the leaf axils were 12 mm in diameter [36]; before they became receptive, uniform ‘Bursa Siyahı’ branches (about 50 cm long) were selected (three temperature treatment × two pollination treatment × three replication × four branches) and covered with mesh. One caprifig fruit of each temperature treatment was placed in an isolation mesh, and caprification was repeated 5 times with 4-day intervals (T1) and 3 times with 8-day intervals (T2). The caprification was carried out in the early morning hours. The isolated mesh was removed from each branch about three weeks after the end of the caprification period.
2.5. Evaluation of Fruit Quality
2.5.1. Initial and Final Fruit Set (%)
The number of fruits on the selected branches was recorded from caprification to harvest to determine the initial fruit set. The final fruit set was found by counting marketable fruits after harvest and comparing them with the number of fruits before caprification.
2.5.2. Time of Ripening (%)
The fruit ripening time was determined according to the change in skin color of the fruits. The ripe fruits were counted from September to November with 4-day intervals, and the proportion of ripe fruits was calculated. The proportion of ripened fruits was evaluated in 4 periods as before—September 15, September 15–October 1, October 1–15 and after October 15–November 1.
2.5.3. Fruit Characteristics
In terms of pomological characteristics, 10 fruits were collected randomly from each storage temperature*pollination frequency in each replication, and fruit weight (g), fruit length (mm), fruit diameter (mm), ostiole diameter (mm), skin thickness (mm) and flesh thickness (mm) were measured. The proportion of fruit weight was evaluated as follows: 40–60, 60–80, 80–100, and more than 100 g in all harvested fruits. Based on the quantity of observed fruit skin damage, ostiole damage was determined in all harvested fruits using the European grading criteria in which there are four categories: none (no damage), slight damage (ostiole-end splitting covering less than one-third of the fruit), moderate damage (ostiole-end splitting covering between one-third and two-thirds of the fruit), and severe damage (ostiole-end splitting covering more than two-thirds of the fruit) [37]. In all harvested fruits, proportion of fruit cavity was classified as very small, small, medium or large [38]. The average number of seeds was calculated by counting the number of fertile and sterile seeds in four fruits per storage temperature and pollination treatment interaction. The ripe fruits were immersed in water to separate fermented flesh from seeds. The dried seeds were then immersed in water. Floating seeds were regarded as sterile, whereas those at the bottom were considered fertile [34].
The incidence of fig endosepsis (internal rot) was evaluated as the presence or absence of damage and calculated as a percentage. Soluble solids content (SSC) (°Brix) was determined with a digital refractometer, (PR-101 ATAGO, Norfolk, VA) and titratable acidity (TA) (g citric acid/100 mL) was found by titrating fig juice with 0.1 M NaOH. Fruit skin and flesh colors in lightness (L), hue (H°) and chroma (C) values were measured using a colorimeter (Chroma Meter CR-400, Minolta, Japan).
2.6. Statistical Analysis
The data obtained in the study were statistically analyzed by two-factor analyses of variance (ANOVA). ANOVA was conducted with the factors of caprifig storage temperature (0, 4, and 8 °C) and storage time (4, 8, 12, 16 and 20 days), with fresh pollen (0 days) as the control. Moreover, caprifig storage temperature and pollination frequency factors were analyzed. The mean values were compared with Duncan’s multiple range test (p < 0.05). Arcsine root square transformation was used for binomial data (pollen viability, germination, initial and fruit set, time of ripening, the proportion of fruit weight, ostiole damage and fruit cavity). ANOVA using the Statistical Package for the Social Sciences (SPSS) software version 23.0 was used and presented in Tables S1–S19. Correlations between experimental variables were determined using Pearson’s correlation coefficient, with the significance level set at p < 0.05. Principle component analysis was performed using the Varimax factor rotation method by JMP version 17, and scatter plots of the first two factors were created.
3. Results
3.1. Number of Blastophaga psenes and Duration of Blastophaga’s Exit
The results indicate that the number of B. psenes was significantly affected by caprifig storage (Table 1 and Table S1). The B. psenes number decreased depending on the caprifig storage time. The highest B. psenes number was obtained on the day of harvest (0 days) (357.29), followed by 8 (266.88) and 4 (243.62) days of caprifig storage. The lowest wasp number was observed in the caprifigs stored for 20 (56.22) days. The B. psenes number was also affected by the caprifig storage temperature. The number of B. psenes in the caprifigs stored at 4 °C (239.42) and 8 °C (225.75) was higher than in those stored at 0 °C (143.27). Moreover, the interaction of storage temperature and time had a significant effect on the number of B. psenes. The B. psenes number decreased at 0 °C by about 50% after 4 (180.22) and 8 (185.22) days of storage and by more than 95% after 16 (19.44) and 20 (1.44) days. At 4 °C, the number of B. psenes decreased by 52% and 47% after 12 (174.11) and 16 (192.66) days, respectively, and more than 85% after 20 (46.55) days. While a 38% decrease in the number of B. psenes was observed after 12 days of caprifig storage at 8 °C (227), for those stored for 20 days, the least B. psenes loss was obtained from 8 °C (120.66) (Figure 1).

Table 1.
The effect of storage temperatures and time on the number of Blastophaga psenes, duration of Blastophaga’s exit, pollen viability, and germination.
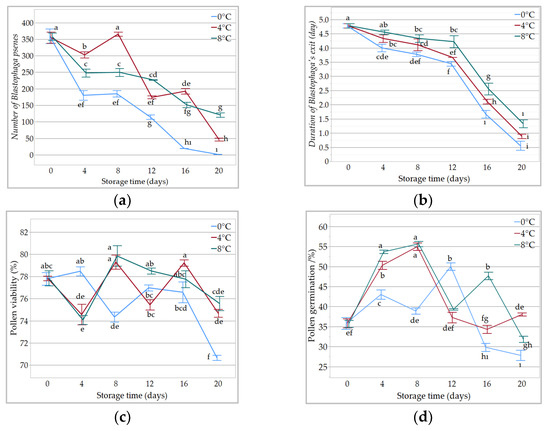
Figure 1.
The effect of storage temperature and time interaction on (a) the number of B. psenes; (b) duration of Blastophaga’s exit; (c) pollen viability; (d) pollen germination. Different small letters indicate a significant difference according to Duncan test, p < 0.05.
The storage temperature and time significantly affected the duration of Blastophaga’s exit, but the interaction effect of storage temperature and time was insignificant (Table 1 and Table S1). B. psenes had the highest exit time (4.77) on the day of harvest (0 days), followed by those stored for 4 (4.29) and 8 days (4.07). The lowest wasp exit time was observed in the caprifigs with a storage time of 20 days (0.92). Storage temperature significantly affected the duration of Blastophaga’s exit. The Blastophaga’s exit time in the caprifigs stored at 8 °C (3.62) was the highest, followed by those stored at 4 °C (3.31).
3.2. Evaluation of the Pollen Viability and Germination
Pollen viability was not affected by whether the pollen was obtained from fresh or stored caprifig but was affected by the storage temperature (Table 1 and Table S2). The pollen viability of the caprifigs stored at 8 °C (77.25%) was the highest, followed by those stored at 4 °C (76.83%). The pollen viability of the caprifigs varied according to the storage time, and the highest pollen viability was obtained at 0 (77.82%) and 8 (77.80%) days, followed by 12 (76.96%) days of storage. Moreover, the interaction of storage temperature and time significantly affected pollen viability. The highest pollen viability was obtained from the caprifigs stored at 8 °C (79.84%) and 4 °C (79.29%) for 8 days, followed by those stored for 16 days at 4 °C (79.22%) (Figure 1 and Figure 2).
