Extraction, Composition and Comparisons–Free Volatile Compounds from Hydrosols of Nine Veronica Taxa
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extractions, Preparation and Analyses of Hydrosols
2.3. PCA Analyses
3. Results
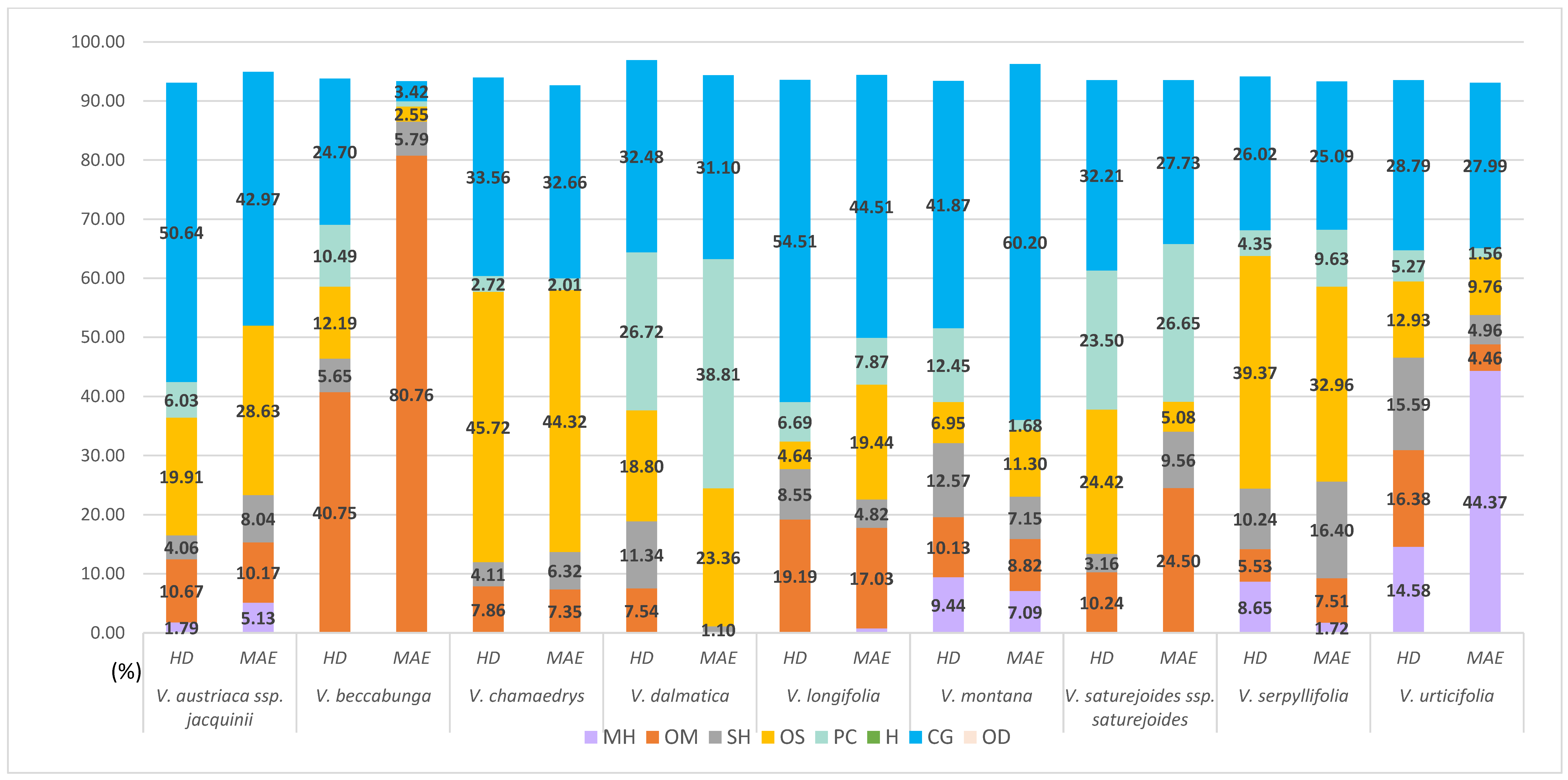
3.1. Extraction of Hydrosol Components from Veronica Taxa
3.1.1. Composition of Veronica Hydrosols Obtained by Hydrodistillation
3.1.2. Composition of Veronica Hydrosols Obtained by Microwave-Assisted Water Extraction
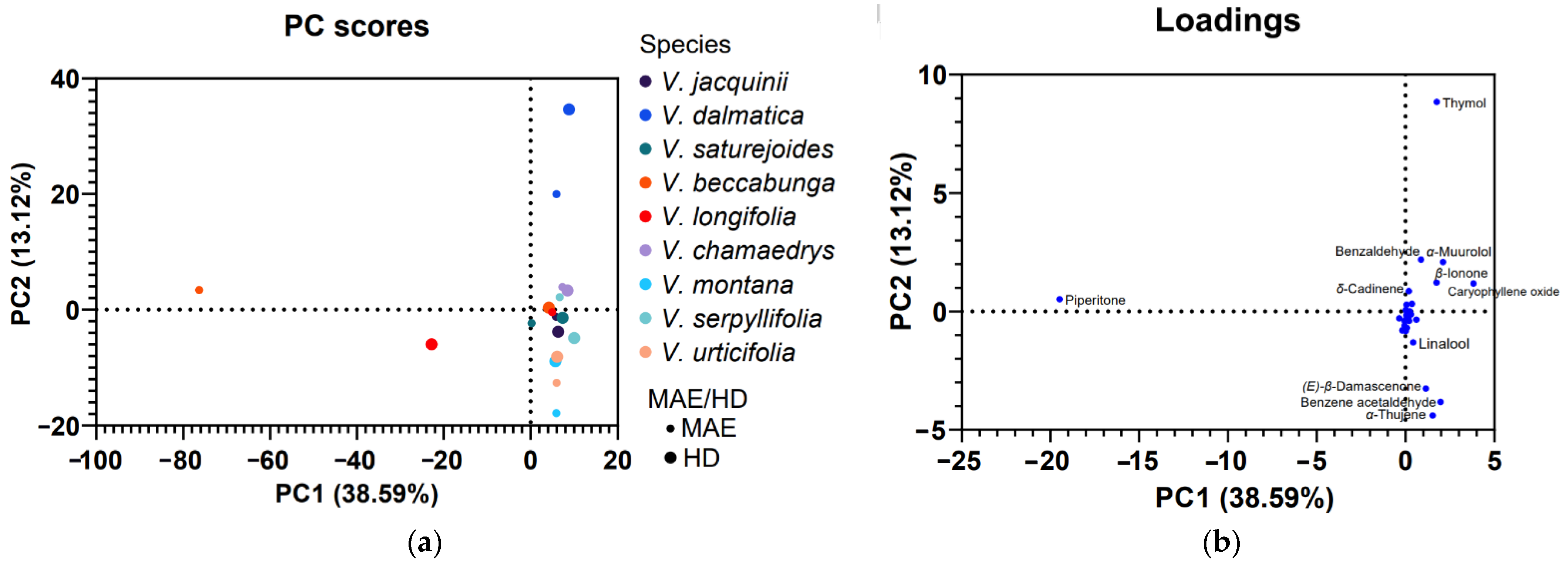
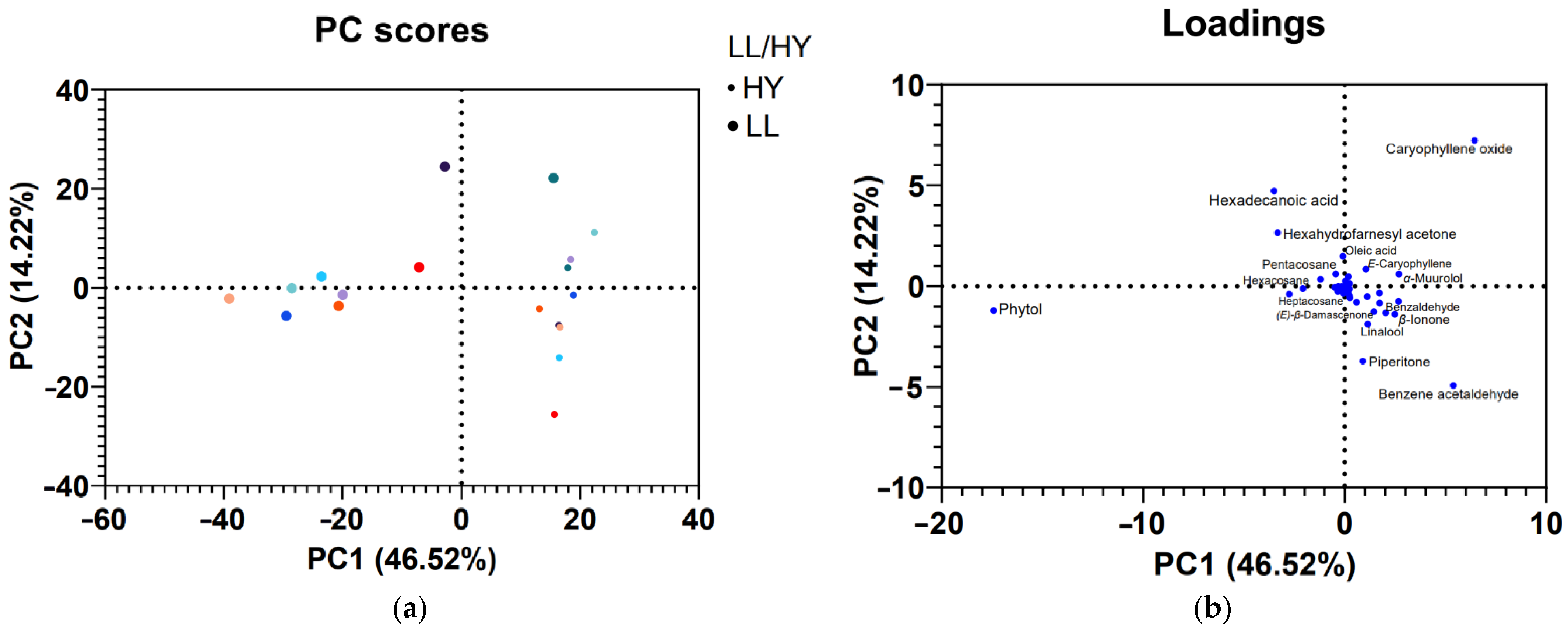
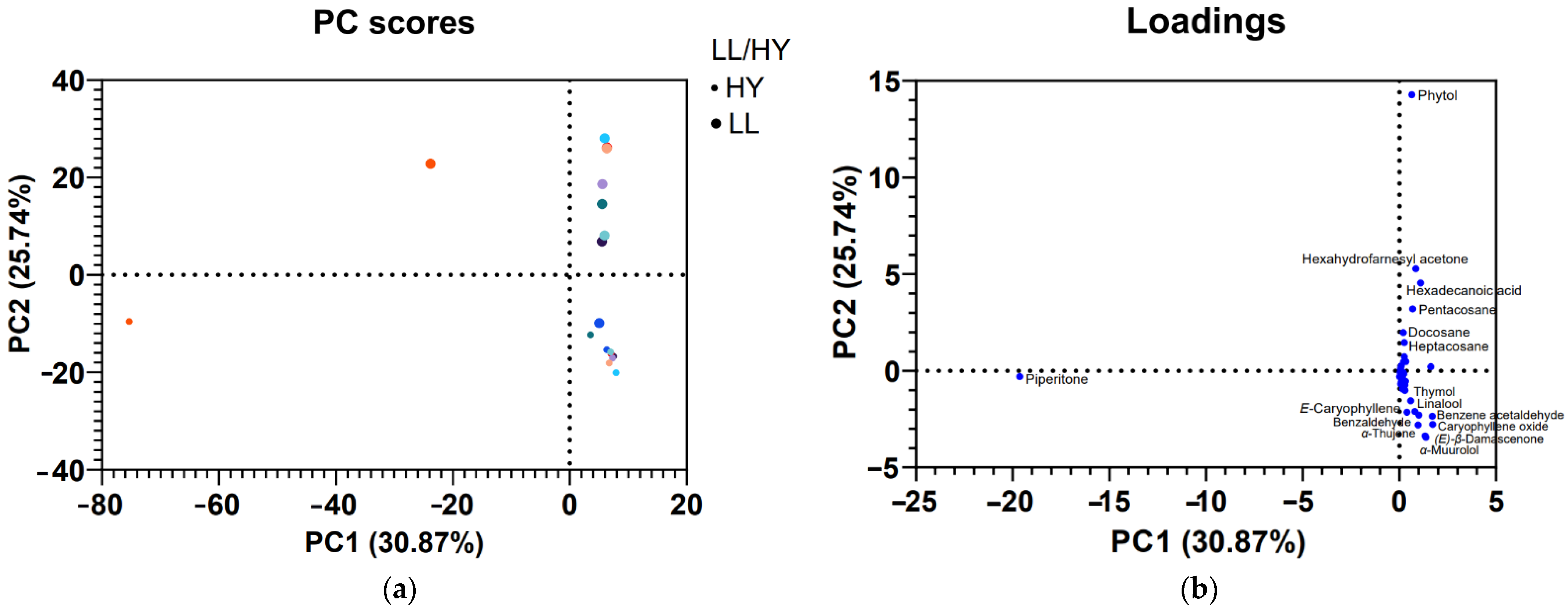
3.2. PCA Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Gupta, A.; Kothari, V.; Naraniwal, M. Modern Extraction Methods for Preparation of Bioactive Plant Extracts. Int. J. Appl. Nat. Sci. 2012, 1, 8–26. [Google Scholar]
- Hidayat, R. Patricia Wulandari Methods of Extraction: Maceration, Percolation and Decoction. Eureka Herba Indones. 2021, 2, 73–79. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Koidis, A. Methods for Extracting Essential Oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2015; pp. 31–38. ISBN 9780124166417. [Google Scholar]
- Capuzzo, A.; Maffei, M.E.; Occhipinti, A. Supercritical Fluid Extraction of Plant Flavors and Fragrances. Molecules 2013, 18, 7194–7238. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason Sonochem 2021, 70, 105325. [Google Scholar] [CrossRef]
