1. Introduction
The recent and rapid expansion of the grape and wine industry in North Dakota was made possible by the development and release of interspecific
Vitis spp. hybrids during the 1990s. ‘Frontenac’, an interspecific hybrid with
V. riparia parentage, was released from the University of Minnesota in 1996. ‘Frontenac’ is currently one of the most common wine grape cultivars in the Upper Midwest [
1] due to its cold hardiness and productivity. However, ‘Frontenac’ challenges winemakers due to high acidity and low pH, which leads to a different acid profile compared to traditional
V. vinifera cultivars [
2,
3,
4,
5]. Work with
V. vinifera has demonstrated that increased irradiance within the fruiting zone can improve fruit quality [
6,
7,
8]. However, interspecific hybrids with
V. riparia lineage may respond differently to canopy management practices.
Grapevine training systems manipulate vine form and contribute to differences in total leaf area, the percentage of leaf area well-exposed to light, and the percentage of leaves located in the interior of the canopy [
9,
10,
11,
12]. Consequently, a grapevine’s capacity to photosynthesize efficiently depends upon its training system and the accompanying light microclimate of its leaves [
10]. Modifications in training may increase the amount of leaf area exposed to high-intensity direct radiation while also increasing the interception of diffuse radiation, thus improving the radiation microclimate of the remaining foliage [
13,
14,
15]. In addition, training may impact numerous other variables such as fruit bud differentiation, cluster exposure, vine water status, and leaf transpiration [
10].
Furthermore, a training system structure that maximizes fruit sunlight exposure, especially in cool climates, may be used to optimize berry growth and composition. Fruit in exposed portions of the canopy generally exhibit higher concentrations of sugars, anthocyanins, and total phenolics, as well as lower levels of malic acid, potassium, and juice pH compared to shaded fruits [
16]. With the appropriate choice of training system, increases in yield and alterations to fruit composition and/or wine sensory have been reported [
17,
18,
19,
20,
21,
22,
23,
24,
25].
Contrastingly, other researchers have failed to identify differences in fruit or wine composition directly attributable to training system selection [
26,
27,
28,
29,
30,
31,
32]. Martinson and Particka stated that maintaining cluster exposure and avoiding shading may be more important than the training system based on work at Clayton, NY on ‘Frontenac’ [
33]. Multiple studies indicate shaded canopies produce fruit with lower sugar concentration and increased pH and TA content [
34,
35,
36]. Therefore, excessively shaded fruits may have compromised composition. The decreased sugar content for shaded fruit may result from a combination of a delay in maturation, or lower light intensity on source leaves, and lower berry temperature [
13,
37,
38].
Decreased pH levels for shaded berries have been associated with the higher fruit concentrations of nitrates and potassium; low light wavelengths (600 nm to 730 nm) in the canopy reduces the activity of the enzyme nitrate reductase leading to an accumulation of nitrate and potassium [
11,
39]. High TA levels in shaded fruit were attributed to a reduction in malate degradation associated with reduced berry temperature [
38,
40,
41,
42]. Excessive shade also produces fruit with reduced aromatic, anthocyanin, and monoterpene levels [
7,
8].
Fruit zone leaf removal is one of the most frequently applied summer canopy management practices in grape growing [
17,
38,
39,
43]. Leaf removal is traditionally conducted between fruit set and veraison; however, early leaf removal is conducted as early as pre-bloom to fruit set [
17]. Early leaf removal is the removal of basal leaves of the main shoots, and occasionally, lateral shoots developed from the basal nodes. Increasingly, work is focusing on early leaf removal practices as a potential alternative to cluster thinning [
44,
45]. Benefits of early leaf removal include reducing the severity of Botrytis rot infection, altering flower development, and modulating fruit and wine composition [
9,
43,
46,
47]. The improved microclimate following leaf removal may influence the berry epidermis, contributing to higher anthocyanin concentrations in wine. Further, wine volatile components are increased by early leaf removal under warm climatic conditions [
48]. Leaf removal effects are dependent on the cultivar, timing, severity, and climate [
39,
49,
50].
