Nutraceutical Potential of Seven “Quelites” Harvested in the Northern Highlands of Puebla-México
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Proximate Analysis and Micronutrient Content
2.4. Nutraceutical Content and Antioxidant Capacity
2.5. Inhibitory Activity on Key Enzymes with Therapeutic Potential
2.6. In Vitro Antimicrobial Activity on Selected Pathogens
2.7. Statistical Analysis
3. Results and Discussion
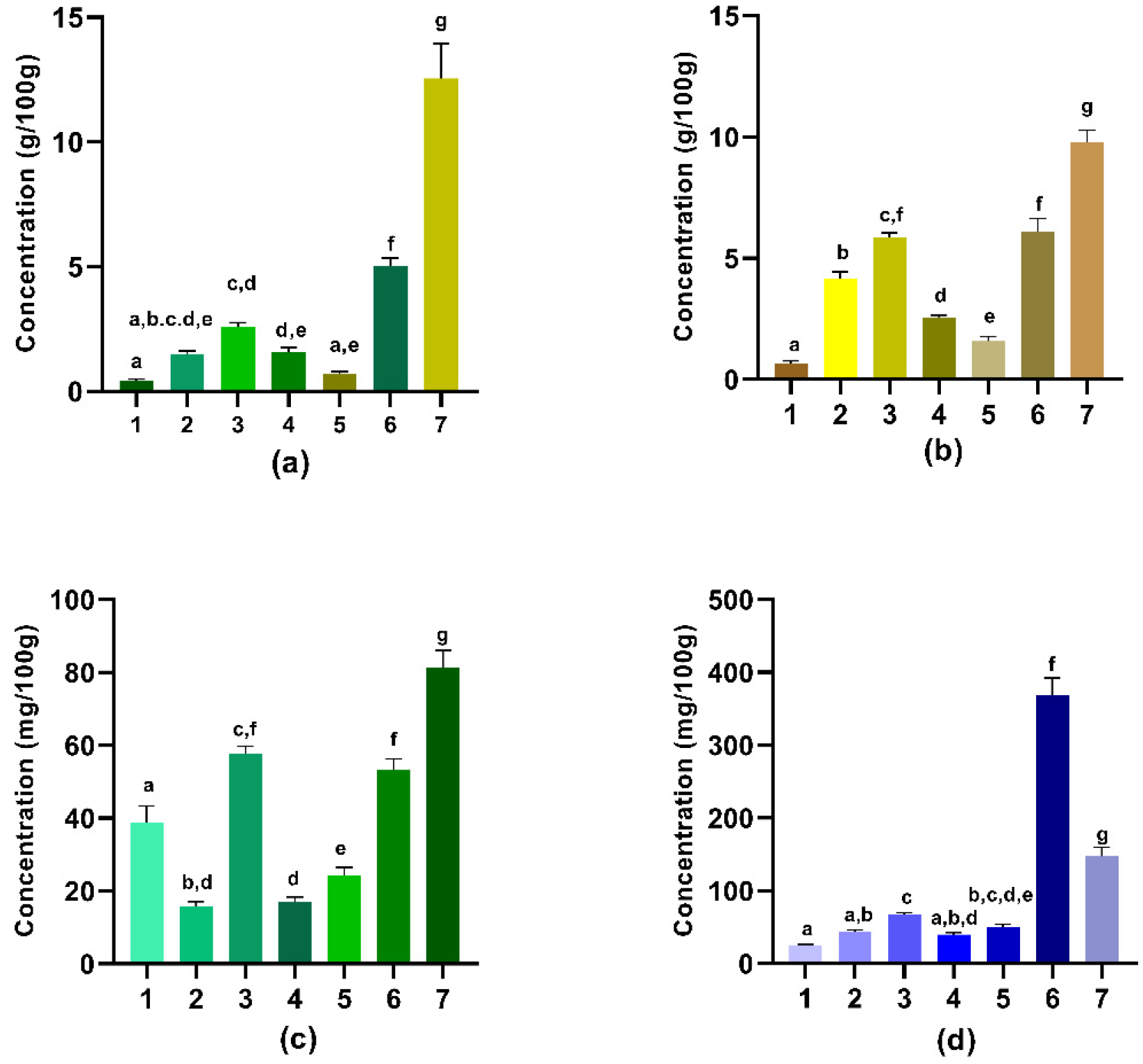
3.1. Nutritional Content
3.2. Selected Vitamin Content
3.3. Mineral Content
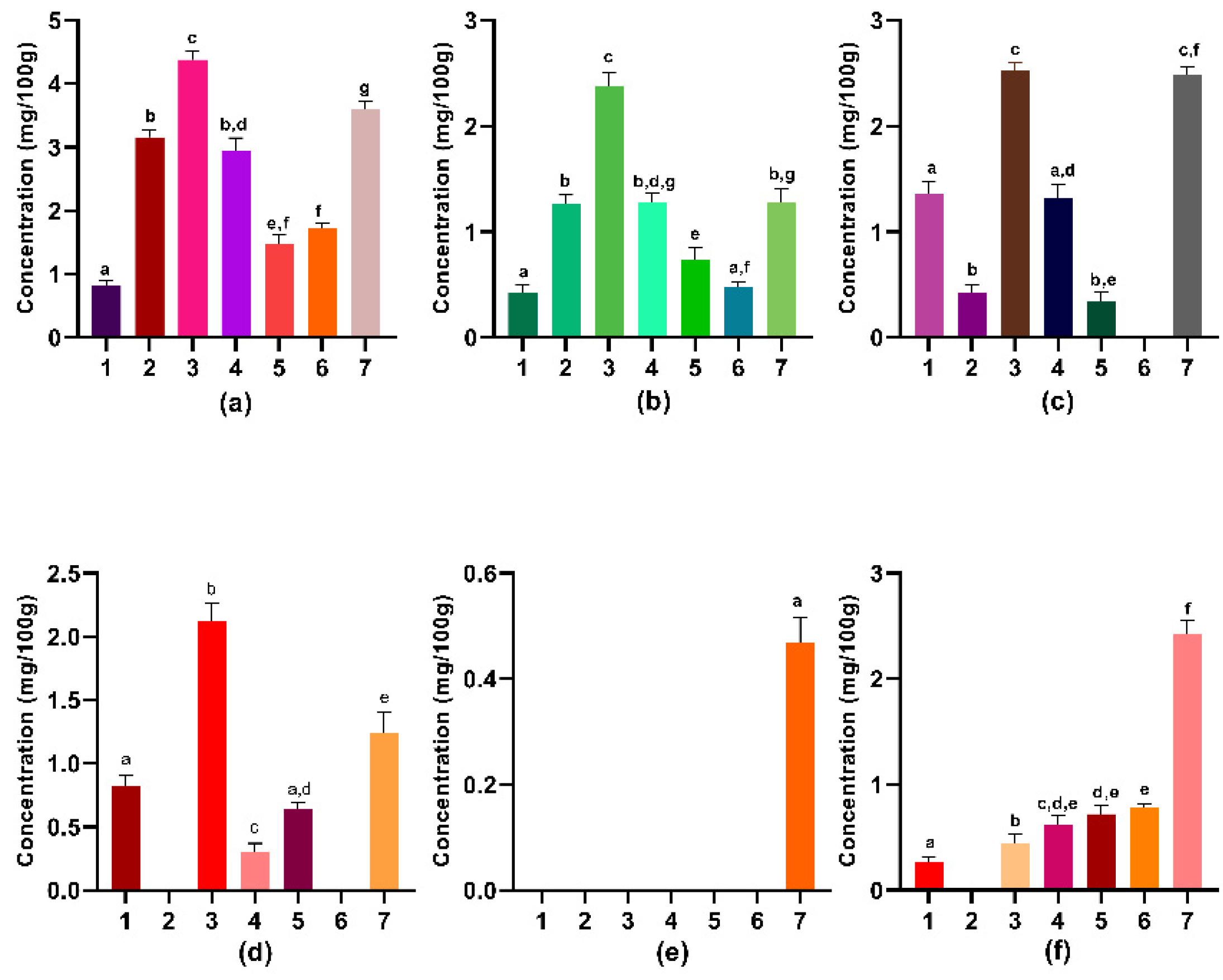
3.4. Molecules with Nutraceutical Activity
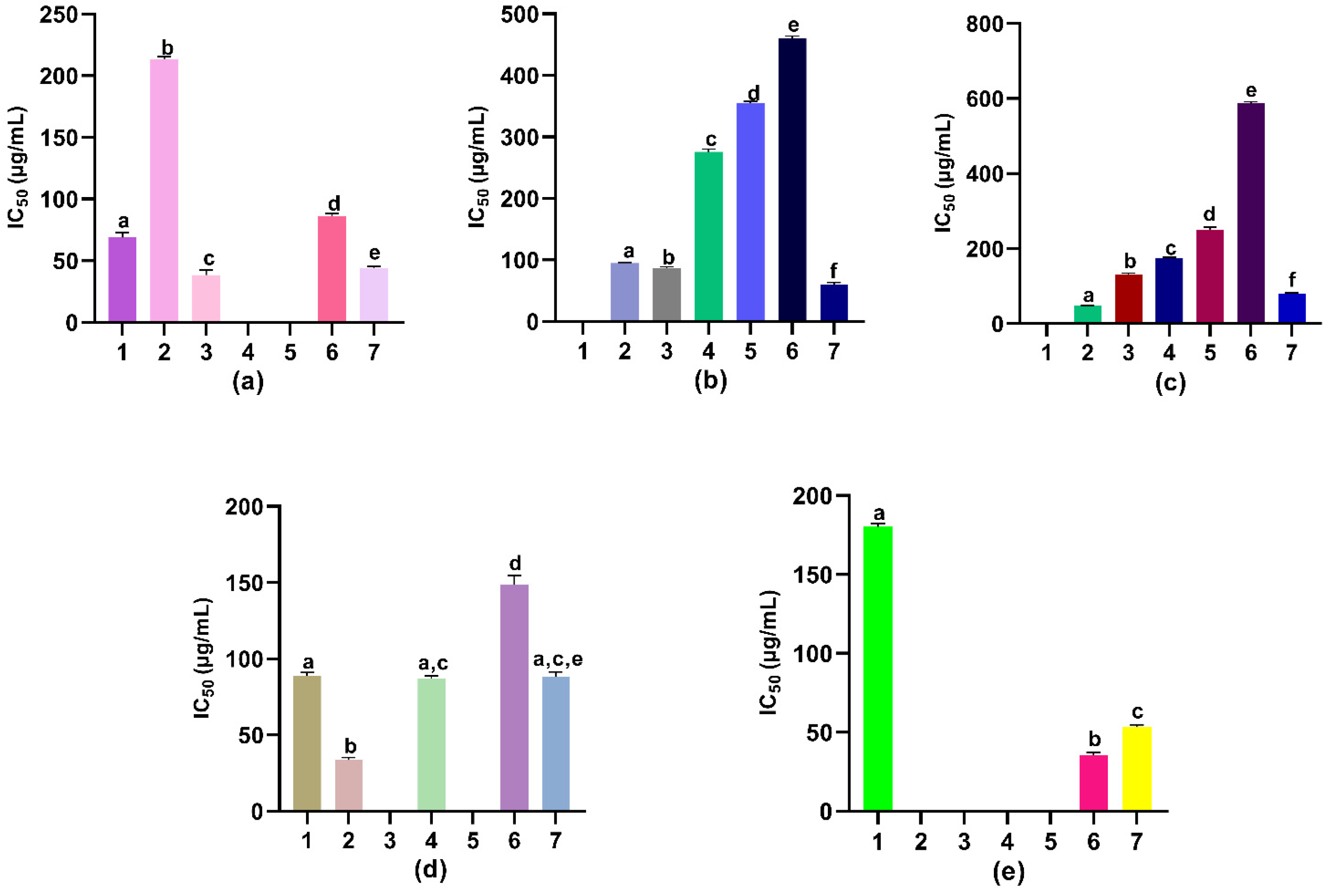
3.5. Antioxidant Potential
3.6. Inhibitory Activity on Key Enzymes
3.7. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz, M.C.; Heike Lindemann, V.K.; Paczka, O.R.; Koch, S.D. Edible greens among Nahuas and Mazatecs in a cloud forest region, state of Puebla, Mexico. In Especies Vegetales Poco Valoradas: Una Alternativa Para la Seguridad Alimentaria, 1st ed.; Mera, L.M., Castro, D., Bye, R., Eds.; UNAM: Mexico City, Mexico, 2011; pp. 85–101. [Google Scholar]
- Peña, B.F.; Alfaro, M.A.M.; Contreras, G.V. Los quelites de la Sierra Norte de Puebla, México: Inventario y formas de preparación. Bol. Soc Bot. Mex. 1998, 62, 49–62. [Google Scholar]
- Romero-Arenas, O.; Pérez-Vázquez, M.A.K.; Rivera, A.; Pacheco-Hernández, Y.; Ramírez-García, S.A.; Landeta-Cortés, G.; Villa-Ruano, N. Volatiles of Zanthoxylum limoncello as antifungal agents against the postharvest rot of manzano pepper triggered by Fusarium temperatum. Horticulturae 2022, 8, 700. [Google Scholar] [CrossRef]
- Martínez, M.A.; Evangelista, V.; Basurto, F.; Mendoza, M.; Cruz, R.A. Flora útil de los cafetales en la Sierra Norte de Puebla, México. Rev. Mex. Biodivers. 2007, 78, 15–40. [Google Scholar] [CrossRef]
