Abstract
The profitability of modern apple orchard plantings depends largely on how rapidly the costs of planting are returned. Tree establishment and growth in the formative years are often can be limited by transplant shock associated with bare-root trees. In this experiment, we examined the effect of two planting systems, air-pruning containers, and field-grown liners, on above- and below-ground growth and development during the first year in the nursery. M9 ‘Nic 29’ bench grafts for three apple cultivars of different vigor classes, ‘Fuji’ (high vigor), ‘Gala’ (moderate vigor), and ‘Honeycrisp’ (low vigor). We hypothesized that air root-pruning containers would alter rooting distribution compared to field-grown liners by increasing fine root production, and this, in turn, would result in improved resource allocation and greater biomass partitioned to above-ground organs. Bench grafts were divided evenly between a field-grown liner bed (for bare root production) and an air-pruning container system. Air-pruning containers produced trees with significantly more root tips and greater total root length per tree than field-grown liners. Importantly, air-pruning containers resulted in a marked and significant increase in roots < 0.4 mm in diameter; however, field-grown liners produced trees had significantly more roots with diameters > 1.5 mm and, thus, significantly greater root dry matter content (DMC). Above-ground parameters (scion length, DMC of wood and leaves, and individual leaf area and DMC) were significantly increased for plants in air-pruning containers. Generally, the growth benefits from air-pruning containers were less pronounced in ‘Gala’ compared to ‘Honeycrisp’ or ‘Fuji’. The percentage of total DMC partitioned to the canopies of air-pruning containerized trees was significantly greater than field-grown liners.
1. Introduction
Orchard profitability depends on several factors such as yield, fruit quality, fruit cultivar, current market price, orchard management practices, and, most notably, tree density [1,2,3,4,5]. A 10–20-fold increase in apple tree density has occurred over the past 50 years [6]. Trees represent a disproportionate fraction of the total production costs when establishing high-density orchards. In the US, tree costs for modern high-density apple orchards total ~$36,000 USD per ha when established at ~3630 trees per ha [7]. Return on investment (ROI) is the most critical factor in modern orchard establishment in the USA [5]; thus, producers need to fill orchard space rapidly and then shift trees to cropping. This reality underscores the need for healthy trees with well-structured root systems capable of filling orchard space quickly.
Because precocity is intimately associated with profitability, orchardists enact several strategies to achieve early production, including increasing tree density, avoidance of water stress and/or nutrient deficits, minimal pruning in the formative years, branch positioning below horizontal orientations, and use of highly branched, feathered nursery trees [8]. Despite implementing these practices, the establishment of orchards with bare-root nursery material has generally remained unchanged despite unrealized growth during the establishment year due to the loss of roots during harvest, storage, transportation, and planting processes [9,10].
Containerized trees undergo comparatively less transplant shock than bare root trees since the entirety of their root system remains intact at the time of transplanting. They may be limited, however, by several other factors: circling and girdling roots, root escape via drainage holes, low root occupancy resulting in root disruption and/or significant root loss during transplanting, and high hydraulic resistance between the soilless media and the native soil resulting in water stress after planting. Air-pruning containers eliminate all but the latter issue due to the generation and branching of new roots following the ‘pruning’ of apical root meristems when root tips are exposed to air. A demonstrable growth advantage after transplanting perennial plants produced in air-pruning containers was purportedly due to a combination of greater absorptive capacity and limited transplant disturbance of their root systems compared to plants produced by alternative production methods [9,10,11].