Previously, research on leaf removal impact demonstrates its variable effect on fruit quality and yield. Leaf removal on ‘Sauvignon Blanc’ from fruit set to veraison with various defoliation rates was found to effectively reduce TA, malic acid, pH, and juice potassium in all leaf removal treatments with no effect on yield [
39]. Similar results found decreases in TA, pH, and potassium with basal leaf removal treatments on
V. vinifera cultivars Bacchus, Pearl of Csaba, Schönburger, and Siegerrebe near veraison [
50]. Basal leaf removal, in
V. vinifera cultivars Graciano and Carignan, at fruit set resulted in decreased malic acid concentration [
51]. Interestingly, defoliation of six basal leaves per shoot pre-bloom in ‘Sangiovese’ caused a decrease in yield, yet increased soluble solids, total anthocyanins, and TA [
49]. Hence, not all attempts to advance maturity or improve grape composition with leaf removal have been successful [
52,
53,
54]. Work performed by Percival, Fisher, and Sullivan (1994) in the Niagara region of Canada reported on leaf removal before veraison on
V. vinifera and found no difference in SS, pH, and TA, and no reduction in yield [
38].
Leaf removal effects can be cultivar-dependent. Three
V. vinifera cultivars were compared by leaf removal treatments over 4 years [
55]. The cultivar Barbera had no significant differences in TA and pH, while cultivars Croatina and Malvasia di Candia aromatica had significant differences in TA [
55]. A report by Portz et al. (2010) on ‘Frontenac Gris’ in Iowa found no significant differences in SS, pH, and TA with leaf removal conducted in early July [
56]. Similarly, leaf removal at veraison on ‘Frontenac’ by Wlordachak et al. (2009) in Illinois found no significant differences in SS, TA, and pH in leaf removal treatments. Therefore, it is of great importance to research leaf removal treatment during bloom, post-bloom, and veraison compared to no leaf removal treatment in North Dakota-specific environments [
57].
The effect of leaf removal on fruit quality and yield is dependent on timing, severity, climate, and cultivar; therefore, it is necessary to conduct experiments on specific cultivars within specific climates to tailor regional best management practices. The objectives of this experiment were to evaluate the effects of training systems and fruit zone leaf removal timings on ‘Frontenac’ grapevines’ fruit composition and vine performance in eastern North Dakota. The effects of these practices are valuable and necessary for growers and winemakers in the Upper Midwest grape and wine regions.
2. Materials and Methods
2.1. Experimental Site and Design
The University of Minnesota interspecific hybrid, ‘Frontenac’, was used to study the effects of training systems and leaf removal on vine performance and fruit composition over two years, 2013 and 2014. The research vineyard used was located at the North Dakota State University (NDSU) research station near Absaraka, ND, USA (Lat: 46°59′22.0986″ Long: −97°21′22.2222″). Soils at the site are Warsing sandy loam, fine-loamy over sandy and sandy-skeletal, mixed, superactive, frigid Oxyaquic Hapludolls with 0–2% slopes. One hundred twenty-eight own-rooted ‘Frontenac’ vines were established in 2006 and spaced 2.6 m apart in rows 3.3 m apart. Rows were oriented North-South with 32 vines per row.
Weed, disease, and pest control were managed according to North Dakota industry standards. Due to low pressure, no fungicide or insecticide applications were conducted during the experimental period. Weed-free strips (0.5 m wide) were maintained below the vine rows using tillage combined with pre-emerge (Flumioxazin, Chateau®, Valent USA, San Ramon, CA, USA) and post-emerge (Glufosinate, Rely®, BASF, Florham Park, NJ, USA; Glyphosate, Roundup®, Monsanto, St. Louis, MO, USA) herbicide applications. Red fescue (Festuca rubra) was grown between rows as a ground cover. Annual petiole tests were used in the research vineyard to determine fertilizer applications prior to the experiment; no fertility alterations were conducted during the experimental period.
2.2. Training Systems and Canopy Management
Vines were originally trained to the Four-Armed-Kniffin (4AK) trellis system. In 2010, vines were retroactively trained to three additional canopy-training systems: Geneva Double Curtain (GDC), High Cordon (HC), and mid-wire with Vertical Shoot Positioning (VSP). Training system treatments were arranged as a randomized complete block design with eight replicates of the four training system treatments and four vines within each training system treatment, resulting in 16 vines per rep and 128 plants total.
Vines in the HC system were trained to bilateral cordons 2 m aboveground. Cordons extended in opposite directions (North-South). Vines in the 4AK system were trained to two bilateral cordons, one at 2 m aboveground and the second at 1.5 m aboveground. Vines in the VSP system were trained to bilateral cordons 1 m aboveground. Throughout the summer, shoots were tucked upward as needed between horizontally running catch wire. GDC vines were trained to two bilateral cordons, each 2 m aboveground with wires 0.6 m apart supported by post extensions. Shoots were combed downward three times during the growing season for GDC, HC, and 4AK vines at three weeks post-bloom, four weeks post-bloom, and veraison.
