On-Tree Fruit Bagging and Cold Storage Maintain the Postharvest Quality of Mango Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit and Bagging Material
2.2. Treatments
2.3. Measurements
2.3.1. Respiration Rate and Ethylene Production
2.3.2. Peel Colour
2.3.3. Fruit Firmness
2.3.4. Weight Loss
2.3.5. Soluble Solids Content
2.3.6. Titratable Acidity and Juice pH
2.3.7. Ascorbic Acid Content
2.3.8. Total Phenolic Content and Total Antioxidant Activity
2.3.9. Determination of Catalase, Peroxidase and Superoxide Dismutase Enzyme Activity
Catalase Activity
Peroxidase Activity
Superoxide Dismutase Activity
2.4. Statistical Analysis
3. Results
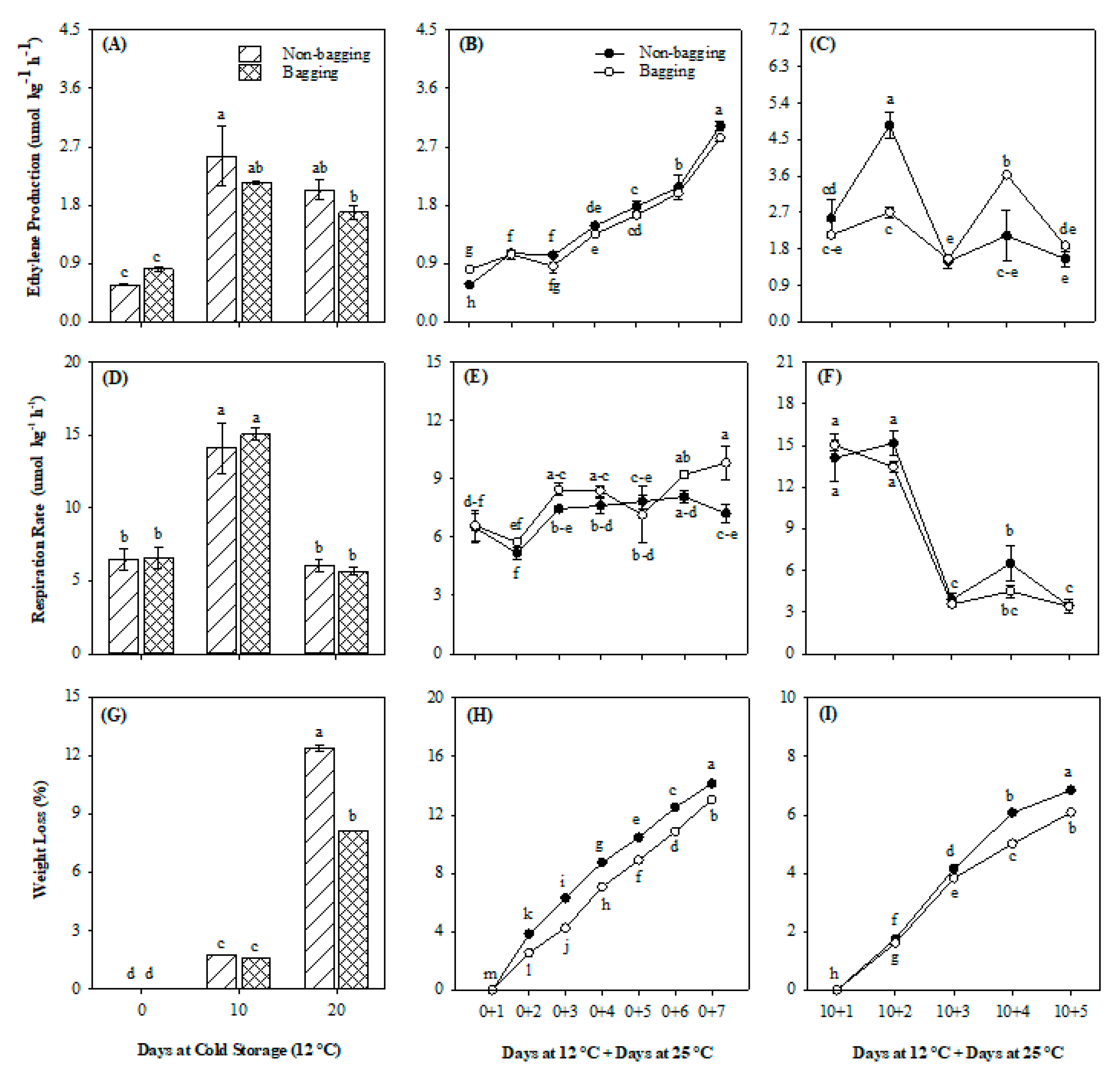
3.1. Ethylene Production, Respiration Rate and Weight Loss
3.2. Fruit Firmness
3.3. Fruit Peel Colour (L*, a* and b*)
3.4. Soluble Solids Content, Titratable Acidity and Juice pH
3.5. Vitamin C, Total Antioxidant Activity and Total Phenolic Contents
3.6. Antioxidative Enzyme Activities (CAT, POD and SOD)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Singh, Z.; Singh, R.K.; Sane, V.A.; Nath, P. Mango-postharvest biology and biotechnology. Crit. Rev. Plant. Sci. 2013, 32, 217–236. [Google Scholar] [CrossRef]
- FAO. Food and Agricultural Organization of United Nations. Available online: http://faostat.fao.org (accessed on 27 August 2019).
- MNFSR. Fruit, Vegetable, and Condiments Statistics of Pakistan (2018–2019); Ministry of National Food Security & Research Economic Wing Islamabad: Islamabad, Pakistan, 2019.
- Rajwana, I.A.; Malik, A.U.; Khan, A.S.; Saleem, B.A.; Malik, S.A. A new mango hybrid shows better shelf life and fruit quality. Pak. J. Bot. 2010, 42, 2503–2512. [Google Scholar]
- Jabbar, A.; Malik, A.U.; Saeed, M.; Malik, O.H.; Amin, M.; Khan, A.S.; Rajwana, I.A.; Saleem, B.A.; Hameed, R.; Mazhar, M.S. Performance of hot water phytosanitary treated mangoes for intended export from Pakistan to Iran and China. Int. J. Agric. Biol. 2011, 13, 645–651. [Google Scholar]
- Hafeez, O.; Malik, A.U.; Khan, A.S.; Rehman, A.; Javaid, Q.A. Impact of different packaging types and low temperature shipping durations on fruit quality and marketability of Pakistani mangoes. Int. J. Agric. Biol. 2012, 14, 47–54. [Google Scholar]