- Delazar, A.; Nahar, L.; Hamedeyazdan, S.; Sarker, S.D. Microwave-Assisted Extraction in Natural Products Isolation. Methods Mol. Biol. 2012, 864, 89–115. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent Extraction Techniques for Natural Products: Microwave-Assisted Extraction and Pressurised Solvent Extraction. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Destandau, E.; Michel, T.; Elfakir, C. Microwave-Assisted Extraction; The Royal Society of Chemistry: London, UK, 2013; pp. 113–156. [Google Scholar] [CrossRef]
- Ferhat, M.A.; Meklati, B.Y.; Chemat, F. Comparison of Different Isolation Methods of Essential Oil from Citrus Fruits: Cold Pressing, Hydrodistillation and Microwave “dry” Distillation. Flavour Fragr. J. 2007, 22, 494–504. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Tuchowska, A.; Janda-Milczarek, K. Plant Hydrolates—Antioxidant Properties, Chemical Composition and Potential Applications. Biomed. Pharmacother. 2021, 142, 112033. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T. Effect of Residual Distillation Water of 15 Plants and Three Plant Hormones on Scotch Spearmint (Mentha × gracilis Sole). Ind Crops Prod. 2011, 33, 704–709. [Google Scholar] [CrossRef]
- Vuko, E.; Dunkić, V.; Maravić, A.; Ruščić, M.; Nazlić, M.; Radan, M.; Ljubenkov, I.; Soldo, B.; Fredotović, Ž. Not Only a Weed Plant—Biological Activities of Essential Oil and Hydrosol of Dittrichia viscosa (L.) Greuter. Plants 2021, 10, 1837. [Google Scholar] [CrossRef] [PubMed]
- Zekri, N.; Handaq, N.; el Caidi, A.; Zair, T.; Alaoui El Belghiti, M. Insecticidal Effect of Mentha pulegium L. and Mentha suaveolens Ehrh. Hydrosols against a Pest of Citrus, Toxoptera aurantii (Aphididae). Res. Chem. Intermed. 2016, 42, 1639–1649. [Google Scholar] [CrossRef]
