Abstract
The application of elicitors enhances grape quality, especially the volatile compounds. There are few studies on the influence of elicitors on the aroma compositions of grapes. Additionally, studies on the amino acids and aroma profiles of ‘Cabernet Gernischt’ grapes are scant. The objective of this work was to evaluate the effect of benzothiadiazole (BTH) treatments on the amino acids and aroma profiles of ‘Cabernet Gernischt’ grapes during berry development. BTH was sprayed on berries at three different stages during grape development; the fruit set period, swelling, and veraison stages. Physicochemical parameters, amino acids, and aroma compounds of the grapes were evaluated. The results showed increased an weight and color quality of treated grapes, while the content of primary metabolites such as sugar and amino acids in treated grapes declined relative to control grapes. However, total concentrations of the various aroma classes were higher in treated grapes, except for carbonyls and terpenoids, which presented higher levels in control grapes than in BTH-treated grapes. The correlation analysis between amino acids and aroma compounds revealed positive correlations in both samples with few negative correlations in BTH samples. The odor activity values (OAVs) affirmed the floral, fruity, and fresh-green nature of ‘Cabernet Gernischt’ grapes. BTH application to ‘Cabernet Gernischt’ berries significantly influenced the compositional qualities of the grapes.
1. Introduction
Benzothiadiazole (BTH) is a chemical elicitor and a functional analog of salicylic acid which functions in inducing protection in plants against pathogens through the establishment of systemic acquired resistance (SAR) [1,2,3]. Most recently, elicitors such as Methyl Jasmonate (MeJ) are used to enhance the metabolite content in grapes. However, others such as chitosan and BTH are used primarily against disease infections such as grey mold (Botrytis cinerea) and powdery mildew (Erysiphe necator) [1]. The defense mechanism of BTH in grapes leads to the activation of several enzymes, triggering the syntheses of bioactive metabolites (e.g., phenolic compounds, antioxidants, etc.) [3,4,5,6]. According to Wang et al. [7], the metabolic cost of the defense, accompanied by increased berry size, is incurred on the accumulation of grape soluble sugars. Total soluble sugars and amino acids are primary metabolites used to measure grape quality at harvest [8]. These primary metabolites also serve as precursors for the synthesis of volatile aroma compounds [9].
Aroma is an essential quality parameter of grapes and wines that influences consumer preferences [10,11]. Several factors such as the grape cultivar, vintage, maturity, climate, and other management practices influence the accumulation and concentration of aromas [12,13]. Grape aroma compounds are derived from the metabolism of fatty acids, sugars, and amino acids through the lipoxygenase (LOX), terpenoid, and amino acid pathways [3,14,15]. However, their syntheses are dependent on the activity and specificity of related enzymes in the pathways [16], which are also affected by elicitors. Several studies have reported the influence of BTH on the compositional qualities of grapes and wines. However, the majority are based on phenolic compounds [17,18,19,20,21] while a handful are on the amino acids and aroma compounds of grapes and wines [3,22,23].
Grape is an essential fruit crop cultivated globally for its economic and health benefits [24,25,26]. According to OIV [27], the production of wine in China for the past four successive years has declined. Findings from the Third National Agricultural Census in China showed that areas under vines have reduced, resulting in decreased grape production, which may be attributed to the difficult climate conditions, management practices, and low productivity. In addition to the function of BTH in protecting crops against pathogens [28], grape growers apply it to maximize yield, as it is also known to increase the weight and size of the elicited berries. However, the impact of BTH on grape aroma quality is still unclear. Moreover, there are fewer studies on the use of only BTH and its influence on the aroma of grapes.
Cabernet Gernischt cv (Vitis vinifera L.) is one of China’s most cultivated varieties in the Northwest region, the main grape and wine production area [29]. ‘Cabernet Gernischt’ is well-known for its appealing color and taste, with intensive fruity, leafy, and vegetative aromatic notes [29]. However, despite its recognition, few studies on the effect of induced resistance on the grape berry and wine volatiles have been reported [30,31,32]. Therefore, this research aimed to assess the impact of BTH on the amino acids and aroma compounds in ‘Cabernet Gernischt’ grapes.
2. Materials and Methods
2.1. Chemicals and Reagents
Unless otherwise stated, all the reagents used for quantification and identification were analytically pure and the water used was purified with a Milli-Q purification system (Molecular, Chongqing, China). Glycine (Gly, 98.5%), phenylalanine (Phe, 98%), tyrosine (Tyr, 98%), arginine (Arg, 98%), proline (Pro, 99%), lysine (Lys, 98%), glutamic acid (Glu, 99%), asparagine (Asp, 99%), histidine (His, 98%), serine (Ser, 98%), alanine (Ala, 98%), threonine (Thr, 98%), cysteine (Cys, 97%), and 2-Octanol were purchased from Sigma-Aldrich (Shanghai, China). Benzothiadiazole (BTH) was purchased from Sigma-Aldrich (Munich, Germany).
2.2. Study Site, Treatment, and Sampling
The field study was conducted in the experimental vineyard of Food Science and Engineering College in Gansu Agricultural University (Lanzhou, China, 103°41′ E, 36°5′ N; 1530 m above sea level) during the 2020 growing season. The site’s annual average temperature and annual rainfalls are 11.2 °C and 367 mm, respectively, the frost-free period is 180 days, and annual sunshine hours are more than 2446 h. The soil was sandy loam with average pH (9.48), organic matter (1.47%), total nitrogen (0.35 g/kg), total phosphorus (1.19 g/kg), total potassium (22.43 g/kg), alkali-hydrolyzed nitrogen (43.78 mg/kg), available phosphorus (54.01 mg/kg), available potassium (366.61 mg/kg), and total boron (37.38 mg/kg) being measured. The experiment was performed on 11-year-old ‘Cabernet Gernischt’ grapevines trained to a vertical trellis system in an east–west orientation with a row spacing of 2.5 m and plant spacing of 0.80 m. The vineyard was managed according to the standard viticulture practices (e.g., pruning, shade controlling, etc.) and irrigated using a drip irrigation system.
The treatment method by Gómez-Plaza et al. [33] was used with slight modifications. The treatments (control and BTH) were performed using 10 vine blocks arranged in a complete randomized design, with three biological replications. Two untreated rows between treatments served as a barrier to prevent contamination. BTH treatment (0.37 mM, Sigma-Aldrich, Munich, Germany) was carried out by preparing aqueous solutions using Tween 80 as a wetting agent and aqueous solutions of Tween 80 only for control vines. The aqueous solutions were applied to the surfaces of the berries using a high-pressure sprayer. Treatments were applied three times during the grape’s development (Fruit set stage (7 June), swelling stage (4 July), and veraison (1 August)). The application schedule was to enable the assessment of the impact of BTH on the formation and accumulation of grape metabolites during development.
The grape berries were sampled every 2 weeks from the fruit set stage to harvest (21 June to 30 August). Samples consisted of several clusters selected randomly. Samples were bagged and taken to the laboratory. The berries were destemmed and wrapped with aluminum foil in small quantities. They were frozen in liquid nitrogen and stored at −80 °C until further use.
