Whole-Canopy Photosynthetic Characterization of Apple Tree and the Effects Induced by Grafting on Rootstocks with Different Vigor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Culture Conditions
2.2. Growth Parameters
2.3. Leaf Photosynthetic Characterizations
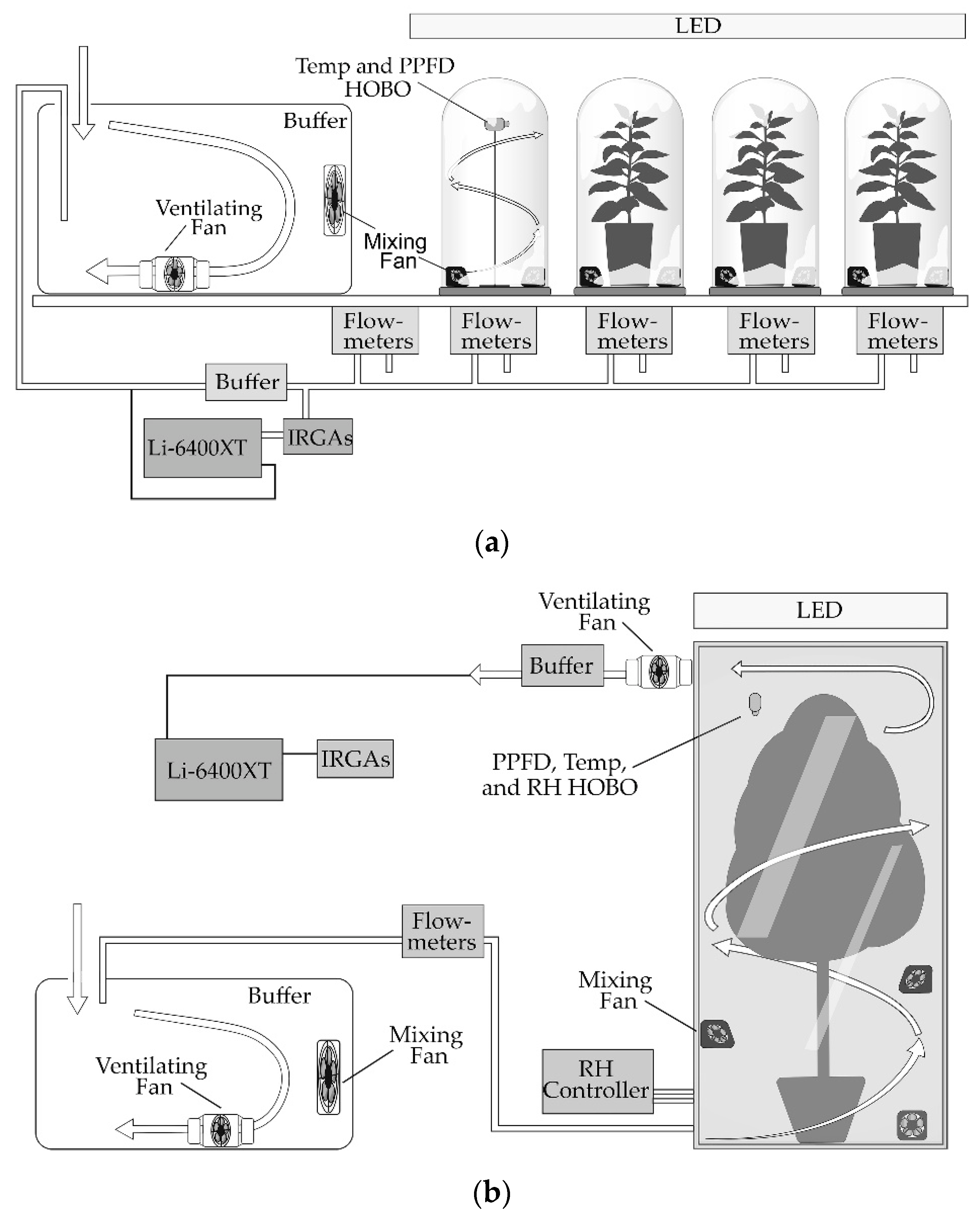
2.4. Canopy Photosynthetic Characterization
2.5. Statistical Analysis
3. Results
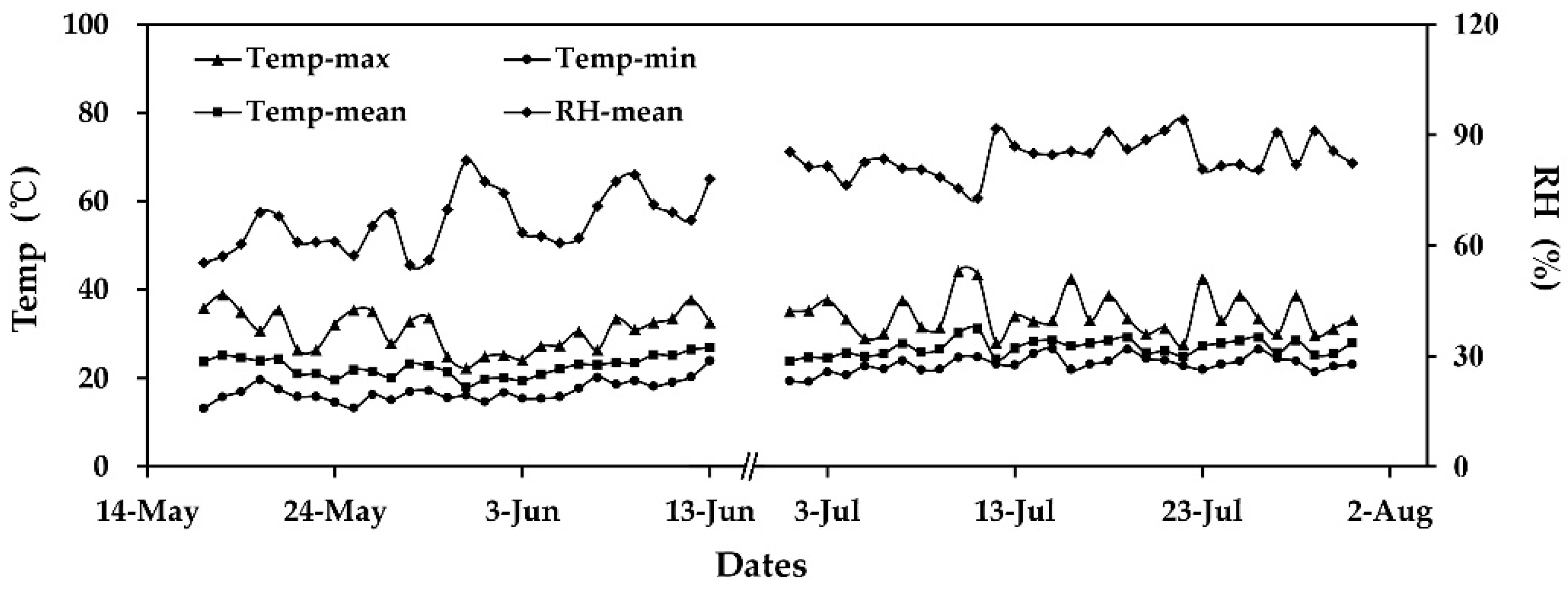
3.1. Environmental Condition
3.2. Photosynthate Accumulation of M.9 Clones Based on Leaf and Canopy Photosynthesis
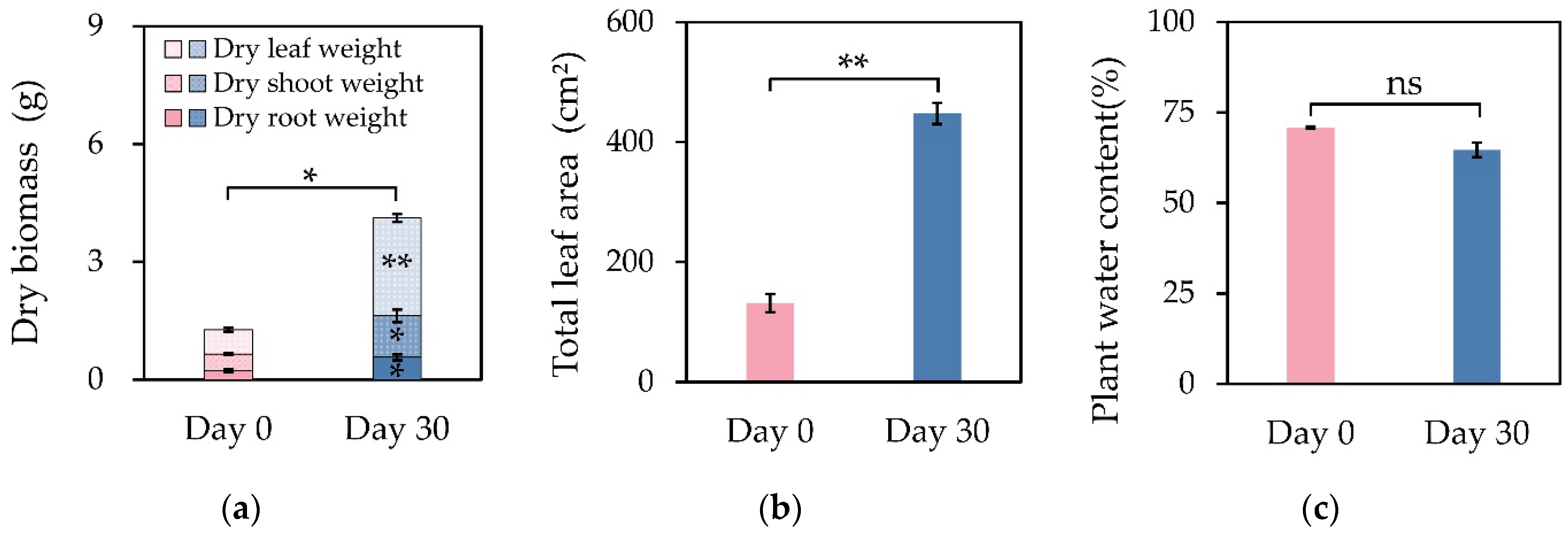
