Abstract
Enzymatic browning is a common limiting factor in the fruit industry because it causes significant losses through fresh product deterioration, affecting taste, flavor, and nutrition. Artocarpus odoratissimus, locally known as terap, is an exotic indigenous fruit to Borneo. This fruit remains underutilized due to its perishability, short shelf-life, and peel browning. Currently, no information has apparently been published on the browning mechanisms of A. odoratissimus. Thus, the present study aims to evaluate the degree of browning and enzymatic activities in relation to the phytochemical contents in A. odoratissimus during postharvest ripening. The experimental design consists of packaging (without packaging and with packaging) and storage temperatures (ambient at 25 °C, and cold storage at 10 °C), which were arranged in a randomized complete block design. Findings showed significantly higher weight loss in fruit stored at 25 °C on days 4 (T1) at 12.20 ± 0.19% and 8 (T5) at 11.09 ± 0.24%. The degree of browning was higher in the fruits stored with packaging at 25 °C, collected on day 4 at 0.48 ± 0.01 and day 8 at 0.51 ± 0.02, and consequently, the phenylalanine ammonia-lyase (671.00 ± 5.25 UE g−1 min−1) and polyphenol oxidase (670.00 ± 2.56 UE g−1 min−1) enzymatic activities were also higher. The lower enzymatic activities were recorded in the fruit stored at 10 °C without packaging, resulting in the least degree of browning. The fruit with the lowest enzymatic activities was stored at 10 °C without packaging, resulting in the least amount of browning. This trend is supported by lower total phenolic content (TPC) and is explained by a strong positive correlation between TPC and PAL (r = 0.927). Low-temperature storage was effective in reducing the effect of browning and deterioration on A. odoratissimus for up to 16 days. The results not only provided insights into the peel browning in A. odoratissimus but also guidance on controlling postharvest fruit browning.
1. Introduction
Postharvest is one of the essential practices contributing to the limiting factor of fruit production development in the Malaysian fruits industry sector. According to Kitinoja and Kader [1], postharvest losses for fruits and vegetables in developing countries range between 20% to 50%, with the majority occurring at the farm, wholesaler, and store levels, respectively. Despite this, Malaysia’s fruit sector exported 41 million metric tons in 2019, yet the nation still needed 80 million metric tons of imports in 2020 [2]. Fresh fruit and vegetables were seen to suffer massive losses due to improper postharvest management throughout the production chain, including pathogen-induced deterioration [3], moisture losses [4], and mechanical injuries [5].
Browning is the most common limiting factor in the food industry, causing significant losses in fresh produce [6,7]. Browning not only affects the color, flavor, and nutritional value of foods, but it also influences the cosmetic looks of fresh produce. This causes substantial economic loss due to lower selling prices as it is not preferred by consumers. Browning is significantly induced by enzymatic activity, whereas browning intensity in fruits and vegetables is primarily determined by the rate of oxidative and solution concentration [6]. Polyphenol oxidase (PPO) and peroxidase (POD) are the primary enzymes involved in browning. Phenylalanine ammonia-lyase (PAL), PPO, and POD enzymatic activities increase in response to tissue stress during cutting, which shortens the shelf-life of the products, as reported by Saltveit [8]. As a result of tissue damage, phenolic chemicals are exposed to air, enabling the oxidation of phenols into quinones to emerge. These quinones and their derivatives immediately polymerize via a sequence of alternate processes to generate melanin, a dark pigment highly impermeable in water [9,10].
Besides, phenolic compounds have a major part in enzymatic browning, as they act as substrates for the enzymes responsible for browning. When plant cells are damaged due to slicing, crushing, bruising, or drying, the enzymatic oxidation of phenolic compounds occurs. This process is also involved in processing plants, where peroxidase, laccase, and polyphenol oxidase catalyze the enzymatic oxidation of phenolic compounds [11]. Despite this, during postharvest handling, wounding, temperature, modified atmosphere, light irradiation, and elicitor treatments are known to be used to regulate the phenolic content in fruits and vegetables. Several phenolic chemicals are found in fruits, which vary according to species, cultivar maturity, weather conditions, and other factors affecting plants [10,11,12]. Previous studies showed that the expression of genes encoding the enzymes and phenolic compounds was changed during the browning of fruit and vegetables. It has also been reported that the enzymatic activities changed during the browning of potato, apple, and peach fruits [6,13,14]. Studies were also conducted to measure the phenolic content, antioxidant capacity, and related enzyme activities in the pericarp browning of pomegranate, longan, and cantaloupe fruits [15,16,17].
Artocarpus odoratissimus, also known as terap, is an indigenous fruit species that belongs to the Moraceae family and is mainly found on Borneo and Mindanao Islands [18]. It is a commonly called marang, madang, timadang, or johey oak. Terap fruits have a strong aroma; their scent comes mainly through their skin and could remind people of durian [18]. The fruit availability lasts between August–October and December–January and has gained popularity among Sarawak locals, as it has a great potential to be commercialized. This fruit is famous for its sweet and juicy flesh, suitable for being eaten raw or making fritters [19]. The flesh can also be fermented to make organic vinegar and ice cream flavors. Because of their nutty flavor, the seeds are boiled or steamed and eaten as snacks [19]. Local communities in Sarawak use the leaves to treat gout, diarrhea, and fever, as well as venomous stings and skin diseases [20]. The authors also reported that, because of their large size, the leaves are also used as a food wrapping. The combination of latex and vinegar promotes the treatment of abscesses, snakebites, and glandular swelling. The trunk wood is used to make light constructions. Dayak local communities in Sarawak used them to cure diarrhea, skin diseases, and asthma [21].
Despite its high nutritional value and enormous health benefits, A. odoratissimus is underutilized and not classified as a commercial crop due to its highly esteemed climacteric properties and shorter shelf-life. This fruit is susceptible to bruising and mechanical injuries, posing a significant issue during postharvest handling and marketing. Similarly, Artocarpus species, such as jackfruit, cempedak, and breadfruits have common traits that degrade quickly after ripening [18]. The Artocarpus species are highly perishable and frequently exhibit an unpleasant taste, tissue softness, and surface browning, all of which contribute to postharvest losses. Similarly, fruits damaged by carbon dioxide will develop brown spots of hypoxia, generally due to low oxygen levels on the fruit surface [12,13].
According to the literature, there is no information on the browning mechanisms in A. odoratissimus concerning enzymatic activities and phytochemical attributes during postharvest ripening. Thus, the present study aims to evaluate the degree of browning, enzymatic activities, and phytochemical contents of A. odoratissimus during postharvest ripening. This study could be the first to provide insights into the mechanism of peel browning in A. odoratissimus, as well as guidance on developing appropriate post-harvest technology, particularly in evaluating anti-browning techniques to extend shelf-life and improve fruit quality and marketability.
2. Materials and Methods
2.1. Sample Collection
The unripe fruits A. odoratissimus that attained optimum maturity (stage 815 BBCH scale -week 12 after anthesis) were collected from the fruit orchard at Batu 10, Bintulu, Sarawak (3°13′08″ N, 113°09′05″ E), and immediately brought to the laboratory. Sarawak has an equatorial climate. The temperature is relatively uniform throughout the year, within the range of 23–32 °C, while the average rainfall received was 268–619 mm.
2.2. Pre-Fruit Characterization
The initial fruit weight, length, and width were recorded prior to postharvest treatments (Table 1). The color of the fruit was assessed visually using a grading system ranging from 0 to 5. The fruit is graded on a scale of 0 to 5, where 0 is light green, 1 is green, 2 is greenish yellow, 3 is greenish brown, 4 is fully brown, and 5 is fully dark brown. Additionally, color coordinates were recorded on the surface of the fruits using a colour reader CR10 (Konica Minolta CR10, Tokyo, Japan). The fruit’s firmness was measured using a penetrometer (FT011, Virginia, USA). Penetrometer readings were converted to newton (N) values. The fruits’ gas measurements for carbon dioxide (CO2), and oxygen (O2), were determined using a portable gas detector (FD-600-HA-CO2-O2, California, USA). The fruit’s moisture was measured using the portable fruit moisture meter (E12211, Guangdong, China).

Table 1.
Pre-fruit characterization of Artocarpus odoratissimus.