- Paolini, J.; Leandri, C.; Desjobert, J.M.; Barboni, T.; Costa, J. Comparison of Liquid-Liquid Extraction with Headspace Methods for the Characterization of Volatile Fractions of Commercial Hydrolats from Typically Mediterranean Species. J. Chromatogr A 2008, 1193, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Vodnar, D.C.; Vlase, L.; Crișan, O.; Gheldiu, A.M.; Crișan, G. Phytochemical Characterization of Veronica officinalis L., V. teucrium L. and V. orchidea Crantz from Romania and Their Antioxidant and Antimicrobial Properties. Int. J. Mol. Sci 2015, 16, 21109–21127. [Google Scholar] [CrossRef]
- Politi, M.; Menghini, L.; Conti, B.; Bedini, S.; Farina, P.; Cioni, P.L.; Braca, A.; de Leo, M. Reconsidering Hydrosols as Main Products of Aromatic Plants Manufactory: The Lavandin (Lavandula × intermedia) Case Study in Tuscany. Molecules 2020, 25, 2225. [Google Scholar] [CrossRef] [PubMed]
- Albach, D.C.; Martínez-Ortega, M.M.; Chase, M.W. Veronica: Parallel Morphological Evolution and Phylogeography in the Mediterranean. In Plant Systematics and Evolution; Springer: Wien, Austria, 2004; Volume 246, pp. 177–194. [Google Scholar]
- Barreira, J.C.M.; Dias, M.I.; Živković, J.; Stojkovic, D.; Soković, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic Profiling of Veronica spp. Grown in Mountain, Urban and Sandy Soil Environments. Food Chem. 2014, 163, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, T. Flora Croatica Database. Available online: http://hirc.botanic.hr/fcd (accessed on 12 May 2022).
