Supplemental Lighting Quality Influences Time to Flower and Finished Quality of Three Long-Day Specialty Cut Flowers
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Culture, and Lighting Treatments
2.2. Data Collection and Analysis
3. Results
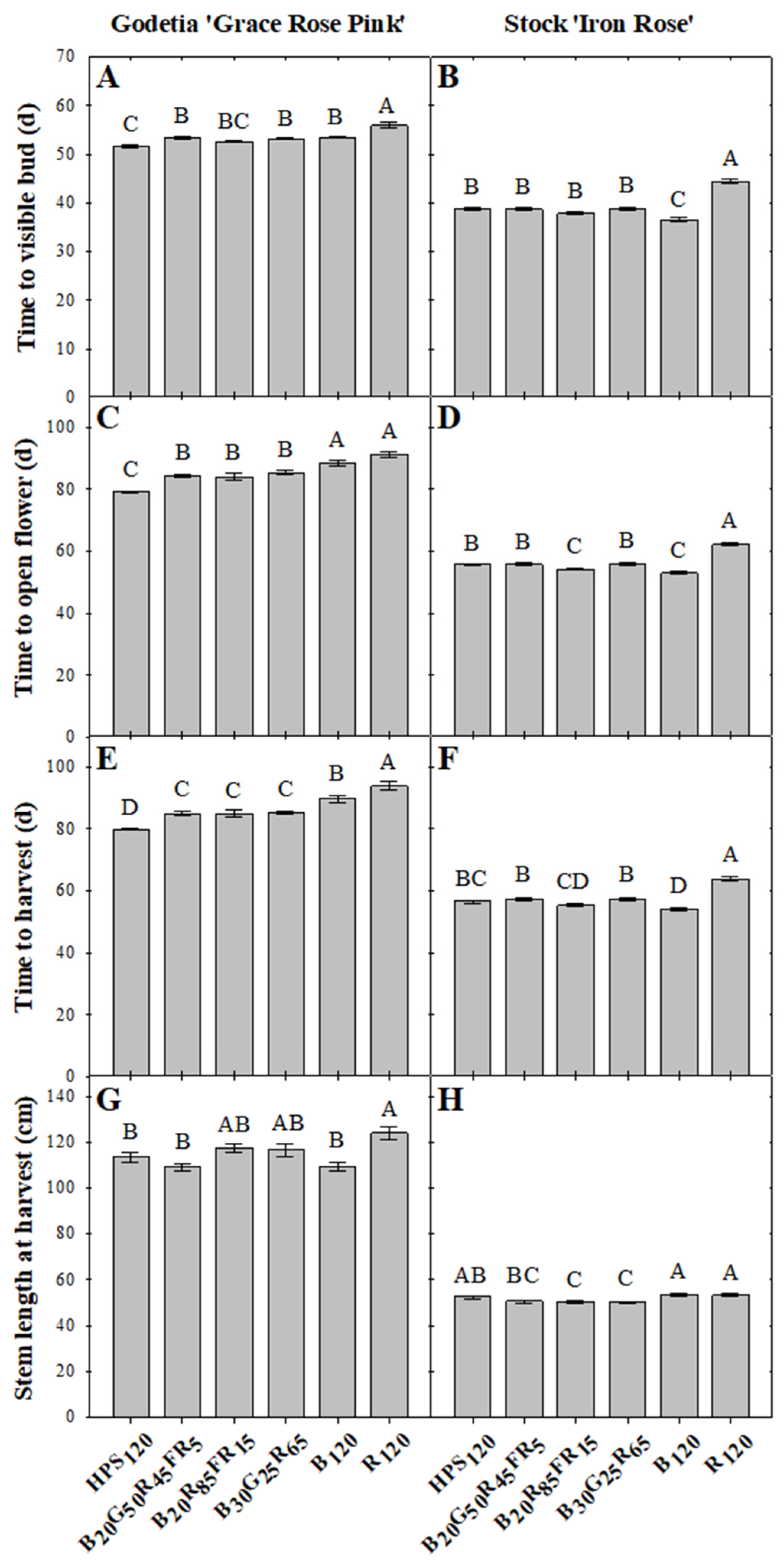
3.1. Time to Visible Flower Bud
3.2. Time to Open Flower
3.3. Time to Harvest
3.4. Cut Flower Morphology at Harvest
3.5. Flower Petal Coloration at Harvest
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prinzing, D. New Findings on Consumer Attitudes toward Domestic & Locally-Grown Cut Flowers. Available online: https://slowflowersjournal.com/new-findings-on-consumer-attitudes-toward-domestic-locally-grown-flowers/ (accessed on 27 April 2021).
