1. Introduction
Container-plant production is intensive and reliant on significant contributions of labor [
1], water [
2,
3], and fertilizers [
4,
5]. Overhead irrigation is common and economical for #7 (21.9–29.3 L) and smaller containers [
3] and applying water soluble fertilizers via irrigation is an easy and effective method for fertilizing plants. However, applying water soluble fertilizers via overhead irrigation is particularly wasteful in container-plant production because of poor interception efficiency from container spacing. Controlled-release fertilizers (CRF) are a recommended best management practice to reduce nutrient leaching and runoff from container-plant production [
6].
Although, CRF are a recommended best management practice to reduce nutrient waste, many studies on CRF nutrient release and leaching in soilless substrate production systems indicated significant leaching of nutrients in the early stages of the production cycle [
4,
7,
8,
9,
10,
11,
12,
13]. Significant quantities of nutrients in leachate from soilless substrates fertilized with CRF could be due to prill release rate exceeding young plant demand, poorly established root systems inability to utilize released nutrients, or failure of prill coatings. “Catastrophic failure” occurs when all nutrients are released at once from a prill and is attributed to osmotic pressure exceeding the threshold of prill coating resistance [
14]. Damage to CRF prill coating can exacerbate this phenomenon through weakening the coating, either visibly or microscopically by allowing liquid water to enter the prill and cause all fertilizer solution to be released at once [
15]. Huett and Morris [
16] monitored nutrient leaching from soilless substrate incorporated with new, old, and damaged Osmocote NPK prills and found that damaged Osmocote leached the greatest amount of nutrients.
Best management practices for utilizing CRF include uniform incorporation of the fertilizer prills into the soilless growing substrate prior to planting [
17]. Mechanization is regularly utilized at nurseries and greenhouses to mix soilless substrate components [
18] and homogeneously incorporate CRF. Soilless substrate mixing equipment may consist of material feeder bins, conveyor belts [
18], rotating tines, and ribbon blenders. Additionally, equipment with spinning augers may be used to remove a portion of soilless substrate from the container so that a smaller plant can be easily transplanted into a larger container. Mechanized soilless substrate mixing and potting equipment is prevalent in the nursery and greenhouse industry because of the short supply [
19] and subsequent cost of agricultural labor. Although mechanization increases production efficiency through reduced labor demand and uniform CRF incorporation, it is possible that mechanically incorporated CRF damages the CRF prill coating and causes undesirable nutrient release.
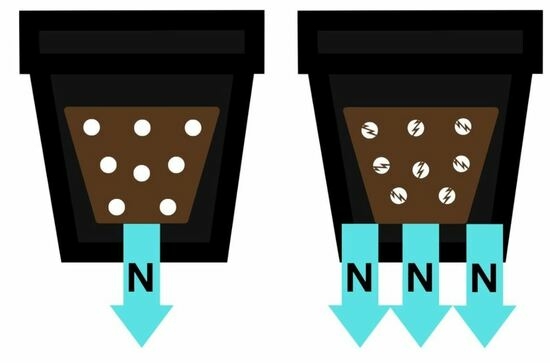
The first six weeks of production accounted for the majority of nitrogen leaching loss from a container-plant production system with Osmocote Plus 15-9-12 mechanically incorporated into soilless substrate [
4]. It was hypothesized that mechanized equipment to incorporate CRF into substrate damaged Osmocote Plus 15-9-12 prills and resulted in excessive nutrient release during the first six weeks of the experiment. To test this hypothesis, Osmocote Plus was mechanically and manually incorporated at the same rate and leachate was collected weekly from soilless substrate in the greenhouse for eleven weeks.