- Valerino-Perea, S.; Lara-Castor, L.; Armstrong, M.E.G.; Papadaki, A. Definition of the traditional Mexican diet and its role in health: A systematic review. Nutrients 2019, 11, 2803. [Google Scholar] [CrossRef] [PubMed]
- Santiago, F.H.; Moreno, J.P.; Cázares, B.X.; Suárez, J.J.A.; Trejo, E.O.; Montes de Oca, G.M.; Aguilar, I.D. Traditional knowledge and use of wild mushrooms by Mixtecs or Ñuu savi, the people of the rain, from Southeastern Mexico. J. Ethnobiol. Ethnomed. 2016, 12, 35. [Google Scholar] [CrossRef]
- Basto-Abreu, A.; López-Olmedo, N.; Rojas-Martínez, R.; Aguilar-Salinas, C.A.; De la Cruz-Góngora, V.; Rivera-Dommarco, J.; Shamah-Levy, T.; Romero-Martínez, M.; Barquera, S.; Villalpando, S.; et al. Prevalence of diabetes and glycemic control in Mexico: National results from 2018 and 2020. Salud Publ. Mex. 2021, 63, 725–733. [Google Scholar] [CrossRef]
- Padilla-Raygoza, N.; Monroy-Torres, R.; Sandoval-Salazar, C.; Vera-Becerra, L.E.; Patiño-López, M.E.; García-Campos, M.L.; Beltrán Campos, V.; Ortega Jiménez, M.C.; Delgado-Sandoval, S.C.; Ramirez-Gomez, X.S.; et al. Cancer prevention programmes in Mexico: Are we doing enough? Ecancer 2020, 14, 997. [Google Scholar] [CrossRef]
- Caponio, G.R.; Lippolis, T.; Tutino, V.; Gigante, I.; De Nunzio, V.; Milella, R.A.; Gasparro, M.; Notarnicola, M. Nutraceuticals: Focus on anti-Inflammatory, anti-Cancer, antioxidant properties in gastrointestinal tract. Antioxidants 2022, 11, 1274. [Google Scholar] [CrossRef]
- Caponio, G.R.; Cofano, M.; Lippolis, T.; Gigante, I.; De Nunzio, V.; Difonzo, G.; Noviello, M.; Tarricone, L.; Gambacorta, G.; Giannelli, G.; et al. Anti-proliferative and pro-Apoptotic effects of digested aglianico grape pomace extract in human colorectal cancer cells. Molecules 2022, 27, 6791. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Pacheco-Hernández, Y.; Cruz-Duran, R.; Lozoya-Gloria, E.; Betancourt-Jiménez, M.B. Seasonal variation in phytochemicals and nutraceutical potential of Begonia nelumbiifolia consumed in Puebla, México. J. Food Sci. Technol. 2017, 54, 1484–1490. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Pacheco-Hernández, Y.; Zárate-Reyes, J.A.; Becerra-Martínez, E.; Lozoya-Gloria, E.; Cruz-Durán. Nutraceutical potential and hypolipidemic properties of the volatiles from the edible leaves of Peperomia maculosa. J. Food Biochem. 2018, 42, e12650. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Pacheco-Hernández, Y.; Zárate-Reyes, J.A.; Cruz-Durán, R.; Lozoya-Gloria, E. Volatile composition and biological activities of the leaf essential oil from Zanthoxylum limoncello grown in Oaxaca, México. Chem. Biodivers. 2019, 16, e1800498. [Google Scholar] [CrossRef] [PubMed]
- Meier, U. Growth Stage of Mono- and Dicotyledoneous Plants-BBCH Monograph, 2nd ed.; Federal Biological Research Centre for Agriculture and Forestry: Grossbeeren, Germany, 2001. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Gaithersburg, MA, USA, 2019. [Google Scholar]
- Awika, J.M.; Rooney, L.W.; Wu, X.; Prior, R.L.; Cisneros-Zevallos, L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J. Agric. Food Chem. 2003, 51, 6657–6662. [Google Scholar] [CrossRef] [PubMed]
- Coyotl-Pérez, W.; Rubio-Rosas, E.; Morales-Rabanales, N.; Ramírez-García, S.A.; Pacheco-Hernández, Y.; Pérez-España, V.H.; Romero-Arenas, O.; Villa-Ruano, N. Improving the shelf life of avocado fruit against Clonostachys rosea with chitosan hybrid films containing thyme essential oil. Polymers 2022, 14, 2050. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Pacheco-Hernández, Y.; Rubio-Rosas, E.; Zárate-Reyes, J.A.; Lozoya-Gloria, E.; Cruz-Durán, R. Chemical profile, nutraceutical and anti-phytobacterial properties of the essential oil from Dalea foliolosa (Fabaceae). Emir. J. Food Agric. 