Peroxidase Activity in Tomato Leaf Cells under Salt Stress Based on Micro-Hyperspectral Imaging Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Slices
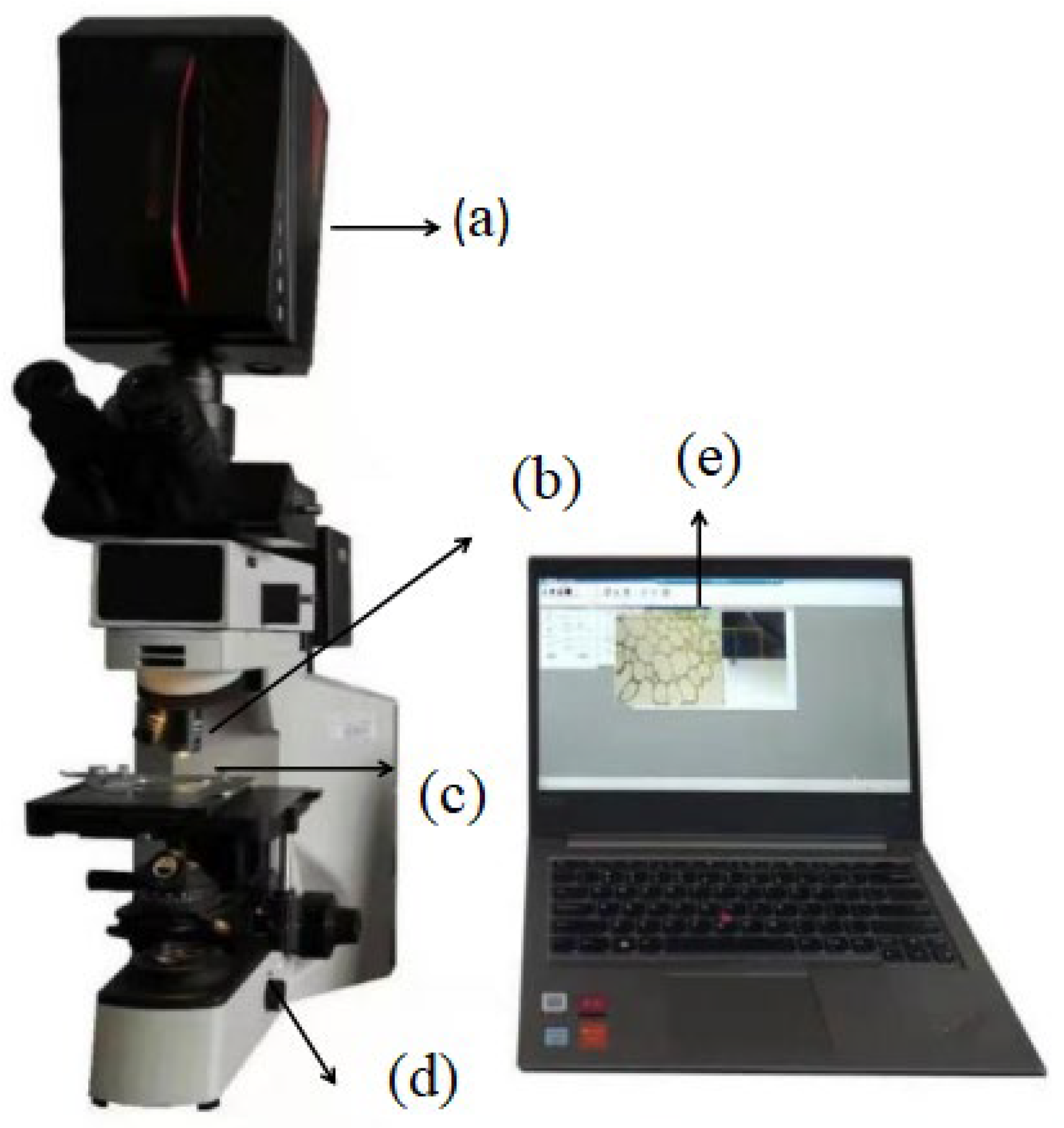
2.3. Acquisition and Correction of Microscopic Hyperspectral Images
2.4. Method for Determination of Peroxidase
2.5. Data Analysis
2.6. Model Evaluation
3. Results
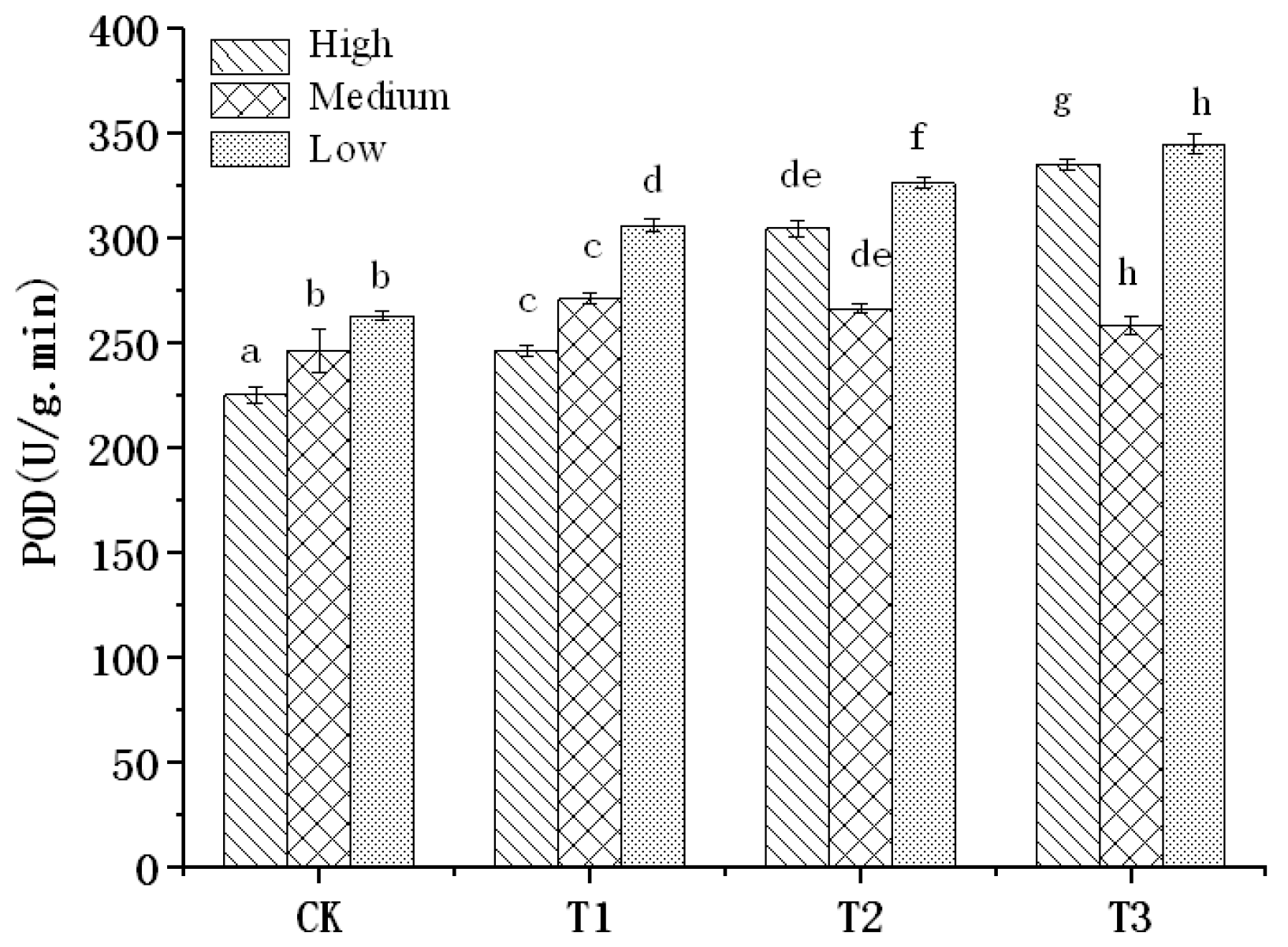
3.1. POD Activity of Tomato Leaves
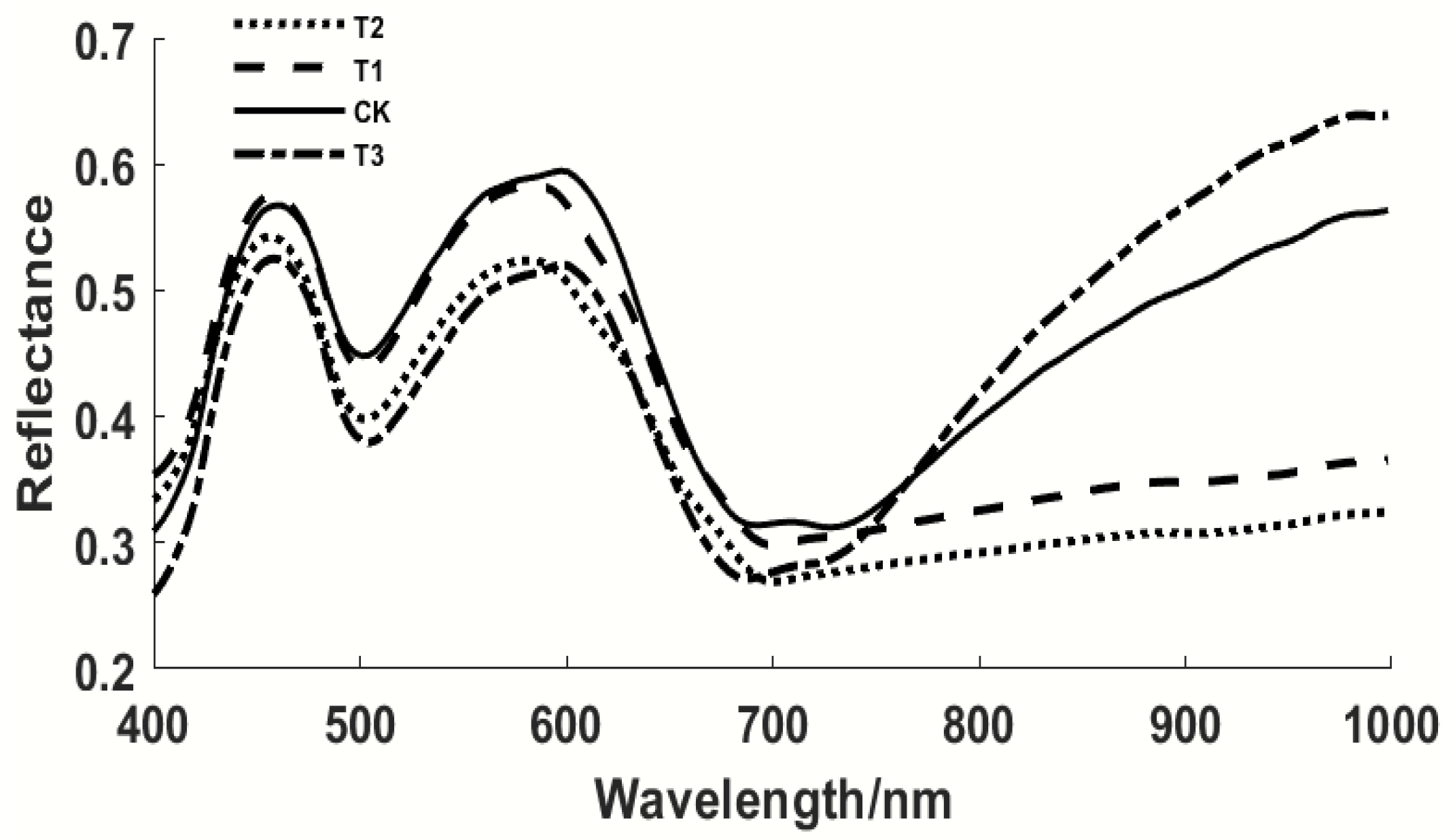
3.2. Spectral Characteristic
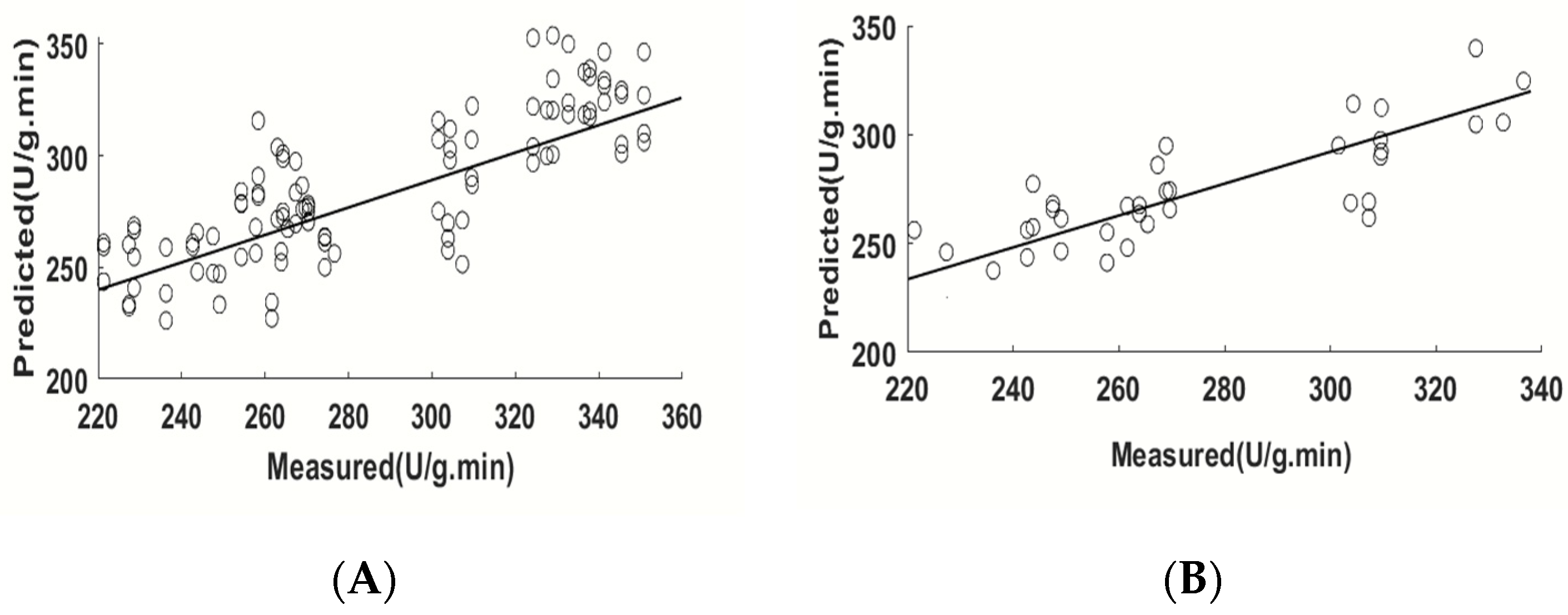
3.3. Modeling Based on Full Spectrum
3.4. Selection of Characteristic Variables
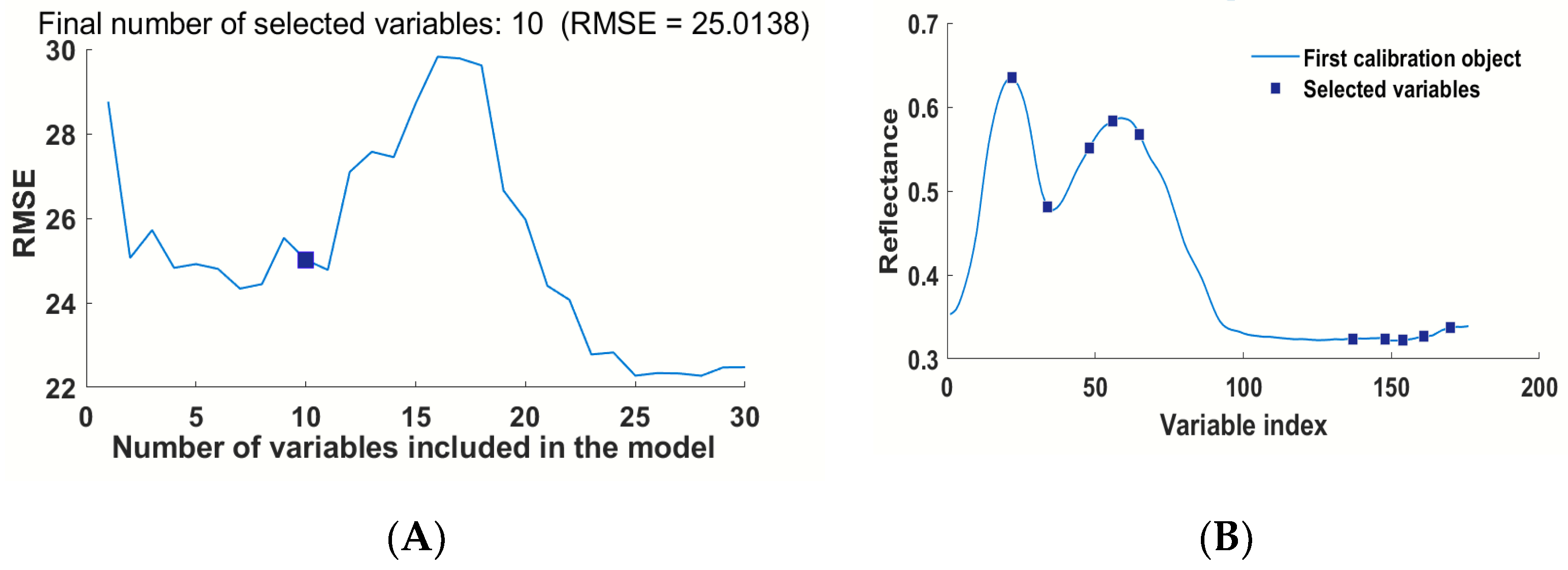
3.4.1. Modeling Based on The Variable Selected by SPA
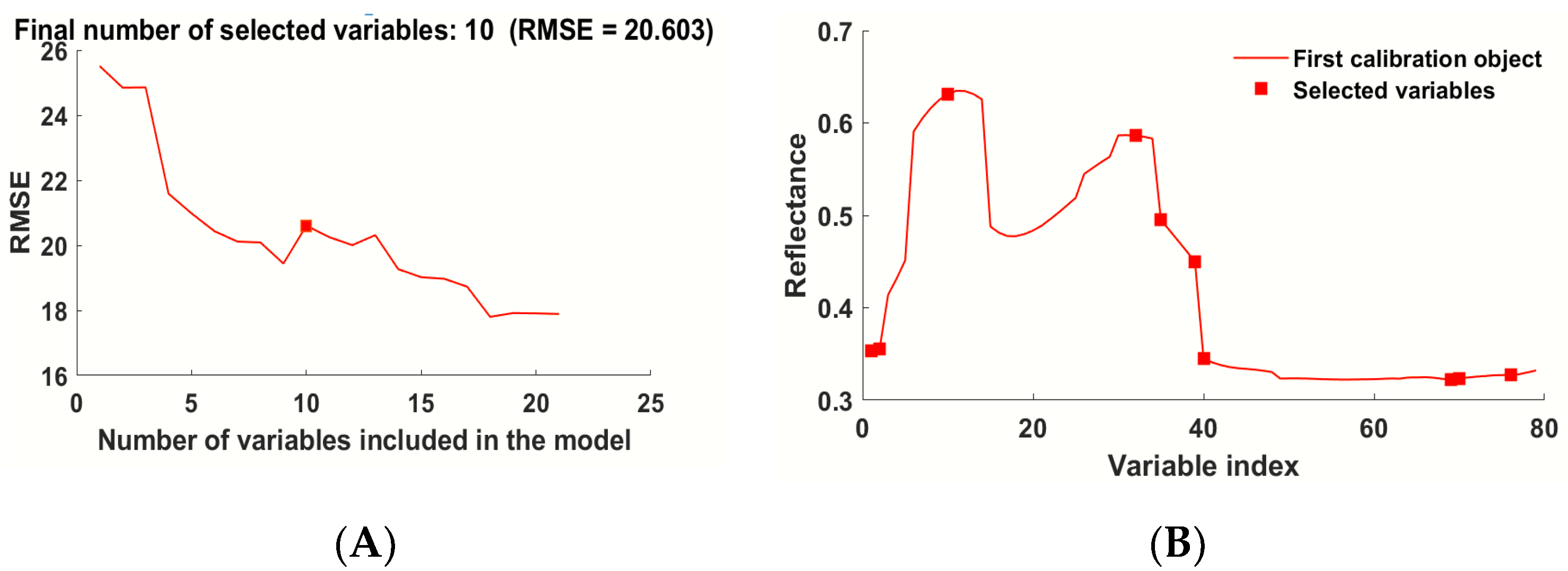
3.4.2. Modeling Based on the Variable Selected by VCPA and IVISSA
3.4.3. Different Methods to Extract Characteristic Wavelengths and Comparative Analysis
3.5. Subsection
3.6. Comparison of Modeling Methods
3.7. Subsubs
3.8. Visualization of Spatial Distribution of Peroxidase in Tomato Leaves
4. Discussion
5. Conclusions
- The POD activity in tomato leaves increased with the increase of salt stress.
- The optimal pretreatment method of enzyme activity in tomato leaves was established based on PLSR model by combining the micro-hyperspectral imaging technology and stoichiometry method.