- Produce Marketing Association and Food Marketing Institute (PMAFMI). Trends in Mass-Market Floral; Produce Marketing Association: Newark, DE, USA, 2016. [Google Scholar]
- Owen, W.G.; Hilligoss, A.; Lopez, R.G. Late-season high tunnel planting of specialty cut flowers in the midwestern United States influences yield and stem quality. HortTechnology 2016, 26, 338–343. [Google Scholar] [CrossRef]
- Ortiz, M.A.; Hyrczyk, K.; Lopez, R.G. Comparison of high tunnel and field production of specialty cut flowers in the Midwest. HortScience 2012, 47, 1265–1269. [Google Scholar] [CrossRef]
- Faust, J.E.; Logan, J. Daily light integral: A research review and high-resolution maps of the United States. HortScience 2018, 53, 1250–1257. [Google Scholar] [CrossRef]
- Lopez, R.G.; Runkle, E.S. Photosynthetic daily light integral during propagation influences rooting and growth of cuttings and subsequent development of new guinea impatiens and petunia. HortScience 2008, 43, 2052–2059. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Reg. 2021, 41, 742–780. [Google Scholar] [CrossRef]
- Kusuma, P.; Pattison, P.M.; Bugbee, B. From physics to fixtures to food: Current and potential LED efficacy. Hortic. Res. 2020, 7, 56. [Google Scholar] [CrossRef]
- Owen, W.G.; Meng, Q.; Lopez, R.G. Promotion of flowering from far-red radiation depends on the photosynthetic daily light integral. HortScience 2018, 53, 465–471. [Google Scholar] [CrossRef]
- Poel, B.R.; Runkle, E.S. Spectral effects of supplemental greenhouse radiation on growth and flowering of annual bedding plants and vegetable transplants. HortScience 2017, 52, 1221–1228. [Google Scholar] [CrossRef]
- Runkle, E.S. Including far red in an LED lighting spectrum. Greenhouse Prod. News 2019, 29, 58. [Google Scholar]
- Hernández, R.; Kubota, C. Light quality and photomorphogenesis. In Light Management in Controlled Environments; Lopez, R.G., Runkle, E.S., Eds.; Meister Media Worldwide: Willoughby, OH, USA, 2017; p. 29. [Google Scholar]
- Goins, G.D.; Yorio, N.C.; Sanwo, M.M.; Brown, C.S. Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting. J. Exp. Bot. 1997, 48, 1407–1413. [Google Scholar] [CrossRef]
- Zhen, S.; van Iersel, M.W.; Bugbee, B. Why far-red photons should be included in the definition of photosynthetic photons and the measurement of horticultural fixture efficacy. Front. Plant Sci. 2021, 22, 1158. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Bugbee, B. Far-red photons have equivalent efficiency to traditional photosynthetic photons: Implications for redefining photosynthetically active radiation. Plant Cell Environ. 2020, 43, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Bugbee, B. Substituting far-red for traditionally defined photosynthetic photons results in equal canopy quantum yield for CO2 fixation and increased photon capture during long-term studies: Implications for re-defining PAR. Front. Plant Sci. 2020, 11, 581156. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Zhen, S.; van Iersel, M.W. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Phys. 2016, 209, 115–122. [Google Scholar] [CrossRef]
- Elkins, C.; van Iersel, M.W. Supplemental far-red light-emitting diode light increases growth of foxglove seedlings under sole-source lighting. HortTechnology 2020, 30, 564–569. [Google Scholar] [CrossRef]
- Craig, D.S.; Runkle, E.S. A moderate to high red to far-red light ratio from light-emitting diodes controls flowering of short-day plants. J. Am. Soc. Hortic. Sci. 2013, 138, 167–172. [Google Scholar] [CrossRef]
- Craig, D.S.; Runkle, E.S. Using LEDs to quantify the effect of the red to far-red ratio of night-interruption lighting on flowering of photoperiodic crops. Acta Hortic. 2012, 956, 179–186. [Google Scholar] [CrossRef]
- Borthwick, H.A.; Hendricks, S.B. Photoperiodism in plants. Science 1960, 132, 1223–1228. [Google Scholar] [CrossRef]