Based on the pre-fruit characterization, the weight of A. odoratissmus fruits that were collected ranged from 505.33 ± 0.67 to 1326.33 ± 0.67 g; according to the Philippine National Standard, the samples are categorized as small fruits. The fruits used for this study had a firmness value of 7.07–8.67 N. At this stage, the fruit hair protuberances were rigid, with gaps in between, and the fruits were odorless. Meanwhile, the fruit exocarp color ranged between 1, i.e., green (L* 26–28, a* 1.30–1.50, b* 23–25), and 2, i.e., greenish-yellow (L* 24–28, a* 1.30–1.57, b* 23–25). In the initial stage, the CO2 production ranged from 101.20 to 148.20 mL kg h−1, while O2 release ranged from 12.40 to 13.80 mL kg h−1.
2.3. Postharvest Treatments and Observation
Two factors are subjected to the postharvest ripening treatments, i.e., factor 1, the temperature, and factor 2, the packaging. Under the temperature factor, there were two levels, i.e., ambient temperature (23–25 °C) and cooling temperature (10 °C), and there were two types of packaging conditions, including control (without packaging) and with packaging (5% poly-perforated bag). The experiment design was arranged in a randomized complete block design (RCBD), with three replicates in each treatment. The fruits were stored for 4 days, 8 days, 12 days, and 16 days. The freshly collected fruit was processed immediately to analyze the physical and chemical changes.
2.4. Enzymatic Browning Quantification
The color of the fruit was assessed visually using a grading system ranging from 0 to 5. Enzymatic browning was assessed using pulp and skin from fruits taken at various ripening conditions. The color coordinates were recorded on the surface of the fruits using a color reader. The total color difference (ΔE) was calculated using the following equation:
where L* is the degree of lightness to darkness, a* is the degree of redness to greenness, b* is the degree of yellowness to blueness, and the subscripts f and I denote the final and initial values, respectively [22].
ΔE = √ (L*f − L*i)2 + (a*f − a*i)2 + (b*f − b*i)2
Chroma = ((a*)2 + (b*)2)0.5
Hue Angle = tan−1 (b*/a*)
2.5. Degree of Browning
The degree of browning was determined using the Brandelli and Lopes [14] method with slight modifications. Ten grams of terap sample was homogenized with 20 mL of distilled water and centrifuged at 800× g for 10 min. After 1 h, 1.5 mL ethanol was added to 1.0 mL of supernatant to the sample in the centrifuge, shaken, and recentrifuged again at 800× g for 10 min. A UV–VIS spectrophotometer (UNICO, Fairfield, NJ, USA) was used to measure the absorbance at 440 nm to evaluate the degree of browning.
2.6. Enzymatic Activities
Ten grams of peel were weighed and homogenized in an ice-cold 0.2 M phosphate buffer with a pH of 7.0 and 1 M NaCl. The supernatants were centrifuged at 10,000× g for 20 min at 4 °C. The supernatants were kept at 4 °C prior to PAL, PPO, and POD determination. The phenylalanine ammonia-lyase (EC 4.3.1.5) was measured following the protocol of Peixoto et al. [23]. The absorbance was read at 290 nm using a spectrophotometer, and the results were expressed in unit moles min−1. The polyphenol oxidase (PPO-EC 1.14.18.1) activity was determined based on the methodology described by Cano et al. [24], and the absorbance was read at 395 nm. The results were expressed in the unit of μmoles transformed into catechol min−1 g−1. The peroxidase content (POD-EC 1.11.1.9) was recorded at 505 nm, and the results were expressed in unit moles of decomposed H2O2 min−1 g−1 [25].
2.7. Phytochemical Analyses
Fifty grams of the peel sample was blended with 100 mL of 75% ethanol and boiled for 5 min under a hotplate. The residue was then heated for 10 min with an additional 100 mL of the 75% ethanol solution to re-extract the phytochemical compounds. The final volume was made up to 250 mL [14]. The extract was used for phytochemical determination. Total phenolic content (TPC) was determined spectrophotometrically using the Folin–Ciocalteu method [26]. The absorbance was measured at 740 nm and expressed as µg gallic acid equivalent (GAE) g−1. The Brandelli and Lopes [14] method was used to determine the chlorogenic acid (CA). The chlorogenic acid concentration was evaluated using a standard curve (10–50 µg g−1) and expressed as µg chlorogenic acid equivalent g−1.
2.8. Statistical Analyses
All the experiments were carried out in triplicate. The SAS 9.4 software was used to analyze the data. Single-factor analysis of variance (ANOVA) was used to compare the means with a post hoc Tukey’s test (p < 0.05), which was used to compare the mean values of A. odoratissimus peel for the degree of browning, enzymatic activities, and phytochemical contents. For browning activities and phytochemical correlation, raw data were used to calculate the Pearson correlation coefficient to model the relationship.
3. Results and Discussion
3.1. Physical Characterization Changes of Artocarpus odoratissimus during Postharvest Ripening
Physiological and structural changes happen when the fruit ripens, resulting in fruit softening, respiration, and the breakdown of chlorophyll [27]. These physical parameters frequently determine the quality and consumer acceptability of A. odoratissimus fruits. The postharvest treatment has a comparable effect on fruit weight, weight loss, firmness, and gas production. However, the fruit size remained constant during storage. The changes in fruit weight, firmness, and gas production resulting from the various postharvest treatments are presented in Table 2. Fruits stored at a cooler temperature (CT) lost less water than fruits stored at room temperature (RT). Among all treatments, fruits stored in RT without packaging resulted in a significantly higher weight loss (p < 0.05) on day 4 (T1) at 12.20 ± 0.19%, followed by on day 8 (T5) at 11.09 ± 0.24%. A decrease in weight was also reported in the fruits stored with the packaging on day 4 (T2) at 10.57 ± 0.11% and subsequently on day 8 (T6) at 10.08 ± 0.11%.

Table 2.
Physical characterization of Artocarpus odoratissimus fruits at different postharvest treatment.
Comparatively, fruits stored at CT showed a slower decline in weight loss from day 4 (8.52 ± 0.14%) to day 16 (5.02 ± 0.05%), and a similar trend was observed for the fruits without packaging from 9.06 ± 0.09% on day 4 to 4.93 ± 0.08% on day 16. Higher weight loss under RT was associated with a higher respiration rate with storage times, causing water to evaporate from the fruit’s surface [28]. Furthermore, the RT storage condition with high humidity gradually influenced the fruit’s water content release. This demonstrates that the A. odoratissimus fruits did not have a shelf-life of more than eight days when stored at room temperature. However, the slower decline in weight loss during postharvest ripening influenced by the lower temperature storage caused the physiological activities to be lower [29]. Cold storage extends the shelf-life of A. odoratissimus fruits by up to 16 days. This finding is consistent with Maniwara et al. [30] and Kan et al. [3] in passion fruit and pear, respectively, where cold storage can extend the fruit shelf-life over storage.
The decrease in weight loss is an important parameter in determining fruit firmness. According to Sajid et al. [31] firmness is one of the most important characteristics that consumers look for. Because A. odoratissimus is a climacteric fruit, the fruit can ripen quickly after being harvested from the tree. According to this finding, the packaged fruits stored in RT had the lowest firmness. The firmness of the packaged fruits decreased by twice the value from 3.10 ± 0.20 N on day 4 (T2) to 1.76 ± 0.35 N on day 8 (T6). This is followed by the fruits kept without packaging, where the firmness decreased from day 4 (T1) at 4.13 ± 0.30 N to day 8 (T5) at 3.40 ± 0.30 N. This finding was comparable with the fruits stored in CT, where a slower declining trend/maintenance in firmness was observed. The lowest firmness in RT was primarily due to decreased pectin content, the reduced cell that affects turgor pressure, and osmosis with moisture concentration from the environment [32], as well as softening of the membrane as a result of evaporation from the fruit [33]. As a result, storing fruits at a lower temperature can preserve fruit firmness and extend the storage life by slowing down the biological reaction rate [34].
An increase in weight loss and a decrease in firmness during storage was associated with the rate of respiration [35]. The respiration rate was higher in the fruits stored at RT than at CT. At RT, the O2 rate was higher in the packaged fruits at day 4 (T2) with 18.00 ± 0.40 mL O2 kg h−1 and decreased on day 8 (T6) to 13.80 ± 0.95 mL O2 kg h−1. The fruits without packaging stored at RT also showed a decrease in oxygen production from day 4 (T3) (17.80 ± 0.30 mL O2 kg h−1) to day 8 (T5) (13.90 ± 0.78 mL O2 kg h−1). Meanwhile, the CO2 concentration in the packaged fruits increased from day 4 (T2) (368.66 ± 5.13 mL CO2 kg h−1) to day 8 (T6) (487.00 ± 8.22 mL CO2 kg h−1), whereas CO2 concentration in the control fruits also increased on day 4 (T1) (352.33 ± 7.02 mL CO2 kg h−1) to day 8 (T5) (466.33 ± 7.93 mL CO2 kg h−1).