- PLSR and PCR models based on characteristic wavelength were established and analyzed, and their performance was evaluated. The Rp and RMSEP of IVISSA + SPA − PLSR were 0.81 and 18.94, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, C.X.; Liu, Z.G.; Jing, Y.D. Effects of water stress on the activity and isoenzyme of key defense Enzymes in Maize leaves. J. Maize Sci. 2018, 11, 63–66. [Google Scholar]
- Yang, Y.; He, Y. Early prediction of antioxidant enzyme values of rice blast based on hyperspectral image. Trans. Chin. Soc. Agric. Eng. 2013, 29, 135–141. [Google Scholar]
- Zhang, Y.J.; Wang, L.; Bai, Y.L.; Yang, L.P.; Lu, Y.L.; Zhang, J.J.; Ge, L. Diagnosis of nitrogen nutrition in tomato leaves based on hyperspectral image technology. J. Jiangsu Univ. 2014, 35, 290–294. [Google Scholar]
- Sui, Y.H.; Wu, X.Y.; Hu, N.B.; Tang, J.B. Activity Analysis and POD Isoenzyme Patterns in Four Cultivars of Capsicum under NaCl Stress. Jiyinzuxue Yu Yingyong Shengwuxue Genom. Appl. Biol. 2018, 37, 5414–5420. [Google Scholar]
- Song, Y.; Cui, X.H.; Zhang, M.; Miao, C.L.; Cui, S.M.; Ye, L.H. Effects of salt stress on physiological characteristics and ion distribution of tomato seedlings. J. North. Agric. 2019, 47, 115–121. [Google Scholar]
- Miguel Juan, G.B.; Aurora Esther, R.H.; Guillermo, L.H. Structural and ultrastructural injuries in leaves of Baccharis conferta and Buddleja cordata broad-leaved species of a forest impacted with ozone. Flora 2022, 291, 152075. [Google Scholar] [CrossRef]