2.3. Determination of Physicochemical Properties
All physicochemical parameters except weight were analyzed as described by OIV [34]. The weight of berries was determined as reported by Yue et al. [35] with a slight modification. Briefly, an empty beaker was weighed and filled with a random set of 100 berries and weighed again. The weight of berries was then expressed as g/100 berries. The color quality of berries was estimated using the red grapes color index as described by Koyama et al. [36] with the equation CIRG = (180 − h°) ÷ (L* + C*). All parameters were analyzed in triplicates (n = 3).
2.4. Amino Acids Analysis
The grape amino acids were determined as described by Wang et al. [37] with a slight modification, using the Ninhydrin post-column derivatization ion-exchange chromatography method. Briefly, 10 thawed grape berries were deseeded and crushed under liquid nitrogen into a fine powder with a grinder. Two grams of powdered sample were hydrolyzed with 10 mL of 6 M hydrochloric acid (HCL) containing 0.1 % phenol at 110 °C for 22 h. The hydrolysate was filtered into a clean tube, evaporated at 40 °C, and buffered with 2 mL of sodium citrate solution (pH 2.2) to dissolve the content before filtering through a 0.22 µm filter membrane into a sampling vial. Free amino acids in the samples were then determined using an automatic amino acid analyzer (Sykam S-433D, Germany) coupled with a sulfonic acid type ion-exchange resin column. Each amino acid peak was detected with a UV-visible spectrophotometric detector at wavelengths 570 nm and 440 nm. Amino acids were then identified and quantified by comparing their retention times with their pure reference standards. Since the samples were all analyzed in triplicates, the results are expressed as the means of the three determinations (n = 3).
2.5. Analysis of Grape Aroma Compounds
The grape aroma compounds were determined using Headspace Solid Phase Microextraction Gas Chromatography–Mass Spectrometry (HS-SPME-GC-MS) as reported by Wu et al. [10] with slight modifications. Approximately 50 g of berries were deseeded, blended, and 5 g of the slurry was weighed into a 20 mL vial. A small magnetic stir bar, sodium chloride (1 g, NaCl), and 10 µL of internal standard (10 ppm, 2-Octanol) were added. The vial was then tightly capped and equilibrated in a water bath at 40 °C for 30 min with agitation at 40 rpm. The volatile aroma in the headspace of the vial was absorbed using a 50/30 µm DVB/CAR/PDMS fiber. The fiber was thermally desorbed in the injector port of the GC-MS for 10 min after extraction. Samples were all determined in triplicate (n = 3).
Volatiles were analyzed using a gas chromatography–mass spectrometer system (TRACE 1310-ISQ, Thermo Fisher Scientific, San Jose, CA, USA) with a DB-WAX column (60 m × 2.5 mm × 0.25 µm, Agilent Technology, Santa Clara, CA, USA). Helium (He) was the carrier gas at a flow rate of 1 mL/minute. The injector temperature was 230 °C and set for splitless injection. The GC temperature program started with an oven temperature of 50 °C for 10 min, a temperature series of 3 °C/min to a final temperature of 180 °C, and a final time of 6 min. The ion source and transfer line temperature were set respectively at 250 and 180 °C. The mass range was 50 m/z to 350 m/z, operated in full scan mode with an electron energy of 70 eV. The volatile compounds detected were identified by comparing their mass spectra with those in the National Institute for Standards and Technology (NIST 14; search version 2.0) library. The retention indices calculated using C6-C21 n-alkane series were compared with those reported in the literature or the NIST database (http://webbook.nist.gov/chemistry/cas-ser.html) (accessed on 10 March 2022). Quantification analysis was carried out only for the volatile compounds identified in at least two of the three replicates. Any others than this were viewed as artifacts and omitted from further analysis. The compounds were analyzed semi-quantitatively by their relative response to the 2-octanol internal standard. Finally, concentrations of volatile compounds were obtained and expressed as 2-octanol equivalent, µg/L.
2.6. Odor Activity Values (OAVs)
Odor activity values of the volatile compounds identified at harvest (SD-6) were quantified, and the contributions of the compounds to the overall grape aroma were estimated. Odor activity values of the aroma compounds are expressed as c/t, with (c) as the total concentration of each compound and (t) the odor threshold value from aqueous solutions found in the literature.
2.7. Statistical Analysis
All data analyses were done in triplicates and reported as average means. The data were analyzed using one- and two-way Analysis of Variance (ANOVA) in IBM SPSS Statistical software program 26 for Windows (SPSS Inc., Chicago, IL, USA), and the mean was compared using the post hoc Tukey’s test (p < 0.05). A correlation analysis of amino acids and derived volatiles was performed using Pearson’s correlation analysis in SPSS. Hierarchical heatmaps of grape amino acids and aroma compounds were generated using the OmicStudio tools at https://www.omicstudio.cn (accessed on 10 March 2022).
3. Results
3.1. Physicochemical Parameters of ‘Cabernet Gernischt’ Grapes
Figure 1 shows the physicochemical properties of control and treated ‘Cabernet Gernischt’ grapes. Generally, the weight of the berries (Figure 1A) increased throughout the study (Sampling Date-1 to Sampling Date-6), with significant differences between BTH-treated grapes and control grapes. BTH-treated grapes were larger than control grapes. The weight gain for both treatments was gradual from SD-1 to SD-3 but increased abruptly from SD-3 to SD-6, observing a final weight of 98.24 and 126.95 g/100 berries, respectively, in control and BTH-treated grapes. The increasing trend of total soluble sugars in both treatments was similar to the weight. Total soluble sugars (Figure 1A) in the samples increased throughout grape development, with the control samples recording slightly higher Brix values than the BTH-treated samples, although the differences were not substantial. For the controls, we recorded a final Brix value of 20.27, compared to 19.62 Brix in BTH-treated samples.
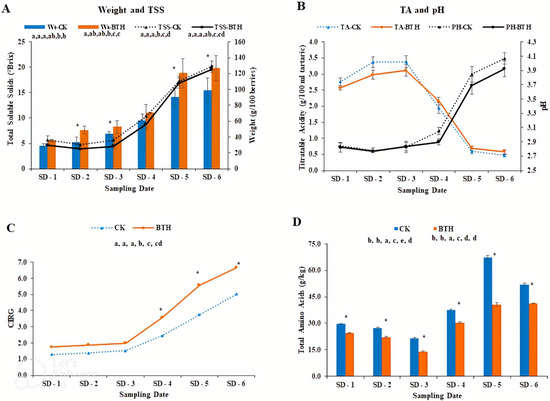
Figure 1.
Physicochemical properties of untreated and treated Cabernet Gernischt cv grapes. For each sampling date, an asterisk (*) indicates a significant difference between treatments (CK and BTH) at p < 0.05 and for each parameter, different alphabets indicate differences between sampling dates at p < 0.05. CK; Control, TSS; Total Soluble Solids, TA; Titratable Acidity, CIRG; Color Index for Red Grapes, SD; Sampling date.