Likewise, storing the fruits at 10 °C reduces the gas production of O2 and CO2. Fruits stored with packaging collected on day 4 had a higher O2 release rate in T4 (11.50 ± 0.79 mL O2 kg h−1) and decreased on day 16 in T12 (7.50 ± 0.55 mL O2 kg h−1). Concurrently, CO2 production was higher on day 4 in T4 (204.00 ± 7.45 mL CO2 kg h−1) and decreased on day 16 in T12 at 139.33 ± 9.06 mL CO2 kg h−1. Fruits collected on day 4 (T3) had a higher O2 rate of 11.33 ± 0.40 mL O2 kg h−1 under low-temperature storage and gradually declined on day 16 (T11) at 7.80 ± 0.65 mL O2 kg h−1. Meanwhile, CO2 release was also higher in packaged fruits on day 4 (T4) at 204.00 ± 7.45 mL CO2 kg h−1 and slightly increased when collected on day 16 in T12 (139.33 ± 9.06 mL CO2 kg h−1).
Fruits stored under 25 °C had a higher respiration rate, particularly the packaged fruit, which is influenced by water excess accumulation inside the packaging, causing the skins of the fruit to turn brown and produce a foul odor due to the degradation of chlorophyll fruit senescence [36]. According to Rattanakran et al. [37], higher temperatures increased the gas exchange rate, making the fruits more susceptible to water loss and deterioration. Hence, lower temperatures slow respiration and prevent fruit softening [28,38]. Conclusively, the lower temperature storage at 10 °C increases the shelf-life of A. odoratissimus fruits by up to 16 days when compared to ambient temperature storage (sustained for 8 days).
3.2. Peel Color Changes of Artocarpus odoratissimus during Postharvest Ripening
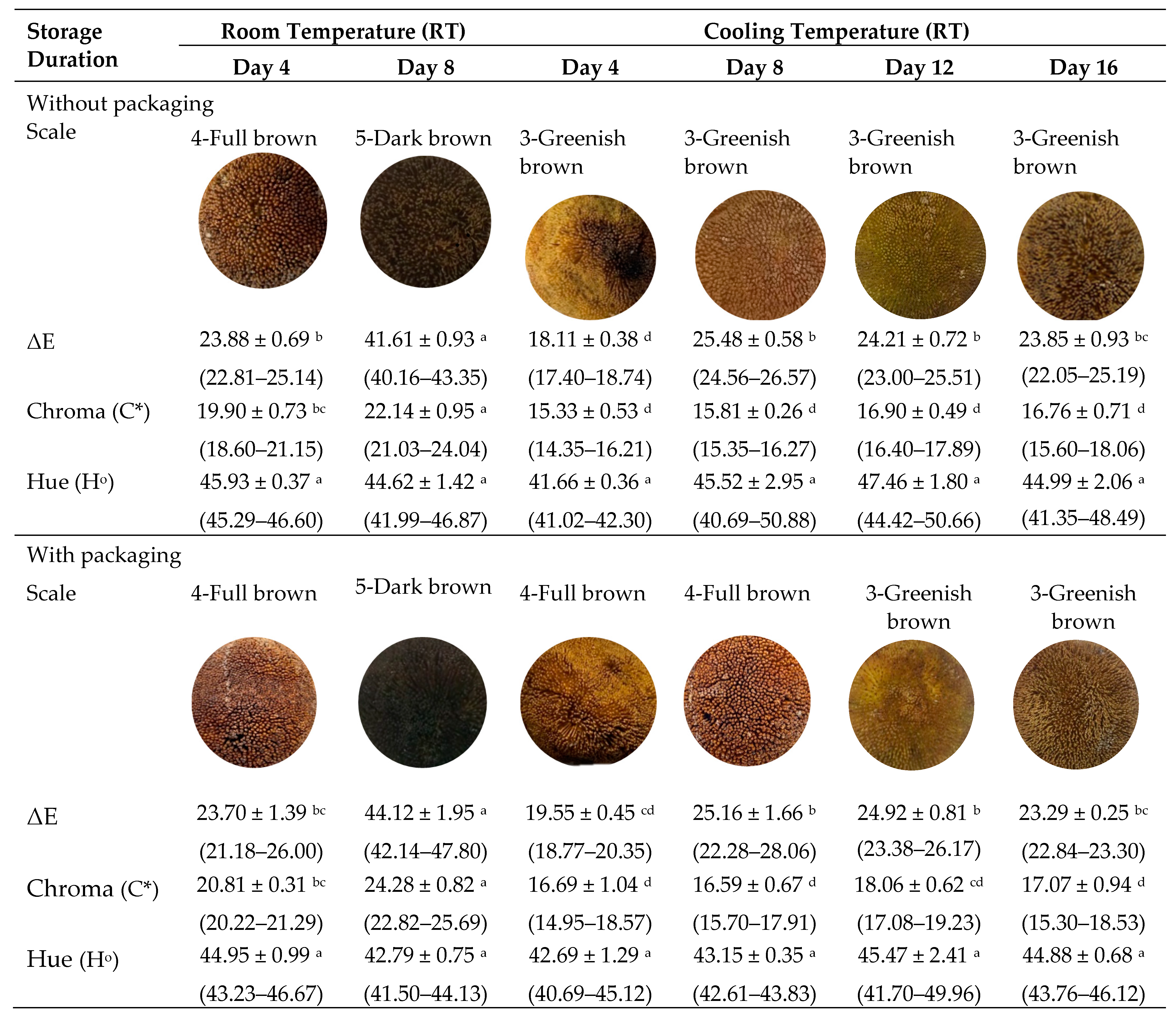
Color is an important quality parameter that has a direct impact on the appearance and acceptability of food products. The increase in L*, a*, b*, total color change (ΔE), chroma (C*), and hue (Ho) values are influenced by the carotenoids and other phytochemicals produced in the fruits [39]. The peel color (L*, a*, b*) of A. odoratissimus changed significantly (p < 0.05) over the course of the two weeks of storage duration (Figure 1). An increase in redness (a*) and yellowness (b*), and darkness (L*) values indicated that the A. odoratissimus peel under RT was over-ripened compared to CT. Fruits stored at RT had more intense peel browning than CT throughout postharvest storage.

Figure 1.
Peel total color changes (ΔE), chroma (C*) and hue (Ho) values of Artocarpus odoratissimus at various postharvest treatments. Different superscript alphabets indicate differences in the same row (with and without packaging) at p < 0.05 (ANOVA, Tukey’s test). Values are given as means ± standard error, and values in parenthesis are the range.
The higher total color changes (ΔE) were obtained in fruits stored with packaging under RT on day 4 at 23.70 ± 1.39 and were twice as high on day 8 at 44.12 ± 1.95. Fruits stored without packaging also increased their ΔE values from 23.88 ± 0.69 to 41.61 ± 0.93. This finding is comparable to CT storage, where browning activity was still observed on the peel, but its intensity gradually decreased. Fruits stored at a lower temperature on day 4 resulted in an ΔE at 18.11 ± 0.38, which gradually increased to 23.85 ± 0.93 on day 16. A similar trend was observed in the fruit with the packaging on day 4 (19.55 ± 0.45) and day 16 (23.29 ± 0.25). The initially green with yellowish A. odoratissimus fruit peel gradually changed to brown and dark brown upon ripening in the packaging under RT due to the influence of the higher respiration rate, as a result of water clogged inside the packaging [5]. Nambi et al. [22] reported that in mango fruit skin, the ripening process might be accelerated, thus, leading to a more remarkable change in all three-color coordinates. Similarly, Qi et al. [15] found that the pericarp browning increased significantly in pomegranate after 3 days of storage at RT, which was influenced by respiration rate. Thus, the peel color changes in A. odoratissimus fruit are associated with pigment loss during postharvest storage due to increased respiration production.