- Gong, Y.X.; Gu, T.H.; Ling, L.; Qiu, R.L.; Zhang, W.X. Visualizing hazardous solids with cryogenic electron microscopy (Cryo-EM). J. Hazard. Mater. 2022, 436, 129192. [Google Scholar] [CrossRef]
- Faylo, J.L.; Christianson, D.W. Visualizing transiently associated catalytic domains in assembly-line biosynthesis using cryo-electron microscopy. J. Struct. Biol. 2021, 213, 107802. [Google Scholar] [CrossRef]
- Thomson Raine, E.S.; Carrera-Pacheco, E.S.; Gillam Elizabeth, M.J. Engineering functional thermostable proteins using ancestral sequence reconstruction. J. Biol. Chem. 2022, 102435. [Google Scholar] [CrossRef]
- Duan, D.D.; Zhao, C.J.; Li, Z.H.; Yang, G.J.; Zhao, Y.; Qiao, X.J.; Zhang, Y.H.; Zhang, L.X.; Yang, W.D. Estimating total leaf nitrogen concentration in winter wheat by canopy hyperspectral data and nitrogen vertical distribution. J. Integr. Agric. 2019, 18, 1562–1570. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Shi, Y.K.; Qin, Y.D.; Xuan, G.T.; Li, J.; Li, Q.K.; Yang, F.J.; Hu, Z.C. A new quantitative index for the assessment of tomato quality using Vis-NIR hyperspectral imaging. Food Chem. 2022, 386, 132864. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, X.; Wu, X.H.; Lu, B.; Dai, C.X.; Shen, J.F. Research and analysis of cadmium residue in tomato leaves based on WT-LSSVR and Vis-NIR hyperspectral imaging. Spectrochim. Acta A 2019, 212, 215–221. [Google Scholar]