- Craig, D.S.; Runkle, E.S. An intermediate phytochrome photoequilibria from night-interruption lighting optimally promotes flowering of several long-day plants. Environ. Exp. Bot. 2016, 121, 132–138. [Google Scholar] [CrossRef]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Am. Soc. Agric. Eng. 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Walters, K.J.; Hurt, A.A.; Lopez, R.G. Flowering, stem extension growth, and cutting yield of foliage annuals in response to photoperiod. HortScience 2019, 54, 661–666. [Google Scholar] [CrossRef]
- Runkle, E.S.; Heins, R.D. Specific functions of red, far red, and blue light in flowering and stem extension of long-day plants. J. Amer. Soc. Hortic. Sci. 2001, 126, 275–282. [Google Scholar]
- Kohler, A.E.; Lopez, R.G. Duration of light-emitting diode (LED) supplemental lighting providing far-red radiation during seedling production influences subsequent time to flower of long-day annuals. Sci. Hortic. 2021, 30, 109956. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation and photosynthetic photon flux density independently regulate seedling growth but interactively regulate flowering. Environ. Exp. Bot. 2018, 155, 206–216. [Google Scholar] [CrossRef]
- Fankhauser, C.; Christie, J.M. Plant phototropic growth. Curr. Biol. 2015, 25, R384–R389. [Google Scholar] [CrossRef] [PubMed]
- Wollaeger, H.M.; Runkle, E.S. Growth and acclimation of impatiens, salvia, petunia, and tomato seedlings to blue and red light. HortScience 2015, 50, 522–529. [Google Scholar] [CrossRef]
- Ashraful Islam, M.; Kuwar, G.; Clarke, J.L.; Blystad, D.-R.; Gislerød, H.R.; Olsen, J.E.; Torre, S. Artificial light from light-emitting diodes (LEDs) with a high portion of blue light results in shorter poinsettias compared to high pressure sodium (HPS) lamps. Sci. Hortic. 2012, 147, 136–143. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Moderate-intensity blue radiation can regulate flowering, but not extension growth, of several photoperiodic ornamental crops. Environ. Exp. Bot. 2017, 134, 12–20. [Google Scholar] [CrossRef]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F.M.; van Ieperen, W. Floral induction in the short-day plant chrysanthemum under blue and red extended long-days. Front. Plant Sci. 2021, 11, 610041. [Google Scholar] [CrossRef]
- Kusuma, P.; Fatzinger, B.; Bugbee, B.; Soer, W.; Wheeler, R. LEDs for Extraterrestrial Agriculture: Tradeoffs between Color Perception and Photon Efficacy (NASA/TM-20210016720); National Aeronautics and Space Administration: Merritt Island, FL, USA, 2021.
- Liu, J.; van Iersel, M.W. Photosynthetic physiology of blue, green, and red light: Light intensity effects and underlying mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Runkle, E.S. Regulation of flowering by green light depends on its photon flux density and involves cryptochromes. Phys. Plantarum 2019, 166, 762–771. [Google Scholar] [CrossRef]
- Zhang, T.; Maruhnich, S.A.; Folta, K.M. Green light induces shade avoidance symptoms. Plant Phys. 2011, 157, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Folta, K.M. Contributions of green light to plant growth and development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef]
- Garcia, C.; Lopez, R.G. Supplemental radiation quality influences cucumber, tomato, and pepper transplant growth and development. HortScience 2020, 55, 804–811. [Google Scholar] [CrossRef]
- Van der Kooi, C.J.; Elzen, J.T.M.; Staal, M.; Stavenga, D.G. How to color a flower: On the optical principles of flower coloration. Proc. Biol. Sci. 2016, 283, 20160429. [Google Scholar]
- Zhao, D.; Tao, J. Recent advances on the development and regulation of flower color in ornamental plants. Front. Plant Sci. 2015, 6, 261. [Google Scholar] [CrossRef]
- Yue, C.; Behe, B.K. Consumer color preferences for single-stem cut flowers on calendar holidays and noncalendar occasions. HortScience 2010, 45, 78–82. [Google Scholar] [CrossRef]
- Sakata Ornamentals. Stock Iron Culture Sheet. Available online: https://sakataornamentals.com/wp-content/uploads/sites/13/2022/02/Stock-Iron-1121-SAKATA.pdf (accessed on 11 November 2022).