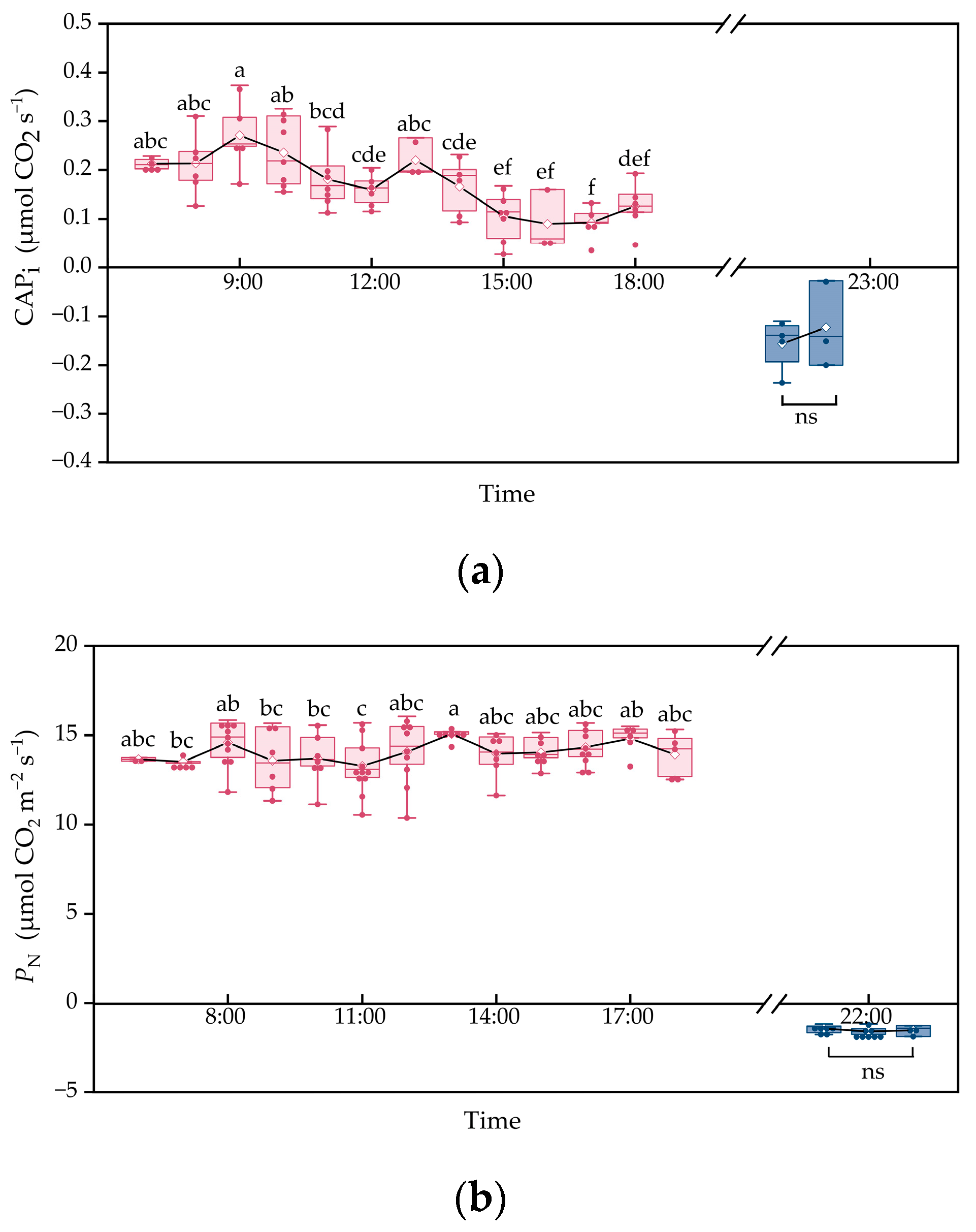
- The dry mass and total leaf area of M.9 clones increased significantly within the 30 days, whereas the plant water content showed no evident change (Figure 3). The CAPi fluctuated over time throughout the day, with a variation of 201.8%, and peaked in the forenoon, with a value of 0.27 μmol CO2 s−1. During the night, the CAPi was maintained at around −0.14 μmol CO2 s−1 (Figure 4a). The PN of functional leaves varied 13.62 % throughout the day, and the respiration rates were about −1.47 μmol CO2 m−2 s−1 during the night (Figure 4b). Compared to the gained dry mass of 2.85 