2017, 29, 724–728. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Pacheco-Hernández, Y.; Zurita-Vásquez, G.; Betancourt-Jiménez, M.B.; Cruz-Duran, R.; Duque-Bautista, O. Anti-lipase and antioxidant properties of 30 medicinal plants used in Oaxaca México. Biol. Res. 2013, 46, 153–160. [Google Scholar] [CrossRef]
- García-Herrera, P.; Morales, P.; Cámara, M.; Fernández-Ruiz, V.; Tardío, J.; Sánchez-Mata, M.C. Nutritional and phytochemical composition of Mediterranean wild vegetables after culinary treatment. Foods 2020, 9, 1761. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Hess, J.M.; Gould, T.J.; Slavin, J.L. Prebiotic dietary fiber and gut health: Comparing the in vitro fermentations beta-glucan, inulin and xylooligosaccharide. Nutrients 2020, 9, 1361. [Google Scholar] [CrossRef]
- Baskan, S.; Daut-Ozdemir, A.; Gunaydın, K.; Erim, F.B. Analysis of anthraquinones in Rumex crispus by micellar electrokinetic chromatography. Talanta 2007, 71, 747–750. [Google Scholar] [CrossRef]
- Shivembe, A.; Ojinnaka, D. Determination of vitamin C and total phenolic in fresh and freeze dried blueberries and the antioxidant capacity of their extracts. Integr. Food Nutr. Metab. 2017, 4, 1–5. [Google Scholar] [CrossRef]
- Orkusz, A. Edible insects versus meat—Nutritional comparison: Knowledge of their composition is the key to good health. Nutrients. Nutrients 2021, 13, 1207. [Google Scholar] [CrossRef] [PubMed]
- Koseoglu, S.Z.A. Determination and evaluation of the pyridoxal, pyridoxine, and pyridoxamine forms of vitamin B6 in plant-based foods in terms of healthy vegetarian nutrition. Progr. Nut. 2020, 22, e2020015. [Google Scholar] [CrossRef]
