Physiological and Yield Performance Is Partially Linked to Water Use Efficiency of Eggplant Genotypes in a High-Tech Glasshouse
Abstract
1. Introduction
2. Materials and Methods
2.1. Glasshouse Facility Descriptions
2.2. Plant Growth and Management
2.3. Plant Growth and Productivity Measurements
2.4. Leaf Gas Exchange Measurements
2.5. Statistical Analysis
3. Results
3.1. Leaf and Stem Growth Parameters
3.2. Leaf Gas Exchange Parameters
3.3. Flower Number and Fruit Growth Parameters
3.4. Yield
3.5. Water Use Efficiency Only Correlates with Photosynthetic Parameters in Certain Weeks
4. Discussion
4.1. Protected Cropping of Eggplants Cultivars Is Sustainable in High-Tech Greenhouse
4.2. Genotypic Difference of Growth and Gas Exchange of Eggplants
4.3. Yield of Eggplants Is a Combination of Genetic and Environmental Factors That May Not Be Directly Linked to Net Photosynthetic Rate
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO (Food and Agriculture Organization of the United Nations); FAOSTAT. FAOSTAT Statistical Database. 2019. Available online: http://www.fao.org/faostat (accessed on 10 December 2022).
- Lo Scalzo, R.; Fibiani, M.; Mennella, G.; Rotino, G.L.; Dal Sasso, M.; Culici, M.; Spallino, A.; Braga, P.C. Thermal Treatment of Eggplant (Solanum melongena L.) Increases the Antioxidant Content and the Inhibitory Effect on Human Neutrophil Burst. J. Agric. Food Chem. 2010, 58, 3371–3379. [Google Scholar] [CrossRef]
- Stommel, J.R.; Whitaker, B.D.; Haynes, K.G.; Prohens, J. Genotype × environment interactions in eggplant for fruit phenolic acid content. Euphytica 2015, 205, 823–836. [Google Scholar] [CrossRef]
- Adamczewska-Sowińska, K.; Krygier, M. Yield quantity and quality of field cultivated eggplant in relation to its cultivar and the degree of fruit maturity. Acta Sci. Pol. 2013, 12, 1323. [Google Scholar]
- Boyacı, H.F. Resistance Resources and Its Inheritance against to Fusarium Wilt in Eggplants; Food and Agriculture Organization of the United Nations: Rome, Italy, 2007. [Google Scholar]
- Ali, Z.; Xu, Z.; Zhang, D.; He, X.; Bahadur, S.; Yi, J. Molecular diversity analysis of eggplant (Solanum melongena) genetic resources. Genet. Mol. Res. 2011, 10, 1141–1155. [Google Scholar] [CrossRef]