For plant management, vines were pruned in late spring to delay early bud break and decrease susceptibility to late spring frosts [
30,
58,
59,
60]. Prunings of one-year-old canes were weighed to determine vine size. Balanced pruning was used to maintain a balance between vegetative vigor and reproductive quality. The base node count was 30 with every additional 0.45 kg of one-year-old pruning wood leading to retention of an additional 10 nodes per vine, with a maximum limit of 60 nodes per vine. Cane pruning weights, cordon lengths, and trunk diameter measurements were taken each spring to determine vine size.
Viable nodes were counted at bud burst. Shoots per node and shoots per plant were counted close to bloom. Shoots were not thinned in an attempt to retain shoots for annual cordon rejuvenation and to reduce gaps between spurs prone to separation. In both years, no cluster thinning was conducted. Shoot tips were only hedged if growth reached the soil surface.
2.3. Leaf Removal and Light Measurements
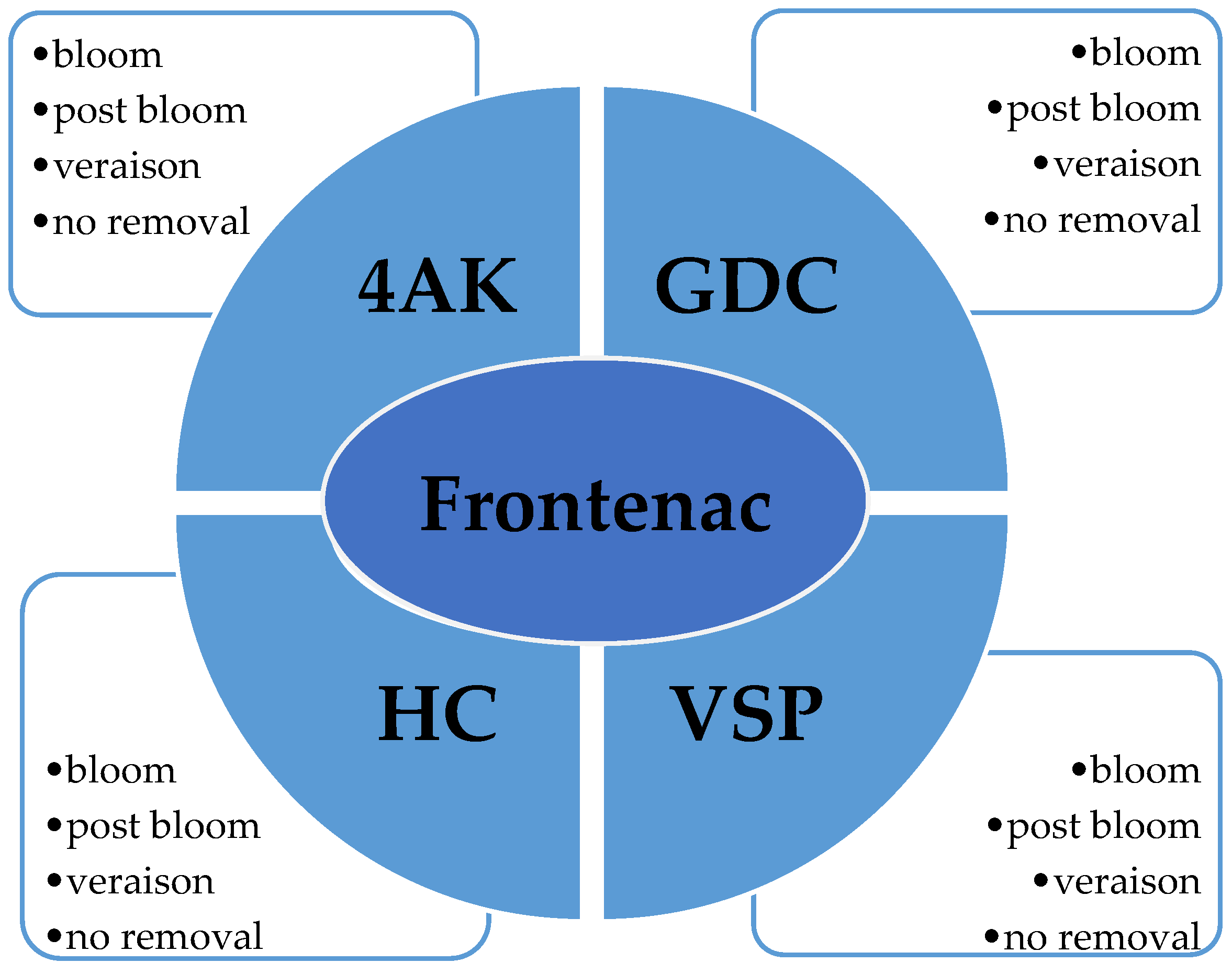
Fruit zone leaf removal treatments were arranged as a split-plot, with training system as the whole-plot and leaf removal as the sub-plot. The four-leaf removal treatments were conducted at bloom, post-bloom, veraison, and no removal (control) (
Figure 1). The treatments administered in 2013 were re-administered to the same vines the following growing season, 2014.
