Abstract
Insect vectors and insect-borne plant viruses seriously endanger the safety of agricultural production. An insecticide is one of the main methods to prevent insect-borne virus transmission. However, the curious relationship between the resistance of insect vectors and arboviruses has been less studied. In this study, the effect of Tomato chlorosis virus (ToCV) on the insecticide resistance of Bemisia tabaci MED was studied. It was found that the detoxification cytochrome P450, glutathione S-transferase, and carboxylesterase-related genes in ToCV-infected B. tabaci were upregulated. The activity of the three detoxification enzymes all increased at the same time, after 48 h of virus acquisition, with the activity of carboxylesterase being the most pronounced. It was found that cytochrome P450 and glutathione S-transferase activity was the least. ToCV led to the reduced sensitivity of B. tabaci MED to pyrethroids and flupyradifurone. Therefore, it was proven that the insect-borne plant virus ToCV shows the possibility of enhancing insect-borne insecticide resistance.
1. Introduction
Tomato chlorosis virus (genus Crinivirus, family Closteroviridae) was first discovered in tomato plants in 1996 [1]. At present, ToCV can be found in more than 20 countries [2,3,4,5,6,7,8]. The hosts of ToCV vary greatly and are known to infect more than 20 plants in seven families. ToCV causes interveinal chlorosis, leaf necrosis, brittleness, and reduced plant vitality and, therefore, reduces vegetable yield [9]. Previous studies have proven that Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) was one of the main insects that effectively transmits ToCV under natural conditions [1,9].
B. tabaci, an herbivorous insect with a piercing and sucking mouthpart, can transmit ToCV while sucking plant juice. A wide range of plants serve as hosts for B. tabaci, with obvious intergenerational alternation [10]. This has caused great harm to agricultural production. In China, B. tabaci MED has replaced B. tabaci MEAM1, which was more efficient at spreading ToCV [11,12]. One of the main measures to control ToCV was to eliminate its transmission vector, in which pesticides played a key role. Pyrethroids and flupyradifurone were the main insecticides controlling B. tabaci. At present, B. tabaci has developed varying degrees of resistance to them [13].
The mechanisms of insect resistance can be roughly divided into metabolic resistance, target resistance, and behavioral resistance, among which the former two are the main mechanisms. The enhanced ability of the detoxification enzymes related to insecticide metabolism is one of the main reasons for insecticide resistance in insects [14]. Recently, our research found that B. tabaci MED infected with ToCV was less sensitive to pyrethroids and flupyradifurone than a healthy whitefly. Studies found that the enhancement of metabolic enzyme activity was considered an important mechanism for B. tabaci resistance, and the overexpression of cytochrome P450 (P450)-related gene CYP6CX4 and glutathione S-transferase (GST)-related gene CSTs2 also played crucial roles in the flupyradifurone resistance of B. tabaci; carboxylesterase (CarE) played an important role in the resistance of B. tabaci to pyrethroids [15,16,17]. In this study, we explored whether ToCV affected the resistance of B. tabaci MED to pyrethroids and flupyradifurone at the genetic and enzyme activity levels. The study aimed to reveal the correlation between ToCV and the pesticide susceptibility of B. tabaci and provide new ideas for studying insect–virus coevolution.
2. Materials and Methods
2.1. Tomato, Cotton and B. tabaci
The whole experiment was done at the Institute of Plant Protection, Hunan Academy of Agricultural Sciences. Tomato (Solanum lycopersicum Mill. cv. Zuanhongmeina) and cotton (Gossypium hirsutum L. cv. Zhongmian–63) plants were grown in separate insect-proof cages (60 cm × 50 cm × 80 cm), which were placed in a greenhouse under controlled conditions: 26 ± 0.5 °C with a long-day photoperiod (L:D = 14:10) and 60–70% relative humidity.
Tomato plants at the three-true-leaf stage were injected with an infectioned ToCV cDNA clone, which was constructed and presented by Zhou Tao, College of Plant Protection, China Agricultural University. In addition, 0.5 mL of the infectious cDNA clone were injected into each plant to achieve systemic infection [18]. The leaves, 15 days after infection, were taken for RNA extraction and reverse transcription and combined with specific primers to determine whether the tomato plant was successfully infected by RT-PCR. The total RNA was extracted by the TRI reagent® (life Technologies, Shanghai, China), in accordance with the instructions of the manufacturer. Hiscript ®ⅡQ RT SuperMix for qPCR Kit (+g DNA wiper) (Vazyme, Nanjing, China) was used for reverse transcription.