2. Materials and Methods
2.1. All Experiments
2.1.1. Preparation of Soilless Substrate
The soilless substrate consisted of 7:1 (v:v) Douglas fir (Pseudotsuga menziesii) bark and washed concrete sand. One batch of the soilless substrate had Osmocote Plus (15-9-12) controlled-release fertilizer (CRF) (Scotts MiracleGro, Marysville, OH, USA) that was mechanically incorporated. The substrate mixing line, engineered by Wurdinger Manufacturing Inc. (Silverton, OR, USA) moved substrate components along a series of three conveyor belts arranged at right angles to each other. Between the belts, rototiller-like tines agitated and mixed the substrate components. Douglas fir bark, sand, and CRF were added sequentially from bins located above the first conveyor belt and passed quickly through two sets of rototiller-like tines. The soilless substrate with manually incorporated CRF had an identical mixing method and traveled the same route but had CRF incorporated by hand in a laboratory at University of California, Davis.
The rate of CRF prills in the substrate with mechanically incorporated CRF was measured by counting the number of prills in nine 500-mL substrate samples. The rate of Osmocote Plus CRF in the Douglas fir bark substrate was determined to be 5.083 kg m−3. Osmocote Plus was 6.6% nitrate-nitrogen (NO3-N) and 8.4% ammonium-nitrogen (NH4-N), which resulted in 0.335 kg NO3-N m−3 and 0.427 kg NH4-N m−3. For the substrate with manually incorporated CRF, Osmocote Plus was added at the same rate as the mechanically incorporated. Manual-incorporation consisted of adding CRF to substrate and mixing with gloved hands in a plastic bin. Nine 500-mL samples of the substrate with manually incorporated CRF were collected to verify that the rate of Osmocote Plus was equal to the substrate with mechanically incorporated prills. Dolomite lime was manually incorporated at the rate of 3.85 kg m−3 to both substrates.
2.1.2. Determining Substrate Physical Properties
Six samples of substrate were used to determine the physical properties. Characterization of the physical properties of the Douglas fir bark substrate included measurement of total porosity, bulk density, mineral and organic content, volumetric-moisture content (P
v), and air-filled porosity (E
a) at container capacity. Bulk density of the substrate was determined using samples that had been placed in cylinders with holes at the bottom (25 cm height × 10 cm diameter). The substrate was packed to mimic the anticipated density during plant production. The total porosity was calculated using the equation,
where Φ is total porosity,
Vb bulk density in g cm
−3,
g is the soil dry weight in grams,
ρ is the particle density in g cm
−3 and the subscripts
m and
o signify the substrate mineral and organic fractions, respectively. The particle densities for silica (2.65 g cm
−3) and cellulose (1.60 g cm
−3) were used for
ρm and
ρo, respectively.
The substrate in the cylinders was weighed and then saturated with water. The substrate was left to drain for two hours, and once drainage was not detectable at the bottom of the cylinder, the substrate was weighed again. Volumetric-moisture content was calculated using the ratio of water volume in the substrate to the bulk volume of the substrate, expressed as a percentage. Air-filled porosity was calculated by subtracting P
v from the total porosity. Six samples of substrate were used to determine the organic and mineral content of the substrate by the loss on ignition method [
20]. Resulting physical properties of the substrate were 76.4% total porosity, 40.7% volumetric-moisture content, and 35.7% air-filled porosity, volumetric-moisture content, and air-filled porosity, respectively. Bulk density was 0.48 g cm
−3 with mineral content 53.3% and organic content 46.7%.
2.1.3. Greenhouse Environmental Conditions
Experiments were conducted from 10 January 2020 until 25 March 2020. During both experiments, the mean temperature and relative humidity in the greenhouse was 20.7 °C and 64.7%, respectively.
2.1.4. Nitrogen Analysis and Load Estimation
Leachate samples were analyzed for NH
4-N and NO
3-N using the spectrophotometric determination with a single reagent [
21]. The volume of leachate and the concentration of NO
3-N and NH
4-N in the leachate samples were used to calculate the mass load of nitrogen leached from each container. Electrical conductivity (dS m
−1) was converted to meq L
−1 [
22] and used with volume of leachate to calculate total salts leached.