- Hori, Y.; Nishidate, K.; Nishiyama, M.; Kanahama, K.; Kanayama, Y. Flowering and expression of flowering-related genes under long-day conditions with light-emitting diodes. Planta 2011, 234, 321–330. [Google Scholar] [CrossRef]
- Zou, Y. Light-Emitting Diode Lighting Quality Effects on Morphology, Growth, Flowering, and Carotenoid Content of Potted Plant, Cut Flower, and Medicinal Flower Production. Master’s Thesis, University of California, Davis, CA, USA, 2018. [Google Scholar]
- Kong, Y.; Stasiak, M.; Dixon, M.A.; Zheng, Y. Blue light associated with low phytochrome activity can promote elongation growth as shade-avoidance response: A comparison with red light in four bedding plant species. Environ. Exp. Bot. 2018, 155, 345–359. [Google Scholar] [CrossRef]
- Collado, C.; Hernández, R. Effects of Light Intensity, Spectral Composition, and Paclobutrazol on the Morphology, Physiology, and Growth of Petunia, Geranium, Pansy, and Dianthus Ornamental Transplants. J. Plant Growth Regul. 2022, 41, 461–478. [Google Scholar] [CrossRef]
- Runkle, E.S.; Park, Y.; Meng, Q. High photosynthetic photon flux density can attenuate effects of light quality. Acta Hortic. 2022, 1337, 333–340. [Google Scholar] [CrossRef]
- Runkle, E.S. Does light quantity trump light quality? Greenhouse Prod. News 2017, 27, 38. [Google Scholar]
- Randall, W.C.; Lopez, R.G. Comparison of supplemental lighting from high-pressure sodium lamps and light-emitting diodes during bedding plant seedling production. HortScience 2014, 49, 589–595. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Tomato seedling growth and morphological responses to supplemental LED lighting red:blue ratios under varied daily solar light integrals. Acta Hortic. 2012, 956, 187–194. [Google Scholar] [CrossRef]