On the other hand, the chroma (C*) in the control and packaged fruits under RT was higher on day 8. Fruits with packaging (T6) had a higher chroma value of 24.28 ± 0.82, while the control (T5) had a value of 22.14 ± 0.95. Meanwhile, cold storage at 10 °C resulted in lower chroma values in fruit packaging and the control throughout the storage period. The present findings are consistent with those of Al-Waili et al. [40] in mango and Venkatachalam [41] in longkong fruit. Color variation in fruit was also observed using the hue angle (Ho). According to the findings, the A. odoratissimus peel color for hue angle rapidly declined in the control of the packaging treatments stored at RT. The hue angle trend in the packaged fruits was higher on day 4 but decreased slightly on day 8 (42.79 ± 0.75) in the packaged fruits compared to the control fruit at T5 (44.62 ± 1.42). Decreased hue angle resulted in the A. odoratissimus peel color changing from green to brown [42]. Sommano et al. [16] discovered that the hue angle decreased when it reached a maximum of 8 days of storage. The decrease in the hue angle was influenced by the packaging and rate of respiration during storage, which affects the appearance of the fruit over longer periods of time. In conclusion, storing fruit at low temperatures causes the peel browning to be lower and the fruits to take longer to ripen than the fruits stored at higher temperatures.
3.3. Enzyme Activities Associated with Browning in Artocarpus odoratissimus Peel at Different Postharvest Treatments
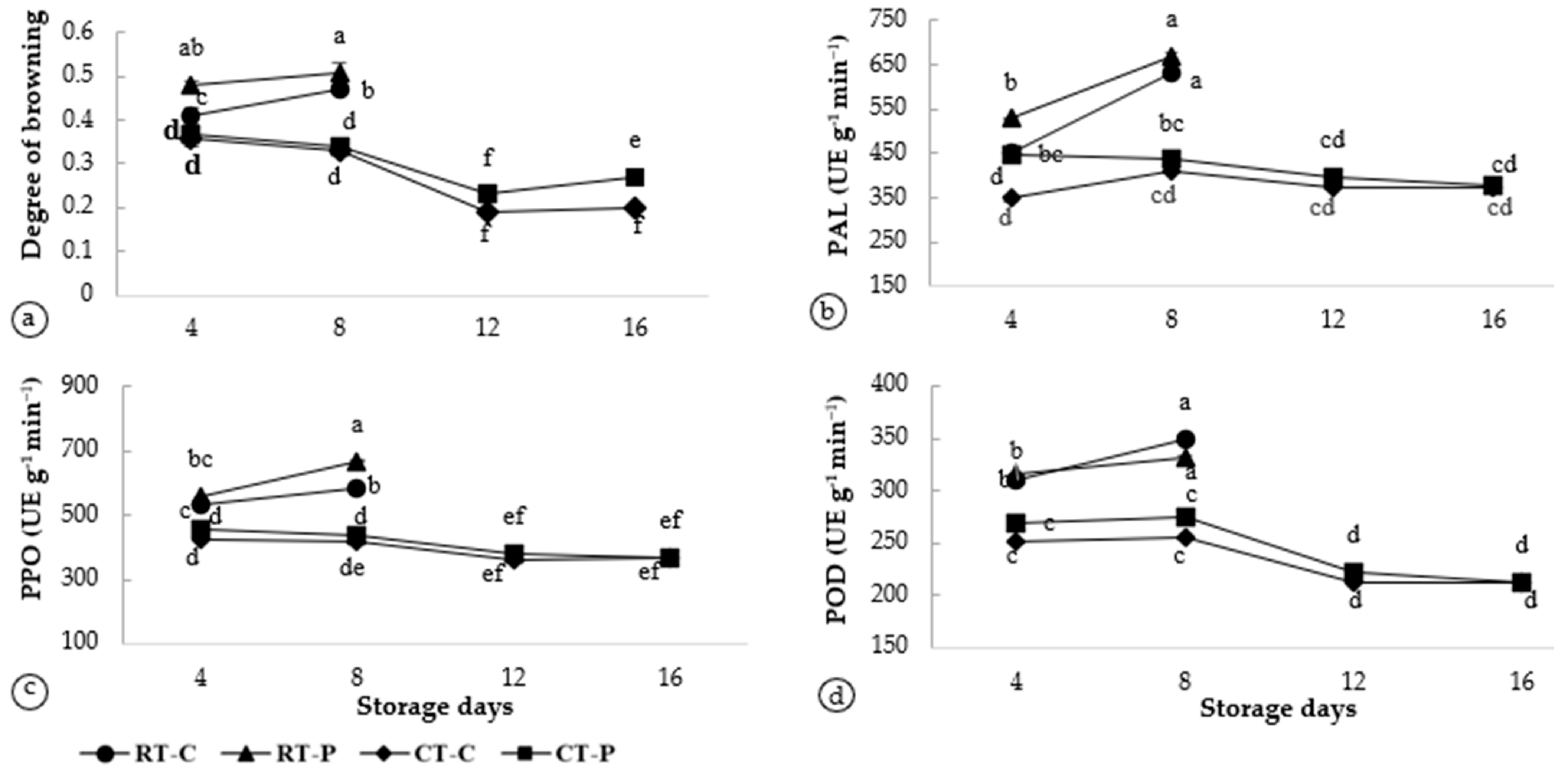
Browning is a significant problem in the fruit industry and is regarded as the primary cause of quality loss during postharvest handling and processing. The enzymatic activity associated with browning in A. odoratissimus peel at different postharvest treatments, including the degree of browning, phenylalanine ammonia-lyase (PAL), polyphenol oxidase (PPO), and peroxidase (POD), is presented in Figure 2.

Figure 2.
Enzyme activities associated with browning in Artocarpus odoratissimus fruit peel at different postharvest treatments. (a) Degree of browning, (b) PAL, (c) PPO, and (d) POD. Different superscript alphabets indicate differences at p < 0.05 (ANOVA, Tukey’s test. RT-C (room temperature and without packaging/control), RT-P (room temperature and with packaging), CT-C (cool temperature and without packaging/control), and CT-P (cool temperature and with packaging).
The degree of browning was used to determine the browning rate potential in the fruit peel at various postharvest treatments. Fruit stored at room temperature, with or without packaging, showed the greatest degree of browning in response to changes in physical characterization. Based on Figure 2a, the packaged fruits had a significantly higher degree of browning on day 4 in T2 at 0.48 ± 0.01 which increased slightly up to day 8 in T6 at 0.51 ± 0.02. A similar trend was also obtained in the control fruits on day 4 in T1 at 0.41 ± 0.02 and increased on day 8 in T5 at 0.47 ± 0.01. This was comparable to the CT, where the fruit stored in control had more browning on day 4 in T3 (0.46 ± 0.50) but less on day 8 in T7 (0.33 ± 0.01) and day 12 in T9 (0.19 ± 0.01), followed by a slight increase on day 16 in T11 (0.20 ± 0.01). A similar trend was also observed in packaged fruit, with the degree of browning was 0.37 ± 0.01 on day 4 (T4), decreasing to 0.34 ± 0.01 on day 8 (T8), and at 0.23 ± 0.01 on day 12 (T10). However, it increased to 0.27 ± 0.01 on day 16 (T12). Mphahlele et al. [33] and Dogan [35] reported that storage temperature has an effect on respiration rate, especially in packaging. Package perforations accelerate the process of fruit deterioration due to high water vapor permeability by increasing the absorption of water from the packaged product via evaporation. A comparable result was obtained in peach [14] and pomegranate fruits [43]. However, either with or without packaging, storing in the CT reduces the browning potential of the fruit. Cool storage decreased the browning potential in A. odoratissimus up to day 16 compared to RT, which could only last 8 days.
The enzymatic activities of phenylalanine ammonia-lyase (PAL), polyphenol oxidase (PPO), and peroxidase (POD) in A. odoratissimus peel were studied to better understand their relationship with the browning activities. Here, PAL is the main enzyme that contributes to lignin cell wall deposition by catalyzing the reaction in the phenylpropanoid biosynthesis pathway [44]. In this study, the PAL activity was significantly higher (p < 0.05) in the RT for fruits stored with the packaging on day 4 in T2 (528.66 ± 0.98 UE g−1 min−1), and increased on day 8 in T6 (671.00 ± 5.25 UE g−1 min−1) compared to the control fruits on day 4 (T1) at 453.33 ± 1.25 UE g−1 min−1 to day 8 (T5) at 633.66 ± 5.17 UE g−1 min−1. However, compared to CT, the PAL activity was also higher in the packaged fruits on day 4 (T4) at 445.66 ± 3.88 UE g−1 min−1 but reduced on day 16 (T12) at 378.00 ± 0.92 UE g−1 min−1 (Figure 2b). This demonstrates that temperature and packaging method both influenced an increase in PAL activity. Fruits with high-temperature packaging trap the vapor inside, causing excess water accumulation injuries in the tissues. According to Vitti et al. [6], increased PAL activity stimulates the exposure of phenolic compound concentration, which are reagents for key enzymes, such as PPO and POD.