The susceptible B. tabaci MED was presented by Youjun Zhang, Chinese Academy of Agricultural Sciences, and reared on cotton in cages for more than 6 generations to establish populations with no exposure to any pesticides. The field B. tabaci MED was collected from the tomato plants in a vegetable plantation field in the suburbs of Changsha, China, in 2021, and maintained on cotton plants in greenhouse.
The biotype of B. tabaci adults was observed to the purity of MED in accordance with the mtCOI enzyme cleaving identification method [19,20,21]. To obtain the mtCOI fragment, the DNA of an adult was isolated and was PCR-amplified using the primers C1-J-2195 and R-BQ-2819 (about 620 bp) (Table 1). The clone result was then enzymed with the restricted endonuclease AseI (Beijing Biolink biotechnology Co., Ltd., Beijing, China). The B. tabaci MED gene fragment contained an AseI endonuclease cleavage site, which allows the enzyme to split the gene fragment into two fragments of differing lengths (498 and 122 bp) (Figure 1). The purity of B. tabaci MED was monitored every 2–3 generations using sequencing of mtCOI gene with sequence-specific primers.

Table 1.
Primers used for study.
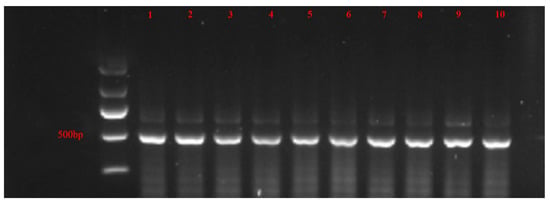
Figure 1.
Nucleic acid electrophoresis of B. tabaci biotypic identification. The size of the gene fragment was roughly 500 bp. Sample numbers 1 to 5 were indoor-sensitive B. tabaci MED results, while sample numbers 6 to 10 were field-resistant results. As an identifying scale, Maker II (Tiangen biotech (Beijing) Co., Ltd., Beijing, China) was employed.
The calculated LC50 value was then used to screen the resistant strains of B. tabaci MED. The feeding conditions were temperature of 26 ± 2 °C, relative humidity of 55 ± 5% and L:D = 14:10 photocycle.
2.2. Pyrethroids and Flupyradifurone Bioassay of Viruliferous and Non-Viruliferous B. tabaci MED
Pyrethroids, EW (2.5%; Antai Chemical, Guangxi, China) and flupyradifurone, SL (17%; Bayer, Beijing, China) were used for all insecticide bioassays. Non-viruliferous B. tabaci MED were collected from cotton. After starving for 2 h, the B. tabaci MED was placed on ToCV-infected tomato plants by being fixed on the leaves through mini-cages. After feeding for 48 h, a total of 25 B. tabaci individuals were chosen for the pyrethroids and flupyradifurone bioassay, which was performed using the leaf dip agar moisturizing method [22]. Then 2% ager solution was added to the bottom of a clean flat glass tube, with a diameter of about 2.2 cm and a length of about 8 cm, until it cools and solidifies dry. Clean and well-developed cotton leaves were taken, while avoiding large leaf veins by choosing a flat leaf part; a perforator was used to beat out a leaf dish with a diameter of 22 cm, which was then soaked in a pre-configured insecticide solution of 20 s, taken out, and naturally dried with the back facing up. The concentration of the insecticide was established according to the suggested concentration in the insecticide instructions for pre-experiment and was diluted with distilled water to obtain five working concentrations and one control concentration (Table 2). Leaves collected from uninfected cotton plants that had never been exposed to any insecticide were cut into leaf discs (22 mm in diameter). The leaf discs were dipped in insecticide or water for 10 s and dried in the air. The abaxial surface of each disc was placed downward on 2% agar (flat) in a glass tube and marked, and then about 25 B. tabaci of mixed sex were blown into glass tubes 48 h after feeding on ToCV-infected tomatoes. Glass tubes were put in an incubator. The settings for the temperature, humidity and light cycle were 26 ± 2 °C, 70 ± 10% and 16 h:8 h (L:D), respectively. The survival of B. tabaci was investigated after 48 h.