2.2. Experiment One
Unplanted containers were prepared by adding Douglas fir bark:sand substrate with either mechanically or manually incorporated CRF into #3 containers (14-L) resulting in the addition of 3.28 g NO3-N and 4.19 g NH4-N on average to each container. No plants were planted in this experiment to quantify total leaching losses from manually or mechanically incorporated CRF. Containers had 10.3–11 L of substrate when filled. There were 10 replicate containers of substrate with each incorporation method. The containers were randomly placed in the greenhouse experimental area spaced at 61 cm centers.
On day one, a plastic tray was placed under the container and substrate was irrigated thoroughly with deionized (DI) water. The containers were left for two hours to drain, then leachate volume was measured and aliquots of leachate were collected to measure the electrical conductivity (EC) and pH. An additional aliquot was frozen and stored for later determination of NH
4-N and NO
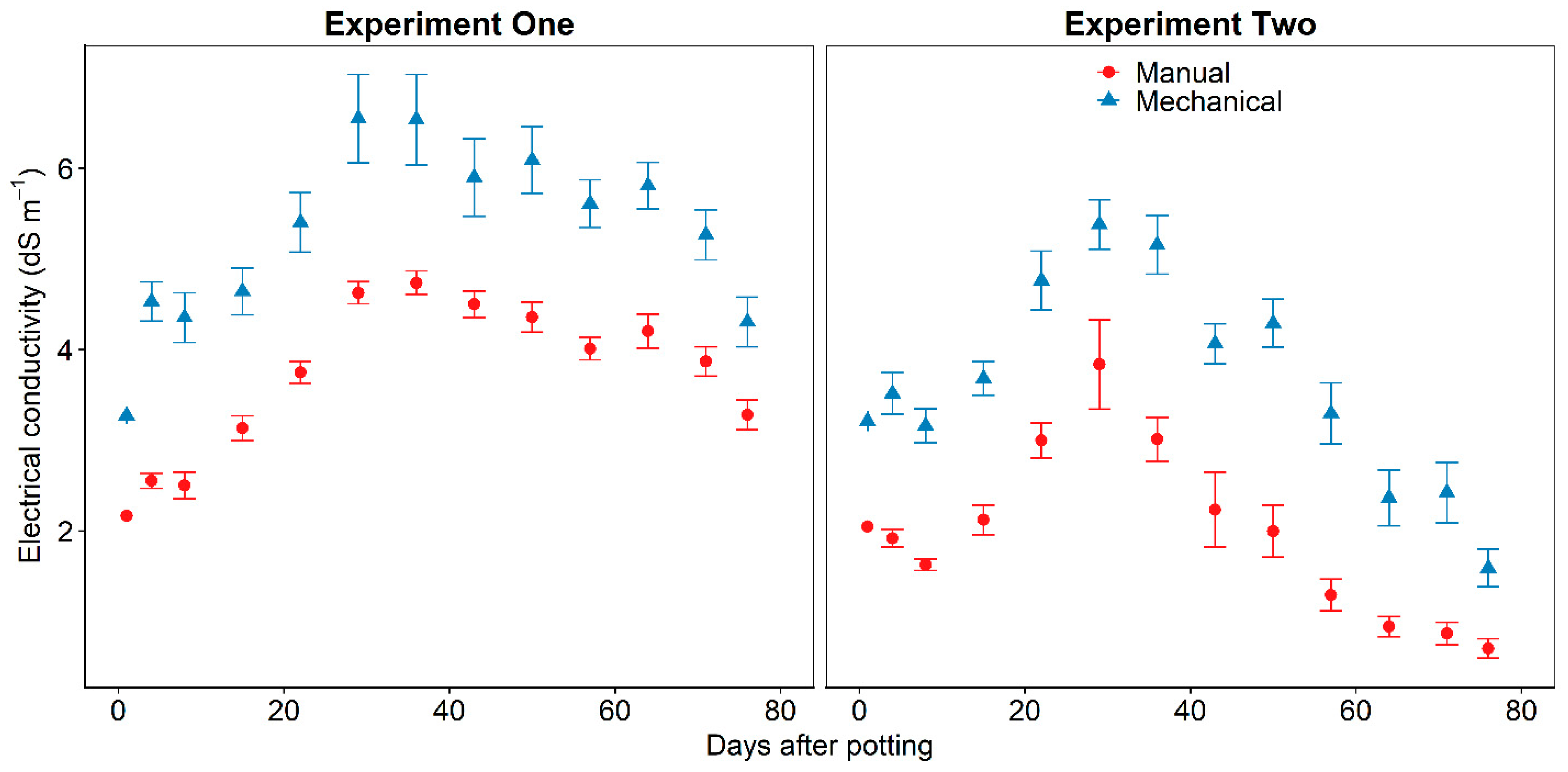
3-N concentration. For subsequent sampling days DI water was slowly added to the substrate surface and the substrate drained for two hours before the leachate was collected. Sampling was performed on days 1, 4, 8, and weekly after day 8. Leachate volume from the substrate with mechanically or manually incorporated CRF was not significantly different for all sampling days except day 50 (
p = 0.0376) (
Figure 1). Total leachate volume during the entire experiment was not significantly different (
p = 0.36) between the unplanted substrate with mechanically or manually incorporated CRF (
Table 1).