- Gu, Q.; Sheng, L.; Zhang, T.H.; Lu, Y.W.; Zhang, Z.J.; Zheng, K.F.; Hu, H.; Zhou, H.K. Early detection of tomato spotted wilt virus infection in tobacco using the hyperspectral imaging technique and machine learning algorithms. Comput. Electron. Agric. 2019, 167, 105066. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Zhang, J.Y.; Li, M.Y.; Zhao, L.J.; Ren, H.R.; Yan, L.G.; Zhao, G.M.; Zhu, C.Z. Rapid determination of spore germinability of Clostridium perfringens based on microscopic hyperspectral imaging technology and chemometrics. J. Food Eng. 2020, 280, 1–11. [Google Scholar] [CrossRef]
- Gao, L.; Smith, R.T. Optical hyperspectral imaging in microscopy and spectroscopy—A review of data acquisition. J. Biophotonics 2015, 8, 441–456. [Google Scholar] [CrossRef]
- Wang, Y.F.; Mao, H.P.; Zhang, X.D.; Liu, Y.; Du, X.X. A Rapid Detection Method for Tomato Gray Mold Spores in Greenhouse Based on Microfluidic Chip Enrichment and Lens-Less Diffraction Image Processing. Foods 2021, 10, 3011. [Google Scholar] [CrossRef]
- Qin, O.Y.; Yang, Y.C.; Park, B.; Kang, R.; Wu, J.Z.; Chen, Q.S.; Guo, Z.M.; Li, H.H. A novel hyperspectral microscope imaging technology for rapid evaluation of particle size distribution in matcha. J. Food Eng. 2020, 272, 109782. [Google Scholar]
- Huang, Y.; Zhang, R.; Wu, D.J.; Lu, H.L. The preparation of frozen sections. Mod. Med. Health 2017, 33, 3358–3359. [Google Scholar]
- Giovenzana, V.; Beghi, R.; Civelli, R.; Guidetti, R. Optical techniques for rapid quality monitoring along minimally processed fruit and vegetable chain. Trends Food Sci. Technol. 2015, 46, 331–338. [Google Scholar] [CrossRef]