- Illuminating Engineering Society (IES). IES Method for Evaluating Light Source Color Rendition (IES TM-30-20); IES: New York, NY, USA, 2020. [Google Scholar]
- Katzin, D.; Marcelis, L.F.M.; van Mourik, S. Energy savings in greenhouses by transition from high-pressure sodium to LED lighting. Appl. Energy 2021, 281, 116019. [Google Scholar] [CrossRef]
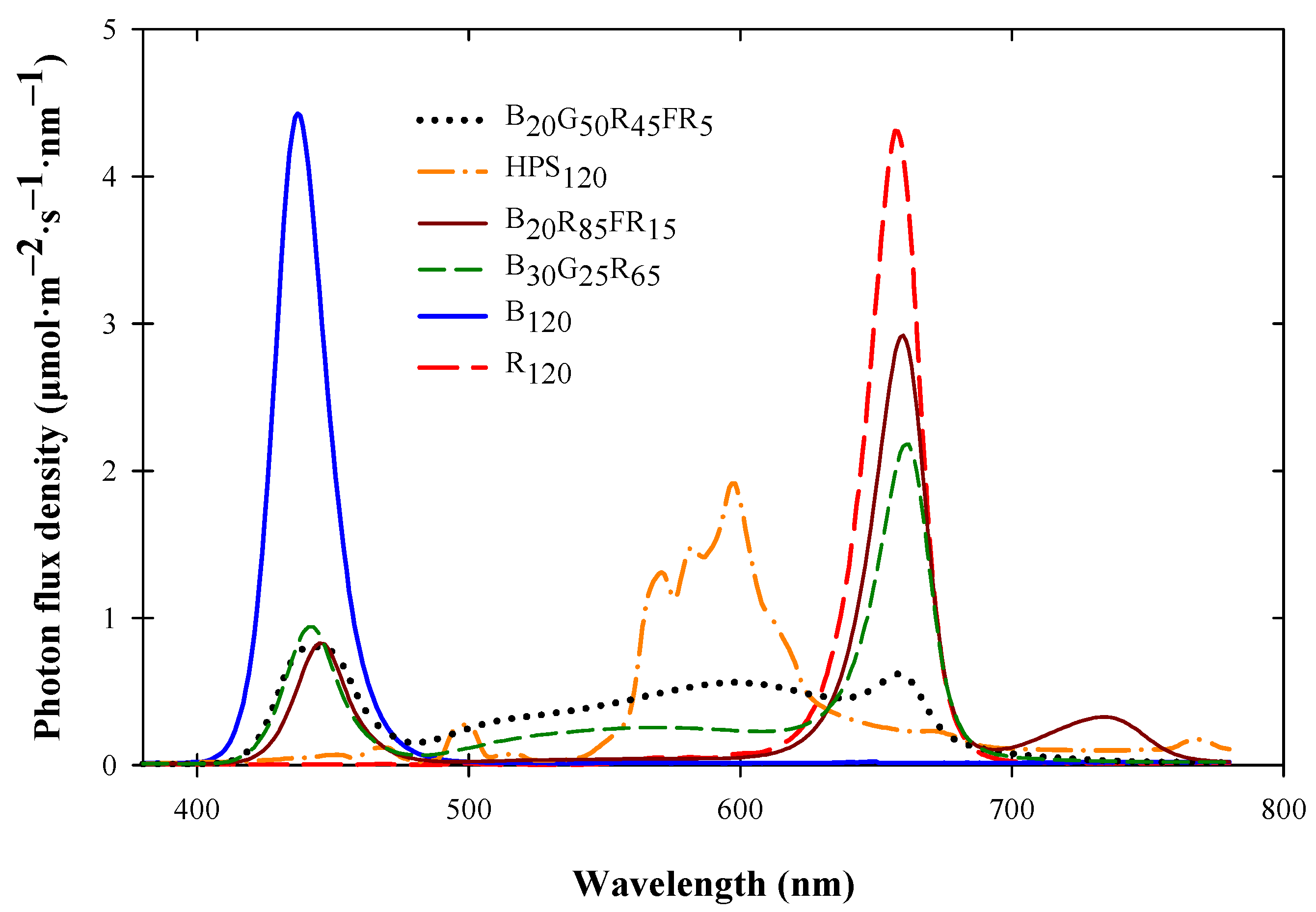


| SL Treatment and Stage | DLI (Mean ± SD (mol·m−2·d−1)) | ADT (Mean ± SD (°C)) | Day Temperature (Mean ± SD (°C)) | Night Temperature (Mean ± SD (°C)) | Leaf Temperature (Mean ± SD (°C)) |
|---|---|---|---|---|---|
| HPS120 | |||||
| VEG | 10.7 ± 2.0 | 15.6 ± 0.6 | 18.4 ± 1.6 | 12.7 ± 0.8 | 17.9 ± 3.5 |
| REP | 15.7 ± 4.5 | 16.1 ± 1.9 | 18.6 ± 2.7 | 13.5 ± 3.2 | 18.3 ± 3.4 |
| B20G50R45FR5 | |||||
| VEG | 10.8 ± 2.0 | 15.6 ± 0.5 | 18.3 ± 0.8 | 12.9 ± 1.0 | 17.0 ± 2.7 |
| REP | 15.6 ± 5.0 | 16.2 ± 1.7 | 18.9 ± 2.5 | 13.4 ± 3.1 | 18.4 ± 3.6 |
| B20R85FR15 | |||||
| VEG | 10.6 ± 2.0 | 15.6 ± 0.4 | 18.2 ± 0.8 | 12.9 ± 0.7 | 17.1 ± 2.9 |
| REP | 15.4 ± 8.9 | 16.2 ± 1.8 | 18.8 ± 2.5 | 16.2 ± 1.8 | 19.9 ± 4.4 |
| B30G25R65 | |||||
| VEG | 10.9 ± 2.1 | 15.5 ± 0.4 | 18.1 ± 0.8 | 12.9 ± 0.6 | 17.6 ± 2.9 |
| REP | 15.1 ± 4.2 | 16.2 ± 1.8 | 18.9 ± 2.6 | 13.5 ± 3.2 | 18.3 ± 2.7 |
| B120 | |||||
| VEG | 10.8 ± 2.1 | 16.1 ± 0.5 | 18.8 ± 1.3 | 13.3 ± 0.8 | 17.6 ± 0.9 |
| REP | 15.0 ± 4.1 | 16.5 ± 1.6 | 19.3 ± 2.5 | 13.7 ± 2.9 | 18.8 ± 2.8 |
| R120 | |||||