2.3. Experiment Two
On day one, Lavandula angustifolia ‘Provence’ plants were individually transplanted from #1 (2.7-L) containers into #3 containers of Douglas fir bark substrate with either mechanically or manually incorporated CRF. Plants were grown in this experiment to determine if nutrient toxicity symptoms occurred in beginning stages of experiment and if nutrient deficiency symptoms occurred in later stages of experiment. However, nutrient deficiency could not be evaluated because the experiment ended earlier than planned due to COVID-19 rules that required the conclusion of all “non-essential” research. The plants were kindly provided by a nursery grower in Sacramento, CA, USA. The plants had spent multiple years in the #1 container so it was assumed that the originally incorporated-CRF had expended all mineral fertilizer prior to transplanting. Surface-applied CRF was removed from the substrate surface of the #1 container before planting. Due to rootball volume, the planted containers had less CRF added than the unplanted, resulting in 2.38 g of NO3-N and 3.03 g of NH4-N on average per container. Containers had 10.5–11.3 L of substrate after filling and transplanting. There were 10 replicate containers of substrate with each incorporation method.
On day one, a plastic tray was placed under the container and substrate was irrigated thoroughly with DI water. The containers were left for two hours to drain, then leachate volume was measured and aliquots of leachate were collected to measure the EC and pH. An additional aliquot was frozen and stored for later determination of NH
4-N and NO
3-N concentration. For each subsequent sampling day, four to six planted containers were weighed to determine the DI water needed to bring the substrate to container capacity. The average volume required to return these four to six containers to container capacity plus additional DI water was slowly added to the substrate surface and the planted substrate drained for two hours before the leachate was collected. Sampling was performed on days 1, 4, 8, and weekly after day 8. Leachate volume from the substrate with mechanically or manually incorporated CRF was significantly different on sampling day 71 (
p = 0.0041) (
Figure 1). Total leachate volume during the entire experiment was not significantly different (
p = 0.21) between the planted substrate with mechanically or manually incorporated CRF (
Table 1).
Plants were visually evaluated and irrigation was applied to ensure plants received adequate water between leaching events. The volume of water added was determined by weighing four to six containers and calculating the volume to return the substrate to container capacity. To prevent leaching during these irrigations, the volume of DI water was below the amount to exceed container capacity. The volume of water added was recorded for each container and used to calculate total weekly irrigation volume to maintain an intended leaching fraction of 20%.
The plants were harvested on day 76. The shoots of the lavender plants were cut at the crown and weighed. The shoots were then dried at 60 °C for 48 h, and weighed again.