- Léchaudel, M.; Joas, J. An overview of preharvest factors influencing mango fruit growth, quality and postharvest behaviour. Braz. J. Plant Physiol. 2007, 19, 287–298. [Google Scholar] [CrossRef]
- Qian, M.; Zhang, D.; Yue, X.; Wang, S.; Li, X.; Teng, Y. Analysis of different pigmentation patterns in ‘Mantianhong’ (Pyrus pyrifolia Nakai) and ‘Cascade’ (Pyrus communis L.) under bagging treatment and postharvest UV-B/visible irradiation conditions. Sci. Hortic. 2013, 151, 75–82. [Google Scholar] [CrossRef]
- Bai, S.; Tuan, P.A.; Saito, T.; Honda, C.; Hatsuyama, Y.; Ito, A.; Moriguchi, T. Epigenetic regulation of MdMYB1 is associated with paper bagging-induced red pigmentation of apples. Planta 2016, 244, 573–586. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Xin, H.; Wang, N.; Guan, L.; Wu, B.-H.; Li, S. Anthocyanin profile and gene expression in berry skin of two red Vitis vinifera grape cultivars that are sunlight dependent versus sunlight independent. Aust. J. Grape Wine Res. 2013, 19, 238–248. [Google Scholar] [CrossRef]
- Karanjalker, G.; Ravishankar, K.; Shivashankara, K.; Dinesh, M. Influence of bagging on color, anthocyanin and anthocyanin biosynthetic genes in peel of red colored mango cv. ‘Lily’. Erwerbs-Obstbau 2018, 60, 281–287. [Google Scholar] [CrossRef]
- Kanzaki, S.; Ichihi, A.; Tanaka, Y.; Fujishige, S.; Koeda, S.; Shimizu, K. The R2R3-MYB transcription factor MiMYB1 regulates light dependent red coloration of ‘Irwin’mango fruit skin. Sci. Hortic. 2020, 272, 109567. [Google Scholar] [CrossRef]
- Ali, M.M.; Anwar, R.; Yousef, A.F.; Li, B.; Luvisi, A.; Bellis, L.D.; Aprile, A.; Chen, F. Influence of Bagging on the Development and Quality of Fruits. Plants 2021, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Nagaharshitha, D.; Haldankar, P. Effect of Preharvest Fruit Bagging on quality of Mango (Mangifera indica) Cv. Alphonso. Int. J. Agric. Sci. 2017, 7, 531–536. [Google Scholar]
- Devalla, N.; Haldankar, P.; Khopkar, R. Effect of bagging on chemical properties of mango (Mangifera indica L.) cv. Alphonso. Int. J. Hortic. Sci. Crop Sci. Res. 2016, 6, 1–8. [Google Scholar]