- Marchenko, A.; Kintya, P.; Wyrzykiewicz, B.; Gorincioi, E. Steroidal Glycosides from Veronica chamaedrys L. Part I. The Structures of Chamaedrosides C, C1, C2, E, E1and E 2. Nat. Prod. Commun. 2012, 7, 565–568. [Google Scholar] [CrossRef]
- Albach, D.C.; Grayer, R.J.; Kite, G.C.; Jensen, S.R. Veronica: Acylated Flavone Glycosides as Chemosystematic Markers. Biochem. Syst. Ecol. 2005, 33, 1167–1177. [Google Scholar] [CrossRef]
- Jensen, S.R.; Gotfredsen, C.H.; Grayer, R.J. Unusual Iridoid Glycosides in Veronica Sects. Hebe and Labiatoides. Biochem. Syst. Ecol. 2008, 36, 207–215. [Google Scholar] [CrossRef]
- Verma, S.; Nizam, S.; Verma, P.K. Biotic and Abiotic Stress Signaling in Plants. In Stress Signaling in Plants: Genomics and Proteomics Perspective; Springer: New York, NY, USA, 2013; Volume 1, pp. 25–50. ISBN 9781461463726. [Google Scholar]
- Aćimović, M.; Tešević, V.; Smiljanić, K.; Cvetković, M.; Stanković, J.; Kiprovski, B.; Sikora, V. Hydrolates: By-Products of Essential Oil Distillation: Chemical Composition, Biological Activity and Potential Uses. Adv. Technol. 2020, 9, 54–70. [Google Scholar] [CrossRef]
- Shen, X.; Chen, W.; Zheng, Y.; Lei, X.; Tang, M.; Wang, H.; Song, F. Chemical Composition, Antibacterial and Antioxidant Activities of Hydrosols from Different Parts of Areca catechu L. and Cocos nucifera L. Ind Crops Prod. 2017, 96, 110–119. [Google Scholar] [CrossRef]
- Ilieva, Y.; Dimitrova, L.; Georgieva, A.; Vilhelmova-Ilieva, N.; Zaharieva, M.M.; Kokanova-Nedialkova, Z.; Dobreva, A.; Nedialkov, P.; Kussovski, V.; Kroumov, A.D.; et al. In Vitro Study of the Biological Potential of Wastewater Obtained after the Distillation of Four Bulgarian Oil-Bearing Roses. Plants 2022, 11, 1073. [Google Scholar] [CrossRef] [PubMed]
- Dunkić, V.; Nazlić, M.; Ruščić, M.; Vuko, E.; Akrap, K.; Topić, S.; Milović, M.; Vuletić, N.; Puizina, J.; Jurišić Grubešić, R.; et al. Hydrodistillation and Microwave Extraction of Volatile Compounds: Comparing Data for Twenty-One Veronica Species from Different Habitats. Plants 2022, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2017; ISBN 978-1-932633-21-4. [Google Scholar]
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/ (accessed on 12 March 2021).
- Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, R.; Malfa, G.A.; di Giacomo, C. Plant-Based Bioactive Molecules in Improving Health and Preventing Lifestyle Diseases. Int. J. Mol. Sci. 2021, 22, 2991. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13. [Google Scholar] [CrossRef]
- Hilgers, F.; Habash, S.S.; Loeschcke, A.; Ackermann, Y.S.; Neumann, S.; Heck, A.; Klaus, O.; Hage-Hülsmann, J.; Grundler, F.M.W.; Jaeger, K.E.; et al. Heterologous Production of Ββ-Caryophyllene and Evaluation of Its Activity against Plant Pathogenic Fungi. Microorganisms 2021, 9, 168. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, H.; Li, Q.; Lin, H.; Wen, X. Identification of VOCs in Essential Oils Extracted Using Ultrasound- and Microwave-Assisted Methods from Sweet Cherry Flower. Sci. Rep. 2021, 11, 1167. [Google Scholar] [CrossRef]
- Rao, B.R.R. Hydrosols and Water-Soluble Essential Oils: Medicinal and Biological Properties. In Recent Progress in Medicinal Plants Essential Oils I; Govil, J.N., Bhattacharya, S., Eds.; Studium Press: Delhi, India, 2013; pp. 120–140. [Google Scholar]
- Salehi, B.; Shetty, M.S.; Anil Kumar, N.v.; Živković, J.; Calina, D.; Docea, A.O.; Emamzadeh-Yazdi, S.; Kılıç, C.S.; Goloshvili, T.; Nicola, S.; et al. Veronica Plants—Drifting from Farm to Traditional Healing, Food Application, and Phytopharmacology. Molecules 2019, 24, 2454. [Google Scholar] [CrossRef]