2.4. Statistics
Leaching volume data from day one for each experiment was analyzed separately from other days to meet model assumptions of Gaussian distribution, homoscedasticity, and linearity. Daily and total experiment leaching volume, daily pH, and total NH4-N, NO3-N, and soluble salts in leachate were analyzed with Student’s T-test to compare mechanically and manually incorporated CRF. Container number was given a random intercept to account for possible dependence among leaching volume over the sampling days. Daily pH data from experiment one had a single outlier on day 29 from the manually incorporated CRF and Shapiro–Wilks test indicated that residuals were not normally distributed (W = 0.77). The outlier was removed from the dataset and Shapiro–Wilks test indicated Gaussian distribution of residuals (W = 0.996). The data without the outlier was used in calculating summary statistics for the leachate pH from substrate with mechanically incorporated CRF and to determine significant difference from manually incorporated CRF. The planted substrates for both manually and mechanically incorporated CRF each had one replicate plant that was visibly smaller. A normal probability plot of plant shoot dry biomass identified these plants as outliers and a Shapiro–Wilk test indicated that the assumption of Gaussian distribution of residuals was not met (W = 0.83). A Student’s T-test was performed on the shoot dry biomass with and without the outliers included in the data. Although, removing the outliers improved the normality of the residual errors (W = 0.96) and reported p-value was different with or without the outliers included, both p-values were greater than significance level (α = 0.05). Therefore, the reported plant shoot dry biomass includes the two abnormally small plants.
4. Discussion