- Ahmed, T.; Hasan, M.; Hassan, K.; Ahmed, J.D.; Ahmed, K.S.D.; Azam, A.; Mondal, M. Fruit bagging of custard apple (Annona reticulata) as an eco-friendly protection approach against mealybug (Phenacoccus solenopsis) infestation in the north-eastern Bangladesh. Int. J. Trop. Insect Sci. 2022, 42, 723–732. [Google Scholar] [CrossRef]
- Islam, M.; Akter, M.; Rahman, M.; Uddin, M.; Bari, M.; Islam, M.; Rahman, M. Effect of bagging on quality and shelf life of mango (Mangifera indica L.) cv. BARI Mango-4. Asian J. Agric. Hortic. Res. 2020, 6, 37–45. [Google Scholar] [CrossRef]
- Hossain, M.; Sarkar, B.; Hossain, M.; Mian, M.; Rajotte, E.; Muniappan, R.; O’Rourke, M. Comparison of biorational management approaches against mango fruit fly (Bactrocera dorsalis Hendel) in Bangladesh. J. Crop Prot. 2020, 135, 104807. [Google Scholar] [CrossRef]
- Akter, M.; Islam, M.; Akter, N.; Amin, M.; Bari, M.; Uddin, M. Pre-harvest fruit bagging enhanced quality and shelf-life of mango (Mangifera indica L.) cv. Amrapali. Asian J. Agric. Hortic. Res. 2020, 5, 45–54. [Google Scholar] [CrossRef]
- Sharma, R.R.; Reddy, S.; Jhalegar, M. Pre-harvest fruit bagging: A useful approach for plant protection and improved post-harvest fruit quality–a review. Int. J. Hortic. Sci. 2014, 89, 101–113. [Google Scholar] [CrossRef]
- Ayele, L.; Bayleyegn, A. Effect of low temperature storage on ripening and quality of Mango (Mangifera indica L.). J. Agric. Sci. 2017, 5, 570–576. [Google Scholar]
- Purwanto, Y.A.; Budiastra, I.W.; Darmawati, E. Effect of low temperature storage on the ripestage eating period of ‘gedong gincu’mango fruits. J. Appl. Eng. Sci. 2016, 11, 10365–10367. [Google Scholar]
- Ahmed, Z.F.; Kaur, N.; Maqsood, S.; Schmeda-Hirschmann, G. Preharvest Applications of Chitosan, Salicylic Acid, and Calcium Chloride Have a Synergistic Effect on Quality and Storability of Date Palm Fruit (Phoenix dactylifera L.). HortScience 2022, 57, 422–430. [Google Scholar] [CrossRef]
- Xylia, P.; Chrysargyris, A.; Ahmed, Z.F.; Tzortzakis, N. Application of rosemary and eucalyptus essential oils and their main component on the preservation of apple and pear fruits. Horticulturae 2021, 7, 479. [Google Scholar] [CrossRef]
- Ruck, J. Chemical Methods for Analysis of Fruits and Vegetables; Canada Department of Agriculture: Ottawa, ON, Canada, 1963.