Table 2.
Susceptibility of B. tabaci MED adults to pyrethroids and flupyradifurone.
2.3. Metabolic Enzyme Assays
The 1.5 mL centrifugal tube was used to collect 200 B. tabaci MED after obtaining the virus 48 h, treated with liquid nitrogen, and ground into powder on the ice. Then, 100 µL of 1 X phosphate buffer was added to the centrifuge tube, and its supernatant was removed by high-speed centrifugation for subsequent experiments. Absorbance was measured in the experiments using a spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA).
The activity of P450 was measured by Insect Cytochrome P450 (CYP450) ELISA Kit (Shanghai Enzyme-linked Biotechnology, Shanghai, China), in accordance with the instructions of the manufacturer. Then, 50 µL of the supernatant were collected; 100 µL of the enzyme-labeled reagent were added, incubated and washed; 50 µL of the dye reagent were added; and sample color development was performed at 37 °C for 15 min in the dark. Finally, 50 µL of termination solution were added, and the absorbance of each well was measured sequentially at 450 nm wavelength. The blank control group was set. The concentration of CYP450 in the samples was then determined by comparing the absorbance of the samples to the standard curve.
The activity of GST was measured by GST assay kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China), in accordance with the instructions of the manufacturer. The change of absorbance was detected at 340 nm. Different reagents were added to 20 µL of the extract, in accordance with the instruction sequence of the manufacturer. The absorbance after 10 s and after 5 min of water bath in 25 or 37 °C were measured using spectrophotometer, and the difference was calculated. One unit of GST activity was defined as the amount of enzyme catalyzing 1 µmol/L of CDNB to form GSH-CDNB per minute per milligram of total protein.
The activity of carboxylesterase was determined in accordance with the instructions of the manufacturer of the carboxylesterase assay kit (Beijing Solarbio Science & Technology Co., Ltd., China). Different reagents were added to 10 µL of the extract, in accordance with the instruction sequence of the manufacturer. The absorbance after 10 s and after 5 min of water bath in 37 °C were measured using spectrophotometer, and the difference was calculated. The change in absorbance was detected at 450 nm. At 37 °C, the increase in catalytic absorbance value of 0.5 per g tissue mass per minute per mL of reaction system was defined as one unit of enzyme activity.
2.4. Expression Analysis of Detoxification Enzyme Related Genes CYP6CX4, GSTs2 and LOC109038667
Target gene LOC109038667 was screened by transcriptome expression analysis (unpublished data), which was associated with CarE. Non-viruliferous B. tabaci MED were collected from cotton. After starving for 2 h, B. tabaci MED was fed on healthy and ToCV-infected tomato plants for 48 h; the relative expression of detoxification enzyme P450, GST and CarE-related genes CYP6CX4, GSTs2 and LOC109038667 in B. tabaci MED (30 per sample) was determined by RT-qPCR. Then, fluorescence quantitative PCR was conducted with gene-specific primers by ChamQ Universal SYBR qPCR Master Mix Kit (Vazyme, Nanjing, China). Actin-R and Actin-F were been used for B. tabaci MED reference genes [23]. The specific primers of the target genes are listed in Table 1. Comparative cycle threshold (2−ΔΔCt) methods were used for calculating the relative gene expression of detoxification-related P450, GST and CarE [21]. The experiment was carried out of three biological replicates, and each biological replicate contained three technical replicates.
2.5. Statistical Analyses
Data of expression analysis of gene and metabolic enzyme assays were statistically analyzed using Tukey’s HSD with α = 0.05. Statistical analyses were performed using IBM SPSS Statistics 20.0 (SPSS Inc., Chicago, IL, USA), and graphs were generated using GraphPad Prism 8.0.2. All obtained data were expressed as the mean ± standard error. The LC50 values (of pyrethroids and flupyradifurone) were calculated using Polo Plus 2.0.
3. Results
3.1. Bioassays
Using the agar moisturizing method, the biometric results of indoor-sensitive and field-resistant populations and their consumptions for 48 h on ToCV-infected tomatoes were found. The indoor-sensitive populations were significantly more sensitive to pyrethroids and flupyradifurone than the field-resistant populations, and, after eating ToCV-infected tomato plants for 48 h, their sensitivities to both pesticides decreased.