The mean greenhouse temperature during both experiments was 20.7 °C and according to the Osmocote Plus 15-9-12 label, the product should last for 8–9 months at average substrate temperature of 21 °C. The experiment lasted two and a half months and Osmocote has a consistent release pattern [
7,
23] which would have resulted in 30% of nitrogen release during the experiment. Therefore, total inorganic nitrogen leached from the unplanted substrate with manually incorporated CRF (
Table 1) was about expected from Osmocote Plus 15-9-12 based on greenhouse temperature and release pattern.
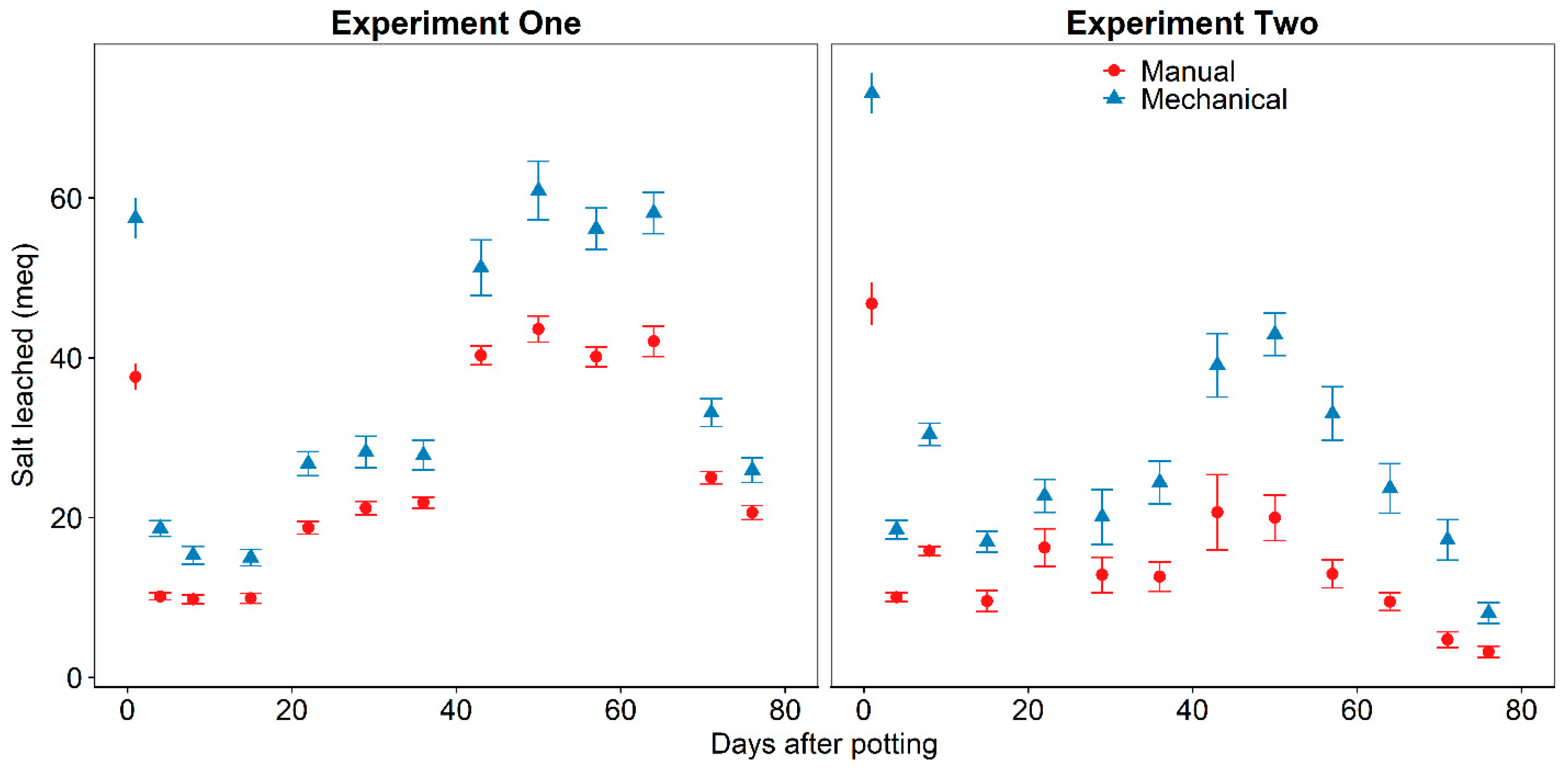
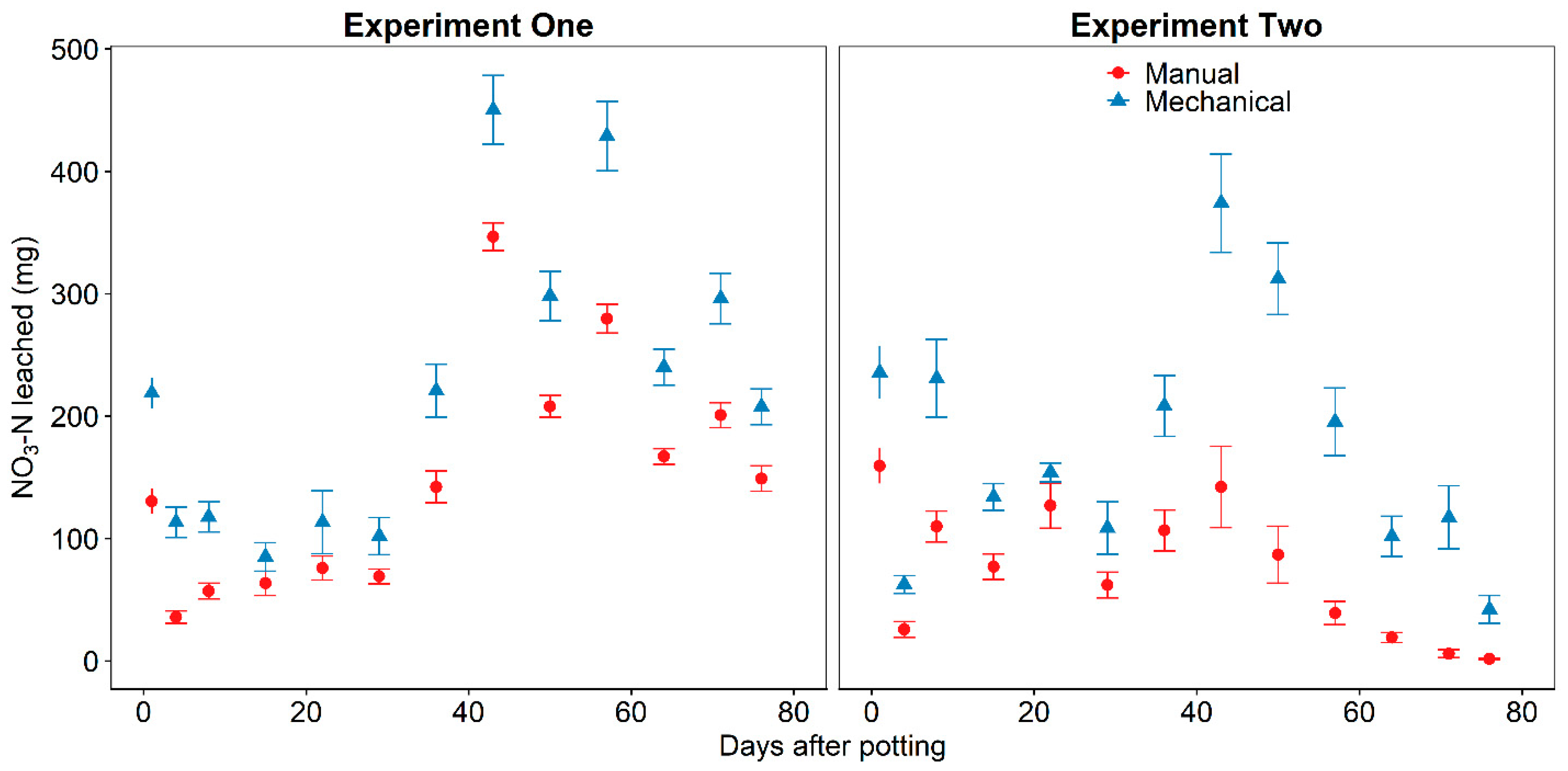
The substrate with mechanically incorporated CRF had significantly more (
p < 0.05) total inorganic nitrogen and salt leached than the substrate with manually incorporated CRF (
Table 1), whether plants were present or not. This suggests that CRF prills are damaged by the mechanical incorporation equipment during the substrate preparation process. Previous research determined visibly damaged Osmocote prills leached more inorganic nitrogen than undamaged prills, especially in the first week of the experiment [
16]. In this experiment, CRF prills were mechanically incorporated with rapidly spinning tines and it is reasonable to expect damage and subsequent coating failure during substrate preparation. It could be expected that damage to the CRF prill coating would result in catastrophic failure and large leaching losses on the first few irrigations. However, throughout the experiment, the mechanically incorporated CRF continued to leach significantly more nitrogen and salts than the manually incorporated CRF. This indicates that prill coating failure may not happen all at once and there are different degrees of damage to the CRF prills resulting in catastrophic failure occurring to a portion of prills each week.