g per plant, the simulated photosynthate based on accumulated leaf photosynthesis was 20.39 g per plant, 231.7 times higher than that simulated by CAPi (3.02 g).
3.3. Leaf and Canopy Photosynthetic Parameters of Scions Influenced by Rootstocks
- Figure 8a visualized the correlation matrix of plant growth and photosynthetic parameters via a correlation heat map. CAPi was positively correlated with gs (r = 0.965, p = 0.044, two-tailed), PN (r = 0.988, p = 0.012, two-tailed), total leaf area (r = 0.972, p = 0.028, two-tailed), and canopy width (r = 0.999, p = 0.001, two-tailed). PN was positively correlated with gs (r = 0.962, p = 0.038, two-tailed), E (r = 0.960, p = 0.040, two-tailed), CE (r = 0.935, p = 0.047, two-tailed), CAPi (r = 0.988, p = 0.012, two-tailed), total leaf area (r = 0.966, p = 0.034, two-tailed), and canopy width (r = 0.986, p = 0.033, two-tailed). According to PCA in Figure 8b, two main comprehensive evaluation factors accounting for 85.3% variation were extracted, including PN, gs, E, total leaf area, and canopy width in PC1, which explained 69% of the total variation, and CAPi, CE, LS, Ci, WUE and plant height in PC2, which explained the 16.3% of the total variation.