- Saleem, M.S.; Anjum, M.A.; Naz, S.; Ali, S.; Hussain, S.; Azam, M.; Sardar, H.; Khaliq, G.; Canan, İ.; Ejaz, S. Incorporation of ascorbic acid in chitosan-based edible coating improves postharvest quality and storability of strawberry fruits. Int. J. Biol. Macromol. 2021, 189, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.A.; El-Ansary, D.O.; Ahmed, Z.F. Mitigation of Salinity Stress on Pomegranate (Punica granatum L. cv. Wonderful) Plant Using Salicylic Acid Foliar Spray. Horticulturae 2022, 8, 375. [Google Scholar] [CrossRef]
- Bambalele, N.L.; Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z. Recent Advances on Postharvest Technologies of Mango Fruit: A Review. Int. J. Fruit Sci. 2021, 21, 565–586. [Google Scholar] [CrossRef]
- Montalvo, E.; García, H.S.; Tovar, B.; Mata, M. Application of exogenous ethylene on postharvest ripening of refrigerated ‘Ataulfo’ mangoes. J. Food Sci. Technol. 2007, 40, 1466–1472. [Google Scholar] [CrossRef]
- Amarante, C.; Banks, N.H.; Max, S. Effect of preharvest bagging on fruit quality and postharvest physiology of pears (Pyrus communis). N. Z. J. Crop Hortic. Sci. 2002, 30, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.R.; Pal, R.K.; Asrey, R.; Sagar, V.R.; Dhiman, M.R.; Rana, M.R. Pre-harvest fruit bagging influences fruit color and quality of apple cv. Delicious. J. Agric. Sci. 2013, 4, 443. [Google Scholar] [CrossRef]
- Narayana, C.; Pal, R.; Roy, S. Effect of pre-storage treatments and temperature regimes on shelf-life and respiratory behaviour of ripe Baneshan mango. J. Food Sci. Technol. 1996, 33, 79–82. [Google Scholar]
- Karki, D. Effect of harvesting states on the quality of tomato (Lycopersicon esculentum Mill CV. Avinash-2, hybrid). J. Trib. Uni. 2005, 25, 141–147. [Google Scholar] [CrossRef]
- Ali, Z.M.; Chin, L.-H.; Lazan, H. A comparative study on wall degrading enzymes, pectin modifications and softening during ripening of selected tropical fruits. J. Plant Sci. 2004, 167, 317–327. [Google Scholar] [CrossRef]
- Gonzalez-Aguilar, G.A.; Celis, J.; Sotelo-Mundo, R.R.; De La Rosa, L.A.; Rodrigo-Garcia, J.; Alvarez-Parrilla, E. Physiological and biochemical changes of different fresh-cut mango cultivars stored at 5 °C. Int. J. Food Sci. Technol. 2008, 43, 91–101. [Google Scholar] [CrossRef]
- Robles-Sánchez, R.; Islas-Osuna, M.; Astiazarán-García, H.; Vazquez-Ortiz, F.; Martín-Belloso, O.; Gorinstein, S.; González-Aguilar, G. Quality index, consumer acceptability, bioactive compounds, and antioxidant activity of fresh-cut “Ataulfo” mangoes (Mangifera indica L.) as affected by low-temperature storage. J. Food Sci. Technol. 2009, 74, S126–S134. [Google Scholar]