- Syfert, M.M.; Castañeda-Álvarez, N.P.; Khoury, C.K.; Särkinen, T.; Sosa, C.C.; Achicanoy, H.A.; Bernau, V.; Prohens, J.; Daunay, M.-C.; Knapp, S. Crop wild relatives of the brinjal eggplant (Solanum melongena): Poorly represented in genebanks and many species at risk of extinction. Am. J. Bot. 2016, 103, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.S.; Karol, K.G.; Little, D.P.; Nee, M.H.; Litt, A. Phylogeographic relationships among Asian eggplants and new perspectives on eggplant domestication. Mol. Phylogen. Evol. 2012, 63, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Plazas, M.; Prohens, J.; Cuñat, A.N.; Vilanova, S.; Gramazio, P.; Herraiz, F.J.; Andújar, I. Reducing Capacity, Chlorogenic Acid Content and Biological Activity in a Collection of Scarlet (Solanum aethiopicum) and Gboma (S. macrocarpon) Eggplants. Int. J. Mol. Sci. 2014, 15, 17221–17241. [Google Scholar] [CrossRef] [PubMed]
- Gramazio, P. Genetics and Genomics of Cultivated Eggplants and Wild Relatives. Ph.D. Thesis, Universitat Politècnica de València: Valencia, Spain, 2018. [Google Scholar] [CrossRef]
- Akpan, N.M.; Ogbonna, P.; Onyia, V.; Okechukwu, E.; Atugwu, A.; Dominic, I.-O. Studies on the Variability and Combining Ability for Improved Growth and Yield of Local Eggplant Genotypes (Solanum melongena L.). Not. Sci. Biol. 2016, 8, 226–231. [Google Scholar] [CrossRef][Green Version]
- Wei, Q.; Du, L.; Wang, W.; Hu, T.; Hu, H.; Wang, J.; David, K.; Bao, C. Comparative Transcriptome Analysis in Eggplant Reveals Selection Trends during Eggplant Domestication. Int. J. Genom. 2019, 2019, 7924383. [Google Scholar] [CrossRef]
- Sulaiman, N.N.M.; Rafii, M.Y.; Duangjit, J.; Ramlee, S.I.; Phumichai, C.; Oladosu, Y.; Datta, D.R.; Musa, I. Genetic Variability of Eggplant Germplasm Evaluated under Open Field and Glasshouse Cropping Conditions. Agronomy 2020, 10, 436. [Google Scholar] [CrossRef]
- Roser, M.; Ritchie, H. Yields and Land Use in Agriculture; Our World in Data: Oxford, UK, 2019. [Google Scholar]
- Rigby, E. Protected Cropping in Subtropical Climates; A Report for Nuffield Australia Farming Solutions: North Sydney, NSW, Australia, 2019. [Google Scholar]
- Chavan, S.G.; Maier, C.; Alagoz, Y.; Filipe, J.C.; Warren, C.R.; Lin, H.; Jia, B.; Loik, M.E.; Cazzonelli, C.I.; Chen, Z.H.; et al. Light-limited photosynthesis under energy-saving film decreases eggplant yield. Food Energy Secur. 2020, 9, e245. [Google Scholar] [CrossRef]
- Katsoulas, N.; Kittas, C. Impact of greenhouse microclimate on plant growth and development with special reference to the Solanaceae. Eur. J. Plant Sci. Biotechnol. 2008, 2, 31–34. [Google Scholar]
- Zhu, X.-G.; Long, S.P.; Ort, D.R. Improving Photosynthetic Efficiency for Greater Yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Wang, R.; Liu, W.; Zhou, P. Experimental performance of evaporative cooling pad systems in greenhouses in humid subtropical climates. Appl. Energy 2015, 138, 291–301. [Google Scholar] [CrossRef]