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brazel, A.J.; O’Maoileidigh, D.S. Photosynthetic activity of reproductive organs. J. Exp. Bot. 2019, 70, 1737–1754. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.X.; Wang, X.C.; Ding, Y.Q.; Lu, W.; Sun, Y. Remote Measurement of Apple Orchard Canopy Information Using Unmanned Aerial Vehicle Photogrammetry. Agronomy 2019, 9, 774. [Google Scholar] [CrossRef]
- Matloobi, M.; Ebrahimzadeh, A.; Khaligi, A.; Hasandokht, M. Training system affects whole canopy photosynthesis of the greenhouse roses (Rosa hybrida ‘Habari’). J. Food Agric. Environ. 2009, 7, 114–117. [Google Scholar]
- Sellin, A.; Taneda, H.; Alber, M. Leaf structural and hydraulic adjustment with respect to air humidity and canopy position in silver birch (Betula pendula). J. Plant Res. 2019, 132, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.S.; Norman, J.M. The description and measurement of plant canopy structure. In Plant Canopies: Their Growth, Form, and Function; Marshall, B., Jarvis, P.G., Eds.; Cambridge University Press: Cambridge, UK, 1989; pp. 1–20. [Google Scholar]
- Zhang, L.X.; Guo, Q.S.; Chang, Q.S.; Zhu, Z.B.; Liu, L.; Chen, Y.H. Chloroplast ultrastructure, photosynthesis and accumulation of secondary metabolites in Glechoma longituba in response to irradiance. Photosynthetica 2015, 53, 144–153. [Google Scholar] [CrossRef]
- Ge, Z.M.; Zhou, X.; Kellomäki, S.; Wang, K.Y.; Peltola, H.; Martikainen, P.J. Responses of leaf photosynthesis, pigments and chlorophyll fluorescence within canopy position in a boreal grass (Phalaris arundinacea L.) to elevated temperature and CO2 under varying water regimes. Photosynthetica 2011, 49, 172–184. [Google Scholar] [CrossRef]
- Inoue, T.; Sunaga, M.; Ito, M.; Yuchen, Q.; Matsushima, Y.; Sakoda, K.; Yamori, W. Minimizing VPD Fluctuations Maintains Higher Stomatal Conductance and Photosynthesis, Resulting in Improvement of Plant Growth in Lettuce. Front. Plant Sci. 2021, 12, 646144. [Google Scholar] [CrossRef]
- Hiratsuka, S.; Suzuki, M.; Nishimura, H.; Nada, K. Fruit photosynthesis in Satsuma mandarin. Plant Sci. 2015, 241, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.F. Apical control of branch growth and angle in woody plants. Am. J. Bot. 2000, 87, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Vemmos, S.; Goldwin, G. The Photosynthetic Activity of Cox’s Orange Pippin Apple Flowers in Relation to Fruit Setting. Ann. Bot. 1994, 73, 385–391. [Google Scholar] [CrossRef]
- Prieto, J.A.; Louarn, G.; Perez Pena, J.; Ojeda, H.; Simonneau, T.; Lebon, E. A functional-structural plant model that simulates whole- canopy gas exchange of grapevine plants (Vitis vinifera L.) under different training systems. Ann. Bot. 2020, 126, 647–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayat, F.; Iqbal, S.; Coulibaly, D.; Razzaq, M.K.; Nawaz, M.A.; Jiang, W.; Shi, T.; Gao, Z. An insight into dwarfing mechanism: Contribution of scion-rootstock interactions toward fruit crop improvement. Fruit Res. 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, J.; Wu, Y.; Huang, R.; Chang, Y.; Lei, X.; Song, X.; Pei, D. Possibility of Increasing the Growth and Photosynthetic Properties of Precocious Walnut by Grafting. Sustainability 2020, 12, 5178. [Google Scholar] [CrossRef]