- Ding, P.; Syakirah, M. Influence of Fruit Bagging on Postharvest Quality of ‘harumanis’ mango (Mangifera Indica L.). Acta Hortic. 2010, 877, 169–174. [Google Scholar] [CrossRef]
- Hofman, P. Mango fruit quality at harvest is affected by production condition. In Proceedings of the Mango 2000 Marketing Seminar and Production Workshop; DPI: Brisbane, Australia, 1995; pp. 199–207. [Google Scholar]
- Wang, H.-C.; Huang, X.-M.; Hu, G.-B.; Yang, Z.-y.; Huang, H.-B. A comparative study of chlorophyll loss and its related mechanism during fruit maturation in the pericarp of fast-and slow-degreening litchi pericarp. J. Sci. Hortic. 2005, 106, 247–257. [Google Scholar] [CrossRef]
- Jia, H.-J.; Araki, A.; Okamoto, G. Influence of fruit bagging on aroma volatiles and skin coloration of ‘Hakuho’ peach (Prunus persica Batsch). Postharvest Biol. Technol. 2005, 35, 61–68. [Google Scholar] [CrossRef]
- Beauvoit, B.; Belouah, I.; Bertin, N.; Cakpo, C.B.; Colombié, S.; Dai, Z.; Gautier, H.; Génard, M.; Moing, A.; Roch, L.; et al. Putting primary metabolism into perspective to obtain better fruits. Ann. Bot. 2018, 122, 1–21. [Google Scholar] [CrossRef]
- Ni, Z.; Zhang, Z.; Gao, Z.; Gu, L.; Huang, L. Effects of bagging on sugar metabolism and the activity of sugar metabolism related enzymes during fruit development of Qingzhong loquat. Afr. J. Biotechnol. 2011, 10, 4212–4216. [Google Scholar]
- Hasan, M.U.; Malik, A.U.; Khan, A.S.; Anwar, R.; Latif, M.; Amjad, A.; Shah, M.S.; Amin, M. Impact of postharvest hot water treatment on two commercial mango cultivars of Pakistan under simulated air freight conditions for China. J. Agric. Sci. 2020, 57, 1381–1391. [Google Scholar]
- Wu, H.; Wang, S.; Shi, S.; Ma, W.; Zhou, Y.; Zhan, R. Effects of bagging on fruit quality in Zill mango. J. Plant Sci. 2009, 26, 644–648. [Google Scholar]
- Anthon, G.E.; LeStrange, M.; Barrett, D.M. Changes in pH, acids, sugars and other quality parameters during extended vine holding of ripe processing tomatoes. J. Sci. Food Agric. 2011, 91, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Sarker, D.; Rahman, M.; Barman, J. Efficacy of different bagging materials for the control of mango fruit fly. Bangladesh J. Agric. Res. 2009, 34, 165–168. [Google Scholar] [CrossRef]
- Zhou, J.; Zhong, G.; Lin, Z.; Xu, H.-l. The effects of bagging on fresh fruit quality of Canarium album. J. Plant Stud. 2012, 10, 505–508. [Google Scholar]