- Shohag, M.J.I.; Wei, Y.Y.; Yu, N.; Zhang, J.; Wang, K.; Patring, J.; He, Z.L.; Yang, X.E. Natural variation of folate content and composition in spinach (Spinacia oleracea) germplasm. J. Agric. Food Chem. 2011, 59, 12520–12526. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.L.G.; Martínez, J.G.; Isasa, M.E.T. Mineral nutrient composition of edible wild plants. J. Food Comp. Anal. 1998, 11, 322–328. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Ventura Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, J.; Ci, F.F.; Pang, H.; Cheng, N.; Xing, A. α-Carotene: A valuable carotenoid in biological and medical research. J. Sci. Food Agric. 2022, 102, 5606–5617. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 101–107. [Google Scholar] [CrossRef]
- Kusumah, D.; Wakui, M.; Murakami, M.; Xie, X.; Yukihito, K.; Maeda, I. Linoleic acid, α-linolenic acid, and monolinolenins as antibacterial substances in the heat-processed soybean fermented with Rhizopus oligosporus. Biosci. Biotechnol. Biochem. 2020, 84, 1285–1290. [Google Scholar] [CrossRef]
- Vidrih, R.; Filip, S.; Hribar, J. Content of higher fatty acids in green vegetables. Czech, J. Food Sci. 2009, 27, S125–S129. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nut. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Grijalva, E.P.; Zamudio-Sosa, V.E.; Contreras-Angulo, L.A.; Leyva-López, N.; Heredia, J.B. Bioaccessibility of phenolic compounds from mistletoe infusions and effect of in vitro digestion on its antioxidant and pancreatic lipase inhibitory Activity. Foods 2022, 23, 3319. [Google Scholar] [CrossRef] [PubMed]
- Caponio, G.R.; Noviello, M.; Calabrese, F.M.; Gambacorta, G.; Giannelli, G.; De Angelis, M. Effects of grape pomace polyphenols and in vitro gastrointestinal digestion on antimicrobial activity: Recovery of bioactive compounds. Antioxidants 2022, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.; Ryden, P.; Edwards, C.H.; Grundy, M.M.-L. Plant cell walls: Impact on nutrient bioaccessibility and digestibility. Foods 2020, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Poovitha, S.; Parani, M. In vitro and in vivo α-amylase and α-glucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L.). BMC Complement. Med. Ther. 2016, 16, 185. [Google Scholar] [CrossRef]