The pH content (Figure 1B) in both treatments increased gradually from SD-1 to SD-6, with no statistical differences between treatments. Control and treated samples reached a pH of 4.07 and 3.92, respectively, on SD-6. On the other hand, titratable acidity (TA) values decreased during growth, with an acidity value of 0.47 g/100 mL tartaric acid in control grapes and 0.59 g/100 mL tartaric acid in treated grapes at harvest (Figure 1B).
BTH-treated samples observed high CIRG than their corresponding controls throughout the study (Figure 1C). The difference in CIRG between treatments from SD-1 to SD-3 was not significant. However, clear distinctions were observed from SD-4 to SD-6. The CIRG values in each treatment on SD-4 to SD-6 were nearly two times higher than the CIRG values in samples preceding SD-4.
Total amino acids concentrations differed significantly at p < 0.05 in both treatments throughout the sampling dates, with control samples being higher than BTH-treated samples (Figure 1D). The total amino acids in each treatment declined from SD-1 to SD-3, at which concentrations in both treatments started increasing, presenting a final value of 51.85 g/kg in controls and 41.27 g/kg in treated samples.
3.2. Amino Acids in ‘Cabernet Gernischt’ Grapes
Thirteen (13) amino acids were identified in this study with varying concentrations (Supplementary Table S1). Some amino acids were found only in one treatment, and some others were not detected throughout development but only on a few sampling dates. The color scale shows that compounds with high concentrations range from black to bright red, while the compounds with low concentrations range from black to light green. Compounds not detected are designated with very bright green colors (Figure 2). Generally, glutamic acid (Glu), arginine (Arg), proline (Pro), and lysine (Lys) were the most concentrated amino acids at harvest (SD-6), followed by glycine (Gly), serine (Ser), threonine (Thr), and phenylalanine (Phe). Alanine (Ala), tyrosine (Tyr), aspartic acid (Asp), and histidine (His) presented lower concentrations, while cysteine (Cys) was not detected in both treatments at harvest.
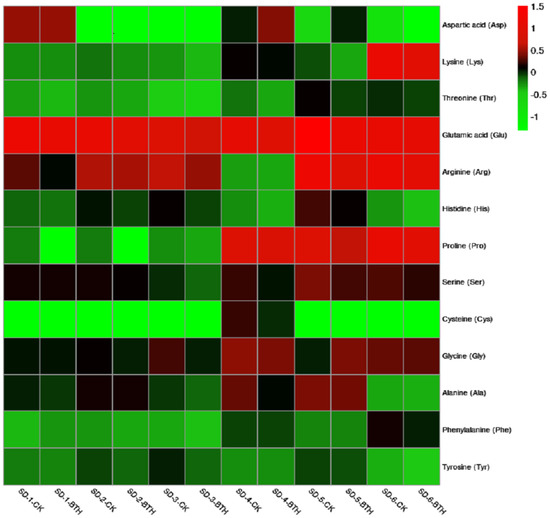
Figure 2.
Hierarchical heatmap of detected amino acids in control (CK) and BTH-treated Cabernet Gernischt cv grapes during development; SD; Sampling Date. The color scale shows that compounds with high concentrations range from black to bright red, while compounds with low concentrations range from black to light green. Compounds not detected are designated with very bright green colors.
Precisely, Glu, Gly, Arg, and Ser were the most abundant compounds detected in both treatments throughout development. Glu was the most concentrated amino acid amongst the four, with the highest concentration in control on SD-5. For proline, we observed higher concentrations in both treatments from SD-4 to SD-6. Thr, Ala, Tyr, Phe, and histidine (His) observed low concentrations throughout the study in both treatments. However, their concentrations in control samples were higher than in BTH-treated samples. Contrary, cysteine was found only on SD-4 in both treatments.
3.3. Concentrations of Aroma Compounds in ‘Cabernet Gernischt’ Grapes
Figure 3 and Figure 4 show the concentrations of volatile compounds detected in this study. A total of 97 volatile compounds were detected consisting of 5 acids, 11 C6 compounds, 17 carbonyls, 27 alcohols, 10 esters, 12 terpenes and norisoprenoids, and 15 other volatiles (Supplementary Table S2). C6 compounds were the most concentrated volatiles, although alcohols were the abundant class. Specifically, the aroma compounds were not all detected in both treatments. Some volatiles were found in samples throughout the growth period, while the concentrations of others were lost in the process, as shown on the hierarchical heatmap (Figure 3). The color scale shows that compounds with high concentrations range from light blue to deep red, while the compounds with low concentrations range from light blue to deep blue.
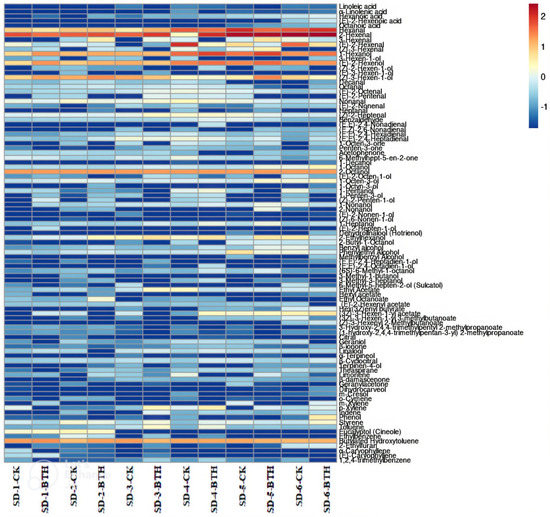
Figure 3.
Hierarchical heatmap of volatile compounds in control (CK) and BTH-treated Cabernet Gernischt cv grapes during development. SD; Sampling Date. The color scale shows that compounds with high concentrations range from light blue to deep red, while the compounds with low concentrations range from light blue to deep blue.
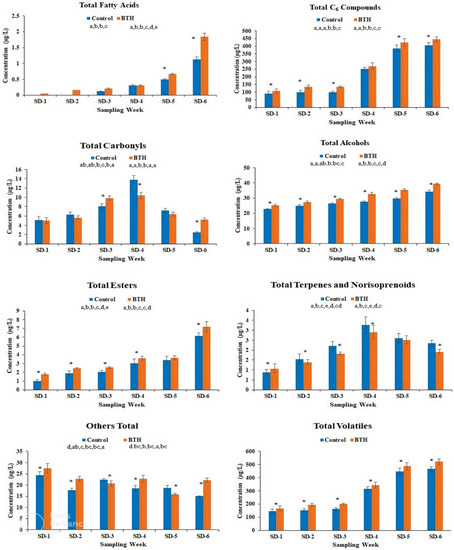
Figure 4.
Total aroma compounds in control and BTH-treated Cabernet Gernischt cv grapes during development. All samples are shown with their standard deviation (n = 3). For each sampling week, an asterisk (*) indicates a significant difference between treatments (CK and BTH) at p < 0.05. For each treatment, different letters indicate differences between sampling dates (p < 0.05). SD; Sampling Date.