- Li, J.B.; Chen, L.P.; Huang, W.Q.; Wang, Q.Y.; Zhang, B.H.; Tian, X.; Fan, S.X.; Li, B. Multispectral detection of skin defects of bi -colored peaches based on Vis-VIR hyperspectral imaging. Postharvest Biol. Technol. 2016, 112, 121–133. [Google Scholar] [CrossRef]
- Li, H.S. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2000; pp. 182–197. [Google Scholar]
- Duan, M.; Yang, W.C.; Mao, X.M. Effects of water deficit on photosynthetic Characteristics and light response models of spring wheat under film mulching conditions. Trans. Chin. Soc. Agric. Mach. 2019, 49, 219–227. [Google Scholar]
- Fan, S.X.; Li, J.B.; Xia, Y.; Tian, X.; Guo, Z.M.; Huang, W.Q. Long-term evaluation of soluble solids content of apples with biological variability by using near-infrared spectroscopy and calibration transfer method. Postharvest Biol. Technol. 2019, 151, 79–87. [Google Scholar] [CrossRef]
- Jie, D.F.; Xie, L.J.; Fu, X.P.; Rao, X.Q.; Ying, Y.B. Variable selection for partial least squares analysis of soluble solids content in watermelon using near-infrared diffuse transmission technique. J. Food Eng. 2013, 118, 387–392. [Google Scholar] [CrossRef]
- Shi, J.Y.; Zhang, F.; Wu, S.B.; Guo, Z.M.; Huang, X.W.; Hu, X.T.; Holmes, M.; Zou, X.B. Noise-free microbial colony counting method based on hyperspectral features of agar plates. Food Chem. 2019, 274, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Emam, P.A. Modulation of oxidative damage due to salt stress using salicylic acid in Hordeum vulgare. Arch. Agron. Soil Sci. 2018, 64, 1268–1277. [Google Scholar]