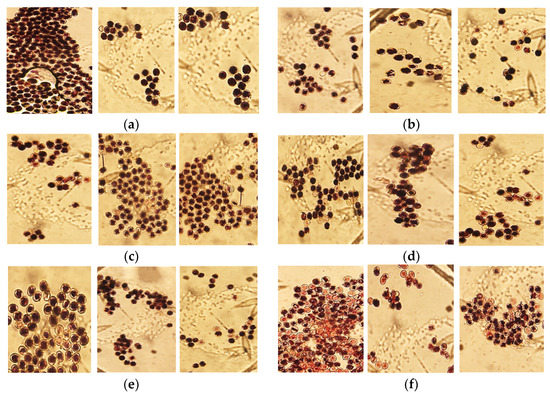
Figure 2.
Comparison of pollen viability from storage temperatures 0, 4 and 8 °C (from the left). Storage time: (a) 0 days; (b) 4 days; (c) 8 days; (d) 12 days; (e) 16 days; (f) 20 days.
The results indicate that pollen germination increased with caprifig storage (Table 1 and Table S2). Moreover, storage temperature and time affected the germination rate. Pollen germination was higher in the caprifigs stored at 8 °C (43.96%), followed by those stored at 4 °C (41.70%). The pollen of caprifigs stored for 8 (49.82%) and 4 (48.98%) days had the highest germination rate, while those stored for 20 (32.55%) days had the lowest. Germination increased continuously until the 12th day of storage but then decreased linearly. The germination rate of the pollen grains was affected by the interaction of storage temperature and time. The pollen obtained from the caprifigs stored for 8 days at 8 (52.62%) and 4 °C (50.11%) had the highest germination rate, and those stored for 20 days at 8 °C (33.44%) and for 16 (32.16%) and 20 (31.04%) days at 0 °C had the lowest germination rate (Figure 1 and Figure 3).
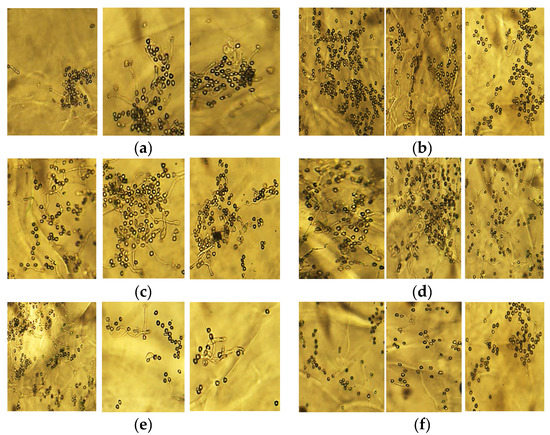
Figure 3.
Comparison of pollen germination from storage temperatures 0, 4 and 8 °C (from the left). Storage time: (a) 0 days; (b) 4 days; (c) 8 days; (d) 12 days; (e) 16 days; (f) 20 days.
3.3. Initial and Final Fruit Set
Storage temperature and pollination frequency affected the initial and final fruit sets, but their interaction was significant only on the final fruit set (Table 2 and Table S3). The initial and final fruit set in the caprifigs stored at 0 °C were lower than in those stored at 4 and 8 °C. Thus, the percentages of the initial and final fruit set were, respectively, 76.77% and 65.44% in the caprifigs stored at 0 °C, and 81.23% and 70.96% in the caprifigs stored at 4 °C. Moreover, the differences in the pollination frequency were statistically significant for the initial and final fruit set. The highest initial and final fruit set was obtained from T1 (4 day intervals), with 80.47% and 71.37%, respectively. The interaction of storage temperature and pollination frequency significantly affected the final fruit set. The highest fruit set was obtained from the caprifigs stored at 4 °C and pollinated 4-day intervals (76.41%), followed by those stored at 8 °C and pollinated 4-day intervals (71.38%).

Table 2.
The effect of storage temperature, pollination frequency, and their interaction on the fruit characteristics of the ‘Bursa Siyahı’ fig cultivar.
3.4. Fruit Characteristics
The effect of pollination frequency on the weight, diameter, length and ostiole diameter of the edible fruits was statistically significant, while caprifig storage temperature did not significantly affect these parameters (Table 2, Tables S4 and S5). In addition, ostiole diameter was affected by caprifig storage temperature. However, the effects of caprifig storage temperature and pollination frequency were insignificant except for fruit weight. The highest fruit weight was recorded for 8-day intervals (T2) pollination (84.89 g). Considering the caprifig storage temperature and pollination frequency, the highest fruit weight was obtained from the caprifigs stored at 8 and 0 °C and pollinated at 8-day intervals (87.16 and 83.79 g, respectively), whereas the lowest value was observed for the caprifigs stored at 8 °C and pollinated at 4-day intervals (78.89 g). The proportion of fruit weight was affected by the interaction of storage temperature and pollination (Figure 4, Tables S6 and S7). When the fruits stored at 8 °C were pollinated at 4-day intervals (18.74%), the proportion of fruits with a weight of 40–60 g increased. When the caprifigs stored at 8 °C were pollinated three times (30.66%), the proportion of fruits weighing more than 100 g increased, whereas it decreased in 4 °C × T1 (14.41%) and 8 °C × T1 (14.78%) (Figure 4). The fruit diameter and length values increased with 8-day-interval pollination (57.34 and 52.67 mm). Moreover, the ostiole diameter value was higher when the fruits were pollinated at 8-day intervals (7.16 mm). In addition, the ostiole diameter increased when the caprifigs stored at 4 °C (7.44 mm) and 8 °C (7.28 mm) were used for pollination, and decreased when those stored at 0 °C (6.43 mm) were used. The proportion of ostiole damage was affected by the interaction of storage temperature and pollination frequency (Tables S8 and S9). The proportion of severe ostiole damage increased (37.24%) when the caprifigs stored at 4 °C were used for 8-day-interval pollination, while it decreased when those stored at 0 °C (20.32%), 4 °C (21.65%) and 8 °C (20.02%) were used for 4-day-interval pollination (Figure 4).
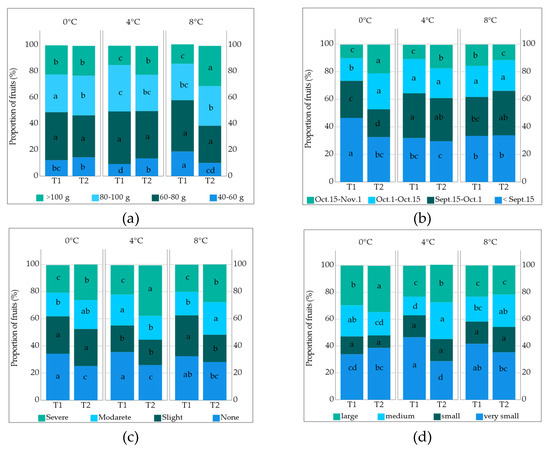
Figure 4.