Similarly, PPO activity increased gradually with storage time under RT. Figure 2c shows the increasing trend of the packaged fruits from day 4 (T2) (557.00 ± 0.60 UE g−1 min−1) to day 8 (T6) (670.00 ± 2.56 UE g−1 min−1) and the control fruit on day 4 (T1) (530.00 ± 0.81 UE g−1 min−1) to day 8 (T5) (586.66 ± 2.33 UE g−1 min−1). Contradicting this, fruits stored in the CT showed a decreasing trend for fruit stored with the packaging as follows: day 4 (T4) at (456.66 ± 1.21 UE g−1 min−1) > day 8 (T8) at 436.66 ± 2.88 UE g−1 min−1 > day 12 (T10) (380.00 ± 0.53 UE g−1 min−1) > day 16 (T12) (370.00 ± 0.53 UE g−1 min−1). Control fruit also exhibited a similar pattern where PPO concentration was 423.66 ± 1.25 UE g−1 min−1 on day 4 (T3) and decreased to 416.66 ± 2.07 UE g−1 min−1 on day 8 (T7) > day 12 (T9) (363.33 ± 1.21 UE g−1 min−1) > day 16 (T11) (366.66 ± 0.81 UE g−1 min−1). This shows that the PPO gradually increased with the increasing storage times. Gandhi et al. [45] and Camargo et al. 45] emphasize that PPO is responsible for the enzymatic browning oxidation that occurs during the handling, preservation, and manufacturing of fruits and vegetables, resulting in gradual discoloration throughout ripening. Hence, this enzyme catalyzes the oxidation of polyphenol substances in raw materials, producing substances that change color as well as sensory and nutritional properties [46].
Consequently, the changes in POD activity are similar to those observed in PAL and PPO enzymatic activities. Based on Figure 2d, fruits stored without packaging had higher POD activity from day 4 to day 8 in the T1 (310.33 ± 0.57 UE g−1 min−1) to T5 (350.00 ± 2.16 UE g−1 min−1). This was followed by packaged fruits in the T2 (315.33 ± 1.09 UE g−1 min−1) and T6 (330.66 ± 2.16 UE g−1 min−1). Meanwhile, fruits stored at 10 °C demonstrated decreasing POD activity in the packaging from day 4 (T4) (270.00 ± 1.26 UE g−1 min−1) to day 16 (T12) (213.00 ± 0.15 UE g−1 min−1). From the observation, an increase in the POD activity resulted in the general deterioration of fruit quality, particularly the enzymatic browning of fruits [11]. This discovery also demonstrated that an increase in POD activity was associated with increased lignification during postharvest storage [40]. Hence, the increase in the POD activity enhanced the oxidation of the enzymatic activity in response to stress conditions.
3.4. Phytochemical Properties Associated with Browning in Artocarpus odoratissimus Peel at Postharvest Treatments
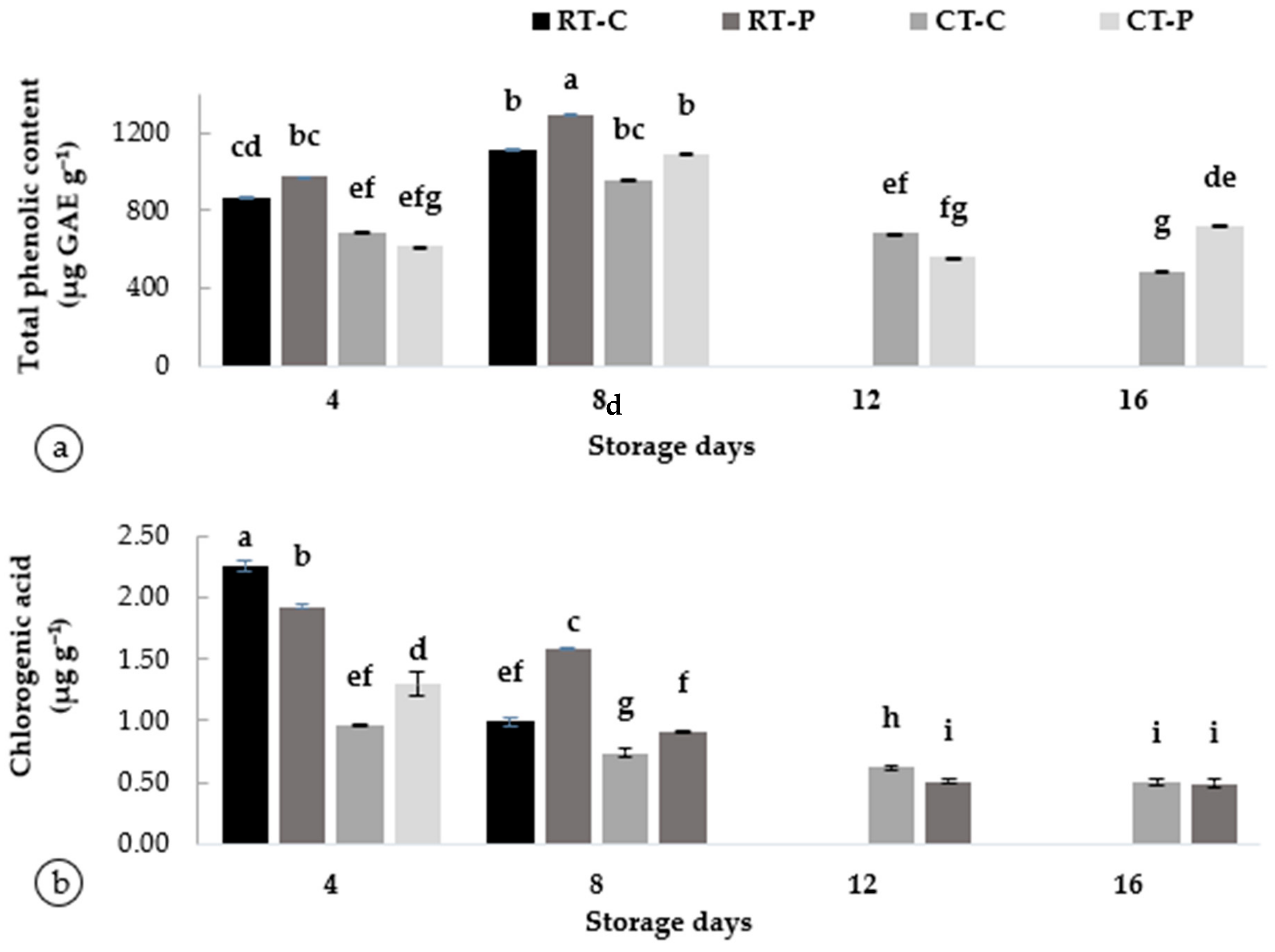
The phytochemical content was analyzed to determine whether the chemical variables are associated with the fruits’ peel browning during postharvest treatments. The phytochemicals, such as TPC and CA, in A. odoratissimus peel in response to varying postharvest changes are presented in Figure 3. Phenolic compounds are secondary metabolites of plants; they not only have pharmacological activities but are plant antitoxins.

Figure 3.
Phytochemical properties: (a) TPC and (b) TFC associated with browning in Artocarpus odoratissimus fruit peel. Different superscript alphabets indicate differences at p < 0.05 (ANOVA, Tukey’s test. Abbreviations are as follows: RT-C (room temperature and without packaging/control), RT-P (room temperature and with packaging), CT-C (cool temperature and without packaging/control), and CT-P (cool temperature and with packaging).