- Abdelaziz, M.E.; Abdelsattar, M.; Abdeldaym, E.A.; Omar, M.A. Piriformospora indicaalters Na+/K+ homeostasis, antioxidant enzymes and LeNHX1expression of greenhouse tomato grown under salt stress. Sci. Hortic. 2019, 256, 108532. [Google Scholar] [CrossRef]
- Insaf, B.; Noomene, S.; Vicente, V.P.; Aurelio, G.C.; Rosa, M.C. Identification and expression of the Cucurbita WRKY transcription factors in response to water deficit and salt stress. Sci. Hortic. 2019, 256, 108562. [Google Scholar]
- Shi, C.; Qian, J.; Zhu, W.; Liu, H.; Han, S.; Yang, X. Nondestructive determination of freshness indicators for tilapia fillets stored at various temperatures by hyperspectral imaging coupled with RBF neural networks. Food Chem. 2019, 275, 497–503. [Google Scholar] [CrossRef]
- Hao, J.; Dong, F.J.; Li, Y.L.; Wang, S.L.; Cui, J.R.; Zhang, Z.F.; Wu, K.N. Investigation of the data fusion of spectral and textural data from hyperspectral imaging for the near geographical origin discrimination of wolfberries using 2D-CNN algorithms. Infrared Phys. Technol. 2022, 125, 104286. [Google Scholar] [CrossRef]

| Set | Number of Samples | Min (U/g·min) | Max (U/g·min) | Average (U/g·min) | SD (U/g·min) |
|---|---|---|---|---|---|
| Total | 139 | 221.28 | 350.82 | 283.46 | 38.29 |