Proportion of fruits (%) according to (a) time of ripening; (b) fruit weight; (c) ostiole damage; (d) fruit cavity at interaction different storage temperature × pollination frequency. Different small letters indicate a significant difference according to Duncan test, p < 0.05 (T1: 4-day intervals, T2: 8-day intervals pollination, Sept.: September, Oct.: October, Nov.: Novermber).
Flesh and skin thickness and fruit cavity were not affected by storage temperature, pollination frequency, or their interaction (Table 2 and Tables S10 and S11). However, the interaction of storage temperature and pollination frequency had a significant effect on the proportion of fruit cavity (Tables S12 and S13). 4 °C × T1 (46.51%, 16.37%) and 8 °C × T1 (41.65%, 16.52%) had the highest proportion of fruits with a very small and small cavities. The large fruit cavity was obtained from the fruits belonging to 0 °C × T2 (34.49%) and 0 °C × T1 (29.67%) interactions. The single seed weight and sterile seeds percentage of the fruits were affected by caprifig storage temperature and pollination frequency, whereas the fertile seeds per fruit was affected by only pollination frequency (Table 2 and Table S14). Moreover, the interaction of storage temperature and pollination frequency was significant except for the single seed weight. The fertile seeds number per fruit increased with 4-day interval pollination (1096.66).
The highest seed weight value was recorded at 4 (1.98 mg) and 8 °C (1.78 mg) and in T2 (1.91 mg) in terms of caprifig storage temperatures and pollination frequency, respectively. Regarding storage temperatures and pollination frequency, the highest percentage of sterile seed was observed at 0 (1.13%) and T2 (1.08%), respectively. For the interaction of storage temperature and pollination frequency, the sterile seeds percentage for the caprifigs stored at 0 and 8 °C and pollinated 8-day intervals were the highest (1.33%, 1.19%). The incidence of fig endosepsis increased with the use of the caprifigs stored at 0 °C. Moreover, pollination frequency and its interaction with caprifig storage temperature affected the percentage of fig endosepsis. The highest percentage of fig endosepsis was recorded in T1 (33.02%) and 0 °C × T1 (37.78%) in terms of storage temperature and the interaction of storage temperature and pollination frequency, respectively. The soluble solids content (SSC) and titratable acidity (TA) values of the fruits were not affected by caprifig storage temperature, pollination frequency or their interaction (Table 2 and Table S15).
3.5. Time of Ripening
The effects of storage temperature, pollination frequency as well as their interaction were significant on the proportion of the fruit ripening (Table 3 and Tables S16 and S17).

Table 3.
Effects of storage temperature, pollination frequency and the interaction of storage temperature × pollination frequency on time of ripening of fruits.
Four-day intervals of pollination (37.59%) and using the caprifigs stored at 0 °C (40.00%) increased the proportion of ripened fruits in the period of September 1–15. It was also observed that 0 °C × T1 (46.77%) had the highest proportion of ripened fruits, while 4 °C × T2 (29.51%) had the lowest proportion of ripened fruits in the period of September 1–15. The highest ripened fruit proportion was obtained with the pollination of the caprifigs stored at 4 °C (31.89%) and 8 °C (29.68%) in the period of September 15 and October 1. From October 15 to November, the highest proportion of ripened fruit was obtained from those pollinated 8-day intervals (16.03%). When the caprifigs stored at 0 °C were pollinated at 8-day intervals (20.84%), the proportion of ripened fruit increased in this period, whereas it decreased in 0 °C × T1 (9.92%) and 4 °C × T1 (10.34%) (Figure 4).
3.6. Fruit Skin and Flesh Color
The results showed that flesh color (hue) was significantly affected by pollination frequency and caprifig storage temperature and pollination frequency interaction (Table 4 and Table S18). However, lightness (L) and chroma (C) values were not affected by pollination frequency or storage temperature. The 4-day interval pollination significantly increased the hue value of ‘Bursa Siyahı’ fruit flesh. On the skin hue value, the effect of pollination frequency, storage temperature and the interaction of storage temperature and pollination frequency was insignificant (Table 4 and Table S19).

Table 4.
The effect of storage temperature, pollination frequency, and their interaction on the skin and flesh color of the ‘Bursa Siyahı’ fig cultivar.
3.7. Correlation Matrix Heatmap
The correlation matrix heatmap shows the values of the Pearson correlation coefficient, which expresses the strength of the linear relationship between two variables. Pollen viability showed a strong and positive correlation with pollen germination and initial fruit set and a moderate positive correlation with the number of B. psenes, ripening in the period of September 15–October 1 and skin thickness (the values of the Pearson correlation coefficient ranged from 0.66 to 0.49) (Figure 5). In contrast, it showed a moderate negative correlation with slight ostiole damage, severe fruit cavity, and sterile seeds (the Pearson correlation coefficients ranged from −0.41 to −0.42). Pollen germination showed a strong positive correlation with small fruit cavity and pollen viability, moderate positive correlation with the number of B. psenes, the duration of Blastophaga’s exit, and skin thickness, whereas it was negatively correlated with large fruit cavity and fig endosepsis.
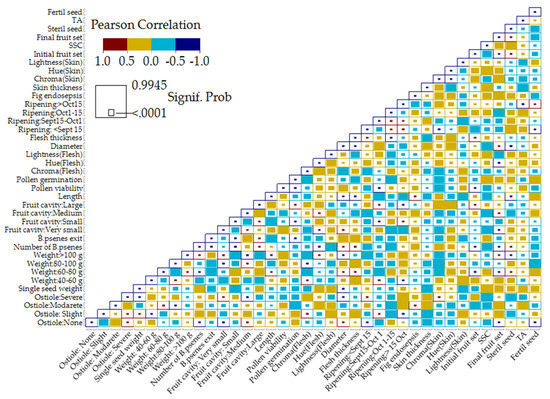
Figure 5.
Heat map showing Pearson’s correlation coefficients for all traits. Self-self correlations are identified in dark blue. Color indicates the direction (positive/negative) and the strength of the correlation, while the size of the circle represents the significance value.
The number of B. psenes showed a strong positive correlation with fruit set, slight ostiole damage, and the proportion of fruit weighing 60–80 g, while it had a moderate positive correlation with the duration of Blastophaga’s exit and the proportion of fruit with a very small and small cavity. In contrast, it was negatively correlated with the proportion of fruit weighing more than 100 g, the proportion of fruit with a large cavity, diameter, length, flesh thickness and sterile seeds. The duration of Blastophaga’s exit showed a positive correlation with fruit set, pollen germination the proportion of fruit with a medium cavity and skin thickness but a negative correlation with the proportion of fruit with a large cavity. The single seed weight was correlated negatively with the proportion of fruits weighing 40–60 g, slight ostiole damage, and the number of fertile seeds, and positively with the proportion of fruits weighing 80–100 g and severe ostiole damage. The number of fertile seeds correlated positively with no ostiole damage, the proportion of fruit with a small cavity and the proportion of fruit ripening before September 15, while it correlated negatively with the proportion of fruit with a large fruit cavity, skin thickness, the proportion of fruit ripening after October 15 and TA.
The percentage of sterile seeds showed a positive correlation with moderate ostiole damage, the proportion of fruit weighing more than 100 g, diameter and length, and a negative correlation with no ostiole damage, the proportion of fruit weighing 60–80 g, pollen viability, the number of B. psenes and fruit sets. Single seed weight, the proportion of fruit with a medium cavity, length, diameter and the percentage of sterile seeds were all correlated positively with the proportion of fruit weighing more than 100 g and negatively with fruit sets, no ostiole damage, the proportion of fruit with very small cavity and flesh hue value. A positive correlation was detected between fig endosepsis and the proportion of large fruit cavity, whereas the correlation was negative between the proportion of small fruit cavity and pollen germination.