3.2. Activities of Metabolic Enzymes
P450: There was no significant difference in the P450 enzymes’ activity between the healthy B. tabaci MED in either the field-resistant populations or the indoor-sensitive populations. However, after 48 h of B. tabaci feeding on tomato seedings infected with ToCV, the activity of the P450 enzymes in its body increased significantly, and the enzyme activity of the field-resistant populations increased more than that of the indoor-sensitive populations (Figure 2A).
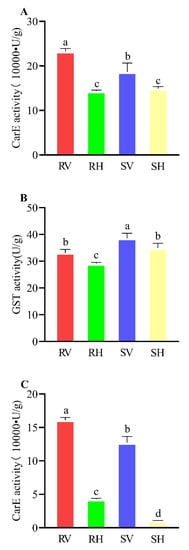
Figure 2.
Effect of ToCV on the activities of three detoxification enzymes. RV, resistant veneniferous B. tabaci MED; RH, resistant healthy B. tabaci MED; SV, sensitive veneniferous B. tabaci MED; SH, sensitive healthy B. tabaci MED. Data represent mean ± SE (n = 3). (A) Activities of P450 metabolic enzymes. (B) Activities of GST metabolic enzymes. (C) Activities of CarE metabolic enzymes. Different uppercase letters above bars indicate significant differences (Tukey’s HSD with α = 0.05).
GST: There was no particularly significant difference or change in the GST enzymes’ activity in the four treatment groups, and the enzyme activity level after 48 h of feeding on ToCV-infected tomato seedings increased slightly compared with healthy adults (Figure 2B).
CarE: The Car enzymes’ activity in the healthy indoor-sensitive populations was at a very low level, and the CarE enzymes’ activity of the healthy field-resistant populations was significantly higher than that of the indoor-sensitive populations Similar to the activity change law of the P450 enzyme, after 48 h of ingesting ToCV-infected tomato plants, the Car enzymes’ activity in the two populations increased significantly, and the Car enzymes’ activity of the field-resistant populations was slightly higher than that of the indoor-sensitive populations (Figure 2C).
3.3. Expression Patterns of Detoxification-Related P450, GST and CarE
The significant difference was observed in the expression of the CYP6CX4 gene between the viruliferous and non-viruliferous B. tabaci after 48 h of feeding. Among them, after 48 h of poisoning for the field-resistant populations, the gene expression was significantly higher than that of the other three cases. Even the CYP6CX4 expression of the healthy field-resistant populations was comparable to that of the indoor-sensitive populations with ToCV 48 h. Compared with the indoor-sensitive populations, the expression of CYP6CX4 after 48 h of toxicity increased significantly (Figure 3A).
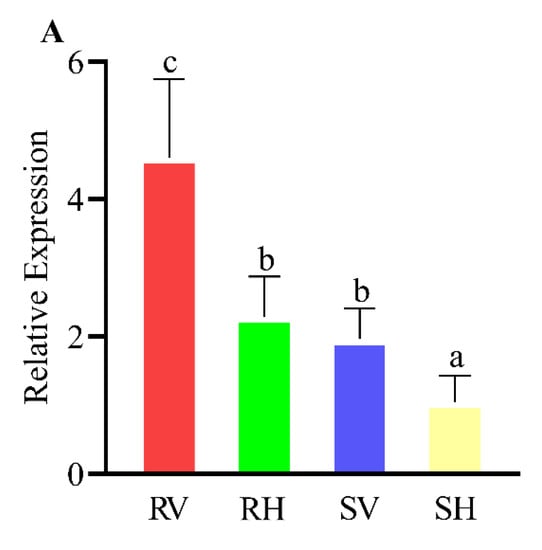
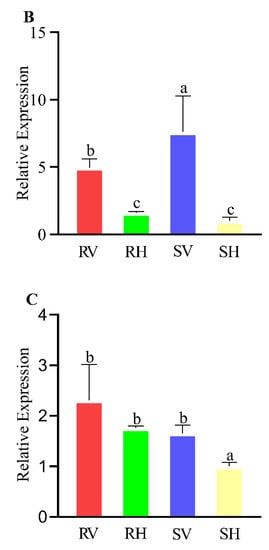
Figure 3.