| VEG | 11.5 ± 2.5 | 15.5 ± 0.8 | 18.4 ± 2.6 | 12.6 ± 0.9 | 16.5 ± 3.8 |
| REP | 15.9 ± 5.3 | 16.0 ± 1.8 | 18.3 ± 3.1 | 13.8 ± 2.8 | 17.9 ± 3.5 |
| SL Treatment and Stage | DLI (Mean ± SD (mol·m−2·d−1)) | ADT (Mean ± SD (°C)) | Day Temperature (Mean ± SD (°C)) | Night Temperature (Mean ± SD (°C)) | Leaf Temperature (Mean ± SD (°C)) |
|---|---|---|---|---|---|
| HPS120 | |||||
| VEG | 11.7 ± 2.2 | 16.0 ± 0.5 | 18.7 ± 2.1 | 13.3 ± 0.8 | 18.0 ± 4.0 |
| REP | 16.1 ± 4.6 | 16.0 ± 1.0 | 18.9 ± 2.1 | 12.9 ± 1.5 | 18.7 ± 4.0 |
| B20G50R45FR5 | |||||
| VEG | 10.9 ± 2.8 | 16.3 ± 0.7 | 19.0 ± 2.4 | 13.6 ± 0.8 | 18.0 ± 3.1 |
| REP | 16.2 ± 5.2 | 15.9 ± 0.8 | 18.9 ± 2.2 | 12.9 ± 1.1 | 18.3 ± 3.2 |
| B20R85FR15 | |||||
| VEG | 11.6 ± 2.6 | 16.0 ± 0.5 | 18.7 ± 2.1 | 13.3 ± 0.8 | 18.3 ± 3.9 |
| REP | 15.9 ± 3.9 | 15.9 ± 1.0 | 18.9 ± 2.4 | 12.9 ± 1.3 | 18.5 ± 3.7 |
| B30G25R65 | |||||
| VEG | 11.6 ± 2.6 | 16.3 ± 0.7 | 19.0 ± 2.4 | 13.6 ± 0.8 | 18.0 ± 3.8 |
| REP | 16.4 ± 4.3 | 16.3 ± 1.4 | 19.3 ± 2.7 | 13.3 ± 1.7 | 18.0 ± 3.1 |
| B120 | |||||
| VEG | 11.6 ± 2.3 | 16.0 ± 1.0 | 19.1 ± 2.5 | 12.7 ± 1.2 | 17.9 ± 3.3 |
| REP | 15.9 ± 5.1 | 15.9 ± 0.9 | 18.9 ± 2.3 | 13.0 ± 1.6 | 18.3 ± 3.0 |
| R120 | |||||
| VEG | 11.8 ± 2.6 | 16.0 ± 1.0 | 19.1 ± 2.4 | 13.6 ± 0.8 | 17.8 ± 3.4 |
| REP | 15.9 ± 6.4 | 16.2 ± 1.4 | 19.5 ± 2.3 | 13.4 ± 1.2 | 18.3 ± 3.4 |
| SL Treatment | ||||||
|---|---|---|---|---|---|---|
| HPS120 | B20G50R45FR5 | B20R85FR15 | B30G25R65 | B120 | R120 | |
| Estimated PPE | 0.85 | 0.85 | 0.84 | 0.87 | 0.50 | 0.89 |
| CFI (Rf) | 44 | 80 | 0 | 55 | <0 | 33 |
| SL Treatment | ||||||
|---|---|---|---|---|---|---|