3.8. Principal Components Analysis
PCA was applied to evaluate the data set and structure for the most important variables. The analysis indicated that the first five principal components had eigenvalues greater than 1.0, and the total variance explained was 75.35%. The variations of the first five principal components were 27.40%, 17.80%, 14.63%, 10.05%, and 5.43%, respectively. The PC1 represented the maximum variation of the data set, and it was positively connected with fruit diameter, weight, length, sterile seed rate and flesh thickness, and negatively connected with the number of Blastophaga, initial fruit set, final fruit set and flesh hue value. Pollen germination, pollen viability, skin thickness and the duration of Blastophaga exit were positively correlated with PC2 and negatively associated with fig endosepsis. The PC3 was positively connected with fertile seeds per fruit, SSC and flesh chroma value. According to the average data between 2020 and 2022, 0 °C × T1, 4 °C × T1, and 8 °C × T1 are located in the positive part of PC1 and PC2 but the negative part of PC3 because of having a higher average fruit weight, fruit size, fruit set, skin thickness and flesh L* value. However, among them, 0 °C T1 had the lowest B. psenes and the highest percentage of sterile seeds, so it was on the farthest side (Figure 6).
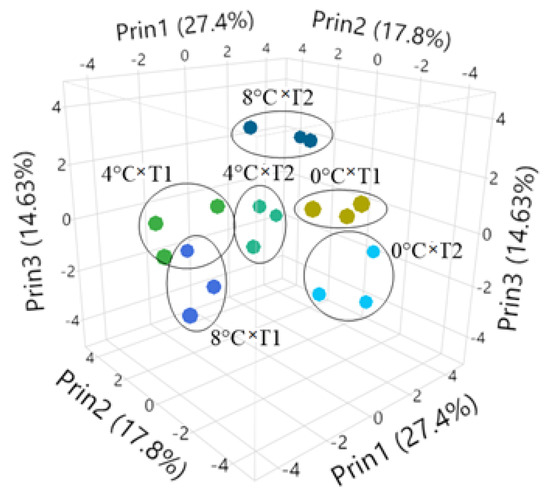
Figure 6.
Three−dimensional scatter diagrams of the relationships among the storage temperature and pollination frequency interaction. (T1: 4-day intervals pollination, T2: 8-day intervals pollination; Prin1: Principal compenent 1, Prin2: Principal compenent 2, Prin3: Principal compenent 3).
4. Discussion
4.1. Number of Blastophaga psenes and Duration of Blastophaga’s Exit
It is important that stored caprifig can maintain their pollen viability, germination and the number of wasps until used in the pollination process. According to the results of the study, the number of wasps decreased depending on the storage period of caprifig fruits. The maximum decrease was detected in the caprifigs stored at 0 °C. Fifty-percent wasp loss was observed in those stored for 4 and 8 days at 0 °C, for 12 days at 4 °C and for 16 days at 8 °C. On the 20th day of storage, a 99% loss of wasps was observed in the caprifig fruits stored at 0 °C, whereas the loss was 68% and 87% in the caprifigs stored at 8 and 4 °C. The minimum temperature at which emergence occurs may be especially important, because fig wasps kept below this critical temperature can develop to pupation but die within their galls (Zavodna and Comptron, unpublished). The number of B. psenes exits per caprifig on the day of harvest ranged between 4.0 and 267.0 in Iran [39], and 119.0 and 480.0 [40] and 26.31 and 263.62 [41] in Turkey. Anjam et al. [13] reported that the Poozdombali genotype had the highest B. psenes number on the harvest day (341.33), but this number gradually decreased in caprifig fruits stored at 8 (106.67), 14 (45.67), 21 (33) and 28 (15) days at 4 °C, and emphasized that an effective caprification requires caprifig trees with a high B. psenes number.
Adult female pollinating wasps are short-lived [5], and they have to find a receptive fig within 1–2 days of their emergence from their natal fig [42]. Thus, the duration of Blastophaga’s exit from the caprifig is essential in determining the frequency of caprification. The study determined that the average duration of Blastophaga’s exit on the day of harvest was 4.77 days. After storage, duration reduced to 2 days with 16 days of storage and 1 day with 20 days of storage. The most prolonged Blastophaga’s exit was in the caprifigs stored at 8 °C, and the shortest one was in the caprifigs stored at 0 °C. Yaman and Caliskan [40] and Çalışkan et al. [15] noted that it ranged between 3 and 7 days. In addition to the exit duration value obtained in laboratory conditions, it has been stated by different researchers that temperature, precipitation and climatic conditions affect the exit duration of B. psenes [2,10]. Caprification carried out at extended intervals without paying attention to the wasp’s exit duration can cause problems in the fruit set [43]. Therefore, it is essential to determine the duration of Blastophaga’s exit and the number of B. psenes in stored caprifigs.
4.2. Evaluation of the Pollen Viability and Germination
Storage of caprifig fruits did not affect pollen viability, and only a 5% decrease was observed at the end of 20 days of storage. When assessed in terms of storage temperature, the decrease was maximum at 0 °C and minimum at 8 °C. However, a reduction in viability was observed after the 16th day of storage in all storage temperatures. No linear decrease or increase in the pollen viability values was found depending on the storage time. The reason for this is that the phenological periods of the anthers in the caprifig fruit cannot be known. Since the ripened caprifig fruits were randomly stored, dehiscence may or may not have occurred in the anthers of some caprifig fruits. According to Gaudet et al. [44], the best growth stage for Cannabis sativa pollen collection is the mid-flowering developmental stage, during which pollen viability begins while the pollen is still present in the anthers. Pollen obtained early may not have fully developed, resulting in a reduced rate of germination [44]. Pollen lifespan has been shown to be extended in several plant species by storing pollen at lower temperatures (4, −20 and −80 °C) for various plants, such as almond [17], cherimoya [18], mango [19], hazel [45], date palm [46,47] and many others. The fact that the pollen was found in the anthers of the caprifig fruit and that the pollen was stored in the caprifig fruit distinguishes the study from previous studies.