Generally, the concentrations of volatile compounds in both treatments varied significantly, as observed in the total volatiles’ concentrations (Figure 4). Total acids, total C6 compounds, total alcohols, and total esters all progressively increased throughout development, with BTH-treated samples having higher concentrations than control samples. Contrary, the overall concentrations of total terpenes and norisoprenoids and total carbonyls in control samples were higher than in BTH-treated samples. The concentrations of these volatile classes in both treatments increased from SD-1 to SD-4 and then started declining, displaying a bell shaped-like pattern. The accumulation of fatty acids, unlike other volatiles, began late in the control treatment when compared to BTH-treated samples. The overall total aroma compounds increased significantly, with higher concentrations in BTH-treated samples.
3.4. Odor Activity Values of Aroma Compounds
Table 1 shows the odor activity values of the aromatic compounds identified in this work. Most of the volatiles found had a profound effect on the overall flavor of the grapes. Volatile compounds with OAVs ≥ 1 are considered vital sources of grape aromas. However, volatiles with OAVs ≤ 1 can also enhance the aroma of grapes through the combination of similar compounds [38]. Therefore, the volatiles displayed in Table 1 are compounds with OAVs ≥ 0.1. Among the total aromatic compounds identified, 14 odor-active volatiles had OAVs above 0.1. Hexanal had the highest OAVs in control (13.30) and BTH-treated samples (22.13) with green, grassy, herbaceous aromatic notes. The level of citrusy and green aromatic notes conferred by nonanal in BTH-treated grapes was twice as in control grapes. When compared with the C6 aldehydes, the C6 alcohols had lower OAVs (OAV ≥ 0.1) and contributed to the overall aroma of the grapes. Another active aroma compound that greatly impacted (OAVs > 1) the overall flavor of the grapes was (E, Z)-2,6-Nonadienal, which contributed fresh green and cucumber notes. The fruity and flowery aromas are usually from esters. Among the esters, Hexyl acetate, (Z)-3-Hexenyl acetate, Hex-(3Z)-enyl butyrate, and (3Z)-3-Hexenyl 3-methylbutanoate were the only active odorants (OAVs ≥ 0.1). Cabernet Gernischt cv grapes, in brief, can be described as a cultivar with floral, fresh grassy, and fruity aromas in great intensity, with BTH-treated samples exceeding control samples.

Table 1.
Odor Activity Values (OAV ≥ 0.1) of untreated (control) and treated Cabernet Gernischt cv grapes at harvest (SD-6).
3.5. Correlation between Amino Acids and Amino Acids-Derived Aroma Compounds
The correlation coefficients between amino acids and aroma compounds in control and BTH-treated samples at the final harvest (SD-6) are shown in Table 2. Significant correlations between the amino acids and aroma compounds observed were generally positive. Lysine (Lys) correlated positively with benzaldehyde in control samples (p < 0.01) and BTH-treated samples (p < 0.05) and positively with 3-Methyl-3-heptanol in control samples only at p < 0.01. Phenylalanine (Phe) correlated positively with benzaldehyde and phenylethyl alcohol in control samples and correlated negatively with phenylethyl alcohol in BTH-treated samples. Phenylethyl alcohol in control samples also correlated strongly with threonine, serine, and glutamic acid. Although phenylethyl alcohol in BTH-treated samples correlated positively with these amino compounds, their correlation coefficients were insignificant. Tyrosine (Tyr) correlated positively with benzaldehyde, 2-Ethylhexanol, and (6S)-6-Methyl-1-octanol in BTH-treated samples and positively with phenylethyl alcohol in control samples. On the other hand, glycine correlated positively (p < 0.01) with phenol in control samples and negatively in treated samples at p < 0.05. Several strong correlations between amino acids and aroma compounds exist, but their coefficients were insignificant.

Table 2.
Correlation coefficients between amino acids and derived volatile compounds from untreated (CK) and treated (BTH) Cabernet Gernischt cv grapes at harvest.
4. Discussion
4.1. Effect of BTH on the Physicochemical Properties of ‘Cabernet Gernischt’ Grapes
As an alternative to fungicides and pesticide treatment, BTH in viticulture has also been exploited for its impact on the quality of grapes and wines from elicited grapes [17,19,20,22,41]. In this work, we studied the influence of multiple BTH applications on the compositional quality of Cabernet Gernischt cv berries.
As shown in Figure 1A, BTH significantly increased the weight of berries and decreased the sugar content of treated grapes when compared to control grapes. Regarding the time of BTH treatment in this study, grape development at that time involved cell division and expansion, and the accumulation of metabolites [16,42]. The increased weight of BTH-treated grapes could be because BTH treatment enhanced the absorption of water molecules into the cells, causing the size to expand, and diluting soluble sugars and other primary metabolites [42]. Gil-Muñoz et al. [19], in their study of BTH and Methyl Jasmonate (MeJ) treatment, reported weight increases in BTH-treated Syrah grapes along with decreased soluble sugars. Similarly, weight gain was observed in Monastrell berries treated with BTH, correlating with a decrease in soluble sugars [17,21,43]. In all these studies, the weight gain in BTH-treated grapes was attributed to high precipitations. However, if BTH treatment had not affected absorption in berry cells, the weight of the BTH-treated grapes may have been statistically similar to the weight of control grapes. This is an indication that the influence of precipitation on berry weight depends on the rate of berry absorption, which may have been accelerated by BTH treatment in the BTH-treated grapes.
Regarding the influence of BTH on the pH and TA content, we found no statistical difference between treated samples and control samples, similar to reports of previous studies [17,18,44].
Grape skin color is a quality component that significantly influences the color quality of the resulting wines. The increase in grape color after BTH treatment has been reported in numerous studies [19,21,41]. The BTH-treated grapes in this study measured high CIRG values when compared with control grapes. According to Beckers and Spoel [45], an increase in grape color is a defense response triggered against biotrophic pathogens since an increase in phenolic compounds inhibits their actions. The grape color increase observed in this work could be attributed to the defense mechanism of BTH in the treated grapes [45].
4.2. Effect of BTH on the Amino Acids Profile of ‘Cabernet Gernischt’ Grapes
Amino acids are vital compounds involved with grapevine metabolism, and their composition and concentration in grapes generally depend on the grape variety and examined berry tissue, among others [46,47]. To the best of our knowledge, this current study is the first to characterize the amino acids of ‘Cabernet Gernischt’ grapes.
The thirteen (13) detected amino acids in this work can be classified into five families based on the amino acids’ biosynthesis pathways [48]. The aspartates (Asp, Lys, and Thr), serines (Ser, Cys, and Gly), glutamates (Glu, Arg, His, and Pro), aromatics (Phe and Tyr), and pyruvates (Ala). The most abundant (Glu, Arg, Pro, and Lys) and least abundant (Cys) detected amino acids in this study are consistent with the most abundant (Glu, Pro, and Arg) and least abundant (Met, Cit, and Cys) amino compounds usually present in grapes [49]. BTH treatment reduced the concentrations of amino acids in the treated samples, which could be due to the defense mechanism of BTH and the larger berry size of the treated samples. The large size of the treated grapes could have diluted the concentrations of the amino acids [7]. Similarly, in a study by Iriti et al. [3], BTH treatment on Merlot grapevines modified the concentrations of amino acids in grape leaves. According to Iriti et al. [3], the differences in concentrations of amino acids were attributed to the induced resistance of Merlot grapevine to gray mold. Thus, induced BTH resistance in grapevines has a significant influence on amino acid concentration [3,7] as well as on berry size, as the skin-to-pulp ratio affects metabolite concentrations [7]. Moreover, the translocation of amino acids from leaves to berries also directly impacts the concentrations of amino acids in grapes [50,51].