- DeGannes, A.; Heru, K.R.; Mohammed, A.; Paul, C.; Rowe, J.; Sealy, L.; Seepersad, G. Tropical Greenhouse Growers Manual for the Caribbean; Cardi: St. Augustine, Trinidad and Tobago, 2014; pp. 1–157. [Google Scholar]
- Ahamed, M.S.; Guo, H.; Tanino, K. Energy saving techniques for reducing the heating cost of conventional greenhouses. Biosyst. Eng. 2019, 178, 9–33. [Google Scholar] [CrossRef]
- Cuce, E.; Harjunowibowo, D.; Cuce, P.M. Renewable and sustainable energy saving strategies for greenhouse systems: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 64, 34–59. [Google Scholar] [CrossRef]
- Marucci, A.; Cappuccini, A. Dynamic photovoltaic greenhouse: Energy efficiency in clear sky conditions. Appl. Energy 2016, 170, 362–376. [Google Scholar] [CrossRef]
- Roslan, N.; Ya’Acob, M.; Radzi, M.; Hashimoto, Y.; Jamaludin, D.; Chen, G. Dye Sensitized Solar Cell (DSSC) greenhouse shading: New insights for solar radiation manipulation. Renew. Sustain. Energy Rev. 2018, 92, 171–186. [Google Scholar] [CrossRef]
- Taki, M.; Rohani, A.; Rahmati-Joneidabad, M. Solar thermal simulation and applications in greenhouse. Inf. Process. Agric. 2018, 5, 83–113. [Google Scholar] [CrossRef]
- Loik, M.E.; Carter, S.A.; Alers, G.; Wade, C.E.; Shugar, D.; Corrado, C.; Jokerst, D.; Kitayama, C. Wavelength-Selective Solar Photovoltaic Systems: Powering Greenhouses for Plant Growth at the Food-Energy-Water Nexus. Earth’s Future 2017, 5, 1044–1053. [Google Scholar] [CrossRef]
- Kwon, J.K.; Khoshimkhujaev, B.; Lee, J.H.; Yu, I.H.; Park, K.S.; Gil Choi, H. Growth and Yield of Tomato and Cucumber Plants in Polycarbonate or Glass Greenhouses. Hortic. Sci. Technol. 2017, 35, 79–87. [Google Scholar] [CrossRef]
- Hao, X.; Papadopoulos, A.P. Effects of supplemental lighting and cover materials on growth, photosynthesis, biomass partitioning, early yield and quality of greenhouse cucumber. Sci. Hortic. 1999, 80, 1–18. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Pierik, R. The shade-avoidance syndrome: Multiple signals and ecological consequences. Plant, Cell Environ. 2017, 40, 2530–2543. [Google Scholar] [CrossRef]
- Ruban, A.V. Plants in light. Commun. Integr. Biol. 2009, 2, 50–55. [Google Scholar] [CrossRef]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-Regulated Plant Growth and Development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar] [PubMed]
- Babla, M.; Cai, S.; Chen, G.; Tissue, D.T.; Cazzonelli, C.I.; Chen, Z.-H. Molecular Evolution and Interaction of Membrane Transport and Photoreception in Plants. Front. Genet. 2019, 10, 956. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T.L. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef] [PubMed]