Pollen germination increased with the storage of the caprifigs, but the increase was not linear, similar to the results obtained in viability. As explained above, the non-linear increase might have been due to the presence of anthers in the caprifig fruits’ phenologically different stages or pollen ages. Consistent with this, Yuan et al. [48] stated that the in vitro germination rate of Phalaenopsis pollen increased gradually with floral stages. According to Rosell et al. [49], cherimoya pollen showed the highest germination rate during anther dehiscence in comparison with at 30 and 5 h before dehiscence or 20 h after dehiscence. Wang et al. [50] revealed that pollen grains of litchi were more abundant in filaments that stretched farther before anther dehiscence. It has been shown that pollen germinability is influenced by anther dehiscence and pollen age [48,51,52]. Compared to the harvest day, the highest increase in the pollen germination was observed in the caprifigs stored at 4 and 8 °C for 4 and 8 days. The germination rate of the caprifigs stored at 4 °C gave the same values as the harvest day, even on the 20th day of storage. Pacini et al. [53] stated that when pollen is presented for dispersal and crosses the atmosphere, it always desiccates under certain limits, except for orchids with pollinia and seagrasses with underwater pollination. This situation may also be an exception for figs, as fig pollen is protected inside the caprifig fruit, carried by the wasp, and not dispersed into the atmosphere; hence, dramatic declines in pollen viability and germination rate may not have been recorded. The amount of pollen from the caprifigs stored at 0 °C increased less than that from those stored at 4 and 8 °C, and after 12 days of storage, the germination rate decreased linearly. Marks et al. [52] reported that the water content should be tested to determine the storability of pollen at low temperatures because excessive moisture reduces the germination rate. At low temperatures, water in pollen crystallizes and eventually leads to pollen alteration. To store recalcitrant pollen (pollen size: 15 to 150 μm), the levels of water content should be higher than those that generate desiccation damage and lower or close to the limit to those in which water can freeze [53,54]. Similarly, Franchi et al. [55] reported that plants such as grasses, spinach, walnut and pumpkin have this kind of pollen, and because of its water content and the lack of mechanisms to prevent the formation of ice crystals, it is difficult to store them at low temperatures. There have been no published reports that classify fig pollen as recalcitrant; however, if fig pollen is classified as resistant pollen due to pollen size [56], the obtained lower viability and germination rate at 0 °C may be explained. In contrast, previous research on several other fruit varieties (e.g., apple, almond, cherry, hazel) revealed that pollen germinability may be optimally maintained in storage at temperatures lower than 4 °C [18,21,22,45].
4.3. Initial and Final Fruit Set
It was observed that the fruit set was affected by the storage temperature of the caprifigs. As a result of pollination with the caprifigs stored at 4 and 8 °C, the initial and final fruit set increased. Tran et al. [23] reported that cool storage reduced pollen viability and compromised fruit set (10–87%) compared with fresh pollen (97%). Metz et al. [57] found that pollen of Hylocereus stored at 4 °C for 3 or 9 months had only 60–70% fruit set, but the pollen stored at sub-freezing temperature had 100% fruit set. According to Marks et al. [52], Dactylorhiza fuchsii pollen stored at −20 °C for 6 years showed lower viability, which influenced seed productivity after pollination and fruit setting. Yuan et al. [48] observed that while the pollen of Phalaenopsis stored at both −20 and −80 °C remained viable after 96 weeks, its pollination ability steadily declined as storage time increased. However, in the study, because the caprifig fruit instead of the pollen was stored, a higher fruit set was obtained at higher temperatures, where the pollen in the caprifigs could sustain its viability and germination rate. When the caprifigs were stored at 4 °C, a 7% increase in the final fruit set was observed compared to 0 °C. According to the correlation analysis performed, as pollen viability and germination rate increased, so did the initial and final fruit sets (Figure 5). Caprification practiced five times with an interval of 4 days increased the fruit set by approximately 9%. The rate of fig wasps entering the edible fig fruit without pollen is very low, so as the number of wasps entering the fruit increases, the amount of pollen entering the fruit increases [58,59,60]. Therefore, pollen-carrying wasps increase with the practice of five times with 4-day-interval pollination, which increases the chance of fertilization. Similarly, Zare [7] reported that pollination performed once every 3 days reduced the fig syconia abscission. Galliche et al. [28] cited that the repetition of caprification four times was the most effective for improving fig fruit set and productivity. Moreover, Gaaliche et al. [29] stated that the highest percentage of fruit set (61.7%) was obtained in five-repetition pollination, whereas it was 58.6% in three repetitions. Şirin [33] reported that five repetitions significantly increased the fig fruit set in comparison with the traditional practice (two–three-repetition pollination). The abscission of fig syconia may be caused by a lack of caprification or a deficiency of caprification, which is obviously influenced by climatic conditions such as temperature and wind, which lower or inhibit pollinator activity [61].
4.4. Fruit Characteristics and Time of Ripening
While the caprifig storage temperature did not affect the edible fig’s fruit weight, diameter and length, the pollination frequency did. Caprification practiced three times with an interval of 8 days increased the fruit weight and size. Moreover, when the caprifigs stored at 8 °C were used for 8-day-interval pollination, the proportion of fruits weighing more than 100 g increased, whereas it decreased in 4 °C × T1 and 8 °C × T1. According to these results, the fruit size might have decreased since the increase in fruit set when the caprifigs stored at 4 °C and 8 °C pollinated five times with an interval of 4 days increased the number of fruits per shoot. Kadri et al. [62] stated that pollination can be delayed for a few days to reduce the crop load for obtaining larger fruits in date palm cultivation. In agreement with the present study, Şirin [33] indicated that three-repetition pollination increased fruit size compared to two, four and five repetitions. Conversely, Gaaliche et al. [28] reported that fruit size increased with the repetition of caprification. Gaaliche et al. [29] stated that differences in fruit quality characteristics were insignificant among the two-, three-, four- and five-repetition caprification. The number of fertile seeds per fruit increased with five times with 4-day interval pollination, but the sterile seeds percentage decreased. The number of sterile seeds increased when the caprifigs stored at 0 °C were used in the pollination process. According to the correlation analysis, finding a moderate correlation between pollen viability and sterile seeds percentage might explain the increase in the number of sterile seeds when the caprifigs stored at 0 °C were used in the pollination process (Figure 5). Gaaliche et al. [28] reported that, as the repetition of pollination increased, the number of fertile seeds also increased. Doi et al. [63] cited that in blueberry, the number of fertile seeds and seed weight decreased with the increase in pollination dilution rate. In contrast to the number of fertile seeds, seed weight increased with three repetitions with 8-day interval caprification. Seed weight may be more appropriate than seed number when assessing seeds’ effect on fruit characteristics [63]. Another reason for the increase in fruit sizes in three repetitions with 8-day interval pollination may be that the number of fertile seeds was low, but the weight of a single seed was high. In fact, as the weight of the single seed increased, the number of fruits weighing more than 100 g and with more ostiole damage increased. However, as the number of germinated seeds increased, the single seed weight and ostiole damage decreased (Figure 5).
The use of pollinators stored at 4 and 8 °C increased ostiole diameter. This situation was considered to be related to the larger seeds of the fruits pollinated by caprifigs stored at these temperatures. On the contrary, it has been reported that an excessive number of fertilized seeds may be a cause of ostiole cracking [9,64]. In the study, severe ostiole damage appeared to be positively correlated with the single seed weight rather than the number of fertile seeds. Moreover, caprification practiced at three dates with an interval of 8 days increased the ostiole diameter of fruits and severe ostiole damage rate obtained from 4 °C × T2 interaction.
The proportion of early ripening fruit increased with 4-day-interval caprification. Fertilized seeds produce a large amount of auxin, which may stimulate ethylene production within the tissue [65]. Caprification conducted using 4-day intervals increased the number of fertile seeds and showed a positive correlation with the proportion of fruits harvested before September 15. Consistent with the results obtained in the present study, Doi et al. [66] indicated that differences in the pollen source or pollen dilution only affect the number of seeds included in a berry, and fruit weight and ripening are affected by the number of seeds. Moreover, the ripening period reduced when the caprifigs stored at 0 °C were used in the caprification process. This might be related to an increase in abscisic acid (ABA) and anthocyanin contents since the caprifigs stored at 0 °C had more fig endosepsis. ABA, which plays an important role in the responses to various abiotic and biotic stresses in plant cells, has been reported to initiate color development by enhancing anthocyanin accumulation [67]. It has been stated in the literature that pathogen attack correlates with anthocyanin content in sweet oranges [68] and grapes [69].