| Calibration set | 103 | 221.28 | 350.82 | 283.45 | 38.58 |
| Prediction set | 36 | 221.28 | 341.29 | 270.92 | 36.00 |
| Spectra Types | PCS | Rc | RMSEC | Rp | RMSEP | RPD |
|---|---|---|---|---|---|---|
| Raw | 7 | 0.86 | 19.70 | 0.76 | 23.96 | 1.50 |
| Average smoothing | 10 | 0.84 | 20.47 | 0.76 | 25.39 | 1.42 |
| Gaussian Filter | 7 | 0.85 | 20.06 | 0.76 | 24.43 | 1.47 |
| Normalize | 6 | 0.86 | 19.15 | 0.77 | 23.84 | 1.51 |
| Baseline | 7 | 0.86 | 19.37 | 0.75 | 25.77 | 1.40 |
| SNV | 7 | 0.87 | 18.81 | 0.76 | 24.05 | 1.50 |
| Detrending | 6 | 0.87 | 18.78 | 0.72 | 26.91 | 1.34 |
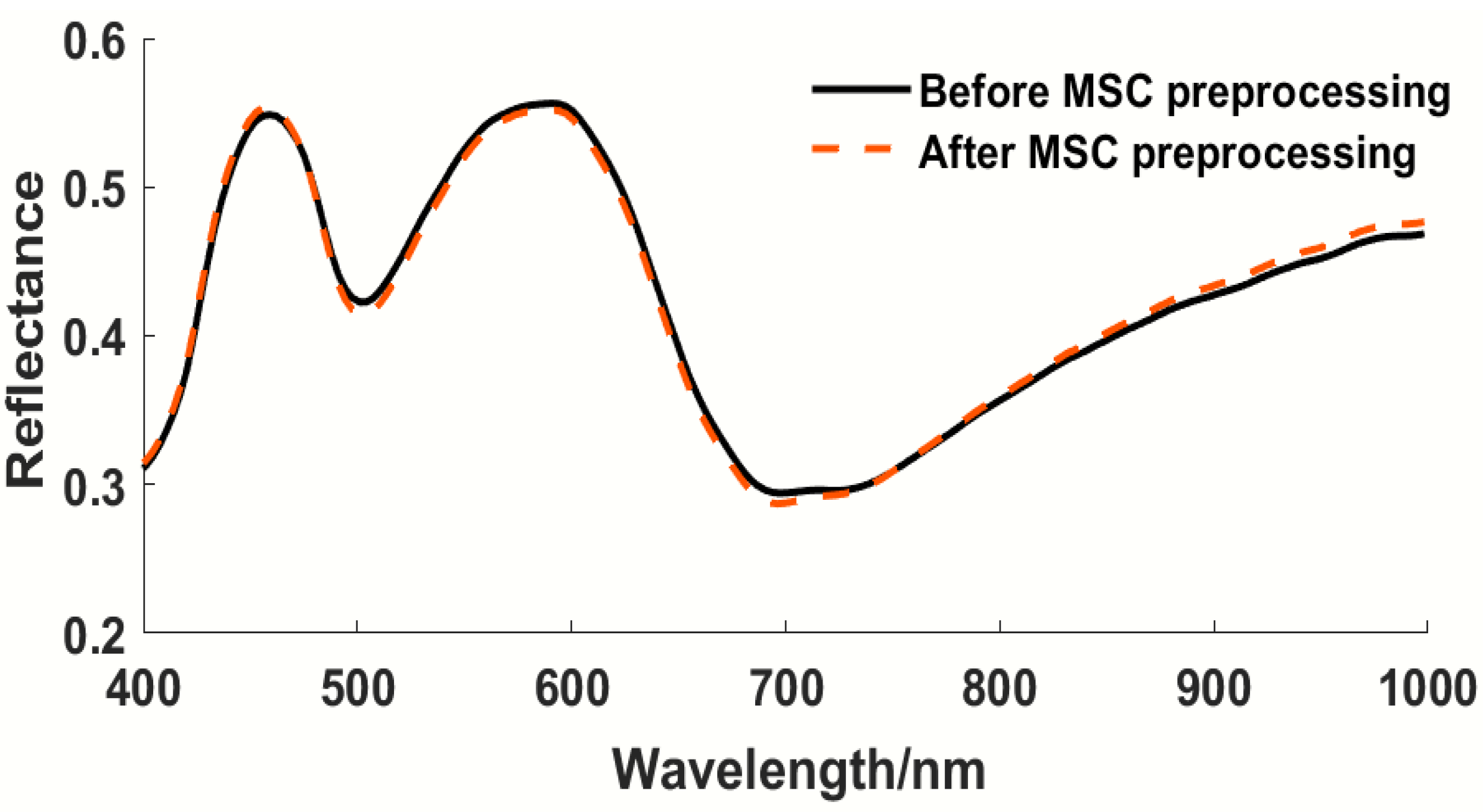
| MSC | 8 | 0.89 | 17.72 | 0.76 | 24.77 | 1.45 |
| Methods. | Numbers | Wavelengths/nm |
|---|---|---|
| SPA | 10 | 456, 495, 541, 567, 597, 850, 890, 912, 938, 972 |
| IVISSA | 79 | 392, 395, 414, 417, 420, 440, 443, 446, 449, 452, 456, 459, 462, 465, 495, 498, 501, 504, 508, 511, 514, 517, 521, 524, 527, 541, 544, 547, 551, 577, 581, 584, 587, 591, 635, 638, 642, 645, 648, 693, 697, 700, 704, 707, 711, 714, 718, 721, 785, 789, 792, 796, 799, 803, 806, 810, 814, 817, 821, 824, 828, 832, 835, 879, 883, 886, 890, 894, 897, 927, 931, 934, 938, 942, 946, 949, 953, 957, 961 |
| IVISSA + SPA | 10 | 392, 417, 488, 498, 511, 514, 611, 614, 635, 728 |
| VCPA | 10 | 392, 401, 544, 551, 564, 567, 571, 635, 679, 897 |
| Model | Method | Band Num | Rc | RMSEC | Rp | RMSEP | RPD |
|---|---|---|---|---|---|---|---|