Effects of ToCV on genes related to three detoxification enzymes. RV, resistant veneniferous B. tabaci MED; RH, resistant healthy B. tabaci MED; SV, sensitive veneniferous B. tabaci MED; SH, sensitive healthy B. tabaci MED. Data represent mean ± SE (n = 3). (A) Effects of ToCV on the upstream regulators of the CYP6CX4 gene. (B) Effects of ToCV on the upstream regulators of the GSTs2 gene. (C) Effects of ToCV on the upstream regulators of the LOC109038667 gene. Different uppercase letters above bars indicate significant differences (Tukey’s HSD with α = 0.05).
There was a significant difference in the expression level of the GSTs2 gene in vivo after the two populations were fed with ToCV-infected tomatoes for 48 h. From Figure 3B, it can be seen that whether it was for field-resistant populations or indoor-sensitive populations, the expression level of the GSTs2 gene in healthy adults remained at a relatively low level, while the GSTs2 gene expression level after the intake of ToCV 48 h increased. The gene expression level of the indoor-sensitive populations increased more significantly, reaching more than five times that of the field-resistant populations (Figure 3B).
Through the transcriptome analysis of the indoor-sensitive feeding of ToCV-virus-infected plant populations in the early laboratory, it was found that the gene LOC109038667 expression increased significantly after 48 h of treatment. Combined with the results of the transcription analysis, the gene was processed 48 h after obtaining ToCV in two populations through the NCBI design primer. Quantitative analysis showed that in healthy insects, the expression of the field-resistant populations was about four times that of the indoor-sensitive populations. However, after spending 48 h on plants infected with ToCV, the expression of the gene in both populations increased significantly, and the field-resistant populations were slightly higher than the indoor-sensitive populations (Figure 3C).
4. Discussion
Pest resistance to pesticides is the main factor hindering pest control and controlling vector transmission. For example, when studying the effect of Cucurbit chlorotic yellows virus (CCYV) on B. tabaci MED, it was found that CCYV enhanced the resistance of B. tabaci to imidacloprid by increasing the expressing of the CYP6CM1 gene, a P450-related gene [24].
ToCV is a semi-retained and semi-persistent plant virus. We found that ToCV enhanced the tolerance of B. tabaci MED to pyrethroids and flupyradifurone. From a comparison of the activity of the three detoxification enzymes in B. tabaci MED, we found that the activity of all three detoxing enzymes increased after B. tabaci MED acquired ToCV for 48 h, and this increase was of a different magnitude. Coincidentally, the activity of the defense enzymes carboxylesterase, superoxide dismutase (SOD) and catalase (CAT) and the detoxification enzymes carboxylesterase (CAE) and glutathione S-transferase (GST) in the adult worms of the small brown planthopper, feeding on rice plants infected with rice black-streaked dwarf virus (RBSDV), was significantly increased [25]. The activity of SOD, peroxidase (POD) and CAT inside the adults and nymphs of black-spotted planthoppers (WBPH) and black-spotted planthoppers (BPH), feeding on rice infected with southern rice black-streaked dwarf virus (SRBSDV), increased with the extension of the feeding duration [26]. However, POD, SOD and CAT activities of the Spring-grain aphid Schizaphis graminum (Hemiptera: Aphididae) were significantly lower when feeding on wheat infected with barley yellow dwarf virus than when feeding on healthy wheat [27]. These differences may be due to the different physiological and biochemical mechanisms of the interactions between different types of plant viruses and different mediator insects.
Then, the results showed that the expression levels of CYP6CX4, GSTs2 and LOC109038667 genes in indoor-sensitive populations of B. tabaci MED were generally lower than that in the field-resistant populations. After obtaining the ToCV virus for 48 h, the expression of the indoor-sensitive and field-resistant populations’ CYP6CX4, GSTs2 and LOC109038667 genes increased. Among them, the gene CYP6CX4 was significantly increased, and the expression of LOC109038667 after the indoor-sensitive populations obtained ToCV for 48 h was even close to that of the healthy field-resistant populations.