On the other hand, a study of MeJ treatment by Gil-Muñoz et al. [52] on the nitrogen composition of Monastrell grapes reported an increase in all amino acids concentrations in elicited grapes when compared to control samples. However, MeJ treatment had no significant influence on amino acids detected in Graciano-treated grapes when compared to the corresponding control samples [49]. This is an indication that the influence of elicitors on amino acid content depends on the type of defense triggered [22,53] and the grape cultivar [49,54].
4.3. Effects of BTH on the Aroma Composition of ‘Cabernet Gernischt’ Grapes
The results of the experiment indicated significant differences (p < 0.05) between the total volatile concentrations of untreated and treated samples. BTH-treated samples presented higher concentrations of total acids, total C6 compounds, total alcohols, and total esters, while control samples observed higher concentrations of total terpenes and norisoprenoids and total carbonyls. This section of the paper will focus only on the final harvest date (SD-6), because samples on this date are the most riped with a favorable sugar level.
As precursors to various aroma compounds, the fatty acids in both samples only accounted for 0.24 and 0.35% of the total volatile compounds in control and treated samples, respectively. Hexanoic acid was the major accumulated acid, whereas linoleic and linolenic acids gave the lowest concentrations. The low concentrations of these two acids suggest their reduction in the formation of C6 compounds as they are the primary precursors in the synthesis of C6 compounds [16]. C6 compounds were the dominant class, accounting for 86.89% (control) and 85.17% (BTH) of the total volatile compounds. C6 aldehydes had relatively higher concentrations in both samples than C6 alcohols, as also reported by Gómez-Plaza et al. [23] in their study of Monastrell grapes and wines. High concentrations of C6 compounds give undesirable herbaceous aromas, requiring much attention during winemaking [55]. However, the degradation of C6 aldehydes into C6 alcohols and subsequently into alcohol esters could reduce the herbaceous green aromatic notes [16,23]. The key enzyme in the biosynthetic pathway of C6 compounds, lipoxygenase (LOX), is reported to be involved in plant protection against biotic and abiotic stresses [55,56,57]. This reason could account for the higher concentrations of C6 compounds in BTH-treated samples relative to control samples, since BTH treatment triggers responses that mimics biotic stresses, indicating the treatment could have upregulated LOX and enhanced its activity.
The most abundant carbonyls were 1-nonanal, (Z)-2-heptenal, (E,E)-2,4-heptadienal, (E)-2-octenal, benzaldehyde, and 6-methylhept-5-en-2-one, representing 70.8 and 63.2% of total carbonyls, respectively, in control and BTH-treated samples. The metabolism of several precursors results in the formation of carbonyl compounds. For instance, (E)-2-octenal is a product of lipid oxidation [56], while benzaldehyde and 6-methylhept-5-en-2-one are produced respectively from amino acid metabolism [58] and carotenoids degradation [59]. In the study of BTH and MeJ effect on volatile compounds of Monastrell grapes and wines, Gómez-Plaza et al. [23] also reported 6-methylhept-5-en-2-one as one of the carbonyl compounds with high concentrations in BTH-treated grapes. The C9 compound (E,Z)-2,6-Nonadienal recorded relatively low concentrations, with no significant differences found between both samples. Xie et al. [32] reported similar findings in their study with the same grape cultivar. Compounds such as octanal, octen-3-one, and penten-3-one were found in BTH-treated samples only, probably because their concentrations in control samples were too low to be detected, or they could have been converted into their respective alcohols by alcohol dehydrogenases [60]. Moreover, BTH treatment could have enhanced their accumulation in a way by improving the activities of related enzymes in their biosynthetic pathways.
Alcohols are derived mainly from aldehydes through the activity of alcohol dehydrogenase, and therefore the concentrations of alcohols depend solely on substrate availability, enzyme activity, and enzyme specificity [10,16]. The total alcohol concentrations in this study differed significantly in both samples, constituting 7.32 and 7.50% of the total volatile compounds detected. Although alcohols were the predominant chemical class in number, their concentrations were relatively low, with 2-ethyl hexanol as the highest alcohol in control samples and 1-octanol the highest in treated samples. Similar observations were reported in the same grape variety by Xie et al. [32], except for the amino acid-derived alcohols, which recorded high concentrations. Amino acid-derived alcohols in this study equally showed higher concentrations in control grapes than in BTH-treated grapes because the concentrations of the precursors (amino acids) were relatively low in BTH-treated grapes, as shown in the Results section.
BTH treatment significantly influenced the concentrations of some ester compounds, resulting in high total concentrations of esters in BTH-treated grapes when compared to control grapes at p < 0.05. An increase in total concentrations of esters in Groppello Gentile and Monastrell grapes treated with BTH was also reported [23,61]. This group of chemical compounds is usually found in grapes at low concentrations because they are mainly products of yeast fermentation [16,62]. The syntheses of acetate esters in grapes are precisely from the degradation of amino acids or carbohydrates through the reaction of acetyl-CoA with higher alcohols [62,63]. The most concentrated ester compound detected in this study was (3Z)-3-Hexen-1-yl acetate, representing 48.14 and 61.51% of the total esters in control and treated grapes, respectively. As a common ester detected in Cabernet cultivars, Kalua and Boss [16] also reported (3Z)-3-Hexen-1-yl acetate as the highest concentrated ester in their study, even though its concentration decreased during development. Other ester compounds found in high concentrations were (3Z)-Hexenyl butyrate and Ethyl acetate, both accounting for 25.12 and 25.10% of total esters in control and BTH-treated samples, presenting higher concentrations in BTH-treated grapes than in control grapes. The high concentrations of ester compounds in BTH-treated grapes could be related to the treatment applied, in the sense that BTH could have stimulated the activity of alcohol acetyltransferase (AAT), enhancing the content of esters in treated samples [16].