The TPC in the peel of A. odoratissimus was determined by an oxidation–reduction reaction using Folic–Ciocalteu reagent. The TPC levels were higher on day 4 in RT on fruit stored with packaging (975.07 ± 1.23 µg GAE g−1) and increased rapidly throughout the maximum on day 8 (T6) at 1297.20 ± 1.61 µg GAE g−1. A similar pattern was observed in the control fruits, where TPC increased from day 4 (T1) (869.74 ± 5.35 µg GAE g−1) and T5 (1111.70 ± 3.68 µg GAE g−1), respectively. As shown in Figure 3a, fruits were stored under CT, and the TPC was initially higher on day 4 (T3) (688.39 ± 1.07 µg GAE g−1 and increased on day 8 (T7) (959.77 ± 2.33 µg GAE g−1); however, it was reduced on day 12 (T9) (683.04 ± 1.68 µg GAE g−1) and day 16 (T11) (489.20 ± 0.57 µg GAE g−1). The metabolic capacity of tissue decreases during storage periods. This caused the contents of phenolic compounds to begin to decrease after day 8. The lower TPC is due to the synthesis of phenolic compounds being inhibited by low temperatures [47,48].
This parameter indicates that the enzymatic activity influenced the phenolic content in fruit peel to form fully darkened, as reported by Vitti et al. [6]. High temperatures may have stimulated plant tissues and accelerated the rate of life metabolism. Plant respiration was aggravated, and the tissues showed severe physiological phenomena and damage [15], which provided conditions for oxygen to enter cells, and the accumulation of phenolic compounds provided a sufficient substrate for PPO [47]. As a result, this enzyme catalyzes the oxidation of polyphenol substances in raw materials, producing substances that change the color of the peel. However, storing products under low temperatures reduced the browning activity. This could be due to the low life activity of the plant tissue and the stable protein structure, which reduces cell tissue damage and also the oxidation of polyphenol substances. The low temperature inhibited the increase in total polyphenol content by inhibiting plant vitality [48].
As for the chlorogenic acid (Figure 3b), the A. odoratissimus fruit that was kept under RT had higher chlorogenic acid on day 4 in T1 (2.26 ± 0.04 µg g−1) but decreased on day 8 in T5 (0.96 ± 0.01 µg g−1). Similar changes were also obtained in treatment with packaging that the chlorogenic acid was reduced during the ripening period from day 4 (T2) (1.93 ± 0.02 µg g−1) to day 8 (T6) (1.30 ± 0.09 µg g−1). Meanwhile, storage under CT also showed a slight decrease in the chlorogenic acid content from day 4 to day 16. For instance, fruits stored with packaging possessed significantly lower chlorogenic acid values (p < 0.05) on day 16 0.49 ± 0.03 µg g−1 (packaging) and 0.50 ± 0.02 µg g−1 (without packaging). Chlorogenic acid was detected as the major hydroxycinnamic acid derivative and the decrease in chlorogenic acid during postharvest storage influenced the rate of PPO activity [17].
3.5. Correlation between Chemical Constituents of Artocarpus odoratissimus Peel
The Pearson correlation revealed a significant and strong correlation (p < 0.05) between some of the variables in A. odoratissimus peel chemical contents and browning activities (Table 3). Weight loss has a significant correlation with most of the parameters. The increased weight loss was strongly correlated with the decrease in the firmness of A. odoratissimus fruits (r = −0.670). The increase in weight loss was also related to the rate of respiration, which increased the O2 (r = 0.716) and CO2 (r = 0.750). Furthermore, a strong correlation was found between weight loss and the degree of browning (r = 0.816). Khan et al. [49] discovered a similar finding in longan, where weight loss has a positive correlation with browning (r = 0.990). The rapid respiration rate influences the higher weight loss in fruit, increasing the possibility of browning due to the increased oxidation. Furthermore, a strong positive correlation was obtained between weight loss and the PPO (r = 0.758), as opposed to the PAL and POD at r = 0.616 and r = 0.595, respectively.

Table 3.
Correlation between chemical constituents of Artocarpus odoratissimus fruit peel.
The degree of browning is also associated with enzymatic activities. A strong positive correlation was found between the degree of browning with PAL (r = 0.791) and PPO (r = 0.896), and a moderate positive correlation was found with POD (r = 0.681). Ding and Yap [50] studied the browning of bananas with PPO based on color space. The authors discovered a strong positive correlation between browning and PPO activity (r = 0.930). This suggests that browning in A. odoratissimus peel was caused by enzymatic activity. The degree of browning, on the other hand, was strongly correlated with TPC content (r = 0.754). A strong correlation between PPO and TPC (r = 0.810) and TFC (r = 0.927) was also observed in the skin. The presence of PPO influenced the phytochemical activity at high temperatures during the storage, causing the browning of the fruit’s peel.
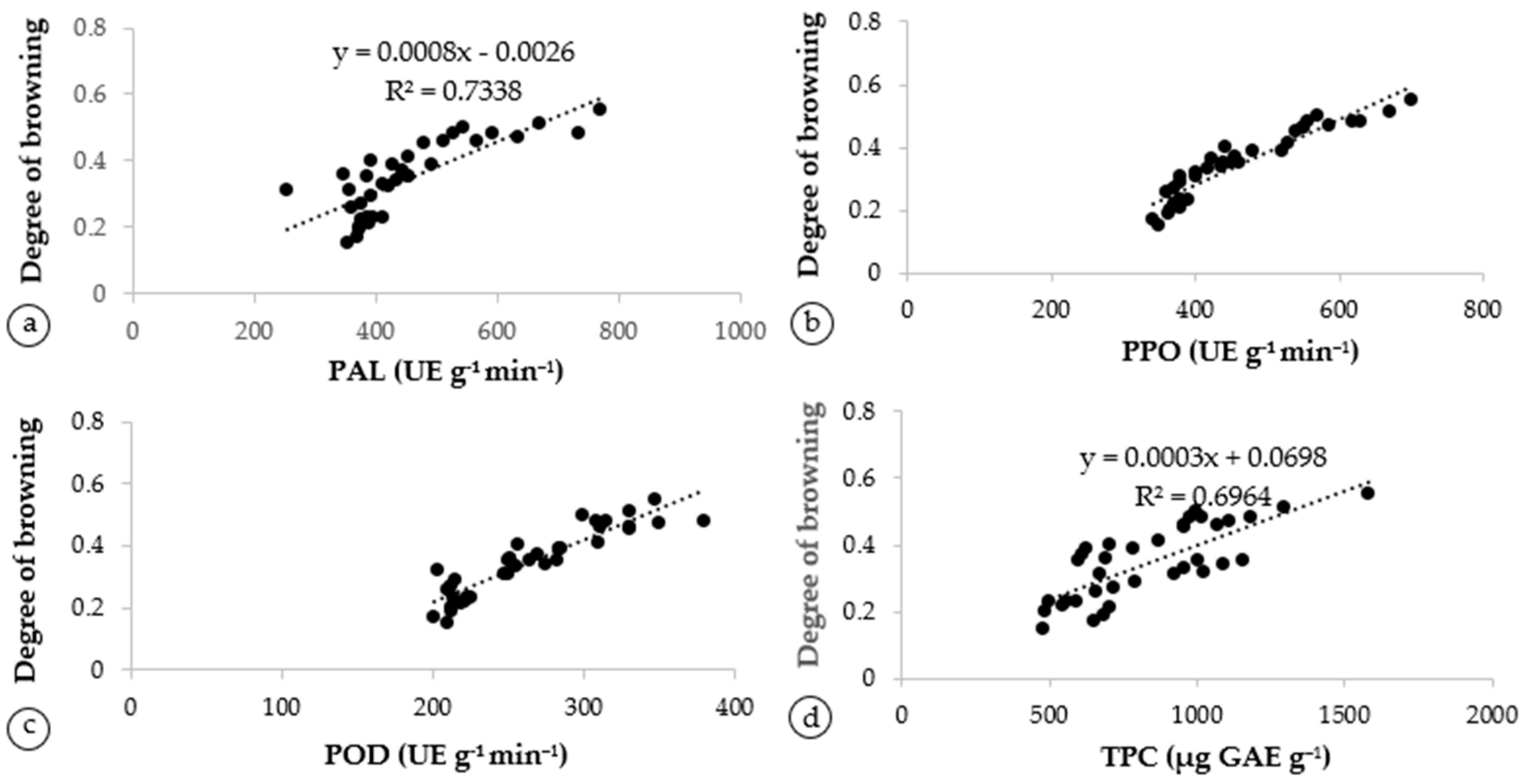
The various correlated attributes were further subject to regression analysis to calculate the regression equation between the degree of browning and polyphenoloxidase shown in Figure 4. There was a highly significant positive correlation between the degree of browning and PAL activity (R2 = 0.734) (Figure 4a) and a significantly positive correlation between the degree of browning and PPO (R2 = 0.876) (Figure 4b). Furthermore, the degree of browning was strongly correlated to the POD (R2 = 0.835) (Figure 4c). Overall, the rapid browning of the fruit’s peel was caused by the higher PAL, PPO, and POD concentrations due to higher storage temperatures. The TPC was found to be related to the degree of browning (R2 = 0.696) (Figure 4d). This suggests that polyphenol oxidase is a key enzyme in the oxidation of phenolics to quinones and the formation of brown pigments [6,7].