Unfortunately, for various reasons, this experiment only focused on two types of insecticides: pyrethroids and flupyradifurone. The detoxification enzymes P450, GST and CarE were only studied in detail, with one gene was selected from each. Whether the results of this project are applicable to other types of pesticides remains to be further verified. The antidase-related genes CYP6CX4, GSTs2 and LOC109038667 were only quantitatively analyzed, the dsRNA was not synthesized, and no further experiments were carried out.
Combined with the biometric results, ToCV could affect the expression level of detoxification-enzyme-related genes in B. tabaci MED, enhance the activity of detoxification enzymes, and ultimately reduce the sensitivity of B. tabaci MED to pyrethroids and flupyradifurone.
Tomato yellow leaf curl virus (TYLCV) is a persistent virus that is also transmitted by B. tabaci MED. Researchers have confirmed the reciprocal relationship between B. tabaci MED and TYLCV. It is now known that B. tabaci MED benefited from feeding on hosts infected with TYLCV. TYLCV could reduce the sensitivity of flupyradifurone in infected B. tabaci MED [28]. Obviously, there was a correlation between virus transmission and insecticide resistance. Plant viruses and their vectors or other differences that may have had different modes of transmission (non-persistence, semi-persistence and persistence) may have such interrelationships between different types and/or degrees. On the other hand, TYLCV infection could benefit its vector B. tabaci MED by improving growth, survival and reproduction [29]. However, ToCV infection could decrease the performance of B. tabaci MED on tomato plants, as measured by declines in longevity and fecundity [30]. In light of the aforementioned findings, it is possible to surmise that while both ToCV and TYLCV were able to decrease the sensitivity of B. tabaci MED to pesticides, their respective mechanisms of resistance were distinct. By boosting the activity of its detoxification enzymes and the expression of the genes associated with its detoxification enzymes, B. tabaci MED was able to deal with the negative effects of ToCV and, to some part, resolve the issue of the insecticide danger to itself.
In addition, there is research demonstrating that the B. tabaci MED that acquired ToCV preferred healthy tomato plants, while healthy B. tabaci MED preferred ToCV-infected tomato plants [12]. This was similar to the cell-wall-lacking bacterium Candidatus Phytoplasma mali, which can modify a host plant (apple) odor, luring its vector (the univoltine psyllid Cacopsylla picta) to infected plants [31]. Obviously, the B. tabaci MED was infected with ToCV, which made it easier for ToCV to spread more readily through B. tabaci MED to more crops and made it difficult for pesticides to control B. tabaci MED. This formed a vicious cycle that was conducive to ToCV transmission and B. tabaci MED harm, which may provide a more appropriate explanation for the severity of the harm to both B. tabaci MED and ToCV.
In this experiment, genes linked to pesticide resistance, established through earlier research, were combined with genes found through transcription group data, and the pertinent detoxifying enzymes were combined to detect recurring changes in the complex scenarios of insects and insect-borne viruses. This gave the researchers a new angle when examining the process behind pesticide resistance in B. tabaci MED. Specifically, investigations of pesticide resistance should take into account whether insects are infected with arboviruses under natural circumstances.
5. Conclusions
To sum up, we found that B. tabaci MED infected with ToCV showed reduced sensitivity to insecticides. It was further found that after 48 h of ToCV acquisition, the enzymatic activities of the three detoxification enzymes of B. tabaci MED were enhanced, and ToCV increased the expression levels of the genes involved in these detoxification enzymes. This study of the changes in the resistance of B. tabaci MED and the role of ToCV in the body of B. tabaci MED is conducive to the effective control of B. tabaci MED and ToCV.
Author Contributions
Conceptualization, J.Z.; methodology, X.S.; software, L.H.; validation, Z.Z. (Zhuo Zhang) and Z.Z. (Zhanghong Zhang); formal analysis, X.S.; investigation, Y.L.; resources, D.Z.; data curation, J.Z.; writing—original draft preparation, J.Z.; writing—review and editing, X.S.; visualization, Z.Z. (Zhuo Zhang); supervision, Y.Z.; project administration, X.S.; funding acquisition, X.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 32030088, 32272535, 32072383, 31901854) and the Agriculture Research System of China (No. CARS-23-D-02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available on request due to institutional restrictions and privacy.