| Parameter | HPS120 | B20G50R45FR5 | B20R85FR15 | B30G25R65 | B120 | R120 |
| Godetia ‘Grace Rose Pink’ | ||||||
| h° | 355.2 NS | 354.9 | 354.5 | 354.3 | 353.6 | 355.2 |
| C | 48.5 NS | 49.1 | 47.7 | 49.4 | 47.4 | 50.1 |
| L* | 43.2 NS | 42.8 | 44.8 | 42.5 | 44.5 | 43.8 |
| a* | 48.3 NS | 48.9 | 47.5 | 49.1 | 47.1 | 49.9 |
| b* | −3.9 NS | −4.2 | −4.5 | −4.4 | −5.2 | −3.7 |
| Snapdragon ‘Potomac Royal’ | ||||||
| h° | 355.1 C | 356.8 A | 357.2 A | 356.3 AB | 356.9 A | 355.7 BC |
| C | 34.4 A | 34.2 AB | 33.0 BC | 33.7 ABC | 32.7 C | 34.3 ABC |
| L* | 19.4 B | 19.7 B | 19.6 B | 20.2 B | 19.8 B | 21.7 A |
| a* | 34.5 A | 34.2 AB | 33.0 BC | 33.7 ABC | 32.7 C | 34.2 ABC |
| b* | −2.7 D | −1.8 BC | −0.9 A | −1.8 B | −1.3 AB | −2.6 CD |
| Stock ‘Iron Rose’ | ||||||
| h° | 336.8 | 337.3 | 337.0 | 336.9 | 337.5 | 337.0 |
| C | 49.6 | 49.2 | 49.5 | 49.1 | 49.9 | 49.8 |
| L* | 33.9 | 33.9 | 34.1 | 33.6 | 34.1 | 33.3 |
| a* | 45.6 | 45.3 | 45.6 | 45.1 | 46.0 | 45.8 |
| b* | −19.5 | −19.0 | −19.3 | −19.3 | −19.1 | −19.5 |
| Parameter | y0 | A | b | R2 |
|---|---|---|---|---|
| Godetia ‘Grace Rose Pink’ | ||||
| Time to visible bud (d) | 136.93 z | −265.74 | 196.25 | 0.206 *** |
| Time to open flower (d) | 324.77 | −746.35 | 543.98 | 0.371 *** |
| Time to harvest (d) | 355.71 | −841.28 | 613.57 | 0.400 *** |
| Stem length at harvest (cm) | 352.60 | −785.44 | 593.81 | 0.151 *** |
| Stock ‘Iron Rose’ | ||||
| Time to visible bud (d) | 163.75 | −410.27 | 309.94 | 0.460 *** |
| Time to open flower (d) | 203.47 | −485.22 | 366.37 | 0.503 *** |
| Time to harvest (d) | 212.48 | −510.96 | 385.82 | 0.422 *** |
| Stem length at harvest (cm) | 104.79 | −160.00 | 113.78 | 0.081 *** |