- Ribeiro, S.M.R.; Queiroz, J.H.; de Queiroz, M.E.L.R.; Campos, F.M.; Sant’Ana, H.M.P. Antioxidant in mango (Mangifera indica L.) pulp. Plant Foods Hum. Nutr. 2007, 62, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.W.; Kenis, K.; Keulemans, J. Genetic control of fruit vitamin C contents. Plant Physiol. 2006, 142, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Tsaniklidis, G.; Delis, C.; Nikoloudakis, N.; Katinakis, P.; Aivalakis, G. Low temperature storage affects the ascorbic acid metabolism of cherry tomato fruits. Plant Physiol. Biochem. 2014, 84, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Rahman, M.; Shamsuzzoha, M.; Alom, R. Influence of pre-harvest bagging on fruit quality of Mango (Mangifera indica L.) cv. Mishribhog. Int. J. Biosci. 2017, 11, 59–68. [Google Scholar]
- Ma, X.; Wu, H.; Liu, L.; Yao, Q.; Wang, S.; Zhan, R.; Xing, S.; Zhou, Y. Polyphenolic compounds and antioxidant properties in mango fruits. Sci. Hortic. 2011, 129, 102–107. [Google Scholar] [CrossRef]
- Ahmed, Z.F.; Al Shaibani, F.Y.; Kaur, N.; Maqsood, S.; Schmeda-Hirschmann, G. Improving fruit quality, bioactive compounds, and storage life of date palm (Phoenix dactylifera L., cv. Barhi) using natural elicitors. Horticulturae 2021, 7, 293. [Google Scholar] [CrossRef]
- Hudina, M.; Stampar, F. Bagging of ‘Concorde’ pears (Pyrus communis L.) influences fruit quality. In Proceedings of the XI International Pear Symposium 909, General Roca, Argentine, 23–26 November 2010; pp. 625–630. [Google Scholar]
- Cunha, M.C.D.; Silva, J.S.; Elias, H.H.D.S.; Carvalho, E.E.N.; Vilas Boas, E.V.D.B. Effects of processing and packaging on bioactive compounds of curriola jelly [Pouteria ramiflora (Mart.) Radlk.] during storage. Food Sci. Technol. 2020, 41, 96–104. [Google Scholar] [CrossRef]
- Zhang, B.B.; Guo, J.Y.; Ma, R.J.; Cai, Z.X.; Yan, J.; Zhang, C.H. Relationship between the bagging microenvironment and fruit quality in ‘Guibao’ peach [Prunus persica (L.) Batsch]. J. Hortic. Sci. Biotechnol. 2015, 90, 303–310. [Google Scholar] [CrossRef]
- Zhu, M.; Fang, W.; Chen, C.; Wang, L.; Cao, K. Effects of Shading by Bagging on Carotenoid Accumulation in Peach Fruit Flesh. J. Plant Growth Regul. 2021, 40, 1912–1921. [Google Scholar] [CrossRef]
- Nagamani, J.; Shivashankara, K.; Roy, T. Role of oxidative stress and the activity of ethylene biosynthetic enzymes on the formation of spongy tissue in ‘Alphonso’ mango. J. Food Sci. Technol. 2010, 47, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, H.; Zhou, Q.; Zhang, X. Effects of bagging on the fruit quality in Litchi chinensis fruit and pesticide residues in it. J. Appl. Ecol. 2003, 14, 710–712. [Google Scholar]
- Razzaq, K.; Khan, A.S.; Malik, A.U.; Shahid, M. Ripening period influences fruit softening and antioxidative system of ‘Samar Bahisht Chaunsa’ mango. Sci. Hortic. 2013, 160, 108–114. [Google Scholar] [CrossRef]
- Ahmed, Z.F.R.; Palta, J.P. Hormone-like effect of a natural lipid, lysophosphatidylethanolamine, can calcium deficiency injury in potato shoot cultures. HortScience 2011, 46, S196. [Google Scholar]