- Sotiropoulos, Τ.E. Performance of the apple (Malus domestica Borkh) cultivar Imperial Double Red Delicious grafted on five rootstocks. Hortic. Sci. 2008, 35, 7–11. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, M.; Yao, J.; Zhou, Y.; Wang, Y.; Zhang, X.; Li, W.; Wu, T.; Han, Z.; Xu, X.; et al. Root architecture characteristics of differing size-controlling rootstocks and the influence on the growth of ‘Red Fuji’ apple trees. Sci. Hortic. 2021, 281, 109959. [Google Scholar] [CrossRef]
- Bagley, J.; Rosenthal, D.M.; Ruiz-Vera, U.M.; Siebers, M.H.; Kumar, P.; Ort, D.R.; Bernacchi, C.J. The influence of photosynthetic acclimation to rising CO2 and warmer temperatures on leaf and canopy photosynthesis models. Glob. Biogeochem. 2015, 29, 194–206. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, X.; Yao, J.; Zhang, Z.; Wang, Y.; Zhang, X.; Li, W.; Wu, T.; Han, Z.; Xu, X.; et al. Morphological and photosynthetic responses differ among eight apple scion-rootstock combinations. Sci. Hortic. 2020, 261, 108981. [Google Scholar] [CrossRef]
- Grau, A. A closed chamber technique for field measurement of gas exchange of forage canopies. N. Z. J. Agric. Res. 2010, 38, 71–77. [Google Scholar] [CrossRef]
- Sinoquet, H.; Le Roux, X.; Adam, B.; Ameglio, T.; Daudet, F.A. RATP: A model for simulating the spatial distribution of radiation absorption, transpiration and photosynthesis within canopies: Application to an isolated tree crown. Plant Cell Environ. 2001, 24, 395–406. [Google Scholar] [CrossRef]
- Takahashi, N.; Ling, P.P.; Frantz, J.M. Considerations for Accurate Whole Plant Photosynthesis Measurement. Environ. Control Biol. 2008, 46, 91–101. [Google Scholar] [CrossRef]
- Song, Q.; Xiao, H.; Xiao, X.; Zhu, X.-G. A new canopy photosynthesis and transpiration measurement system (CAPTS) for canopy gas exchange research. Agric. For. Meteorol. 2016, 217, 101–107. [Google Scholar] [CrossRef]
- Li, K.-T.; Lakso, A.N.; Piccioni, R.; Robinson, T. Summer pruning reduces whole-canopy carbon fixation and transpiration in apple trees. J. Hortic. Sci. Biotechnol. 2003, 78, 749–754. [Google Scholar] [CrossRef]
- Untiedt, R.; Blanke, M.M. Effects of fungicide and insecticide mixtures on apple tree canopy photosynthesis, dark respiration and carbon economy. Crop Prot. 2004, 23, 1001–1006. [Google Scholar] [CrossRef]
- Li, P.; Weng, J.; Zhang, Q.; Yu, L.; Yao, Q.; Chang, L.; Niu, Q. Physiological and Biochemical Responses of Cucumis melo L. Chloroplasts to Low-Phosphate Stress. Front. Plant Sci. 2018, 9, 1525. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Pan, D.; Zhang, Y.; Luo, B.; Ji, J. Effects of drought stress on photosynthesis and chlorophyll fluorescence images of soybean (Glycine max) seedlings. Int. J. Agric. Biol. Eng. 2018, 11, 196–201. [Google Scholar] [CrossRef]
- Thornley, J.H.M. Dynamic Model of Leaf Photosynthesis with Acclimation to Light and Nitrogen. Ann. Bot. 1998, 81, 421–430. [Google Scholar] [CrossRef]
- Burgess, A.J.; Gibbs, J.A.; Murchie, E.H. A canopy conundrum: Can wind-induced movement help to increase crop productivity by relieving photosynthetic limitations? J. Exp. Bot. 2019, 70, 2371–2380. [Google Scholar] [CrossRef]
- Tang, Y.; Rosewarne, G.M.; Li, C.; Wu, X.; Yang, W.; Wu, C. Physiological Factors Underpinning Grain Yield Improvements of Synthetic-Derived Wheat in Southwestern China. Crop Prot. 2015, 55, 98–112. [Google Scholar] [CrossRef]