Figure 4.
Linear regression between chemical constituents of Artocarpus odoratissimus fruit peel, (a) degree of browning with PAL, (b) degree of browning with PPO and (c) degree of browning with POD and (d) degree of browning and TPC.
4. Conclusions
The current study found that A. odoratissimus fruit can only be stored at 25 °C for up to 8 days. The packaging (5% poly-perforated bag) does not help to extend the shelf-life of the fruit because of water accumulation caused by a higher respiration rate trapping the vapour inside, resulting in rapid deterioration. Contrary, fruit stored at 10 °C can extend its shelf-life by up to 16 days, with or without packaging. Browning in A. odoratissmus peel was strongly influenced by PPO activity and phenolic content. The degree of browning was found to be positively related to enzymatic and phytochemical factors. This suggests that PPO catalyzes the oxidation reaction of phenol compounds in A. odoratissimus peel tissue browning. Fruit stored at a cool temperature had a lower incidence of browning. Cold storage improved external quality attributes and extended shelf-life without decay, resulting in lower postharvest losses.
Author Contributions
H.A.I. and I.R. were the leading researchers for this project who contributed to the experiment setup, data collection, and interpretation of the results. They have taken the lead in writing the manuscript. S.D.R. conceived the presented idea, provided critical feedback, and helped shape the research, analysis, and manuscript writing. M.H.Z. and S.Y.L. helped supervise the project, provided critical feedback, and helped shape the research, analysis, and manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was granted with funding support by Sarawak Research Development Council (SRDC) under RDCRG/RIF/2019/15.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are thankful to Universiti Putra Malaysia for the facilities and support provided for this project and we gratefully acknowledge the professional and valuable comments of the editor and the reviewers.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Kitinoja, L.; Kader, A.A. Measuring postharvest losses of fresh fruits and vegetables in developing countries. PEF White Paper 2015, 15, 1–26. [Google Scholar] [CrossRef]
- Department of Statistics Malaysia. Available online: https://www.dosm.gov.my/v1/index.php?r=column/cthemeByCat&cat=164&bul_id=Tm5OaVh6RFpFM2VGOTIrZzltbWg3QT09&menu_id=Z0VTZGU1UHBUT1VJMFlpaXRRR0xpdz09 (accessed on 20 January 2022).
- Kan, C.; Gao, Y.; Wan, C.; Chen, M.; Zhao, X.; Liu, S.; Chen, J. Influence of different cold storage times on quality of ‘Cuiguan’ pear fruits during shelf life. J. Food Process. Preserv. 2019, 43, e14245. [Google Scholar] [CrossRef]
- Lufu, R.; Ambaw, A.; Opara, U.L. Water loss of fresh fruit: Influencing pre-harvest, harvest and postharvest factors. Sci. Hortic. 2020, 272, 109519. [Google Scholar] [CrossRef]
- Hussain, I.; Rab, A.; Khan, S.M.; Naveed, K.; Ali, S.; Hussain, I.; Sajid, M. Physiochemical changes in oranges during different storage durations and temperatures. Pure Appl. Biol. 2017, 6, 394–401. [Google Scholar] [CrossRef]
- Vitti, M.C.D.; Sasaki, F.F.; Miguel, P.; Kluge, R.A.; Moretti, C.L. Activity of enzymes associated with the enzymatic browning of minimally processed potatoes. Braz. Arch. Biol. Technol. 2011, 54, 983–990. [Google Scholar] [CrossRef]
- Ioannou, I.; Ghoul, M. Prevention of enzymatic browning in fruit and vegetables. Eur. J. Sci. 2013, 9, 310–341. [Google Scholar] [CrossRef]
- Saltveit, M.E. Wound induced changes in phenolic metabolism and tissue browning are altered by heat shock. Postharvest Biol. Technol. 2000, 21, 61–69. [Google Scholar] [CrossRef]
- Glagoleva, A.Y.; Shoeva, O.Y.; Khlestkina, E.K. Melanin pigment in plants: Current knowledge and future perspectives. Front. Plant Sci. 2020, 11, 770. [Google Scholar] [CrossRef]
- Moon, K.M.; Kwon, E.B.; Lee, B.; Kim, C.Y. Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef]
- Singh, B.; Suri, K.; Shevkani, K.; Kaur, A.; Kaur, A.; Singh, N. Enzymatic browning of fruit and vegetables: A review. In Enzymes in Food Technology; Academic Press: Cambridge, MA, USA, 2018; pp. 63–78. [Google Scholar] [CrossRef]
- Cukrov, D. Progress toward understanding the molecular basis of fruit response to hypoxia. Plants 2018, 7, 78. [Google Scholar] [CrossRef]
- Mellidou, I.; Buts, K.; Hatoum, D.; Ho, Q.T.; Johnston, J.W.; Watkins, C.B.; Schaffer, R.J.; Gapper, N.E.; Giovannoni, J.J.; Rudell, D.R. Transcriptomics events associated with internal browning of apple during postharvest storage. BMC Plant Biol. 2014, 14, 328. [Google Scholar] [CrossRef]
- Brandelli, A.; Lopes, C.H. Polyphenoloxidase activity, browning potential and phenolic content of peaches during postharvest ripening. J. Food Biochem. 2005, 29, 624–637. [Google Scholar] [CrossRef]
- Qi, X.; Zhao, J.; Jia, Z.; Cao, Z.; Liu, C.; Li, J.; Su, Y.; Pan, Y.; He, C.; Xu, Y.; et al. Potential metabolic pathways and related processes involved in pericarp browning for postharvest pomegranate fruits. Horticulturae 2022, 8, 924. [Google Scholar] [CrossRef]
- Sommano, S.; Kanphet, N.; Siritana, D.; Ittipunya, P. Correlation between browning index and browning parameters during the senescence of longan peel. Int. J. Fruit Sci. 2011, 11, 197–205. [Google Scholar] [CrossRef]
- Vella, F.M.; Calandrelli, R.; Laratta, B. Influence of ripening on polyphenolic content, degradative, and browning enzymes in Cantaloupe varieties. Horticulturae 2021, 7, 421. [Google Scholar] [CrossRef]
- Bakar, F.I.A.; Bakar, M.F.A. odoratissimus. In Exotic Fruits; Academic Press: Cambridge, MA, USA, 2018; pp. 413–418. [Google Scholar] [CrossRef]
- Shaffiq, S.; Sidik, B.J.; Harah, Z.M.; Devi, R.S. Marketable wild fruits of Sarawak, Borneo: Their mode of consumption, uses and sugar profiles. Indian J. Tradit. Knowl. 2013, 12, 195–201. [Google Scholar]
- Naspiah, N.; Pratama, M.R.F. Xanthine oxidase inhibition activity and ADMET properties of terap (Artocarpus odoratissimus Blanco) leaves metabolites: Phytochemical screening and in silico studies. Pharmacogn. J. 2021, 13, 1150–1160. [Google Scholar] [CrossRef]
- Lim, T.K. Artocarpus odoratissimus. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Nambi, V.E.; Thangavel, K.; Shahir, S.; Chandrasekar, V. Color kinetics during ripening of Indian mangoes. Int. J. Food Prop. 2016, 19, 2147–2155. [Google Scholar] [CrossRef]
- Peixoto, P.H.P.; Cambraia, J.; Sant’Anna, R.; Mosquim, P.R.; Moreira, M.A. Aluminum effects on lipid peroxidation and on the activities of enzymes of oxidative metabolism in sorghum. Rev. Bras. Fisiol. Veg. 1999, 11, 137–143. [Google Scholar]
- Cano, M.P.; de Ancos, B.; Matallana, M.C.; Cámara, M.; Reglero, G.; Tabera, J. Differences among Spanish and Latin-American banana cultivars: Morphological, chemical and sensory characteristics. Food Chem. 1997, 59, 411–419. [Google Scholar] [CrossRef]
- Rossi, C.; Lima, G.P.P.; Hakvoort, D.M.R. Ativiade de Peroxidases e teor de prolina em feijoeiro Phaseolus vulgaris L. Cultivado em condições de salinidade. Sci. Agric. 1997, 54, 123–127. [Google Scholar] [CrossRef]
- Ramaiya, S.D.; Lee, H.H.; Xiao, Y.J.; Shahbani, N.S.; Zakaria, M.H.; Bujang, J.S. Organic cultivation practices enhanced antioxidant activities and secondary metabolites in giant granadilla (Passiflora quadrangularis L.). PLoS ONE 2021, 16, e0255059. [Google Scholar] [CrossRef] [PubMed]
- Shewa, A.G.; Gobena, D.A.; Ali, M.K. Review on postharvest quality and handling of apple. J. Agric. Sc. Food Technol. 2022, 8, 028. [Google Scholar] [CrossRef]
- Rahmadhanni, D.S.D.; Reswandha, R.; Rahayoe, S.; Bintoro, N.; Prasetyatama, Y.D.; Karyadi, J.N.W. The effect of cold storage temperatures on respiration rate and physical quality of crownless pineapple (Ananas comosus L.). IOP Conf. Ser. Earth Environ. Sci. 2020, 542, 012006. [Google Scholar] [CrossRef]
- Perez-Lopez, U.; Sgherri, C.; Miranda-Apodaca, J.; Micaelli, F.; Lacuesta, M.; Mena-Petite, A.; Quartacci, M.F.; Muñoz-Rueda, A. Concentration of phenolic compounds is increased in lettuce grown under high light intensity and elevated CO2. Plant Physiol. Biochem. 2018, 123, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Maniwara, P.; Boonyakiat, D.; Poonlarp, P.B.; Natwichai, J.; Nakano, K. Changes of postharvest quality in passion fruit (Passiflora edulis Sims) under modified atmosphere packaging conditions. Int. Food Res. J. 2015, 22, 1596–1606. Available online: http://www.ifrj.upm.edu.my (accessed on 30 June 2022).