- Baskaran, G.; Salvamani, S.; Ahmad, S.A.; Shaharuddin, N.A.; Pattiram, P.D.; Shukor, M.Y. HMG-CoA reductase inhibitory activity and phytocomponent investigation of Basella alba leaf extract as a treatment for hypercholesterolemia. Drug. Des. Devel. Ther. 2015, 14, 509–517. [Google Scholar] [CrossRef]
- Wei, H.; Tye, L.; Bresnick, E.; Birt, D.F. Inhibitory effect of apigenin, a plant Flavonoid, on epidermal ornithine decarboxylase and skin tumor promotion in mice. Cancer Res. 1990, 50, 499–502. [Google Scholar]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]





| Quelite | Scientific Name | Edible Organ | Harvesting Stage * | Collection Date |
|---|---|---|---|---|
| Tepeilite | Rhamnus pompana | Berries | Stage 8 of BBCH-Scale | August 2021 |
| Hoja santa | Piper auritum | Fresh leaves | Stage 5 of BBCH-Scale | February 2021 |
| Hierba mora | Solanum nigrescens | Berries | Stage 8 of BBCH-Scale | May 2021 |
| Quintoniles | Amarathus hybridus | Fresh leaves | Stage 5 of BBCH-Scale | February 2021 |
| Guias de erizo | Sechium edule | Terminal stalks with tendrils | Anthesis | June 2021 |
| Lengua de vaca | Rumex obstusifolia | Fresh leaves | Stage 5 of BBCH-Scale | October 2021 |
| Flor de Izote | Yucca aloifolia | Young inflorescences | Anthesis | November 2021 |
| S. nigrescen | S. edule | R. obtusifolius | A. hybridus | P. auritum | R. pompana | Y. aloifolia | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | AE | EE | AE | EE | AE | EE | AE | EE | AE | EE | AE | EE | AE | EE |
| Escherichia coli ATCC 25922 | >500 | >500 | 457.2 | 361.5 | 472.8 | 341.7 | 497.2 | 398.5 | >500 | >500 | >500 | 346.9 | 376.2 | 246.8 |