There was no significant effect of storage temperature or pollination frequency on fruit cavity. However, there were differences in the classification made based on fruit cavity dimensions. When the caprifigs stored at 4 and 8 °C were pollinated five times with 4-day intervals, the fruit cavity was very small or small in more than 60% of the fruits. Nonpollinated fig fruits whose fruit cavity is large and with sterile flowers rapidly dehisce from the plant [31]. Similarly, in the present study, small fruit cavity positively correlated with pollen germination and viability. Fusarium, which was presumed to be brought by the fig wasp in the first stage, was found in the cavity of the figs with a lack of pollination [31]. Consistent with this, in the present study, large fruit cavity had a positive correlation with fig endosepsis. Fig endosepsis increased when the caprifigs stored at 0 °C were pollinated five times every 4 days. The incidence of endosepsis in edible figs takes place as a result of the entrance of many wasps from caprifigs hanging on female trees to female syconia [31,70]. The flesh thickness, skin thickness, soluble solids content (SSC) and titratable acidity (TA) of the fruits were not affected by storage temperature or pollination frequency. Moreover, Gaaliche et al. [28] reported that pollination frequency did not affect SSC, TA, or skin thickness values of figs.
5. Conclusions
To our knowledge, this is the first report on the effect of caprifig storage on pollen viability, germination and the duration of Blastophaga’s exit. Furthermore, no published study was found in which caprifigs were stored at temperatures alternative to 4 °C to determine the change in the number of wasps. The present study provides valuable information about the possibility of caprifig storage for up to 20 days and using these caprifigs in caprification. The preservation of pollen in the caprifig fruit, rather than being dispersed in the atmosphere, shows that if fruits are harvested in the appropriate phenological period, pollen can maintain viability and germination when the caprifig is stored at higher temperatures. The number of Blastophaga, Blastophaga exit times, and pollen viability were higher in the caprifigs stored at 4 and 8 °C than those stored at 0 °C. It is possible to store caprifig fruits at 0 °C for approximately 12 days, considering pollen viability, the number of wasps and wasps’ exit duration. Although the decrease in pollen viability and germination rate after 12 days may be tolerable, the number of Blastophaga and the duration of Blastophaga exit considerably decreased. However, caprifigs can be stored at 4 or 8 °C for 16 days for pollination without much loss of quality. The fruit set increased when the caprifigs stored at 4 and 8 °C were used in the caprification. Caprification practiced five times with an interval of 4 days was found to be the most appropriate to provide a high fruit set and quality. Ostiole damage and the proportion of fruits with large fruit cavity increased with practicing caprification with 8-day intervals. However, it was determined that caprification repeated five times with 4-day intervals increased the proportion of fig endosepsis. For this reason, it is recommended to sanitize the caprifig fruits and use them in five-repetition caprification with an interval of 4 days.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/horticulturae9010078/s1, Table S1: Analysis of variance for number of Blastophaga psenes and duration of Blastophaga’s exit; Table S2: Analysis of variance for pollen viability and pollen germination; Table S3: Analysis of variance for initial fruit set and final fruit set; Table S4: Analysis of variance for fruit weight and fruit diameter; Table S5: Analysis of variance for fruit length and ostiole diameter; Table S6: Analysis of variance for proportion of the fruit weight 1; Table S7: Analysis of variance for proportion of the fruit weight 2; Table S8: Analysis of variance for proportion of the ostiole damage 1; Table S9: Analysis of variance for proportion of the ostiole damage 2; Table S10: Analysis of variance for flesh and skin thickness; Table S11: Analysis of variance for fruit cavity and fertile seeds per fruit; Table S12: Analysis of variance for proportion of the fruit cavity 1; Table S13: Analysis of variance for proportion of the fruit cavity 2; Table S14: Analysis of variance for single seed weight and sterile seeds; Table S15: Analysis of variance for fig endosepsis, SSC and TA; Table S16: Analysis of variance for proportion of time of ripening 1; Table S17: Analysis of variance for proportion of time of ripening 2; Table S18: Analysis of variance for skin color; Table S19: Analysis of variance for flesh color.
Author Contributions
Conceptualization, D.A.K. and Ü.E.; methodology, D.A.K.; software, D.A.K.; validation, D.A.K. and Ü.E.; formal analysis, D.A.K.; investigation, D.A.K.; resources, D.A.K. and Ü.E.; data curation, D.A.K.; writing—original draft preparation, D.A.K.; writing—review and editing, D.A.K. and Ü.E.; visualization, D.A.K.; supervision, Ü.E.; project administration, D.A.K. and Ü.E.; funding acquisition, D.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data are presented in this manuscript. The data used in the present study were from Dilan Ahi Koşar’s doctoral thesis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Faostat. QCL. 2021. Available online: https://www.fao.org/faostat/en/#data/ (accessed on 10 September 2022).
- Condit, I.J. The Fig; Chronica Botanica: Waltham, MA, USA, 1947. [Google Scholar]
- Aksoy, U.; Balci, B.; Can, H.Z.; Hepaksoy, B. Some significant results of the research-work in Turkey on fig. Acta Hortic. 2003, 605, 173–180. [Google Scholar] [CrossRef]
- Beck, N.G.; Lord, E.M. Breeding system in Ficus carica, the common fig. I. Floral diversity. Am. J. Bot. 1988, 75, 1904–1912. [Google Scholar] [CrossRef]
- Kjellberg, F.; Doumesche, B.; Bronstein, J.L. Longevity of a fig wasp (Blastophaga psenes). Proc. K. Ned. Akad. Van Wet. 1988, 91, 117–122. [Google Scholar]