- Xiao, Y.; He, J.; Zeng, J.; Yuan, X.; Zhang, Z.; Wang, B. Application of Citronella and Rose Hydrosols Reduced Enzymatic Browning of Fresh-Cut Taro. J. Food Biochem 2020, 44, e13283. [Google Scholar] [CrossRef]
- Rodríguez, A.; Peris, J.E.; Redondo, A.; Shimada, T.; Peña, L. Principal Component Analysis (PCA) of Volatile Terpene Compounds Dataset Emitted by Genetically Modified Sweet Orange Fruits and Juices in Which a D-Limonene Synthase Was Either up- or down-Regulated vs. Empty Vector Controls. Data Brief 2016, 9, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; He, X.; Li, C.; Yang, L.; Fu, Y.; Wang, K.; Zhang, Y.; Ni, Y. A Microwave-Assisted Simultaneous Distillation and Extraction Method for the Separation of Polysaccharides and Essential Oil from the Leaves of Taxus chinensis Var. mairei. Appl. Sci. 2016, 6, 19. [Google Scholar] [CrossRef]
- Elyemni, M.; Louaste, B.; Nechad, I.; Elkamli, T.; Bouia, A.; Taleb, M.; Chaouch, M.; Eloutassi, N. Extraction of Essential Oils of Rosmarinus officinalis L. by Two Different Methods: Hydrodistillation and Microwave Assisted Hydrodistillation. Sci. World J. 2019, 2019, 3659432. [Google Scholar] [CrossRef] [PubMed]
| Taxa | Locality | Latitude | Longitude | Altitude a.s.l. (m) | Voucher No. |
|---|---|---|---|---|---|
| V. austriaca ssp. jacquinii | Brač Island | 43°19′07.3″ N | 16°36′08.5″ E | 564 | CROVeS-02-2021 |
| V. beccabunga | Baške Oštarije | 44°31′32.1″ N | 15°10′34.2″ E | 908 | CROVeS-08-2021 |
| V. chamaedrys | Radoboj | 46°09′49.4″ N | 15°55′36.1″ E | 260 | CROVeS-13-2021 |
| V. dalmatica | Dubrovnik | 42°39′19.1″ N | 18°04′56.9″ E | 58 | CROVeS-04-2021 |
| V. longifolia | Oštarije | 45°13′36.1″ N | 15°16′18.2″ E | 311 | CROVeS-10-2021 |
| V. montana | Papuk Mt | 45°30′38.1″ N | 17°39′57.2″ E | 761 | CROVeS-15-2021 |
| V. saturejoides ssp. saturejoides | Dinara Mt | 44°03′11.3″ N | 16°23′29.7″ E | 1697 | CROVeS-05-2021 |
| V. serpyllifolia | Zagreb | 45°49′40.3″ N | 15°58′59.5″ E | 192 | CROVeS-20-2021 |
| V. urticifolia | Plešivica Mt | 45°45′05.7″ N | 15°42′28.3″ E | 350 | CROVeS-21-2021 |
| V. austriaca ssp. jacquinii | V. beccabunga | V. chamaedrys | V. dalmatica | V. longifolia | V. montana | V. saturejoides ssp. saturejoides | V. serpyllifolia | V. urticifolia | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Component | RI a | LRI | VC ± SD | VC ± SD | VC ± SD | VC ± SD | VC ± SD | VC ± SD | VC ± SD | VC ± SD | |
| Monoterpene hydrocarbons | 1.79 | - | - | - | - | 9.44 | - | 8.65 | 14.58 | ||
| α-Thujene | 924 | 924 | 1.79 ± 0.01 d | - | - | - | - | 9.44 ± 0.01 b | - | 8.65 ± 0.01 c | 14.58 ± 0.01 a |
| Oxygenated monoterpenes | 10.67 | 40.75 | 7.86 | 7.54 | 19.19 | 10.13 | 10.24 | 5.53 | 16.38 | ||
| γ-Terpinene | 1057 | 1054 | - | - | - | 1.15 ± 0.01 b | - | - | - | 0.68 ± 0.01 c | 1.61 ± 0.01 a |
| Linalool | 1095 | 1095 | 1.42 ± 0.1 f | - | 1.03 ± 0.01 g | 3.64 ± 0.01 d | 9.43 ± 0.01 b | 5.45 ± 0.01 c | - | 3.52 ± 0.01 e | 10.87 ± 0.01 a |
| Terpinen-4-ol | 1174 | 1174 | 1.77 ± 0.04 d | 1.87 ± 0.03 c | 1.93 ± 0.1 c | - | 3.82 ± 0.01 a | 1.91 ± 0.01 c | - | 0.32 ± 0.05 e | 3.13 ± 0.01 b |
| Borneol | 1176 | 1165 | 6.72 ± 0.01 | - | - | - | - | - | - | - | |
| α-Terpineol | 1184 | 1186 | 0.76 ± 0.12 c | - | - | - | 0.82 ± 0.05 c | 2.77 ± 0.1 a | - | 1.01 ± 0.01 b | 0.77 ± 0.01 c |