- Salvatori, N.; Giorgio, A.; Muller, O.; Rascher, U.; Peressotti, A. A low-cost automated growth chamber system for continuous measurements of gas exchange at canopy scale in dynamic conditions. Plant Methods 2021, 17, 69. [Google Scholar] [CrossRef]
- Singsaas, E.L.; Ort, D.R.; DeLucia, E.H. Diurnal regulation of photosynthesis in understory saplings. New Phytol. 2000, 145, 39–49. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C.; Santos, M.G.; Oliveira, R.F. Photosynthesis and water relations of well-watered orange plants as affected by winter and summer conditions. Photosynthetica 2009, 47, 215–222. [Google Scholar] [CrossRef]
- Klages, K.; Donnison, H.; Wünsche, J.; Boldingh, H. Diurnal changes in non-structural carbohydrates in leaves, phloem exudate and fruit in ‘Braeburn’ apple. Funct. Plant Biol. 2001, 28, 131–139. [Google Scholar] [CrossRef]
- Boonman, A.; Prinsen, E.; Gilmer, F.; Schurr, U.; Peeters, A.J.; Voesenek, L.A.; Pons, T.L. Cytokinin import rate as a signal for photosynthetic acclimation to canopy light gradients. Plant Physiol. 2007, 143, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Whitney, S. Photosynthesis: Getting it together for CO2 fixation. Nat. Plants 2015, 1, 15130. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, C.; Kilburn, M.R.; Clode, P.L.; Fuchslueger, L.; Koranda, M.; Cliff, J.B.; Solaiman, Z.M.; Murphy, D.V. Exploring the transfer of recent plant photosynthates to soil microbes: Mycorrhizal pathway vs direct root exudation. New Phytol. 2015, 205, 1537–1551. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Else, M. Understanding how rootstocks dwarf fruit trees. Compact Fruit Tree 2001, 34, 46–49. [Google Scholar]
- Goncalves, B.; Moutinho-Pereira, J.; Santos, A.; Silva, A.P.; Bacelar, E.; Correia, C.; Rosa, E. Scion-rootstock interaction affects the physiology and fruit quality of sweet cherry. Tree Physiol. 2006, 26, 93–104. [Google Scholar] [CrossRef]
- Yang, W.J.; Chen, H.F.; Zhu, F.Y.; Hu, M.Q.; Jiang, D.A. Low concentration of bisulfite enhances photosynthesis in tea tree by promoting carboxylation efficiency in leaves. Photosynthetica 2008, 46, 615–617. [Google Scholar] [CrossRef]
- Lu, Z.; Pan, Y.; Hu, W.; Cong, R.; Ren, T.; Guo, S.; Lu, J. The photosynthetic and structural differences between leaves and siliques of Brassica napus exposed to potassium deficiency. BMC Plant Biol. 2017, 17, 240. [Google Scholar] [CrossRef]
- Long, S.P.; Farage, P.K.; Garcia, R.L. Measurement of leaf and canopy photosynthetic CO2 exchange in the field. J. Exp. Bot. 1996, 47, 1629–1642. [Google Scholar] [CrossRef]
- Ma, X.; Feng, J.; Guan, H.; Liu, G. Prediction of Chlorophyll Content in Different Light Areas of Apple Tree Canopies based on the Color Characteristics of 3D Reconstruction. Remote Sens. 2018, 10, 429. [Google Scholar] [CrossRef]
- Vu, J.C.V.; Newman, Y.C.; Allen, L.H.; Gallo-Meagher, M.; Zhang, M.Q. Photosynthetic acclimation of young sweet orange trees to elevated growth CO2 and temperature. Tree Physiol. 2002, 159, 147–157. [Google Scholar] [CrossRef]
- Dai, Y.; Dickinson, R.E.; Wang, Y.P. A Two-Big-Leaf Model for Canopy Temperature, Photosynthesis, and Stomatal Conductance. J. Clim. 2004, 17, 2281–2299. [Google Scholar] [CrossRef]
- Talwara, S.; Grout, B.W.W.; Toldam-Andersen, T.B. Leaf Micro-Environment Influences the Altered Foliar Phenotype of Columnar Apple (Malus × Domestica Borkh.) Trees. Acta Hortic. 2015, 1099, 835–841. [Google Scholar] [CrossRef]