- Weiblen, G.D. Correlated evolution in fig pollination. Syst. Biol. 2004, 53, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Zare, H. Comparison of fig caprification vessels, period and caprifig cultivar usable in Iran. Acta Hortic. 2008, 798, 233–239. [Google Scholar] [CrossRef]
- Pourghayoumi, M.; Bakhshi, D.; Rahemi, M.; Jafari, M. Effect of pollen source on quantitative and qualitative characteristics of dried figs (Ficus carica L.) Cvs’ Payves’ and ‘Sabz’ ın Kazerun, Iran. Sci. Hortic. 2012, 147, 98–104. [Google Scholar] [CrossRef]
- Ferguson, L.; Michailides, T.J.; Shorey, H.H. The California Fig Industry. Hortic. Rev. 1990, 12, 409–490. [Google Scholar] [CrossRef]
- Özbek, S. Özel Meyvecilik Ders Kitabı; Çukurova Üniv. Ziraat Fak. Yayınları: Adana, Turkey, 1978. [Google Scholar]
- Küden, A.; Tanriver, E. Plant genetic resources and selection studies on figs in the east mediterranean and south east anatolia regions. Acta Hortic. 1998, 480, 49–54. [Google Scholar] [CrossRef]
- Ilgın, M.; Ergenoğlu, F.; Çağlar, S. Viability, germination and amount of pollen in selected caprifig types. Pak. J. Bot. 2007, 39, 9–14. [Google Scholar]
- Anjam, K.; Khadivi-Khub, A.; Sarkhosh, A. The potential of caprifig genotypes for sheltering Blastophaga psenes for caprification of edible figs. Erwerbs-Obstbau 2017, 59, 45–49. [Google Scholar] [CrossRef]
- Gaaliche, B.; Majdoub, A.; Trad, M.; Mars, M. Assessment of pollen viability, germination, and tube growth in eight Tunisian caprifig (Ficus carica L.) cultivars. ISRN Agron. 2013, 2013, 207434. [Google Scholar] [CrossRef]
- Çalışkan, O.; Bayazit, S.; Ilgin, M.; Karataş, N. Morphological diversity of caprifig (Ficus carica var. caprificus) accessions in the eastern Mediterranean region of Turkey: Potential utility for caprification. Sci. Hortic. 2017, 222, 46–56. [Google Scholar] [CrossRef]
- Essid, A.; Alijane, F.; Ferchichi, A. Morphological characterization and pollen evaluation of some Tunisian ex situ planted caprifig (Ficus carica L.) ecotypes. S. Afr. J. Bot. 2017, 111, 134–143. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Gradziel, T.M.; Ortega, E.; Dicenta, F. Low temperature storage of almond. HortScience 2002, 37, 691–692. [Google Scholar] [CrossRef]
- Lora, J.; Perez de Oteyza, M.A.; Fuentetaja, P.; Hormaza, J.I. Low temperature storage and in vitro germination of cherimoya (Annona cherimola Mill.) pollen. Sci. Hortic. 2006, 108, 91–94. [Google Scholar] [CrossRef]
- Dutta, S.K.; Srivastav, M.; Chaudhary, R.; Lal, K.; Patil, P.; Singh, S.K.; Singh, A.K. Low temperature storage of mango (Mangifera indica L.) pollen. Sci. Hortic. 2013, 161, 193–197. [Google Scholar] [CrossRef]
- Ozcan, A. Effect of Low-temperature storage on sweet cherry (Prunus avium L.) pollen quality. Hortscience 2020, 55, 258–260. [Google Scholar] [CrossRef]
- Ćalić, D.; Milojević, J.; Belić, M.; Miletić, R.; Zdravković-Korać, S. Impact of Storage Temperature on Pollen Viability and Germinability of Four Serbian Autochthon Apple Cultivars. Front. Plant Sci. 2021, 12, 1480. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Lombardini, L. In vitro viability and germination of Carya illinoinensis pollen under different storage conditions. Sci. Hortic. 2021, 275, 109662. [Google Scholar] [CrossRef]
- Tran, X.T.; Parks, S.E.; Nguyen, M.H.; Roach, P.D. Reduced pollination efficiency compromises some physicochemical qualities in gac (Momordica cochinchinensis Spreng.) fruit. Agronomy 2021, 11, 190. [Google Scholar] [CrossRef]
- Ðordevic, M.; Vujović, T.; Cerović, R.; Glišić, I.; Milošević, N.; Marić, S.; Radicević, S.; Fotirić Akšić, M.; Meland, M. In vitro and ın vivo performance of plum (Prunus domestica l.) pollen from the anthers stored at distinct temperatures for different periods. Horticulturae 2022, 8, 616. [Google Scholar] [CrossRef]
- Jeong, N.R.; Park, K.Y. Rose pollen management methods to ımprove productivity. Agronomy 2022, 12, 1285. [Google Scholar] [CrossRef]
- Çalışkan, O.; Polat, A.A. Fruit characteristics of fig cultivars and genotypes grown in Turkey. Sci. Hortic. 2008, 115, 360–367. [Google Scholar] [CrossRef]
- Çalışkan, O. Present status and future of table fig cultivation in Turkey. J. Agric. Fac. Uludağ Univ. 2012, 26, 71–87. [Google Scholar]
- Gaaliche, B.; Trad, M.; Mars, M. Effect of pollination ıntensity, frequency and pollen source on fig (Ficus carica L.) productivity and fruit quality. Sci. Hortic. 2011, 130, 737–742. [Google Scholar] [CrossRef]
- Gaaliche, B.; Hfaiedh, L.; Trad, M.; Mars, M. Caprification efficiency of three Tunisian fig (Ficus carica L.) cultivars. JNPR 2011, 1, 20–25. [Google Scholar]
- Göçmez, A.; Seferoğlu, H.G. Fresh fig and dry fig quality parameters and the effective factors on quality. Turk. J. Agric. Res. 2014, 1, 98–108. [Google Scholar] [CrossRef]
- Michailides, T.J.; Morgan, D.P. Spread of Endosepis in Calimyrna Fig Orchard. Popul. Ecol. 1998, 88, 637–647. [Google Scholar] [CrossRef]
- Şimşek, E.; Kılıç, D.; Çalışkan, O. Phenotypic variation of fig genotypes (Ficus carica L.) in the Eastern Mediterranean of Turkey. Genetica 2020, 52, 957–972. [Google Scholar] [CrossRef]
- Şirin, A. The Effect of Caprıfıcatıon Frequency on Fruit Yield and Quality of Fıg Trees Applıed Dıfferent Traınıng Systems. Master’s Thesis, Aydın Adnan Menderes University, Aydın, Turkey, 2021. [Google Scholar]
- Storey, W.B. Figs Advances İn Fruit Breeding. In Advances in Fruit Breeding; Janick, J., Moore, J.N., Eds.; Purdue Univ.: West Lafayette, IN, USA, 1975; pp. 568–589. [Google Scholar]
- Eti, S. The Pollen Viability And Germination Ratios İn The Some Fruit Species and Cultivars by İn Vitro Tests. Cukurova Univ. J. Agric. Fac. 1991, 6, 69–80. [Google Scholar]
- Zhang, F.P.; Peng, Y.Q.; Compton, S.G.; Yang, D.R. Floral character-istics of Ficus curtipes and the oviposition behavior of its polli-nator fig wasp. Ann. Entomol. Soc. Am. 2009, 102, 556–559. [Google Scholar] [CrossRef]
- United Nations Economic Comission for Europe (UNECE). UNECE Standard FFV-17 Concerning the Marketing and Commercial Quality Control of Fresh Figs. Available online: http://www.unece.org/fileadmin/DAM/trade/agr/standard/fresh/FFV-Std/English/17FreshFigs2010.pdf (accessed on 15 October 2022).