| trans-p-Mentha-1(7),8-dien-2-ol | 1187 | 1187 | - | 10.30 ± 0.01 a | 3.16 ± 0.03 c | - | 5.12 ± 0.01 b | - | 10.24 ± 0.01 a | - | - |
| β-Cyclocitrat | 1233 | 1217 | - | - | 1.74 ± 0.1 | - | - | - | - | - | - |
| Piperitone | 1250 | 1249 | - | 28.15 ± 0.01 | - | - | - | - | - | - | - |
| Menthyl acetate | 1294 | 1294 | - | 0.43 ± 0.07 b | - | 2.75 ± 0.02 a | - | - | - | - | - |
| Sesquiterpene hydrocarbons | 4.06 | 5.65 | 4.11 | 11.34 | 8.55 | 12.57 | 3.16 | 10.24 | 15.59 | ||
| E-Caryophyllene * | 1424 | 1417 | 2.02 ± 0.01 g | 3.41 ± 0.1 f | 2.04 ± 0.01 g | 5.51 ± 0.01 c | 3.95 ± 0.01 e | 5.82 ± 0.01 b | 0.56 ± 0.15 h | 5.12 ± 0.01 d | 9.88 ± 0.01 a |
| allo-Aromadendrene | 1465 | 1458 | 0.76 ± 0.01 d | 0.32 ± 0.01 e | 0.37 ± 0.01 e | - | 0.83 ± 0.01 d | 1.44 ± 0.01 c | - | 1.54 ± 0.01 b | 2.14 ± 0.01 a |
| Germacrene D | 1481 | 1484 | 1.28 ± 0.1 f | 1.92 ± 0.12 e | 0.64 ± 0.01 h | 5.83 ± 0.01 a | 2.32 ± 0.05 d | 4.52 ± 0.01 b | 0.72 ± 0.01 g | 0.66 ± 0.1 h | 2.53 ± 0.01 c |
| δ-Selinene | 1492 | 1492 | - | - | 1.06 ± 0.1 d | - | 1.45 ± 0.01 c | 0.79 ± 0.07 f | 1.88 ± 0.03 b | 2.92 ± 0.01 a | 0.94 ± 0.01 e |
| Oxygenated sesquiterpenes | 19.91 | 12.19 | 45.72 | 18.8 | 4.64 | 6.95 | 24.42 | 39.37 | 12.93 | ||
| Spathulenol | 1577 | 1577 | - | - | - | 1.05 ± 0.01 b | 0.74 ± 0.01 c | 1.21 ± 0.01 a | - | - | 0.43 ± 0.01 d |
| Caryophyllene oxide * | 1581 | 1582 | 9.92 ± 0.01 d | 8.21 ± 0.01 e | 21.11 ± 0.01 b | 8.13 ± 0.01 f | 0.66 ± 0.01 h | 4.88 ± 0.01 g | 21.56 ± 0.01 b | 37.03 ± 0.01 a | 10.32 ± 0.01 c |
| Viridiflorol | 1592 | 1592 | 0.43 ± 0.02 e | 1.45 ± 0.01 a | - | 0.78 ± 0.01 c | 0.86 ± 0.05 b | - | 0.57 ± 0.01 d | 0.54 ± 0.01 d | |
| γ-Eudesmol | 1632 | 1630 | 0.45 ± 0.01 | - | - | - | - | - | - | - | - |
| α-Muurolol | 1645 | 1644 | 8.75 ± 0.01 c | 3.55 ± 0.01 d | 23.16 ± 0.01 a | 9.62 ± 0.01 b | 2.76 ± 0.01 e | - | 1.88 ± 0.01 f | 1.24 ± 0.01 h | 1.64 ± 0.01 g |
| α-Bisabolol | 1685 | 1685 | - | - | - | - | - | - | - | 0.83 ± 0.01 | - |
| Hexahydrofarnesyl acetone * | 1839 | - | 0.79 ± 0.03 b | - | - | - | - | - | 0.98 ± 0.1 a | - | - |
| Phenolic compounds | 6.03 | 10.49 | 2.72 | 26.72 | 6.69 | 12.45 | 23.5 | 4.35 | 5.27 | ||
| Thymol * | 1289 | 1289 | - | 4.11 ± 0.01 c | - | 26.72 ± 0.01 a | - | 4.55 ± 0.01 b | - | 1.58 ± 0.01 e | 3.64 ± 0.05 d |
| p-Vinyl guaicol | 1313 | 1309 | - | - | - | - | 4.25 ± 0.01 a | 1.73 ± 0.01 c | - | 2.42 ± 0.1 b | 0.55 ± 0.01 d |
| Methyl eugenol | 1403 | 1403 | 2.31 ± 0.1 e | 5.82 ± 0.01 c | 2.72 ± 0.01 d | - | 2.44 ± 0.01 e | 6.17 ± 0.01 b | 22.76 ± 0.01 a | 0.35 ± 0.01 g | 1.08 ± 0.01 f |
| (Z)-Methyl isoeugenol | 1451 | 1451 | 3.72 ± 0.01 a | 0.56 ± 0.07 c | - | - | - | - | 0.74 ± 0.01 b | - | - |
| Common group | 50.64 | 24.7 | 33.56 | 32.48 | 54.51 | 41.87 | 32.21 | 26.02 | 28.79 | ||
| (E)-2-Hexenal | 846 | 846 | 3.44 ± 0.02 | - | - | - | - | - | - | - | - |
| Isopentyl acetate | 863 | 869 | - | - | - | - | - | - | - | - | - |
| Benzaldehyde | 952 | 952 | 10.38 ± 0.02 a | 1.42 ± 0.02 e | 3.51 ± 0.01 b | 3.53 ± 0.02 b | 10.33 ± 0.01 a | - | 3.25 ± 0.01 c | - | 1.52 ± 0.01 d |
| Benzene acetaldehyde | 1036 | 1036 | 18.43 ± 0.01 c | 13.23 ± 0.01 e | 8.64 ± 0.01 h | 10.46 ± 0.01 f | 22.27 ± 0.01 b | 25.33 ± 0.01 a | 9.13 ± 0.01 g | 16.44 ± 0.01 d | 18.68 ± 0.01 c |
| n-Nonanal | 1100 | 1100 | - | 0.82 ± 0.1 d | 0.34 ± 0.01 g | 5.92 ± 0.01 a | 3.42 ± 0.01 b | 1.56 ± 0.01 c | 0.45 ± 0.01 f | 0.53 ± 0.01 e | 0.38 ± 0.01 fg |
| Hexyl 2-methyl butanoate | 1233 | 1229 | - | - | 1.72 ± 0.01 | - | - | - | - | - | - |