The large amount of applied inorganic nitrogen leached from substrate with mechanically incorporated CRF (
Table 1) suggests that plants produced at a commercial nursery may not have sufficient nitrogen throughout the production cycle. Nearly half of applied nitrogen was leached from the substrate with mechanically incorporated CRF, irrespective of planting status, and this could result in the CRF longevity not matching the longevity rating on the label. Nutrient symptoms were not detected in any plants because COVID-19 restrictions required that the experiment end prematurely before symptoms developed. Reduced CRF longevity may require additional fertilizer application which could be applied as surface-applied CRF or water-soluble fertilizers through irrigation. Surface-application of CRF after initial transplanting requires significant labor which may already be scarce [
19] and applying water-soluble fertilizers, especially via overhead irrigation, negate some of the benefit of using CRF to reduce NO
3-N leaching from container-plant production [
6].
Nitrate-N accounted for 86–89% of total inorganic nitrogen leached from all combinations of CRF incorporation method and planting (
Table 1). This result was expected and consistent with previous studies on CRF leaching when prills were incorporated [
10,
23] or surface-applied [
16,
23,
24] to growing substrate. The large amount of NO
3-N leaching from the substrate is attributed to nitrification in Douglas fir bark substrate since Osmocote Plus 15-9-12 was 6.6% NH
4-N and 8.4% NO
3-N by weight. Greater NO
3-N content leachate poses more environmental risk than NH
4-N because of the NO
3-N ions propensity to readily leach through soils and subsequent contamination of groundwater. Therefore, reducing damage to CRF prills becomes more important when leachate water has the opportunity to infiltrate into groundwater aquifers.