- IPGRI; CIHEAM. Descriptors for Figs; International Plant Genetic Resources Institute: Rome, Italy; International Centre For Advanced Mediterranean Agronomic Studies: Paris, France, 2003. [Google Scholar]
- Khadivi-Khub, A.; Anjam, K. Characterization and evaluation of male fig (caprifig) accessions in Iran. Plant Syst. Evol. 2014, 10, 2177–2189. [Google Scholar] [CrossRef]
- Yaman, S.; Çalışkan, O. Pollinizer characteristics of some caprifig genotypes (Ficus carica var. caprificus) selected from Hatay. Anadolu J. Agric. Sci. 2016, 31, 315–320. [Google Scholar] [CrossRef]
- Ahi Koşar, D.; Aktepe Tangu, N.; Gencer, N.S.; Durgut, E.; Ertürk, U. Some characteristics and caprification potentials of caprifigs (Ficus carica var. caprificus L.) grown in Bursa, Turkey. In Ficus carica: Production, Cultivation and Uses; Dalkılıç, Z., Ed.; Nova Science Publishers: New York, NY, USA, 2022; pp. 97–101. [Google Scholar] [CrossRef]
- Ware, A.B.; Compton, S.G. Dispersal of adult female fig wasps: 2. Movements between trees. Entomol. Exp. Appl. 1994, 73, 231–238. [Google Scholar] [CrossRef]
- Yaman, S. Determınatıon of Phenologıcal, Pomologıcal and Bıologıcal Characterıstıcs of Some Caprıfıg Genotypes (Ficus carica var. caprificus). Master’s Thesis, Mustafa Kemal University, Hatay, Turkey, 2015. [Google Scholar]
- Gaudet, D.; Singh Yadav, N.; Sorokin, A.; Bilichak, A.; Kovalchuk, I. Development and optimization of a germination assay and long-term storage for Cannabis sativa pollen. Plants 2020, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Novara, C.; Ascari, L.; La Morgia, V.; Reale, L.; Genre, A.; Siniscalco, C. Viability and germinability in long term storage of Corylus avellana pollen. Sci. Hortic. 2017, 214, 295–303. [Google Scholar] [CrossRef]
- Mesnoua, M.; Roumani, M.; Salem, A. The effect of pollen storage temperatures on pollen viability, fruit set and fruit quality of six date palm cultivars. Sci. Hortic. 2018, 236, 279–283. [Google Scholar] [CrossRef]
- Kadri, K.; Elsafy, M.; Makhlouf, S.; Awad, M.A. Effect of pollination time, the hour of daytime, pollen storage temperature and duration on pollen viability, germinability, and fruit set of date palm (Phoenix dactylifera L.) cv “Deglet Nour”. Saudi J. Biol. Sci. 2022, 29, 1085–1091. [Google Scholar] [CrossRef]
- Yuan, S.C.; Chin, S.; Lee, C.Y.; Chen, F.C. Phalaenopsis pollinia storage at sub-zero temperature and its pollen viability assessment. Bot. Stud. 2018, 59, 1. [Google Scholar] [CrossRef]
- Rosell, V.; Galán Saúco, V.; Herrero, M. Pollen germination as affected by pollen age in cherimoya. Sci. Hortic. 2006, 109, 97–100. [Google Scholar] [CrossRef]
- Wang, L.; Wu, J.; Chen, J.; Fu, D.; Zhang, C.; Cai, C.; Ou, L. A simple pollen collection, dehydration, and long-term storage method for litchi (Litchi chinensis Sonn.). Sci. Hortic. 2015, 188, 78–83. [Google Scholar] [CrossRef]
- Bellusci, F.; Musacchio, A.; Stabile, R.; Pellegrino, G. Differences in pollen viability in relation to different deceptive pollination strategies in Mediterranean orchids. Ann. Bot. 2010, 106, 769–774. [Google Scholar] [CrossRef]
- Marks, T.R.; Seaton, P.T.; Pritchard, H.W. Desiccation tolerance, longevity and seed-siring ability of entomophilous pollen from UK native orchid species. Ann. Bot. 2014, 114, 561–569. [Google Scholar] [CrossRef]
- Pacini, E.; Jacquard, C.; Clement, C. Pollen vacuoles and their significance. Planta 2011, 234, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Pacini, E.; Franchi, G.G. Pollen biodiversity—Why are pollen grains different despite having the same function? A review. Bot. J. Linn Soc. 2020, 193, 141–164. [Google Scholar] [CrossRef]
- Franchi, G.G.; Nepi, M.; Dafni, A.; Pacini, E. Partially hydrated pollen: Taxonomic distribution, ecological and evolutionary significance. Plant Syst. Evol. 2002, 234, 211–227. [Google Scholar] [CrossRef]
- Calıskan, O.; Bayazit, S.; Kılıc, D.; Ilgın, M.; Karatas, N. Pollen morphology and variability of caprifig (Ficus carica var. caprificus) genetic resources in Turkey using multivariate analysis. Sci. Hortic. 2021, 287, 1–10. [Google Scholar] [CrossRef]
- Metz, C.; Nerd, A.; Mizrahi, Y. Viability of pollen of two fruit crop cacti of the genus Hylocereus is affected by temperature and duration of storage. HortScience 2000, 35, 22–24. [Google Scholar] [CrossRef]
- Weiblen, G.D.; Bush, G.L. Speciation in fig pollinators and parasites. Mol. Ecol. 2002, 11, 1573–1578. [Google Scholar] [CrossRef]
- Wang, R.W.; Sun, B.F.; Zheng, Q.; Shi, L.; Zhu, L. Asymmetric interaction and indeterminate fitness correlation between cooperative partners in the fig-fig wasp mutualism. J. R. Soc. Interface 2011, 8, 1487–1496. [Google Scholar] [CrossRef]
- Jandér, K.C.; Herre, E.A. Host sanctions and pollinator cheating in the fig tree–fig wasp mutualism. Proc. Biol. Sci. 2010, 277, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Oukabli, A.; Mamouni, A.; Laghezali, M.; Ater, M.; Roger, J.P.; Khadiri, B. Local Caprifig tree characterization and analysis of interest for pollination. Acta Hortic. 2003, 605, 61–64. [Google Scholar] [CrossRef]
- Kadri, K.; Ahmed, O.; Souhaila, M.; Mohamed, S.C.; Abdelhamid, C.; Amani, T. Contribution to the study of the effect of pollination mode on fruit set rate and yield in the date palm (Phoenix dactylifera L.) in the Oases of Tozeur (Tunisia). Int. J. Agric. Innov. Res. 2019, 7, 533–537. [Google Scholar]
- Doi, K.; Inoue, R.; Iwasaki, N. Seed weight mediates effects of pollen on berry weight, ripening, and anthocyanin content in highbush blueberry. Sci. Hortic. 2021, 288, 110313. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Bremer, V.; Stover, E. Fig (Ficus carica L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.E., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2011; pp. 134–158. [Google Scholar]
- Ryugo, K. Fruit Culture: Its Science and Art; John Wiley and Sons: New York, NY, USA, 1988. [Google Scholar]
- Doi, K.; Nozaki, R.; Takahashi, K.; Iwasaki, N. Effects of the number of seeds per berry on fruit growth characteristics, especially on the duration of stage II in blueberry. Plants 2018, 7, 96. [Google Scholar] [CrossRef]
- Lama, K.; Harlev, G.; Shafran, H.; Peer, R.; Flaishman, M.A. Anthocyanin accumulation is initiated by abscisic acid to enhance fruit color during fig (Ficus carica L.) ripening. J. Plant Physiol. 2020, 251, 153192. [Google Scholar] [CrossRef]
- Sicilia, A.; Catara, V.; Scialò, E.; Lo Piero, A.R. Fungal Infection Induces Anthocyanin Biosynthesis and Changes in DNA Methylation Configuration of Blood Orange [Citrus sinensis L. (Osbeck)]. Plants 2021, 10, 244. [Google Scholar] [CrossRef]
- Villegasa, D.; Handford, M.; Alcaldea, J.A.; Perez-Donosoa, A. Exogenous application of pectin-derived oligosaccharides to grape berries modifies anthocyanin accumulation, composition and gene expression. Plant Physiol. Biochem. 2016, 104, 125–133. [Google Scholar] [CrossRef]
- Michailides, T.J.; Morgan, D.P. Dynamics of Blastophaga psenes populations, availability of caprifigs and fig endosepsis caused by Fusarium moniliforme. Phytopathology 1994, 84, 1254–1263. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).