- Cazzonelli, C.I.; Hou, X.; Alagoz, Y.; Rivers, J.; Dhami, N.; Lee, J.; Marri, S.; Pogson, B.J. A cis-carotene derived apocarotenoid regulates etioplast and chloroplast development. eLife 2020, 9, e45310. [Google Scholar] [CrossRef]
- O‘Carrigan, A.; Hinde, E.; Lu, N.; Xu, X.-Q.; Duan, H.; Huang, G.; Mak, M.; Bellotti, B.; Chen, Z.-H. Effects of light irradiance on stomatal regulation and growth of tomato. Environ. Exp. Bot. 2013, 98, 65–73. [Google Scholar] [CrossRef]
- O‘Carrigan, A.; Babla, M.; Wang, F.; Liu, X.; Mak, M.; Thomas, R.; Bellotti, B.; Chen, Z.-H. Analysis of gas exchange, stomatal behaviour and micronutrients uncovers dynamic response and adaptation of tomato plants to monochromatic light treatments. Plant Physiol. Biochem. 2014, 82, 105–115. [Google Scholar] [CrossRef]
- Barker, J.C.; Welles, G.W.H.; Van Uffelen, J.A.M. The effects of day and night humidity on yield and quality of glasshouse cucumbers. J. Hortic. Sci. 1987, 62, 363–370. [Google Scholar] [CrossRef]
- Katsoulas, N.; Savvas, D.; Tsirogiannis, I.; Merkouris, O.; Kittas, C. Response of an eggplant crop grown under Mediterranean summer conditions to greenhouse fog cooling. Sci. Hortic. 2009, 123, 90–98. [Google Scholar] [CrossRef]
- Kaminski, K.P.; Kørup, K.; Nielsen, K.L.; Liu, F.; Topbjerg, H.B.; Kirk, H.G.; Andersen, M.N. Gas-exchange, water use efficiency and yield responses of elite potato (Solanum tuberosum L.) cultivars to changes in atmospheric carbon dioxide concentration, temperature and relative humidity. Agric. For. Meteorol. 2014, 187, 36–45. [Google Scholar] [CrossRef]
- Fierro, A.; Gosselin, A.; Tremblay, N. Supplemental Carbon Dioxide and Light Improved Tomato and Pepper Seedling Growth and Yield. HortScience 1994, 29, 152–154. [Google Scholar] [CrossRef]
- Dorais, M.; Yelle, S.; Gosselin, A. Influence of extended photoperiod on photosynthate partitioning and export in tomato and pepper plants. N. Z. J. Crop Hortic. Sci. 1996, 24, 29–37. [Google Scholar] [CrossRef]
- Cushman, K.E.; Tibbitts, T.W.; Sharkey, T.D.; Wise, R.R. Constant-light Injury of Potato: Temporal and Spatial Patterns of Carbon Dioxide Assimilation, Starch Content, Chloroplast Integrity, and Necrotic Lesions. J. Am. Soc. Hortic. Sci. 1995, 120, 1032–1040. [Google Scholar] [CrossRef]
- Murage, E.N.; Sato, Y.; Masuda, M. Relationship between dark period and leaf chlorosis, potassium, magnesium and calcium content of young eggplants. Sci. Hortic. 1996, 66, 9–16. [Google Scholar] [CrossRef]