The decrease in pH (
Figure 3) for all substrates beginning on day 29 was attributed to nitrification because there was a decrease in NH
4-N (
Figure 5) and increase in NO
3-N (
Figure 6) in leachate at the same time. The net reaction of nitrification generates two hydrogen ions per mole of NH
4-N [
25] and would explain the substrate acidification. Niemiera and Wright [
26] recorded greater acidification when more NH
4-N was applied to pine bark substrate and the larger amount of NH
4-N released by broken prills explained the significantly lower (
p > 0.05) pH of the leachate beginning on day 29 from the substrate with mechanically incorporated CRF. The large amount of NH
4-N in leachate and subsequent nitrification in experiment one compared to experiment two explained the continued acidification after day 29. The increase in pH on day 43 in experiment two was attributed to dolomite lime incorporation and increased uptake of NH
4-N by established plant roots and subsequent reduction in nitrification. This is consistent with other research which determined that the pH of peat-based substrates with dolomite lime incorporated had increased pH when fertilized solely with NO
3-N but had a decrease in pH when fertilized with NH
4-N after 42 days [
27].
Leaching volume increased (
Figure 1) on day 43 and subsequent days because irrigation volume to meet plant water requirements increased. The increase in total salts (
Figure 4) and NO
3-N (
Figure 6) in leachate from the planted substrate with mechanically incorporated CRF and the unplanted substrates on day 43 is explained by the increased leaching volume. Greater leachate volume is known to increase leaching of nutrients from soilless substrates [
28,
29] and the results from this experiment confirm this.
The EC of substrate leachate (
Figure 2) was consistently greater for the mechanically than the manually incorporated CRF but there was no effect on plant growth (
p = 0.70). English Lavender is moderately salt tolerant [
30] and plants were grown for an extended amount of time in 2.8-L pots before transplanting into the 14-L pots. Nutrient toxicity symptoms were not observed on any plants from either treatment. Perhaps if a less salt tolerant species or younger plants were grown, plant growth from the substrate with mechanically incorporated CRF may have been noticeably reduced. Growers should consider the potential for salt damage to young plants when transplanted into substrate with mechanically incorporated CRF and may need to implement practices to leach excess salts soon after planting.
Although, there is an abundance of published studies describing leaching losses from soilless substrates incorporated with CRF, only a few reported the method of incorporation [
7,
8,
31]. Of the three research papers that reported CRF incorporation method, two utilized a portable cement mixer [
7,
8] and the other was by hand [
31]. It is possible that the majority of researchers studying leaching losses from CRF-fertilized soilless substrates incorporated prills by hand because they were usually working with a smaller number of plants than commercial container-plant producers and mechanized equipment may not have been available or necessary. However, CRF prill incorporation methods that are gentler than typically used by industry could reduce the applicability of scientific results. Therefore, it is important that researchers report incorporation method when publishing plant growth and nutrient leaching results from soilless substrate production systems fertilized with CRF.
There is a wide array of mechanical equipment used for incorporating CRF and preparing soilless substrates and a single substrate-mixing line from a single manufacturer was employed in this research. Additionally, a single CRF coating technology was evaluated and there are many different commercially available products that utilize other organic polymers for coating water-soluble fertilizers. Future work should evaluate a greater variety of substrate preparation and potting equipment and different CRF coatings to determine if the results presented here are unique to the combination of mechanized equipment and CRF coating tested.
Manually incorporating CRF into soilless substrate resulted in less salt and inorganic nitrogen leaching, which has implications for container-plant producers and researchers. Container-plant producers should add CRF prills to soilless substrate at the last possible moment that still allows for homogeneous incorporation and minimizes CRF contact with mechanical equipment that could damage prills. This research highlights the necessity for container-plant producers to ensure the compatibility of mechanized substrate-mixing equipment with their CRF of choice to protect the integrity of the prill’s coating. Furthermore, designers and fabricators of mechanized substrate mixing equipment need to understand the potential consequences of machinery on CRF prill coating. Researchers should report methods used for incorporating CRF prills into soilless substrate to help the container-plant production industry determine applicability of results from studies evaluating nutrient leaching losses.