| PLSR | SPA | 10 | 0.78 | 24.94 | 0.78 | 21.24 | 1.70 |
| IVISSA | 79 | 0.83 | 22.97 | 0.81 | 19.45 | 1.85 | |
| IVISSA + SPA | 10 | 0.79 | 24.41 | 0.81 | 18.94 | 1.90 | |
| VCPA | 10 | 0.82 | 22.10 | 0.80 | 20.26 | 1.78 | |
| PCR | SPA | 10 | 0.78 | 24.94 | 0.78 | 21.35 | 1.69 |
| IVISSA | 79 | 0.81 | 23.30 | 0.79 | 20.35 | 1.77 | |
| IVISSA + SPA | 10 | 0.79 | 24.41 | 0.81 | 19.04 | 1.90 | |
| VCPA | 10 | 0.82 | 22.74 | 0.81 | 19.45 | 1.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Jiang, Q.; Zhang, Y.; Du, M.; Ma, L.; Ma, Y. Peroxidase Activity in Tomato Leaf Cells under Salt Stress Based on Micro-Hyperspectral Imaging Technique. Horticulturae 2022, 8, 813. https://doi.org/10.3390/horticulturae8090813
Wu L, Jiang Q, Zhang Y, Du M, Ma L, Ma Y. Peroxidase Activity in Tomato Leaf Cells under Salt Stress Based on Micro-Hyperspectral Imaging Technique. Horticulturae. 2022; 8(9):813. https://doi.org/10.3390/horticulturae8090813
Chicago/Turabian StyleWu, Longguo, Qiufei Jiang, Yao Zhang, Minghua Du, Ling Ma, and Yan Ma. 2022. "Peroxidase Activity in Tomato Leaf Cells under Salt Stress Based on Micro-Hyperspectral Imaging Technique" Horticulturae 8, no. 9: 813. https://doi.org/10.3390/horticulturae8090813
APA StyleWu, L., Jiang, Q., Zhang, Y., Du, M., Ma, L., & Ma, Y. (2022). Peroxidase Activity in Tomato Leaf Cells under Salt Stress Based on Micro-Hyperspectral Imaging Technique. Horticulturae, 8(9), 813. https://doi.org/10.3390/horticulturae8090813