The findings in this experiment showed that total concentrations of terpenes and norisoprenoids differed significantly between control and treated grapes, with control grapes observing higher concentrations than BTH-treated grapes. Neutral grapes are generally low in terpenes and norisoprenoids [16,64], which is why their concentrations in this study were low, accounting for 0.50% (control) and 0.37% (treated) of total volatile compounds. Moreover, the high volatile nature of these compounds [65] and their role as messenger signals in plants could contribute to the low concentrations observed [22,66,67]. Six terpenes and three norisoprenoids were detected at maturity, with no significant differences in the concentrations of individual compounds between control and treated samples, except for theaspirane and β-cyclocitral, which differed significantly at p < 0.05 between control and treated samples. The major norisoprenoid, β-cyclocitral, accounted for 69.31% (BTH-treated) and 59.18% (control) of total norisoprenoids, while theaspirane, the highest terpenoid, accounted for 29.89% (control) and 29.67% (BTH-treated) of the total terpenes. Although both samples recorded relatively high levels of linalool, there was no significant difference between them, similar to the report by Gómez-Plaza et al. [23]. According to Gómez-Plaza et al. [23], BTH-treated grapes presented high contents of limonene, β-ionone, and β-damascenone. Contrary to their observation, this study discovered high concentrations of limonene and β-damascenone in control grapes. The disparity between these two studies could be attributed to the grape cultivar, since the treatment was the same (BTH treatment). Moreover, according to research, the composition and content of terpenes and norisoprenoids are solely grape-cultivar-dependent, although several other factors may influence their concentrations [12,55,68]. Moreover, the low levels of terpenes and norisoprenoids detected in the BTH-treated samples could be that BTH treatment downregulated the activities of related enzymes in the terpenoid and carotenoid biosynthetic pathways, reducing the concentrations in treated grapes [61,69,70].
4.4. Correlation Analysis of Amino Acids and Derived Aroma Compounds
Generally, the results depict positive correlations between amino acids and aroma compounds with high coefficients. The aromatic amino acids correlated with most of the aroma compounds. Phenylalanine correlated significantly to syntheses of phenylethyl alcohol, benzyl alcohol, and benzaldehyde in control samples. The positive coefficients indicate that the accumulation of these compounds is influenced directly by the status of phenylalanine. Thus, an increase or decrease in phenylalanine content increases or decreases levels of the aroma compounds. However, phenylalanine in BTH-treated grapes negatively correlated with phenylethyl alcohol at p < 0.05, with a coefficient of −0.892, indicating the inverse impact of BTH on phenylalanine which changed the proportional relationship between phenylalanine accumulation and consumption. Tyrosine correlated significantly at p < 0.05 with benzaldehyde, 2-Ethylhexanol, and (6S)-6-Methyl-1-octanol in BTH-treated grapes. Lysine and glycine contributed significantly (p < 0.01) to the syntheses of 3-Methyl-3-heptanol and phenol in control grapes, correlating strongly with coefficients of 0.927 and 0.924, respectively. However, glycine negatively correlated with phenol at p < 0.05 in BTH-treated grapes, with a coefficient of −0.812. All significant negative correlations were observed in treated grapes, indicating the impact of BTH treatment on amino acids (precursors), as illustrated in the Results section.
5. Conclusions
Our results showed that BTH treatment affected the weight and color of the samples positively while reducing the sugar content. Additionally, amino acid contents in BTH-treated samples were influenced negatively, with significant differences between control and treated samples. The low amounts of amino precursors accounted for low concentrations of amino acid-derived aromas in treated grapes. However, BTH treatment enhanced the accumulation of fatty acids, C6 compounds, alcohols, and esters, presenting higher total volatile concentrations of these compounds in treated grapes compared to their respective control grapes. On the other hand, BTH-treated samples observed lower concentrations of total terpenes and norisoprenoids and total carbonyls. The sequence of concentrations of each volatile class observed in the study suggests that BTH treatment affected the activities of enzymes related to the synthesis of these aroma compounds. Future studies, therefore, could explore the impact of BTH treatment on the expression of aroma-related enzymes in ‘Cabernet Gernischt’ grapes. Additional research to enhance the amino acids profile of ‘Cabernet Gernischt’ grapes with BTH treatment is recommended.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8090812/s1, Table S1: Concentrations of amino acids obtained from untreated and treated Cabernet Gernischt grapes during development; Table S2: Concentrations of aroma compounds of untreated and treated Cabernet Gernischt grapes during development.
Author Contributions
Conceptualization, R.S. and Y.J.; Formal analysis, R.S.; Funding acquisition, Y.J.; Investigation, R.S., Y.J., L.B., Z.Z., L.F. and J.L.; Methodology, R.S. and Y.J.; Resources, Y.J.; Supervision, Y.J.; Writing—original draft, R.S.; Writing—review & editing, R.S., Y.J., L.B., Z.Z., L.F. and J.L.; R.S. and Y.J. contributed equally to this paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 32060514.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salifu, R.; Chen, C.; Sam, F.E.; Jiang, Y. Application of Elicitors in Grapevine Defense: Impact on Volatile Compounds. Horticulturae 2022, 8, 451. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Y.; Li, C.; Wang, B.; Ma, L.; Ren, Y.; Bi, Y.; Li, Y.; Xue, H.; Prusky, D. The effect of benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester (BTH) treatment on regulation of reactive oxygen species metabolism involved in wound healing of potato tubers during postharvest. Food Chem. 2020, 309, 125608. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Rossoni, M.; Borgo, M.; Ferrara, L.; Faoro, F. Induction of resistance to gray mold with benzothiadiazole modifies amino acid profile and increases proanthocyanidins in grape: Primary versus secondary metabolism. J. Agric. Food Chem. 2005, 53, 9133–9139. [Google Scholar] [CrossRef]
- Ge, Y.; Tang, Q.; Li, C.; Duan, B.; Li, X.; Wei, M.; Li, J. Acibenzolar-S-methyl treatment enhances antioxidant ability and phenylpropanoid pathway of blueberries during low temperature storage. LWT-Food Sci. Technol. 2019, 110, 48–53. [Google Scholar] [CrossRef]
- Miliordos, D.E.; Tsiknia, M.; Kontoudakis, N.; Dimopoulou, M.; Bouyioukos, C.; Kotseridis, Y. Impact of application of abscisic acid, benzothiadiazole and chitosan on berry quality characteristics and plant associated microbial communities of vitis vinifera l var. Mouhtaro plants. Sustainability 2021, 13, 5802. [Google Scholar] [CrossRef]