| Escherichia coli ATCC 11303 | >500 | >500 | 379.8 | 216.7 | 227.1 | 187.3 | >500 | >500 | >500 | >500 | >500 | >500 | 444.6 | 385.1 |
| Staphylococcus aureus ATCC 25923 | 251.3 | 132.6 | >500 | >500 | 189.4 | 111.6 | 267.8 | 211.7 | >500 | >500 | 376.8 | 281.5 | 265.1 | 116.3 |
| Enterococcus faecalis ATCC 29212 | 333.7 | 258.1 | >500 | >500 | 236.9 | 91.3 | >500 | >500 | >500 | >500 | 255.3 | 459.1 | >500 | >500 |
| Salmonella typhi ATCC 6539 | >500 | >500 | >500 | >500 | >500 | >500 | 461.8 | 345.9 | >500 | >500 | >500 | >500 | 473.9 | 399.7 |
| Candida albicans ATCC 90028 | 125.8 | 86.4 | 365.8 | 244.3 | 156.9 | 78.3 | >500 | >500 | 471.4 | 358.2 | 211.5 | 184.2 | 198.2 | 101.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco-Hernández, Y.; Lozoya-Gloria, E.; Becerra-Martínez, E.; Villa-Ruano, N. Nutraceutical Potential of Seven “Quelites” Harvested in the Northern Highlands of Puebla-México. Horticulturae 2023, 9, 18. https://doi.org/10.3390/horticulturae9010018
Pacheco-Hernández Y, Lozoya-Gloria E, Becerra-Martínez E, Villa-Ruano N. Nutraceutical Potential of Seven “Quelites” Harvested in the Northern Highlands of Puebla-México. Horticulturae. 2023; 9(1):18. https://doi.org/10.3390/horticulturae9010018
Chicago/Turabian StylePacheco-Hernández, Yesenia, Edmundo Lozoya-Gloria, Elvia Becerra-Martínez, and Nemesio Villa-Ruano. 2023. "Nutraceutical Potential of Seven “Quelites” Harvested in the Northern Highlands of Puebla-México" Horticulturae 9, no. 1: 18. https://doi.org/10.3390/horticulturae9010018
APA StylePacheco-Hernández, Y., Lozoya-Gloria, E., Becerra-Martínez, E., & Villa-Ruano, N. (2023). Nutraceutical Potential of Seven “Quelites” Harvested in the Northern Highlands of Puebla-México. Horticulturae, 9(1), 18. https://doi.org/10.3390/horticulturae9010018