- Blanke, M.M.; Lenz, F. Fruit photosynthesis. Plant Cell Environ. 1989, 12, 31–46. [Google Scholar] [CrossRef]
- DeJong, T.M.; Walton, E.F. Carbohydrate requirements of peach fruit growth and respiration. Tree Physiol. 1989, 5, 329–335. [Google Scholar] [CrossRef]
- Dikilitas, M.; Guldur, M.E.; Deryaoglu, A.; Erel, O. Antioxidant and Oxidant Levels of Pepper (Capsicum annuum cv. ‘Charlee’) Infected with Pepper Mild Mottle Virus. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 58–63. [Google Scholar] [CrossRef] [Green Version]




| Rootstock | Latin Name | Dwarfing Class | Origin | Parentage |
|---|---|---|---|---|
| M.9 | Malus pumila Mill. | Dwarfing | East Malling Research Station, Kent, United Kingdom | Natural seedlings |
| M.26 | Malus pumila Mill. | Semi-dwarfing | East Malling Research Station, Kent, United Kingdom | M.16 × M.9 |
| Chistock-1 | Malus xiaojinensis | Semi-dwarfing | China Agricultural University, Beijing, China | Natural seedlings |
| Baleng | Malus robusta Rehd. | Vigorous | Huailai, Hebei, China | Natural seedlings |
| Rootstock | Plant Height (cm) | Canopy Width (cm) | Total Leaf Area (cm2) | SPAD | ||||
|---|---|---|---|---|---|---|---|---|
| M.9 | 137.4 ± 4.3 | c | 58.4 ± 10.2 | b | 1796.8 ± 72.1 | c | 45.7 ± 2.4 | c |
| M.26 | 148.2 ± 7.6 | bc | 76.4 ± 10.4 | ab | 2790.0 ± 281.6 | b | 47.6 ± 0.6 | bc |
| Chistock-1 | 157.3 ± 3.3 | b | 83.3 ± 4.6 | ab | 3580.0 ± 235.9 | ab | 51.5 ± 0.9 | ab |
| Baleng | 179.1 ± 2.5 | a | 93.3 ± 8.7 | a | 3829.9 ± 425.3 | a | 56.7 ± 0.5 | a |
| Rootstock | PNMAX (μmol CO2 m−2 s−1) | RD (μmol CO2 m−2 s−1) | LCP (μmol m−2 s−1) | LSP (μmol m−2 s−1) | φ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M.9 | 15.2 ± 0.5 | b | 0.62 ± 0.12 | c | 17.3 ± 2.7 | b | 1137.3 ± 59.6 | a | 0.037 ± 0.004 | b |
| M.26 | 15.2 ± 0.2 | b | 0.95 ± 0.15 | bc | 25.3 ± 5.8 | ab | 1136.0 ± 56.0 | a | 0.037 ± 0.003 | b |
| Chistock-1 | 16.8 ± 0.8 | ab | 1.49 ± 0.05 | a | 30.7 ± 1.3 | a | 1088.0 ± 64.2 | a | 0.049 ± 0.003 | a |
| Baleng | 18.5 ± 0.9 | a | 1.11 ± 0.13 | ab | 25.3 ± 1.3 | ab | 1250.7 ± 97.8 | a | 0.043 ± 0.002 | ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhang, Z.; Guo, P.; Ji, G.; Zhang, X.; Qi, Q.; Xu, X.; Zhang, X.; Li, W.; Han, Z.; et al. Whole-Canopy Photosynthetic Characterization of Apple Tree and the Effects Induced by Grafting on Rootstocks with Different Vigor. Horticulturae 2022, 8, 816. https://doi.org/10.3390/horticulturae8090816
Li M, Zhang Z, Guo P, Ji G, Zhang X, Qi Q, Xu X, Zhang X, Li W, Han Z, et al. Whole-Canopy Photosynthetic Characterization of Apple Tree and the Effects Induced by Grafting on Rootstocks with Different Vigor. Horticulturae. 2022; 8(9):816. https://doi.org/10.3390/horticulturae8090816
Chicago/Turabian StyleLi, Mengke, Zifan Zhang, Pengjing Guo, Guofei Ji, Xi Zhang, Qi Qi, Xuefeng Xu, Xinzhong Zhang, Wei Li, Zhenhai Han, and et al. 2022. "Whole-Canopy Photosynthetic Characterization of Apple Tree and the Effects Induced by Grafting on Rootstocks with Different Vigor" Horticulturae 8, no. 9: 816. https://doi.org/10.3390/horticulturae8090816
APA StyleLi, M., Zhang, Z., Guo, P., Ji, G., Zhang, X., Qi, Q., Xu, X., Zhang, X., Li, W., Han, Z., & Qiu, C. (2022). Whole-Canopy Photosynthetic Characterization of Apple Tree and the Effects Induced by Grafting on Rootstocks with Different Vigor. Horticulturae, 8(9), 816. https://doi.org/10.3390/horticulturae8090816