| n-Decanol | 1266 | 1266 | - | - | - | - | 2.86 ± 0.01 | - | - | - | - |
| Bornyl acetate | 1285 | 1287 | - | - | 4.54 ± 0.01 | - | - | - | - | - | - |
| (E)-β-Damascenone | 1384 | 1383 | 8.92 ± 0.01 a | 3.11 ± 0.01 g | 5.01 ± 0.01 d | 5.17 ± 0.01 d | 7.42 ± 0.01 b | 4.43 ± 0.01 e | 6.17 ± 0.01 c | 3.73 ± 0.01 f | 0.39 ± 0.1 h |
| β-Ionone | 1487 | 1487 | 9.49 ± 0.01 c | 6.12 ± 0.01 h | 9.37 ± 0.01 d | 7.40 ± 0.01 g | 8.21 ± 0.01 e | 10.55 ± 0.01 b | 13.21 ± 0.01 a | 5.32 ± 0.01 i | 7.82 ± 0.01 f |
| Hexadecanoic acid * | 1959 | 1959 | - | - | 0.43 ± 0.01 | - | - | - | - | - | - |
| Total identification (%) | 93.1 | 93.73 | 93.97 | 96.88 | 93.58 | 93.41 | 93.53 | 94.16 | 93.54 |
| V.a austriaca ssp. jacquinii | V. becabunga | V. chamaedrys | V. dalmatica | V. longifolia | V. montana | V. saturejoides ssp. saturejoides | V. serpyllifolia | V. urticifolia | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Component | RI | LRI | VC ± SD | VC ± SD | VC ± SD | VC ± SD | VC ± SD | VC ± SD | VC ± SD | VC ± SD | VC ± SD |
| Monoterpene hydrocarbons | 5.13 | - | - | - | 0.75 | 7.09 | - | 1.72 | 44.37 | ||
| α-Thujene | 924 | 924 | - | - | - | - | - | 6.38 ± 0.01 b | - | 1.72 ± 0.01 c | 39.73 ± 0.01 a |
| α-Pinene * | 935 | 932 | 5.13 ± 0.01 a | - | - | - | 0.75 ± 0.01 c | 0.71 ± 0.01 c | - | - | 4.64 ± 0.1 b |
| Oxygenated monoterpenes | 10.17 | 80.76 | 7.35 | - | 17.03 | 8.82 | 24.5 | 7.51 | 4.46 | ||
| γ-Terpinene | 1057 | 1054 | 9.54 ± 0.01 a | - | - | - | - | - | 3.96 ± 0.02 b | 1.49 ± 0.01 c | - |
| Linalool | 1095 | 1095 | - | - | 3.15 ± 0.01 c | - | 7.74 ± 0.01 a | 4.64 ± 0.01 b | - | 4.84 ± 0.01 b | 2.31 ± 0.01 d |
| Terpinen-4-ol | 1174 | 1174 | 0.63 ± 0.1 g | 0.77 ± 0.01 f | 2.45 ± 0.01 d | - | 4.22 ± 0.03 a | 3.91 ± 0.1 b | 3.43 ± 0.01 c | 0.43 ± 0.01 h | 2.15 ± 0.01 e |
| α-Terpineol | 1184 | 1186 | - | - | - | - | 0.43 ± 0.01 b | 0.27 ± 0.01 c | - | 0.75 ± 0.01 a | - |
| trans-p-Mentha-1(7),8-dien-2-ol | 1187 | 1187 | - | 0.13 ± 0.04 d | 1.75 ± 0.01 c | - | 4.64 ± 0.01 b | - | 17.11 ± 0.01 a | - | - |
| Piperitone | 1250 | 1249 | - | 79.86 ± 0.01 | - | - | - | - | - | - | - |
| Menthyl acetate | 1294 | 1294 | - | - | - | - | - | - | - | - | |
| Sesquiterpene hydrocarbons | 8.04 | 5.79 | 6.32 | 1.1 | 4.82 | 7.15 | 9.56 | 16.4 | 4.96 | ||
| E-Caryophyllene * | 1424 | 1417 | 3.54 ± 0.01 e | 4.43 ± 0.01 d | 3.31 ± 0.01 f | 0.42 ± 0.01 i | 2.33 ± 0.01 g | 6.24 ± 0.01 b | 8.49 ± 0.01 a | 5.25 ± 0.01 c | 0.75 ± 0.01 h |
| allo-Aromadendrene | 1465 | 1458 | 1.31 ± 0.01 b | 0.81 ± 0.01 d | 0.42 ± 0.01 g | 0.68 ± 0.07 f | 1.10 ± 0.01 c | 0.71 ± 0.05 ef | 0.75 ± 0.01 e | 3.77 ± 0.01 a | 0.48 ± 0.01 g |
| Germacrene D | 1481 | 1484 | 1.67 ± 0.01 b | 0.55 ± 0.01 e | 0.76 ± 0.01 c | - | 0.65 ± 0.07 d | 0.56 ± 0.01 e | 0.32 ± 0.1 f | 0.24 ± 0.01 g | 3.73 ± 0.01 a |
| δ-Selinene | 1492 | 1492 | 1.52 ± 0.1 c | - | 1.83 ± 0.01 b | - | 0.74 ± 0.01 e | 1.64 ± 0.01 d | - | 5.12 ± 0.01 a | - |
| δ-Cadinene | 1517 | 1522 | - | - | - | - | - | - | - | 2.02 ± 0.1 | - |
| Oxygenated sesquiterpenes | 28.63 | 2.55 | 44.32 | 23.36 | 19.44 | 11.3 | 5.08 | 32.96 | 9.76 | ||
| Spathulenol | 1577 | 1577 | 0.43 ± 0.01 d | - | 1.15 ± 0.01 a | - | 0.63 ± 0.01 c | 0.82 ± 0.01 b | - | 0.57 ± 0.01 c | 0.33 ± 0.04 e |
| Caryophyllene oxide * | 1581 | 1582 | 5.75 ± 0.01 e | 1.79 ± 0.01 h | 18.16 ± 0.01 a | 13.72 ± 0.02 b | 2.27 ± 0.01 g | 8.14 ± 0.01 d | 2.43 ± 0.01 f | 18.83 ± 0.01 a | 9.08 ± 0.01 c |