- Bektas, Y.; Eulgem, T. Synthetic plant defense elicitors. Front. Plant Sci. 2015, 5, 804. [Google Scholar] [CrossRef]
- Wang, K.; Liao, Y.; Cao, S.; Di, H.; Zheng, Y. Effects of benzothiadiazole on disease resistance and soluble sugar accumulation in grape berries and its possible cellular mechanisms involved. Postharvest Biol. Technol. 2015, 102, 51–60. [Google Scholar] [CrossRef]
- Poni, S.; Gatti, M.; Palliotti, A.; Dai, Z.; Duchêne, E.; Truong, T.T.; Ferrara, G.; Matarrese, A.M.S.; Gallotta, A.; Bellincontro, A.; et al. Grapevine quality: A multiple choice issue. Sci. Hortic. 2018, 234, 445–462. [Google Scholar]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.; Tavares, R.; Sousa, M.; Agasse, A.; Delrot, S.; Gerós, H. Biochemical changes throughout grape berry development and fruit and wine quality. Food 2007, 1, 1–22. [Google Scholar]
- Wu, Y.; Zhang, W.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, L.; Wang, S. Evolution of volatile compounds during the development of Muscat grape ‘Shine Muscat’ (Vitis labrusca × V. vinifera). Food Chem. 2020, 309, 125778. [Google Scholar] [CrossRef]
- Gonda, I.; Bar, E.; Portnoy, V.; Lev, S.; Burger, J.; Schaffer, A.A.; Tadmor, Y.; Gepstein, S.; Giovannoni, J.J.; Katzir, N.; et al. Branched-chain and aromatic amino acid catabolism into aroma volatiles in Cucumis melo L. fruit. J. Exp. Bot. 2010, 61, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Alem, H.; Rigou, P.; Schneider, R.; Ojeda, H.; Torregrosa, L. Impact of agronomic practices on grape aroma composition: A review. J. Sci. Food Agric. 2019, 99, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. Vineyard practice. In Wine Science; Academic Press: Cambridge, MA, USA, 2020; pp. 151–330. ISBN 9780128161180. [Google Scholar]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Maoz, I.; Rikanati, R.D.; Schlesinger, D.; Bar, E.; Gonda, I.; Levin, E.; Kaplunov, T.; Sela, N.; Lichter, A.; Lewinsohn, E. Concealed ester formation and amino acid metabolism to volatile compounds in table grape (Vitis vinifera L.) berries. Plant Sci. 2018, 274, 223–230. [Google Scholar] [CrossRef]
- Kalua, C.M.; Boss, P.K. Evolution of volatile compounds during the development of cabernet sauvignon grapes (Vitis vinifera L.). J. Agric. Food Chem. 2009, 57, 3818–3830. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Fernández-Fernández, J.I.; Moreno-Olivares, J.D.; Bleda-Sánchez, J.A.; Gómez-Martínez, J.C.; Martínez-Jiménez, J.A.; Gil-Muñoz, R. Application of elicitors in two ripening periods of Vitis vinifera L. Cv monastrell: Influence on anthocyanin concentration of grapes and wines. Molecules 2021, 26, 1689. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Romero-Cascales, I.; Bautista-Ortín, A.B.; Gil-Muñoz, R.; Martínez-Cutillas, A.; Gómez-Plaza, E. Increasing bioactive phenolic compounds in grapes: Response of six monastrell grape clones to benzothiadiazole and methyl jasmonate treatments. Am. J. Enol. Vitic. 2013, 64, 459–465. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Bautista-Ortín, A.B.; Ruiz-García, Y.; Fernández-Fernández, J.I.; Gómez-Plaza, E. Improving phenolic and chromatic characteristics of monastrell, merlot and syrah wines by using methyl jasmonate and benzothiadiazole. Oeno One 2017, 51, 17–27. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Gil-Muñoz, R.; López-Roca, J.M.; Martínez-Cutillas, A.; Romero-Cascales, I.; Gómez-Plaza, E. Increasing the Phenolic Compound Content of Grapes by Preharvest Application of Abcisic Acid and a Combination of Methyl Jasmonate and Benzothiadiazole. J. Agric. Food Chem. 2013, 61, 3978–3983. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bautista-Ortín, A.B.; Gil-Muñoz, R. Influence of methyl jasmonate and benzothiadiazole on the composition of grape skin cell walls and wines. Food Chem. 2019, 277, 691–697. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; López-Roca, J.M.; Bautista-Ortín, A.B.; Gil-Muñoz, R.; Gómez-Plaza, E. Effect of combined use of benzothiadiazole and methyl jasmonate on volatile compounds of monastrell wine. Am. J. Enol. Vitic. 2014, 65, 238–243. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Mestre-Ortuño, L.; Ruiz-García, Y.; Fernández-Fernández, J.I.; López-Roca, J.M. Effect of benzothiadiazole and methyl jasmonate on the volatile compound composition of Vitis vinifera L. Monastrell grapes and wines. Am. J. Enol. Vitic. 2012, 63, 394–401. [Google Scholar] [CrossRef]
- FAO and OIV Food and Agriculture Organization of the United Nations and the International Organisation of Vine and Wine 2016. 2016. p. 64. Available online: https://www.fao.org/publications/card/es/c/709ef071-6082-4434-91bf-4bc5b01380c6 (accessed on 10 March 2022).
- Kupe, M.; Ercisli, S.; Baron, M.; Sochor, J. Sustainable viticulture on traditional ‘baran’ training system in eastern turkey. Sustainability 2021, 13, 10236. [Google Scholar] [CrossRef]
- Taskesenlioglu, M.Y.; Ercisli, S.; Kupe, M.; Ercisli, N. History of Grape in Anatolia and Historical Sustainable Grape Production in Erzincan Agroecological Conditions in Turkey. Sustain. 2022, 14, 31496. [Google Scholar] [CrossRef]
- OIV International Organisation of Vine and Wine (OIV). OIV State of the World Vitivinicultural Sector in 2020; OIV International Organisation of Vine and Wine (OIV): Paris, France, 2021; pp. 1–19. [Google Scholar]
- Thakur, M.; Sohal, B.S. Role of Elicitors in Inducing Resistance in Plants against Pathogen Infection: A Review. ISRN Biochem. 2013, 2013, 762412. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y.; Jiang, W.; Li, J. Identification and quantification of impact aroma compounds in 4 nonfloral Vitis vinifera varieties grapes. J. Food Sci. 2010, 75, S81–S88. [Google Scholar] [CrossRef]
- Sun, W.X.; Hu, K.; Zhang, J.X.; Zhu, X.L.; Tao, Y.S. Aroma modulation of Cabernet Gernischt dry red wine by optimal enzyme treatment strategy in winemaking. Food Chem. 2018, 245, 1248–1256. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Li, J.; Xu, Y. Different influences of β-glucosidases on volatile compounds and anthocyanins of Cabernet Gernischt and possible reason. Food Chem. 2013, 140, 245–254. [Google Scholar] [CrossRef]
- Xie, S.; Tang, Y.; Wang, P.; Song, C.; Duan, B.; Zhang, Z.; Meng, J. Influence of natural variation in berry size on the volatile profiles of Vitis vinifera L. Cv. Merlot and Cabernet Gernischt grapes. PLoS ONE 2018, 13, e0201374. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Bautista-Ortín, A.B.; Ruiz-García, Y.; Fernández-Fernández, J.I.; Gil-Muñoz, R. Effect of elicitors on the evolution of grape phenolic compounds during ripening period. J. Sci. Food Agric. 2017, 3, 977–983. [Google Scholar] [CrossRef]
- OIV International Organisation of Vine and Wine (OIV). Compendium of International Methods of Wine and Must Analysis; OIV International Organisation of Vine and Wine (OIV): Paris, France, 2016; Volume 1. [Google Scholar]
- Yue, X.; Ju, Y.; Tang, Z.; Zhao, Y.; Jiao, X.; Zhang, Z. Effects of the severity and timing of basal leaf removal on the amino acids profiles of Sauvignon Blanc grapes and wines. J. Integr. Agric. 2019, 18, 2052–2062. [Google Scholar] [CrossRef]