Acknowledgments
The authors would like to thank everyone who contributed to this article and the field and laboratory work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wisler, G.C.; Li, R.H.; Liu, H.Y.; Lowry, D.S.; Duffus, J.E. Tomato chlorosis virus: A new whitefly-transmitted, phloem-limited, bipartite closterovirus of tomato. Phytopathology 1998, 88, 402–409. [Google Scholar] [CrossRef]
- Wisler, G.C.; Duffus, J.E.; Liu, H.Y.; Li, R.H. Ecology and epidemiology of whitefly-transmitted closteroviruses. Plant Dis. 1998, 82, 270–280. [Google Scholar] [CrossRef]
- Accotto, G.P.; Vaira, A.M.; Vecchiati, M.; Sialer, M.M.F.; Gallitelli, D.; Davino, M. First report of Tomato chlorosis virus in Italy. Plant Dis. 2001, 85, 1208. [Google Scholar] [CrossRef]
- Segev, L.; Wintermantel, W.M.; Polston, J.E.; Lapidot, M. First report of Tomato chlorosis virus in Israel. Plant Dis. 2004, 88, 1160. [Google Scholar] [CrossRef]
- Hirota, T.; Natsuaki, T.; Murai, T.; Nishigawa, H.; Niibori, K.; Goto, K.; Hartono, S.; Suastika, G.; Okuda, S. Yellowing disease of tomato caused by Tomato chlorosis virus newly recognized in Japan. J. Gen. Plant Pathol. 2010, 76, 168–171. [Google Scholar] [CrossRef]
- Arruabarrena, A.; Rubio, L.; González-Arcos, M.; Maeso, D.; Fonseca, M.E.N.; Boiteux, L.S. First report of Tomato chlorosis virus infecting tomato crops in Uruguay. Plant Dis. 2014, 98, 1445. [Google Scholar] [CrossRef]
- Fiallo-Olivé, E.; Hamed, A.A.; Moriones, E.; Navas-Castillo, J. First report of Tomato chlorosis virus infecting tomato in Sudan. Plant Dis. 2011, 95, 1592. [Google Scholar] [CrossRef]
- Zhao, R.N.; Wang, R.; Wang, N.; Fan, Z.F.; Zhou, T.; Shi, Y.C.; Chai, M. First report of Tomato chlorosis virus in China. Plant Dis. 2013, 97, 1123. [Google Scholar] [CrossRef]
- Wintermantel, W.M.; Wisler, G.C. Vector specificity, host range, and genetic diversity of Tomato chlorosis virus. Plant Dis. 2006, 90, 814–819. [Google Scholar] [CrossRef]
- Wu, T.; Yang, D.Q. Biological characteristics and prevention and treatment of Bemisia tabaci. Hunan Agric. Sci. 2005, 01, 56–58. [Google Scholar]
- Zheng, H.X.; Xie, W.; Fu, B.L.; Xiao, S.; Tan, X.; Ji, Y.; Cheng, J.X.; Wang, R.; Liu, B.M.; Yang, X. Annual analysis of field-evolved insecticide resistance in Bemisia tabaci across China. Pest Manag. Sci. 2021, 77, 2990–3001. [Google Scholar] [CrossRef]
- Shi, X.B.; Tang, X.; Zhang, X.; Zhang, D.Y.; Li, F.; Yan, F.; Zhang, Y.J.; Zhou, X.G.; Liu, Y. Transmission efficiency, preference and behavior of Bemisia tabaci MEAM1 and MED under the influence of Tomato chlorosis virus. Front. Plant Sci. 2018, 8, 2271. [Google Scholar] [CrossRef]
- Ahmad, M.; Arif, M.I.; Ahmad, Z.; Denholm, I. Cotton whitefly (Bemisia tabaci) resistance to organophosphate and pyrethroid insecticides in Pakistan. Pest Manag. Sci. 2002, 58, 203–208. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Chi, G.T.; Zhang, J.L. The progress of detoxification enzyme systems and resistance of insecticide. J. Hebei Agric. Univ. 2002, 25, 193–195. [Google Scholar]
- Wang, R.; Wang, J.D.; Zhang, J.S.; Che, W.N.; Feng, H.L.; Luo, C. Characterization of flupyradifurone resistance in the whitefly Bemisia tabaci Mediterranean (Q biotype). Pest Manag. Sci. 2020, 76, 4286–4292. [Google Scholar] [CrossRef]