- Sajid, M.; Basit, A.; Ullah, Z.; Shah, S.T.; Ullah, I.; Mohamed, H.I.; Ullah, I. Chitosan-based foliar application modulated the yield and biochemical attributes of peach (Prunus persica L.) cv. Early Grand. Bull. Natl. Res. Cent. 2020, 44, 150. [Google Scholar] [CrossRef]
- Huang, W.; Shi, Y.; Yan, H.; Wang, H.; Wu, D.; Grierson, D.; Chen, K. The calcium-mediated homogalacturonan pectin complexation in cell walls contributes the firmness increase in loquat fruit during postharvest storage. J. Adv. Res. 2022. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Caleb, O.J.; Ngcobo, M.E. Effects of packaging and duration on quality of minimally processed and unpitted litchi cv. ‘Mauritius’ under low storage temperature. Heliyon 2020, 6, 03229. [Google Scholar] [CrossRef]
- Leng, F.; Wang, C.; Sun, L.; Li, P.; Cao, J.; Wang, Y.; Zhang, C.; Sun, C. Effects of different treatments on physicochemical characteristics of ‘Kyoho’ grapes during storage at low temperature. Horticulturae 2022, 8, 94. [Google Scholar] [CrossRef]
- Dogan, A. Effects of different oxygen levels with high-carbon dioxide atmosphere on postharvest quality of fresh fig under Palliflex storage systems. Horticulturae 2022, 8, 353. [Google Scholar] [CrossRef]
- Ding, C.K.; Chachin, K.; Hamauzu, Y.; Ueda, Y.; Imahori, Y. Effects of storage temperatures on physiology and quality of loquat fruit. Postharvest Biol. Technol. 1998, 14, 309–315. [Google Scholar] [CrossRef]
- Rattanakaran, J.; Saengrayap, R.; Prahsarn, C.; Kitazawa, H.; Chaiwong, S. Application of room cooling and thermal insulation materials to maintain quality of okra during storage and transportation. Horticulturae 2021, 7, 188. [Google Scholar] [CrossRef]
- Mitalo, O.W.; Tokiwa, S.; Kondo, Y.; Otsuki, T.; Galis, I.; Suezawa, K.; Kataoka, I.; Doan, A.T.; Nakano, R.; Ushijima, K.; et al. Low temperature storage stimulates fruit softening and sugar accumulation without ethylene and aroma volatile production in kiwifruit. Front. Plant Sci. 2019, 10, 888. [Google Scholar] [CrossRef]
- Thole, V.; Vain, P.; Yang, R.Y.; Almeida Barros da Silva, J.; Enfissi, E.M.; Nogueira, M.; Price, E.J.; Alseekh, S.; Fernie, A.R.; Fraser, P.D.; et al. Analysis of tomato post-harvest properties: Fruit color, shelf life, and fungal susceptibility. Curr. Protoc. plant Biol. 2020, 5, 20108. [Google Scholar] [CrossRef]
- Al-Waili, N.; Opara, U.L.; Al-Yahyai, R.; Al-Ani, M.; Al-Mahdhori, A.; Annamalai, M. Physiological responses and postharvest quality of banana as affected by storage conditions. Int. J. Fruit Sci. 2011, 13, 373–388. [Google Scholar]
- Venkatachalam, K. Changes in Quality and Enzymes of Longkong (Aglaia dookkoo Griff.) Fruit During Storages as Affected by Maturation, Package and Methyl Jasmonate Treatment. Ph.D. Thesis, Prince of Songkla University, Songkhla, Thailand, 2013. [Google Scholar]
- Kortei, N.K.; Akonor, P.T. Correlation between hue-angle and colour lightness of gamma irradiated mushrooms. Ann. Food Sci. Technol. 2015, 16, 98–103. [Google Scholar]
- Zhang, Y.L.; Zhang, R.G. Study on the mechanism of browning of pomegranate (Punica granatum L. cv. Ganesh) peel in different storage conditions. ASC 2008, 7, 65–73. [Google Scholar] [CrossRef]
- Assis, J.S.; Maldonado, R.; Muñoz, T.; Escribano, M.I.; Merodio, C. Effect of high carbon dioxide concentration on PAL activity and phenolic contents in ripening cherimoya fruit. Postharvest Biol. Technol. 2001, 23, 33–39. [Google Scholar] [CrossRef]
- Gandhi, K.D.; Faldu, P.R.; Patel, K.G.; Solanki, V.H.; Kansara, R.V.; Singh, S.; Vyas, T.K. Plant polyphenol oxidase: Biochemical properties and browning of fruits and vegetables. Indian J. Agric. Biochem. 2018, 31, 1–8. [Google Scholar] [CrossRef]
- Camargo, J.M.; Dunoyer, A.T.; García-Zapateiro, L.A. The effect of storage temperature and time on total phenolics and enzymatic activity of sapodilla (Achras sapota L.). Rev. Fac. Nac. Agron. 2016, 69, 7955–7963. [Google Scholar] [CrossRef]
- Zhao, K.; Xiao, Z.; Zeng, J.; Xie, H. Effects of different storage conditions on the browning degree, PPO activity, and content of chemical components in fresh Lilium bulbs (Lilium brownie F.E.Brown var. viridulum Baker.). Agriculture 2021, 11, 184. [Google Scholar] [CrossRef]
- Ahad, T.; Gull, A.; Nissar, J.; Masoodi, L.; Rather, A.H. Effect of storage temperatures, packaging materials and storage periods on antioxidant activity and non-enzymatic browning of antioxidant treated walnut kernels. J. Food Sci. Technol. 2020, 57, 3556–3563. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Huang, C.; Durrani, Y.; Muhammad, A. Chemistry of enzymatic browning in longan fruit as a function of pericarp pH and dehydration and its prevention by essential oil, an alternative approach to SO2 fumigation. PeerJ 2021, 9, 11539. [Google Scholar] [CrossRef]
- Ding, P.; Lee, Y.L. Use of essential oils for prolonging postharvest life of fresh fruits and vegetables. Int. Food Res. J. 2019, 26, 363–366. Available online: http://www.ifrj.upm.edu.my (accessed on 15 December 2021).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).