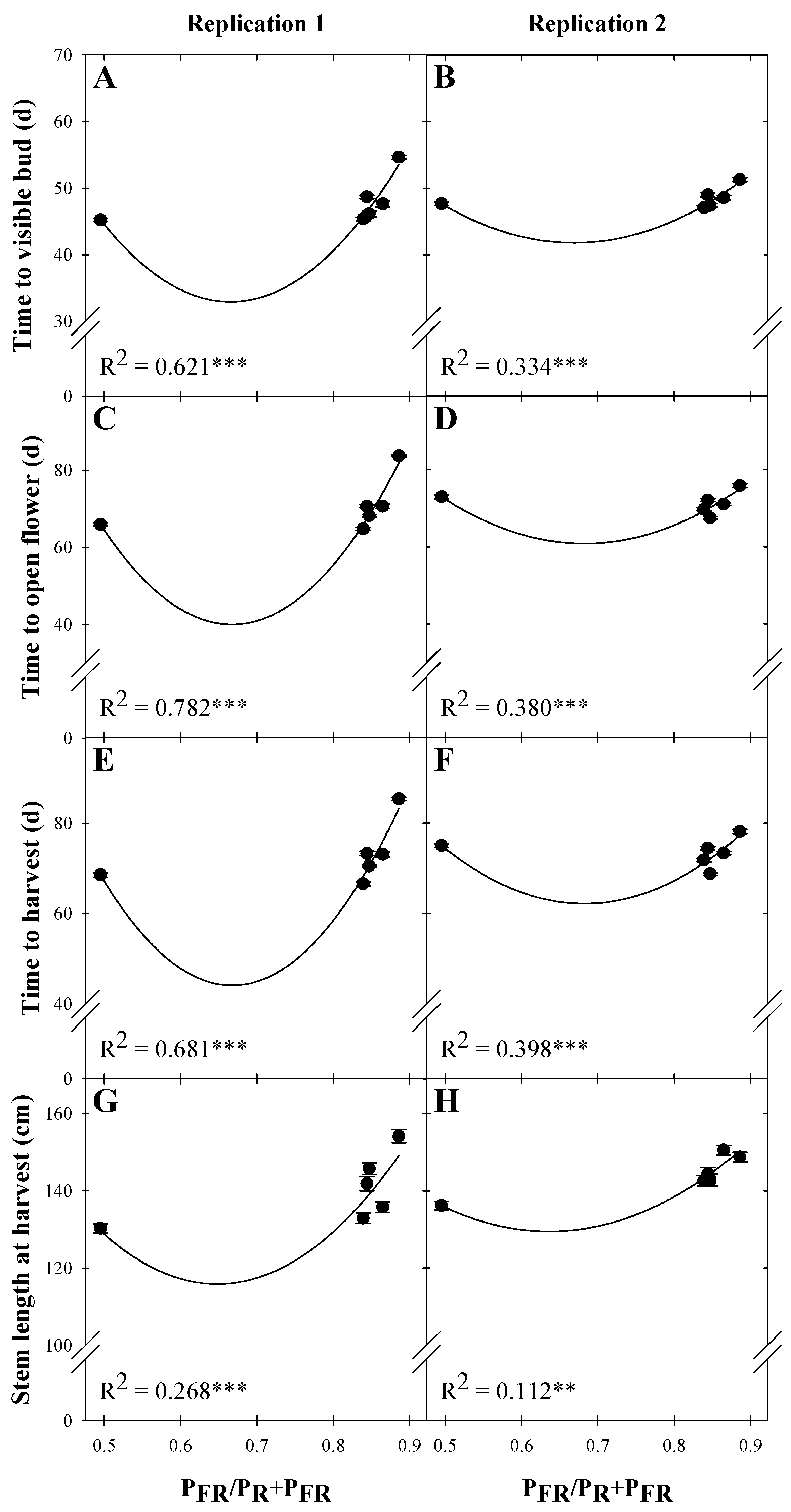
| Snapdragon ‘Potomac Royal’ | ||||
| Time to visible bud (d) | ||||
| Rep. 1 | 220.56 | −563.92 | 423.74 | 0.621 *** |
| Rep. 2 | 128.57 | −259.47 | 193.97 | 0.334 *** |
| Time to open flower (d) | ||||
| Rep. 1 | 429.16 | −1166.49 | 874.18 | 0.782 *** |
| Rep. 2 | 221.23 | −469.85 | 344.12 | 0.380 *** |
| Time to harvest (d) | ||||
| Rep. 1 | 410.51 | −1097.96 | 822.36 | 0.681 *** |
| Rep. 2 | 232.81 | −500.12 | 366.46 | 0.398 *** |
| Stem length at harvest (cm) | ||||
| Rep. 1 | 362.45 | −760.81 | 586.91 | 0.268 *** |
| Rep. 2 | 264.58 | −424.62 | 333.72 | 0.112 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spall, C.E.; Lopez, R.G. Supplemental Lighting Quality Influences Time to Flower and Finished Quality of Three Long-Day Specialty Cut Flowers. Horticulturae 2023, 9, 73. https://doi.org/10.3390/horticulturae9010073
Spall CE, Lopez RG. Supplemental Lighting Quality Influences Time to Flower and Finished Quality of Three Long-Day Specialty Cut Flowers. Horticulturae. 2023; 9(1):73. https://doi.org/10.3390/horticulturae9010073
Chicago/Turabian StyleSpall, Caleb E., and Roberto G. Lopez. 2023. "Supplemental Lighting Quality Influences Time to Flower and Finished Quality of Three Long-Day Specialty Cut Flowers" Horticulturae 9, no. 1: 73. https://doi.org/10.3390/horticulturae9010073
APA StyleSpall, C. E., & Lopez, R. G. (2023). Supplemental Lighting Quality Influences Time to Flower and Finished Quality of Three Long-Day Specialty Cut Flowers. Horticulturae, 9(1), 73. https://doi.org/10.3390/horticulturae9010073