| Treatment | Fruit Firmness (N) | |
|---|---|---|
| Non-Bagging | Bagging | |
| a. Days at 12 °C | ||
| 0 | 142.67 ± 0.33 b | 150.00 ± 0.58 a |
| 10 | 79.33 ± 0.88 d | 94.23 ± 0.50 c |
| 20 | 39.00 ± 0.58 e | 37.17 ± 0.73 f |
| b. Days at 12 °C + Days at 25 °C | ||
| 0 + 0 | 142.67 ± 0.33 b | 150.00 ± 0.58 a |
| 0 + 7 | 27.30 ± 1.37 d | 94.80 ± 0.79 c |
| 10 + 0 | 79.33 ± 0.88 b | 94.23 ± 0.50 a |
| 10 + 5 | 38.47 ± 0.36 d | 46.93 ± 1.90 c |
| 20 + 0 | 39.00 ± 0.58 b | 37.17 ± 0.73 a |
| 20 + 1 | D | D |
| Treatment | SSC (%) | TA (%) | Juice pH | |||
|---|---|---|---|---|---|---|
| Non-Bagging | Bagging | Non-Bagging | Bagging | Non-Bagging | Bagging | |
| a. Days at 12 °C | ||||||
| 0 | 7.55 ± 0.38 e | 8.33 ± 0.06 e | 0.62 ± 0.01 a | 0.45 ± 0.02 b | 4.63 ± 0.09 c | 4.27 ± 0.06 d |
| 10 | 14.53 ± 0.24 d | 15.63 ± 0.35 c | 0.59 ± 0.00 a | 0.41 ± 0.02 bc | 5.44 ± 0.09 a | 4.65 ± 0.06 c |
| 20 | 23.03 ± 0.33 b | 25.50 ± 0.55 a | 0.38 ± 0.00 cd | 0.35 ± 0.03 d | 5.19 ± 0.02 b | 4.27 ± 0.0 d |
| b. Days at 12 °C + Days at 25 °C | ||||||
| 0 + 0 | 7.55 ± 0.38 d | 8.33 ± 0.06 c | 0.62 ± 0.01 a | 0.45 ± 0.02 b | 4.63 ± 0.09 b | 4.27 ± 0.06 b |
| 0 + 7 | 25.42 ± 0.42 b | 27.03 ± 0.27 a | 0.29 ± 0.04 c | 0.19 ± 0.01 d | 5.42 ± 0.08 a | 5.74 ± 0.16 a |
| 10 + 0 | 14.53 ± 0.24 c | 15.63 ± 0.35 c | 0.59 ± 0.00 a | 0.41 ± 0.02 b | 5.44 ± 0.09 a | 4.65 ± 0.06 c |
| 10 + 5 | 22.29 ± 0.35 b | 25.10 ± 0.36 a | 0.40 ± 0.01 b | 0.26 ± 0.02 c | 4.64 ± 0.01 c | 4.86 ± 0.03 b |
| 20 + 0 | 23.03 ± 0.33 b | 25.50 ± 0.55 a | 0.38 ± 0.00 a | 0.35 ± 0.03 a | 5.19 ± 0.02 a | 4.27 ± 0.0 b |
| 20 + 1 | D | D | D | D | D | D |
| Treatment | Vitamin C (mg 100 mL−1 Juice) | TAC (% Inhibition) | TPC (mg GAE 100 g−1) | |||
|---|---|---|---|---|---|---|
| Non-Bagging | Bagging | Non-Bagging | Bagging | Non-Bagging | Bagging | |
| a. Days at 12 °C | ||||||
| 0 | 32.98 ± 0.42 b | 38.40 ± 0.67 a | 83.97 ± 0.17 a | 87.68 ± 0.50 a | 72.26 ± 0.67 bc | 83.03 ± 3.53 a |
| 10 | 21.23 ± 0.18 d | 27.80 ± 0.26 c | 85.71 ± 1.05 a | 83.83 ± 3.44 a | 67.71 ± 0.66 c | 76.16 ± 2.48 b |
| 20 | 12.30 ± 0.46 f | 15.70 ± 0.26 e | 81.83 ± 2.76 a | 82.31 ± 1.23 a | 74.96 ± 1.15 b | 85.55 ± 1.40 a |
| b. Days at 12 °C + Days at 25 °C | ||||||
| 0 + 0 | 32.98 ± 0.42 c | 38.40 ± 0.67 a | 83.97 ± 0.17 b | 87.68 ± 0.50 a | 72.26 ± 0.67 bc | 83.03 ± 3.53 a |
| 0 + 7 | 34.70 ± 1.21 bc | 36.88 ± 0.48 ab | 84.49 ± 0.78 b | 86.98 ± 0.54 a | 67.44 ± 0.74 c | 80.32 ± 3.98 ab |
| 10 + 0 | 21.23 ± 0.18 c | 27.80 ± 0.26 a | 85.71 ± 1.05 a | 83.83 ± 3.44 a | 67.71 ± 0.66 c | 76.16 ± 2.48 b |
| 10 + 5 | 15.77 ± 0.26 d | 26.67 ± 0.42 b | 86.98 ± 0.17 a | 88.95 ± 0.77 a | 78.77 ± 2.29 ab | 85.13 ± 1.66 a |