Four training systems were evaluated: Four-Arm Kniffin (4AK), Geneva Double Curtain (GD), High Cordon (HC), and Vertical Shoot Positioned (VSP) within each training system, and four-leaf removal treatment subplots were examined, bloom, post-bloom, veraison, and no removal treatment (control).
Canopy density of each training system was maintained during the growing season, with shoot positioning appropriate for each training system. Leaf removal treatments were applied at bloom, three or four weeks post-bloom (once 289 GDDs accumulated post-bloom), and veraison. Leaves were removed from the basal three nodes on all shoots arising from the cordon and spurs.
Point quadrant data were collected to supplement the understanding of canopy density. Point quadrant is the use of a thin rode inserted into the fruit zone of the canopy of a single vine 50 times, 25 from each side of the row with the rod parallel to the ground [
17]. At the insertion, contacts with leaves and other vine parts are recorded. The data collected give the ability to calculate percent gaps, leaf layer number, percent interior leaves, and percent interior cluster. These values were compared to optimum values to elucidate canopy structure.
2.4. Harvest Indices and Fruit Composition
Fruit was sampled weekly from veraison to predict the harvest. At the final sampling date, fruit were harvested and weighed on a per-plant basis for yield results. Cluster weight was determined by weighing a random sample of three clusters per vine. Berry weight and diameter were determined by weighing and measuring a 100-berry sample from the three-cluster sample. Fruit characteristics were determined by a 15-berry sample per vine. Total Soluble Solids (TSS) were measured twice per sample by a portable pocket refractometer (PAL-1, ATAGO, Tokyo, Japan). Juice TA and pH were measured in triplicate for each experimental unit and were determined using standard methods with an Orion star A111 pH meter (Thermo Scientific, Beverly, MA, USA). The date of harvest each year was determined by inclement weather, availability of vineyard help, and fruit composition.
2.5. Statistical Analysis
Data were analyzed across years as a split-plot in time using Proc Mixed SAS statistical analysis software (SAS version 9.3, SAS Institute Inc., Cary, NC, USA). Only the whole-plot main effect, the training system, was significant for variables evaluated. Differences among training systems were determined by pairwise t-tests, and the significance of these differences were determined based on a 95% level of confidence for all comparisons.
5. Conclusions
Within our research, leaf removal had no significant effect on ‘Frontenac’ fruit quality; however, training system selection impacted vine performance across numerous metrics. Due to inconsistent results among different trellis treatments in the two years, more research must focus on choosing the best management practices for ‘Frontenac’. Further studies on the effects of canopy management practices to improve fruit quality need to be completed to develop a standard set of recommended viticultural practices for this cultivar and other new cultivars to optimize fruit quality for winemaking for the Upper Midwest and North Dakota producers.
While ‘Frontenac’ showed no response to leaf removal in our experiments, other grapevine cultivars may be more responsive [
66]. Correctly pairing cultivars with management practices will improve fruit and wine composition in cold climates, such as North Dakota. Likewise, although bunch rots were not observed for ‘Frontenac’ within this experimental period, in the future, leaf removal can be an important tool in reducing rots for low input farming systems with susceptible cultivars [
89,
90,
91].
Overall, the use of leaf removal practices and trellis system selection remains an important decision for grape growers to make. In other cool climates, such as Wisconsin, exposure of fruit has resulted in the acid reduction in must at harvest [
92,
93]. Similarly, trellis has demonstrated effects on fruit composition of ‘Frontenac’ in Nebraska with GDC deemed favorable [
2,
18]. In this study, ‘Frontenac’ grape berry components were not substantially altered by management practices; only one occurrence of differences in pH was attributed to trellis selections. The yield was greatest on GDC trellis in 2014, despite no alteration in target bud number. Growers of ‘Frontenac’ should account for the lack of measurable benefits from leaf removal (no observed differences in fruit composition or yield attributes during 2013 or 2014) before incorporating them as standard practices in eastern North Dakota.