- Stutte, G.W.; Yorio, N.C.; Wheeler, R.M. Interacting Effects of Photoperiod and Photosynthetic Photon Flux on Net Carbon Assimilation and Starch Accumulation in Potato Leaves. J. Am. Soc. Hortic. Sci. 1996, 121, 264–268. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lelandais, M.; Kunert, K.J. Photooxidative stress in plants. Physiol. Plant. 1994, 92, 696–717. [Google Scholar] [CrossRef]
- Arias, I.; Pinker, I.; Böhme, M. Cultivation of different eggplant (Solanum melongena L.) cultivars under greenhouse conditions. In Proceedings of the VII International Symposium on Protected Cultivation in Mild Winter Climates: Production, Pest Management and Global Competition 659, Kissimmee, FL, USA, 23–27 March 2004; pp. 403–409. [Google Scholar]
- Inthichack, P.; Nishimura, Y.; Fukumoto, Y. Diurnal temperature alternations on plant growth and mineral absorption in eggplant, sweet pepper, and tomato. Hortic. Environ. Biotechnol. 2013, 54, 37–43. [Google Scholar] [CrossRef]
- Uzun, S. Effect of light and temperature on the phenology and maturation of the fruit of eggplant (Solanum melongena) grown in greenhouses. N. Z. J. Crop Hortic. Sci. 2007, 35, 51–59. [Google Scholar] [CrossRef][Green Version]
- Sinanoglou, V.J.; Kavga, A.; Strati, I.F.; Sotiroudis, G.; Lantzouraki, D.; Zoumpoulakis, P. Effects of Infrared Radiation on Eggplant (Solanum melongena L.) Greenhouse Cultivation and Fruits’ Phenolic Profile. Foods 2019, 8, 630. [Google Scholar] [CrossRef] [PubMed]
- Tani, E.; Kizis, D.; Markellou, E.; Papadakis, I.; Tsamadia, D.; Leventis, G.; Makrogianni, D.; Karapanos, I. Cultivar-Dependent Responses of Eggplant (Solanum melongena L.) to Simultaneous Verticillium dahliae Infection and Drought. Front. Plant Sci. 2018, 9, 1181. [Google Scholar] [CrossRef] [PubMed]
- Babla, M.H.; Tissue, D.T.; Cazzonelli, C.I.; Chen, Z.-H. Effect of high light on canopy-level photosynthesis and leaf mesophyll ion flux in tomato. Planta 2020, 252, 1–15. [Google Scholar] [CrossRef]
- Aied, K.Y.; Wahab, Z.; Kamaruddin, R.H.; Shaari, A. Growth response of eggplant (Solanum melongena L.) to shading and cultivation inside greenhouse in a tropical region. Int. J. Sci. Eng. Res. 2017, 8, 89–101. [Google Scholar]
- Karanisa, T.; Amato, A.; Richer, R.; Majid, S.A.; Skelhorn, C.; Sayadi, S. Agricultural Production in Qatar’s Hot Arid Climate. Sustainability 2021, 13, 4059. [Google Scholar] [CrossRef]
- Marucci, A.; Campiglia, E.; Colla, G.; Pagniello, B. Environmental impact of fertilization and pesticide application in vegetable cropping systems under greenhouse and open field conditions. J. Food Agric. Environ. 2011, 9, 840–846. [Google Scholar]