| 20 + 0 | 12.30 ± 0.46 b | 15.70 ± 0.26 a | 81.83 ± 2.76 a | 82.31 ± 1.23 a | 74.96 ± 1.15 b | 85.55 ± 1.40 a |
| 20 + 1 | D | D | D | D | D | D |
| Treatment | CAT (U mg−1 Protein) | POD (U mg−1 Protein) | SOD (U mg−1 Protein) | |||
|---|---|---|---|---|---|---|
| Non-Bagging | Bagging | Non-Bagging | Bagging | Non-Bagging | Bagging | |
| a. Days at 12 °C | ||||||
| 0 | 3.97 ± 0.19 c | 8.39 ± 1.24 a | 0.16 ± 0.01 b | 2.79 ± 0.48 a | 53.41 ± 1.63 c | 86.78 ± 3.23 a |
| 10 | 4.18 ± 0.70 c | 7.29 ± 0.28 ab | 0.59 ± 0.19 b | 0.59 ± 0.36 b | 75.25 ± 2.25 b | 86.61 ± 2.60 a |
| 20 | 3.62 ± 0.40 c | 5.27 ± 1.12 bc | 0.73 ± 0.43 b | 0.93 ± 0.36 b | 81.32 ± 0.76 ab | 87.06 ± 3.06 a |
| b. Days at 12 °C + Days at 25 °C | ||||||
| 0 + 0 | 3.97 ± 0.19 c | 8.39 ± 1.24 a | 0.16 ± 0.0 c | 2.79 ± 0.48 a | 53.41 ± 1.63 c | 86.78 ± 3.23 a |
| 0 + 7 | 6.66 ± 1.19 ab | 9.86 ± 1.23 a | 1.23 ± 0.48 bc | 2.05 ± 0.45 ab | 69.54 ± 2.92 b | 83.88 ± 1.64 a |
| 10 + 0 | 4.18 ± 0.70 b | 7.29 ± 0.28 a | 0.59 ± 0.19 a | 0.59 ± 0.36 a | 75.25 ± 2.25 b | 86.61 ± 2.60 a |
| 10 + 5 | 3.05 ± 0.15 b | 8.12 ± 1.10 a | 0.66 ± 0.08 a | 1.04 ± 0.10 a | 70.13 ± 1.74 c | 74.84 ± 2.79 bc |
| 20 + 0 | 3.62 ± 0.40 a | 5.27 ± 1.12 a | 0.73 ± 0.43 a | 0.93 ± 0.36 a | 81.32 ± 0.76 a | 87.06 ± 3.06 a |
| 20 + 1 | D | D | D | D | D | D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadeem, A.; Ahmed, Z.F.R.; Hussain, S.B.; Omar, A.E.-D.K.; Amin, M.; Javed, S.; Ali, A.; Ullah, S.; Razzaq, K.; Rajwana, I.A.; et al. On-Tree Fruit Bagging and Cold Storage Maintain the Postharvest Quality of Mango Fruit. Horticulturae 2022, 8, 814. https://doi.org/10.3390/horticulturae8090814
Nadeem A, Ahmed ZFR, Hussain SB, Omar AE-DK, Amin M, Javed S, Ali A, Ullah S, Razzaq K, Rajwana IA, et al. On-Tree Fruit Bagging and Cold Storage Maintain the Postharvest Quality of Mango Fruit. Horticulturae. 2022; 8(9):814. https://doi.org/10.3390/horticulturae8090814
Chicago/Turabian StyleNadeem, Atif, Zienab Fawzy Reiad Ahmed, Syed Bilal Hussain, Alaa El-Din K. Omar, Muhammad Amin, Saqib Javed, Amjad Ali, Sami Ullah, Kashif Razzaq, Ishtiaq A. Rajwana, and et al. 2022. "On-Tree Fruit Bagging and Cold Storage Maintain the Postharvest Quality of Mango Fruit" Horticulturae 8, no. 9: 814. https://doi.org/10.3390/horticulturae8090814
APA StyleNadeem, A., Ahmed, Z. F. R., Hussain, S. B., Omar, A. E.-D. K., Amin, M., Javed, S., Ali, A., Ullah, S., Razzaq, K., Rajwana, I. A., Nayab, S., Ziogas, V., Alam-Eldein, S. M., & Mira, A. M. (2022). On-Tree Fruit Bagging and Cold Storage Maintain the Postharvest Quality of Mango Fruit. Horticulturae, 8(9), 814. https://doi.org/10.3390/horticulturae8090814