| Viridiflorol | 1592 | 1592 | 1.17 ± 0.01 b | - | 0.78 ± 0.01 c | - | 2.65 ± 0.1 a | 0.34 ± 0.05 d | - | - | - |
| α-Muurolol | 1645 | 1644 | 18.75 ± 0.01 b | - | 22.45 ± 0.02 a | 9.64 ± 0.01 e | 13.11 ± 0.01 d | - | 1.88 ± 0.01 f | 10.36 ± 0.01 c | - |
| α-Bisabolol | 1685 | 1685 | 1.56 ± 0.01 b | - | 0.83 ± 0.01 c | - | - | - | - | 2.42 ± 0.01 a | 0.35 ± 0.1 d |
| α-Bisabolol oxide | 1748 | 1748 | - | - | - | - | - | - | 0.78 ± 0.01 | - | |
| Hexahydrofarnesyl acetone * | 1839 | - | 0.97 ± 0.01 a | 0.76 ± 0.01 b | 0.95 ± 0.01 a | - | 0.78 ± 0.1 b | - | 0.77 ± 0.05 b | - | - |
| Phenolic compounds | - | 0.85 | 2.01 | 38.81 | 7.87 | 1.68 | 26.65 | 9.63 | 1.56 | ||
| Thymol * | 1289 | 1289 | - | 0.85 ± 0.01 d | - | 38.81 ± 0.01 a | - | 1.03 ± 0.03 c | - | 2.54 ± 0.01 b | 0.65 ± 0.12 e |
| p-Vinyl guaicol | 1313 | 1309 | - | - | 1.15 ± 0.01 c | - | 3.11 ± 0.01 a | 0.65 ± 0.01 e | 2.42 ± 0.01 b | 0.66 ± 0.01 e | 0.91 ± 0.05 d |
| Methyl eugenol | 1403 | 1403 | - | - | 0.86 ± 0.05 d | - | 4.76 ± 0.01 c | - | 24.23 ± 0.01 a | 6.43 ± 0.01 b | - |
| Common group | 42.97 | 3.42 | 32.66 | 31.1 | 44.51 | 60.2 | 27.73 | 25.09 | 27.99 | ||
| Isopentyl acetate | 863 | 869 | 5.25 ± 0.03 a | - | - | - | - | - | - | - | 4.93 ± 0.01 b |
| Benzaldehyde | 952 | 952 | 7.86 ± 0.01 e | 1.51 ± 0.01 g | 2.11 ± 0.13 f | 15.32 ± 0.01 b | 13.05 ± 0.01 d | - | 18.52 ± 0.01 a | - | 13.32 ± 0.01 c |
| Benzene acetaldehyde | 1036 | 1036 | 19.02 ± 0.01 b | 0.43 ± 0.1 h | 5.43 ± 0.01 e | 5.77 ± 0.01 e | 10.23 ± 0.01 c | 19.52 ± 0.01 a | 3.77 ± 0.01 f | 4.33 ± 0.01 g | 6.15 ± 0.03 d |
| n-Nonanal | 1100 | 1100 | 1.57 ± 0.01 b | - | - | 0.26 ± 0.01 d | 0.65 ± 0.01 c | - | - | 3.93 ± 0.01 a | - |
| Hexyl-2-methyl butanoate | 1233 | 1229 | - | - | 2.76 ± 0.01 | - | - | - | - | - | - |
| n-Decanol | 1266 | 1266 | - | - | - | - | 0.72 ± 0.01 c | 4.64 ± 0.01 a | - | 0.42 ± 0.01 d | 1.47 ± 0.01 b |
| Bornyl acetate | 1285 | 1287 | - | - | 11.85 ± 0.01 | - | - | - | - | - | - |
| (E)-β-Damascenone | 1384 | 1383 | 0.45 ± 0.01 e | - | 3.35 ± 0.01 d | - | 8.32 ± 0.01 b | 36.04 ± 0.01 a | - | 4.95 ± 0.01 c | - |
| β-Ionone | 1487 | 1487 | 7.04 ± 0.01 c | 1.48 ± 0.01 f | 7.16 ± 0.01 c | 9.75 ± 0.01 b | 11.54 ± 0.01 a | - | 5.44 ± 0.02 d | 11.46 ± 0.01 a | 2.12 ± 0.01 e |
| Hexadecanoic acid * | 1959 | 1959 | 0.78 ± 0.07 | - | - | - | - | - | - | - | |
| Total identification (%) | 93.94 | 93.37 | 92.66 | 94.37 | 94.42 | 96.04 | 93.52 | 93.31 | 93.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazlić, M.; Kremer, D.; Akrap, K.; Topić, S.; Vuletić, N.; Dunkić, V. Extraction, Composition and Comparisons–Free Volatile Compounds from Hydrosols of Nine Veronica Taxa. Horticulturae 2023, 9, 16. https://doi.org/10.3390/horticulturae9010016
Nazlić M, Kremer D, Akrap K, Topić S, Vuletić N, Dunkić V. Extraction, Composition and Comparisons–Free Volatile Compounds from Hydrosols of Nine Veronica Taxa. Horticulturae. 2023; 9(1):16. https://doi.org/10.3390/horticulturae9010016
Chicago/Turabian StyleNazlić, Marija, Dario Kremer, Karla Akrap, Snježana Topić, Nenad Vuletić, and Valerija Dunkić. 2023. "Extraction, Composition and Comparisons–Free Volatile Compounds from Hydrosols of Nine Veronica Taxa" Horticulturae 9, no. 1: 16. https://doi.org/10.3390/horticulturae9010016
APA StyleNazlić, M., Kremer, D., Akrap, K., Topić, S., Vuletić, N., & Dunkić, V. (2023). Extraction, Composition and Comparisons–Free Volatile Compounds from Hydrosols of Nine Veronica Taxa. Horticulturae, 9(1), 16. https://doi.org/10.3390/horticulturae9010016