- D‘Odorico, P.; Chiarelli, D.D.; Rosa, L.; Bini, A.; Zilberman, D.; Rulli, M.C. The global value of water in agriculture. Proc. Natl. Acad. Sci. USA 2020, 117, 21985–21993. [Google Scholar] [CrossRef]
- Lages Barbosa, G.; Almeida Gadelha, F.D.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.M.; Halden, R.U. Comparison of Land, Water, and Energy Requirements of Lettuce Grown Using Hydroponic vs. Conventional Agricultural Methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef]
- Castilla, N.; Baeza, E. Greenhouse Site Selection in Good Agricultural Practices (GAPs) Principles for Greenhouse Vegetable Production in the Mediterranean Region; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; pp. 21–33. [Google Scholar]
- Shi, J.; Zuo, J.; Xu, D.; Gao, L.; Wang, Q. Effect of low-temperature conditioning combined with methyl jasmonate treatment on the chilling resistance of eggplant (Solanum melongena L.) fruit. J. Food Sci. Technol. 2019, 56, 4658–4666. [Google Scholar] [CrossRef]
- Nisen, A.; Grafiadellis, M.; Jiménez, R.; La Malfa, G.; Martínez-García, P.; Monteiro, A.; Verlodt, H.; Villele, O.; Zabeltit, C.; Denis, J. Cultures Protégées en Climat Méditerranéen; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Stanghellini, C. Horticultural production in greenhouses: Efficient use of water. In Proceedings of the International Symposium on Growing Media and Soilless Cultivation 1034, Leiden, The Netherlands, 17–21 June 2013; pp. 25–32. [Google Scholar]
- Lin, T.; Goldsworthy, M.; Chavan, S.; Liang, W.; Maier, C.; Ghannoum, O.; Cazzonelli, C.I.; Tissue, D.T.; Lan, Y.-C.; Sethuvenkatraman, S.; et al. A novel cover material improves cooling energy and fertigation efficiency for glasshouse eggplant production. Energy 2022, 251, 123871. [Google Scholar] [CrossRef]
- Cockshull, K.E.; Graves, C.J.; Cave, C.R.J. The influence of shading on yield of glasshouse tomatoes. J. Hortic. Sci. 1992, 67, 11–24. [Google Scholar] [CrossRef]
- Uzun, S. The Quantitative Effects of Temperature and Light Environment on the Growth, Development and Yield of Tomato (Lysopersicon esculentum, Mill) and Aubergine (Solanum melongena L.). Ph.D. Thesis, University of Reading, Reading, UK, 1996. [Google Scholar]
- Aied, K.Y.; Wahab, Z.; Kamaruddin, R.H. Effect of shading and cultivation inside greenhouse on some flowering and fruit characteristics of brinjal (Solanum melongena L.). Int. J. Sci. Tech. Res. Eng. 2017, 2, 10–20. [Google Scholar]