- Koyama, R.; Roberto, S.R.; de Souza, R.T.; Borges, W.F.S.; Anderson, M.; Waterhouse, A.L.; Cantu, D.; Fidelibus, M.W.; Blanco-Ulate, B. Exogenous abscisic acid promotes anthocyanin biosynthesis and increased expression of flavonoid synthesis genes in Vitis vinifera × Vitis labrusca table grapes in a subtropical region. Front. Plant Sci. 2018, 9, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xu, J.; Chen, H.; Zhang, H.; Li, S. Determination of Volatile Substances and Amino Acids in Yali Pear Fruit. Food Sci. Technol. 2002, 71–73. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Abingdon, UK, 2005; ISBN 0849330343. [Google Scholar]
- Pino, J.A.; Quijano, C.E. Study of the volatile compounds from plum (Prunus domestica L. cv. Horvin) and estimation of their contribution to the fruit aroma. Food Sci. Technol. 2012, 32, 76–83. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Romero-Cascales, I.; Gil-Muñoz, R.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. Improving grape phenolic content and wine chromatic characteristics through the use of two different elicitors: Methyl jasmonate versus benzothiadiazole. J. Agric. Food Chem. 2012, 60, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Coombe, B.G.; Bishop, G.R. Development of the grape berry. II * Changes in diameter and deformability during veraison. Aust. J. Agric. Res. 1980, 31, 499–509. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bleda-Sánchez, J.A.; Martínez-Moreno, A.; Gil-Muñoz, R. Elicitors and pre-fermentative cold maceration: Effects on polyphenol concentration in monastrell grapes and wines. Biomolecules 2019, 9, 9110671. [Google Scholar] [CrossRef]
- Fernandez-Marin, M.I.; Guerrero, R.F.; Puertas, B.; Garcia-Parrilla, M.C.; Collado, I.G.; Cantos-Villar, E. Impact of preharvest and postharvest treatment combinations on increase of stilbene content in grape. J. Int. Sci. Vigne Vin 2013, 47, 203–212. [Google Scholar] [CrossRef]
- Beckers, G.J.M.; Spoel, S.H. Fine-tuning plant defence signalling: Salicylate versus jasmonate. Plant Biol. 2006, 8, 1–10. [Google Scholar] [CrossRef]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Filipe-Ribeiro, L.; Mendes-Faia, A. Validation and comparison of analytical methods used to evaluate the nitrogen status of grape juice. Food Chem. 2007, 3, 1272–1277. [Google Scholar] [CrossRef]
- Guan, L.; Wu, B.; Hilbert, G.; Li, S.; Gomès, E.; Delrot, S.; Dai, Z. Cluster shading modifies amino acids in grape (Vitis vinifera L.) berries in a genotype- and tissue-dependent manner. Food Res. Int. 2017, 98, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gamboa, G.; Pérez-Álvarez, E.P.; Rubio-Bretón, P.; Garde-Cerdán, T. Foliar application of methyl jasmonate to Graciano and Tempranillo vines: Effects on grape amino acid content during two consecutive vintages. Oeno One 2019, 53, 1–19. [Google Scholar] [CrossRef]
- Lalonde, S.; Tegeder, M.; Throne-Holst, M.; Frommer, W.B.; Patrick, J.W. Phloem loading and unloading of sugars and amino acids. Plant Cell Environ. 2003, 26, 37–56. [Google Scholar] [CrossRef]
- Kliewer, W.M. Changes in the concentration of free amino acids in grape berries during maturation. Am. J. Enol. Vitic. 1968, 19, 166–174. [Google Scholar]
- Gil-Muñoz, R.; Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Bleda-Sánchez, J.A.; Fernández-Fernández, J.I.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M. Effect of methyl jasmonate doped nanoparticles on nitrogen composition of monastrell grapes and wines. Biomolecules 2021, 11, 1631. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Portu, J.; Santamaría, P.; López, R.; Garde-Cerdán, T. Effects on grape amino acid concentration through foliar application of three different elicitors. Food Res. Int. 2017, 99, 688–692. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Lorenzo, C.; Lara, J.F.; Prado, F.; Ancín-Azplicueta, C.; Salinas, M.R. Study of the Evolution of Nitrogen Compounds during Grape Ripening. Application to Differentiate. J. Agric. Food Chem. 2009, 57, 2410–2419. [Google Scholar] [CrossRef]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of Grape and Wine Aroma. Part 1. Chemical Components and Viticultural Impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef]
- El Hadi, M.A.M.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed]
- Aragüez, I.; Valpuesta Fernández, V. Metabolic engineering of aroma components in fruits. Biotechnol. J. 2013, 8, 1144–1158. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.G.; Olías, R.; Luaces, P.; Sanz, C. Biosynthesis of strawberry aroma compounds through amino acid metabolism. J. Agric. Food Chem. 2002, 50, 4037–4042. [Google Scholar] [CrossRef]
- Rosati, C.; Diretto, G.; Giuliano, G. Biosynthesis and engineering of carotenoids and apocarotenoids in plants: State of the art and future prospects. Biotechnol. Genet. Eng. Rev. 2009, 26, 139–162. [Google Scholar] [CrossRef] [PubMed]
- García, E.; Chacón, J.L.; Martínez, J.; Izquierdo, P.M. Changes in volatile compounds during ripening in grapes of Airén, Macabeo and Chardonnay white varieties grown in La Mancha region (Spain). Food Sci. Technol. Int. 2003, 9, 33–41. [Google Scholar] [CrossRef]
- Vitalini, S.; Ruggiero, A.; Rapparini, F.; Neri, L.; Tonni, M.; Iriti, M. The application of chitosan and benzothiadiazole in the vineyard (Vitis vinífera L. cv Groppello Gentile) changes the aromatic profile and sensory attributes of wine. Food Chem. 2014, 162, 192–205. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Perestrelo, R.; Fernandes, A.; Albuquerque, F.F.; Marques, J.C.; Câmara, J.S. Analytical characterization of the aroma of Tinta Negra Mole red wine: Identification of the main odorants compounds. Anal. Chim. Acta 2006, 563, 154–164. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Seaweed foliar applications at two dosages to Tempranillo blanco (Vitis vinifera L.) grapevines in two seasons: Effects on grape and wine volatile composition. Food Res. Int. 2020, 130, 108918. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From central to specialized metabolism: An overview of some secondary compounds derived from the primary metabolism for their role in conferring nutritional and organoleptic characteristics to fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gamboa, G.; Pérez-Álvarez, E.P.; Rubio-Bretón, P.; Garde-Cerdán, T. Changes on Grape Volatile Composition through Elicitation with Methyl Jasmonate, Chitosan, and a Yeast Extract in Tempranillo (Vitis vinifera L.) Grapevines; Elsevier: Amsterdam, The Netherlands, 2019; Volume 244, pp. 257–262. [Google Scholar]
- Marais, J.; Wyk, C.; Rapp, A. Effect of sunlight and shade on norisoprenoid levels in maturing Weisser Riesling and Chenin blanc grapes and Weisser Riesling wines. S. Afr. J. Enol. Vitic. Stellenbosch 1992, 13, 23–32. [Google Scholar] [CrossRef]
- Martin, D.M.; Gershenzon, J.; Bohlmann, J. Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant Physiol. 2003, 132, 21196. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).