- Li, X.C.; Schuler, M.R.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- He, Y.X.; Huang, J.; Yang, X.J.; Weng, Q.Y. Pyrethroid resistance mechanisms in Bemisia tabaci (Gennadius). Acta Entomol. Sin. 2007, 50, 241–247. [Google Scholar]
- Orílio, A.F.; Fortes, I.M.; Navas-Castillo, J. Infectious cDNA clones of the crinivirus Tomato chlorosis virus are competent for systemic plant infection and whitefly-transmission. Virology 2014, 464, 365–374. [Google Scholar] [CrossRef]
- Khasdan, V.; Levin, I.; Rosner, A. DNA markers for identifying biotypes B and Q of Bemisia tabaci (Hemiptera: Aleyrodidae) and studying population dynamics. Bull. Entomol. Res. 2005, 95, 605–613. [Google Scholar] [CrossRef]
- Chu, D.; Wan, F.H.; Zhang, Y.J. Change in the biotype composition of Bemisia tabaci in Shandong Province of China from 2005 to 2008. Environ. Entomol. 2010, 39, 1028–1036. [Google Scholar] [CrossRef]
- Chu, D.; Zhang, Y.J.; Wan, F.H. Cryptic Invasion of the Exotic Bemisia tabaci Biotype Q Occurred Widespread in Shandong Province of China. Fla. Entomol. 2010, 93, 203–207. [Google Scholar] [CrossRef]
- Liang, P.; Guo, Y.J.; Zhou, X.G.; Gao, X.W. Expression profiling in Bemisia tabaci under insecticide treatment: Indicating the necessity for custom reference gene selection. PLoS ONE 2014, 9, e87514. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Feng, Y.T.; Wu, Q.J.; Xu, B.Y.; Wang, S.L.; Chang, X.L.; Xie, W.; Zhang, Y.J. Fitness costs and morphological change of laboratory-selected thiamethoxam resistance in the B-type Bemisia tabaci (Hemiptera: Aleyrodidae). J. Appl. Entomol. 2009, 133, 466–472. [Google Scholar] [CrossRef]
- Xu, H.X.; He, X.C.; Zheng, X.S.; Yang, Y.J.; Lu, Z.X. Influence of rice black streaked dwarf virus on the ecological fitness of non-vector planthopper Nilaparvata lugens (Hemiptera: Delphacidae). Insect Sci. 2011, 25, 654–658. [Google Scholar]
- Chen, C.; Jiang, D.C.; Yang, H.; Jin, D.C. Effects of southern rice black streaked dwarf virus on defense enzymes in brown planthopper and white-blacked planthopper. J. Environ. Entomol. 2016, 38, 113–118. [Google Scholar]
- Meng, L.Q.; Li, D.D.; Su, D.; Zhao, H.Y.; Hu, Z.Q. Activities of protective and detoxifying enzymes in spring-grain aphid schizaphis graminum (Hemiptera: Aphididae) fed on wheat infected with barley yellow dwarf virus. J. Plant. Protect. 2019, 46, 707–708. [Google Scholar]
- Yan, M.H.; He, H.F.; Zhang, Z.L.; Zhang, B.B.; Zhu, C.Q.; Yan, W.L. Molecular Basis of Mutual Benefits Between Cucurbit Chlorotic Yellows Virus (CCYV) Transmission and Imidacloprid Resistance in Bemisia tabaci. 2022. Available online: https://www.researchsquare.com/article/rs-1469019/v1 (accessed on 5 November 2022). [CrossRef]
- Su, Q.; Preisser, E.L.; Zhou, X.M.; Xie, W.; Liu, B.M.; Wang, S.L.; Wu, Q.J.; Zhang, Y.J. Manipulation of host quality and defense by a plant virus improves performance of whitefly vectors. J. Econ. Entomol. 2015, 108, 11. [Google Scholar] [CrossRef]
- Li, J.; Ding, T.B.; Chi, H.; Chu, D. Effects of tomato chlorosis virus on the performance of its key vector, Bemisia tabaci, in China. J. Appl. Entomol. 2018, 142, 296–304. [Google Scholar] [CrossRef]
- Mayer, C.J.; Vilcinskas, A.; Gross, J. Phytopathogen lures its insect vector by altering host plant odor. J. Chem. Ecol. 2008, 34, 1045–1049. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).