- Huang, C.J.; Wei, G.; Jie, Y.C.; Xu, J.J.; Anjum, S.A.; Tanveer, M. Effect of shade on plant traits, gas exchange and chlorophyll content in four ramie cultivars. Photosynthetica 2016, 54, 390–395. [Google Scholar] [CrossRef]
- Jenabiyan, M.; Pirdashti, H.; Yaghoubian, Y. The combined effect of cold and light intensity stress on some morphological and physiological parameters in two soybean (Glycine max L.) cultivars. Int. J. Biosci. 2014, 5, 189–197. [Google Scholar]
- Gruda, N.; Tanny, J. Protected crops. In Horticulture: Plants for People and Places; Springer: Dordrecht, The Netherlands, 2014; Volume 1, pp. 327–405. [Google Scholar]
- Kikuchi, K.; Honda, I.; Matsuo, S.; Fukuda, M.; Saito, T. Stability of fruit set of newly selected parthenocarpic eggplant lines. Sci. Hortic. 2008, 115, 111–116. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; van Meeteren, U. Can prolonged exposure to low VPD disturb the ABA signalling in stomatal guard cells? J. Exp. Bot. 2013, 64, 3551–3566. [Google Scholar] [CrossRef]
- Nejad, A.R.; Harbinson, J.; Van Meeteren, U. Dynamics of spatial heterogeneity of stomatal closure in Tradescantia virginiana altered by growth at high relative air humidity. J. Exp. Bot. 2006, 57, 3669–3678. [Google Scholar] [CrossRef]
- Arve, L.E.; Terfa, M.T.; Gislerød, H.R.; Olsen, J.E.; Torre, S. High relative air humidity and continuous light reduce stomata functionality by affecting the ABA regulation in rose leaves. Plant Cell Environ. 2013, 36, 382–392. [Google Scholar] [CrossRef]
- Xu, X.; Wu, P.; Song, H.; Zhang, J.; Zheng, S.; Xing, G.; Hou, L.; Li, M. Identification of candidate genes associated with photosynthesis in eggplant under elevated CO2. Biotechnol. Biotechnol. Equip. 2020, 34, 1166–1175. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Gramazio, P.; Yan, H.; Hasing, T.; Vilanova, S.; Prohens, J.; Bombarely, A. Whole-Genome Resequencing of Seven Eggplant (Solanum melongena) and One Wild Relative (S. incanum) Accessions Provides New Insights and Breeding Tools for Eggplant Enhancement. Front. Plant Sci. 2019, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ispizua, E.; Calatayud, Á.; Marsal, J.I.; Mateos-Fernández, R.; Díez, M.J.; Soler, S.; Valcárcel, J.V.; Martínez-Cuenca, M.-R. Phenotyping Local Eggplant Varieties: Commitment to Biodiversity and Nutritional Quality Preservation. Front. Plant Sci. 2021, 12, 696272. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.A. Selectable traits to increase crop photosynthesis and yield of grain crops. J. Exp. Bot. 2000, 51, 447–458. [Google Scholar] [CrossRef]
- Slatyer, R.O. Studies of the water relations of crop plants grown under natural rainfall in northern Australia. Aust. J. Agric. Res. 1955, 6, 365–377. [Google Scholar] [CrossRef]
- Fu, Q.S.; Yang, R.C.; Wang, H.S.; Zhao, B.; Zhou, C.L.; Ren, S.X.; Guo, Y.-D. Leaf morphological and ultrastructural performance of eggplant (Solanum melongena L.) in response to water stress. Photosynthetica 2013, 51, 109–114. [Google Scholar] [CrossRef]
- Vethamoni, P.I.; Natarajan, S. Cultivation of sweet pepper cultivars (Capsicum annuum var. grossum L.) under shade net in tropical plains of Tamil Nadu. Asian J. Hortic. 2008, 3, 372–376. [Google Scholar]
- Nock, C.A.; Baker, P.J.; Wanek, W.; Leis, A.; Grabner, M.; Bunyavejchewin, S.; Hietz, P. Long-term increases in intrinsic water-use efficiency do not lead to increased stem growth in a tropical monsoon forest in western Thailand. Glob. Chang. Biol. 2011, 17, 1049–1063. [Google Scholar] [CrossRef]
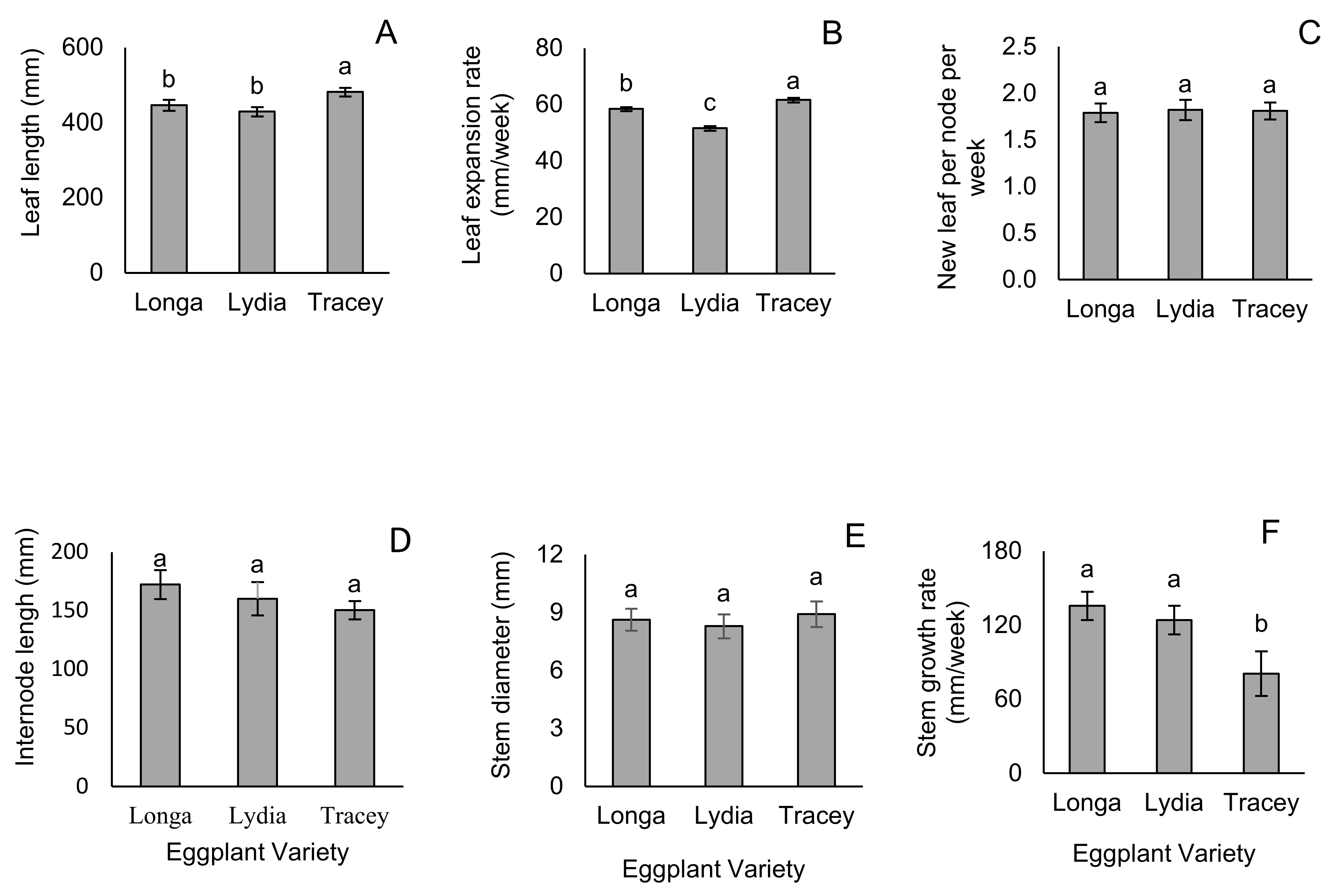
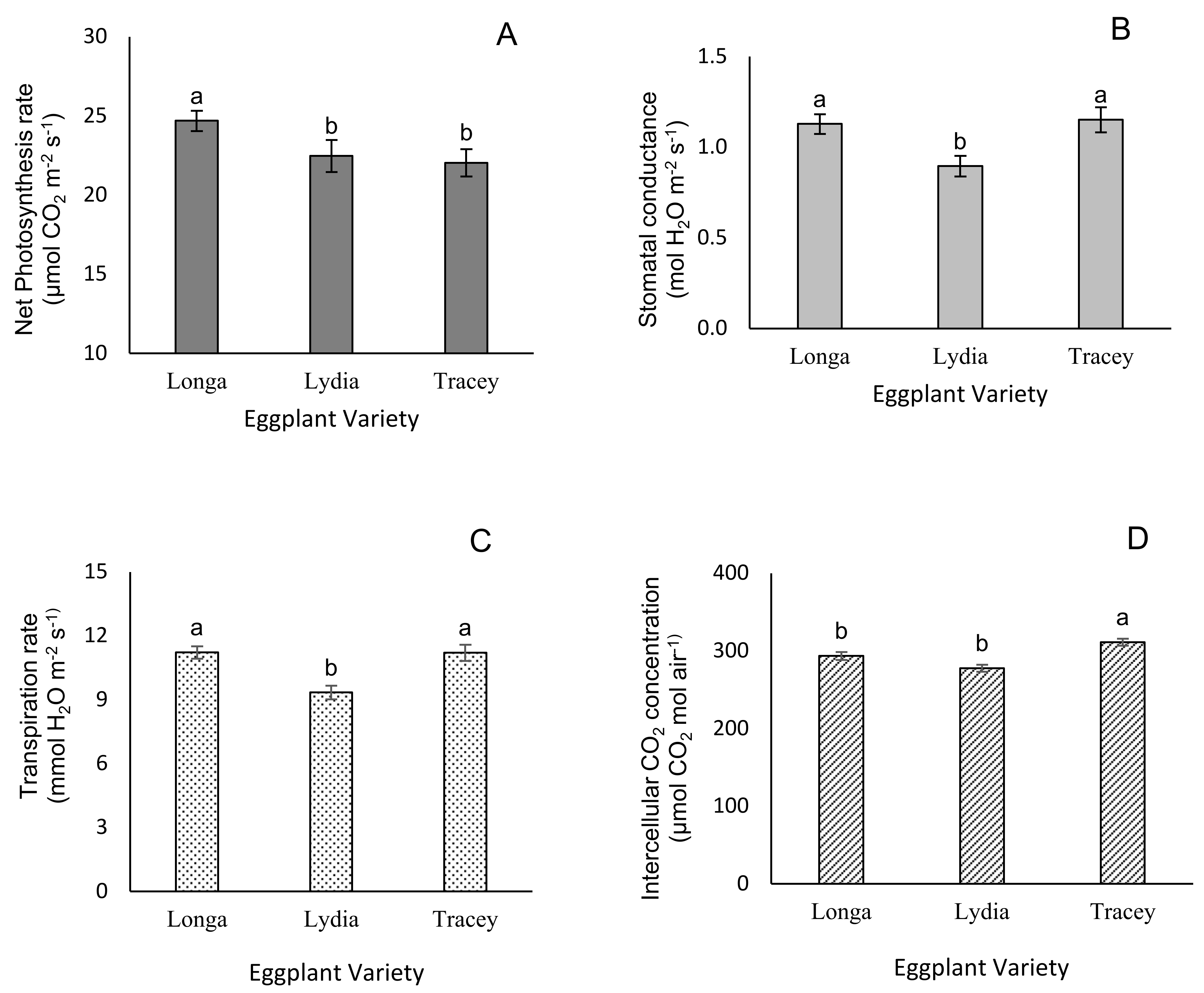
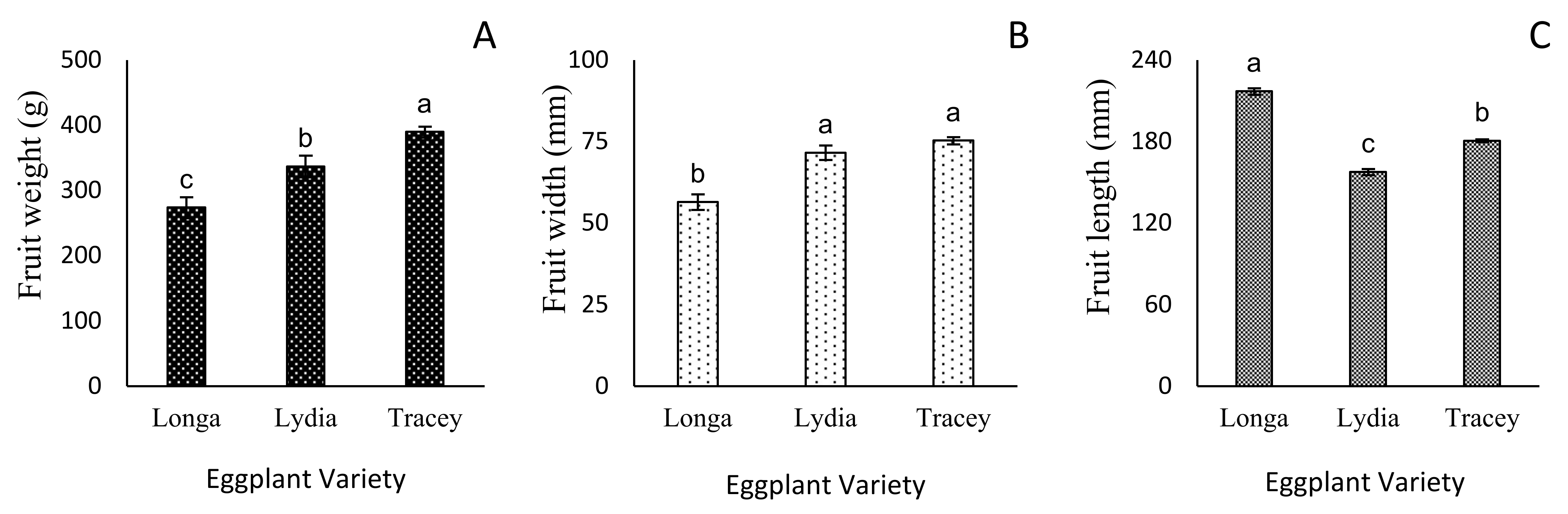

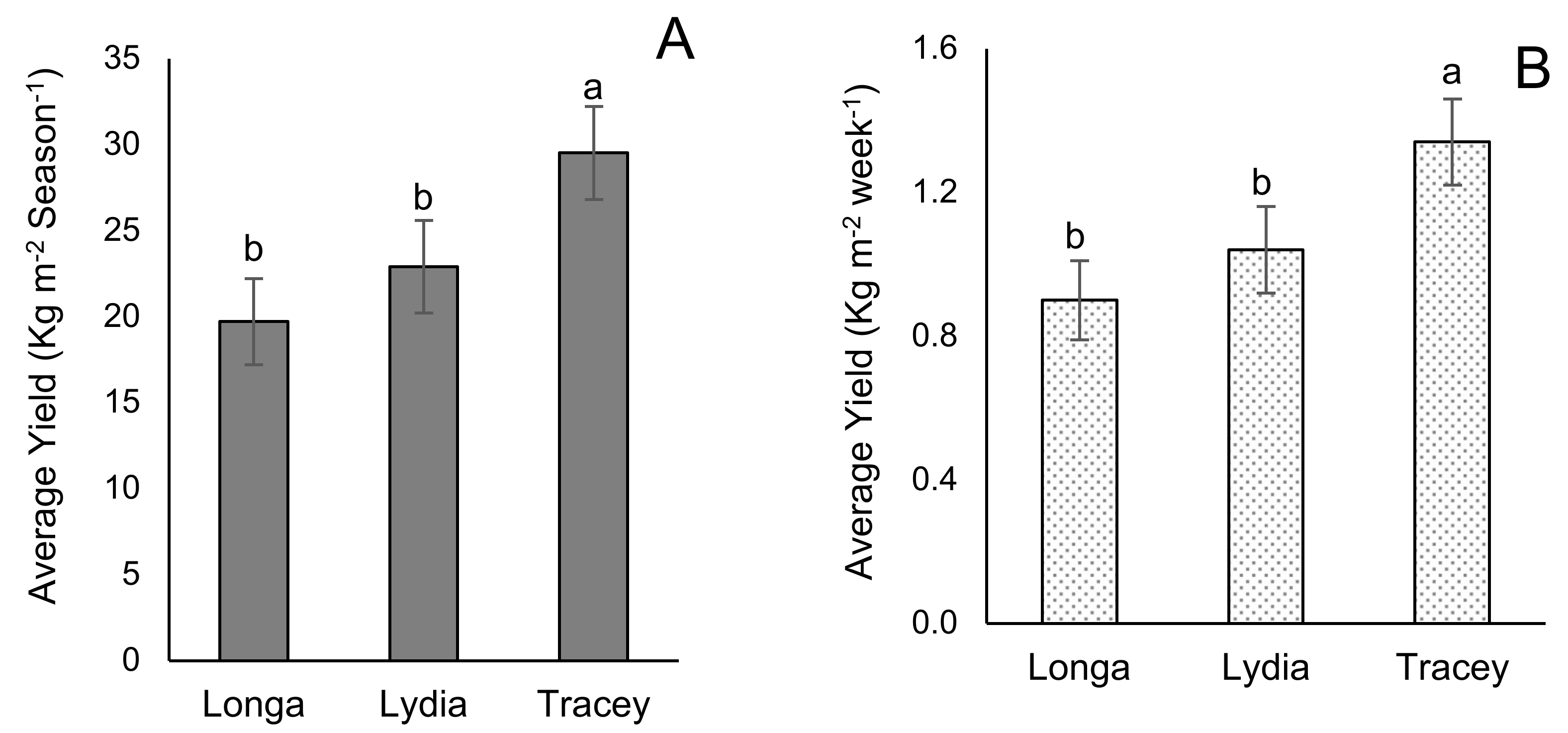

| Reproductive Growth | Longa | Lydia | Tracey |
|---|---|---|---|
| Weekly new flower per node | 1.49 ± 0.12 | 1.27 ± 0.07 | 1.13 ± 0.06 * |
| Average fruit numbers (m−2 week−1) | 2.92 ± 0.36 | 3.2 ± 0.4 | 3.55 ± 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasouli, F.; Babla, M.; Li, L.; Liang, W.; Yong, M.-T.; Ahmed, T.; Tissue, D.; Huda, S.; Chen, Z.-H. Physiological and Yield Performance Is Partially Linked to Water Use Efficiency of Eggplant Genotypes in a High-Tech Glasshouse. Horticulturae 2023, 9, 19. https://doi.org/10.3390/horticulturae9010019
Rasouli F, Babla M, Li L, Liang W, Yong M-T, Ahmed T, Tissue D, Huda S, Chen Z-H. Physiological and Yield Performance Is Partially Linked to Water Use Efficiency of Eggplant Genotypes in a High-Tech Glasshouse. Horticulturae. 2023; 9(1):19. https://doi.org/10.3390/horticulturae9010019
Chicago/Turabian StyleRasouli, Fatemeh, Mohammad Babla, Lihua Li, Weiguang Liang, Miing-Tiem Yong, Talaat Ahmed, David Tissue, Samsul Huda, and Zhong-Hua Chen. 2023. "Physiological and Yield Performance Is Partially Linked to Water Use Efficiency of Eggplant Genotypes in a High-Tech Glasshouse" Horticulturae 9, no. 1: 19. https://doi.org/10.3390/horticulturae9010019
APA StyleRasouli, F., Babla, M., Li, L., Liang, W., Yong, M.-T., Ahmed, T., Tissue, D., Huda, S., & Chen, Z.-H. (2023). Physiological and Yield Performance Is Partially Linked to Water Use Efficiency of Eggplant Genotypes in a High-Tech Glasshouse. Horticulturae, 9(1), 19. https://doi.org/10.3390/horticulturae9010019