Fine roots of tree species are relatively short-lived (i.e., several months) and account for most of the total root length [12,13]. Fine, non-woody roots with diameters < 2 mm are generally associated with nutrient and water acquisition for a range of species [14,15], including apple [16,17,18]. More recently, studies on root function argue that a 2 mm cutoff is non-discriminative for segregating absorptive from structural or pioneer roots and, thus, highly overestimates the actual percentage of absorptive roots [19]. Functional data support differences between ephemeral, low-order roots from higher-order, structural roots when comparing roots of similar diameter [19]. While root function may be more tightly associated with root order than root diameter, the former measure is far more time consumptive [19,20,21]. Ref. [20] proposes that diameter cutoffs to discriminate root function are not at all implicit unless functional correlations have been performed from species-specific data. For apple, an analysis of multiple functional parameters such as root longevity, survival, and neighboring root analyses suggest that roots with diameters > 0.5 mm should not be considered absorptive, even though roots of smaller diameters comprise several root orders (1st through 3rd) [20]. The longevity of absorptive roots of apple is from 20 to 144 days [16,21], which is relatively short compared to turnover rates of other species [22]. Many root studies for apple have focused on small samples (destructively harvested or via minirhizotron) taken from few and relatively mature fruiting trees; however, limited or no root research has been conducted on entire root systems from non-seedling plants less than one-year-old, as is the case for nursery production of asexually produced crops such as apple.
The primary objective of this experiment was to characterize the effects of air-pruning containers in the initial nursery year of growth for bench-grafted apple plants on root production, canopy growth, and total plant (DMC). We hypothesized that greater fine root production of air-pruning containers will lead to improved canopy growth compared to field-grown liners produced trees.
2. Materials and Methods
2.1. Plant Material and Experimental Design
In late May 2017, ~200 uniform ‘Fuji’, ‘Gala’ and ‘Honeycrisp’ dormant benchgrafts (using hardwood cuttings of M.9 Nic29 rootstock) were received from Sierra Gold Nurseries (Yuba City, CA, USA) and randomly divided into two different planting systems; field-grown liners and air-pruning containers (Ellepot USA, Blackmore Company, Belleville, MI, USA). Benchgrafts were callused with limited root initiation at the time of planting. The experiment was performed at the Michigan State University Horticulture Teaching and Research Center (HTRC) in Holt, Michigan. Planting systems were organized in completely randomized designs of ~100 benchgrafts per cultivar in each system with four replicates. Bamboo stakes were installed adjacent to individual plants in each system, and leaders were attached to the stakes ~5 cm below the apex using flexible tree tape throughout the entire season. The root-pruning container media was 80% Peat, 10% fine perlite, 10% coir (Starter Mix, Ellepot USA, Blackmore Company, Belleville, MI, USA), compressed in a heat-welded paper membrane (AP standard paper, Ellepot USA, Blackmore Company, Belleville, MI, USA). The container volume was 785 cm3 (100 mm in diameter by 280 mm in height). The soil of the field liner plot was a Marlette series fine loam.
2.2. Irrigation and Fertilization
Irrigation was provided to keep soil moisture above 75% field capacity (FC) and commenced after bud break. For air-pruning containers, irrigation was supplied daily until saturation using an impact sprinkler system that uniformly wetted the entire land area. Media moisture content was determined using Meter soil moisture sensors (EC-5) connected to EM50 data loggers from which data were downloaded using ECH2O software (Meter Environment, Pullman, WA, USA). Four sensors per treatment (one per replicate) were inserted at 15 cm depth. FC was estimated to be 100% after complete drainage following irrigation events using containers instrumented with soil moisture sensors (Figure S1).
Irrigation for field-grown liners was provided every other day using microsprinklers (Supernet, Netafim USA, Fresno, CA, USA) to replace 100% of ET using a kc value of 0.25 because (1) very low transpiration/leaf area existed for the first two months following planting, (2) water penetrable weed fabric would have reduced soil evaporation, and (3) a physical determination of the wetting front after replacing 25% ET over a three day period exceeded 15 cm profile depth through the fabric. ET data were downloaded from a weather station located within 100 m of the experimental site via the MSU Enviro-weather meteorological online network https://mawn.geo.msu.edu/station.asp?id=htc (accessed on 19 July 2022). In late July, the kc value was increased to 0.5, and soil moisture sensors were instrumented at 15 cm depths in the field (one per replicate) to confirm that the ET replacement method of irrigation was providing a roughly equivalent percent of FC as container plants (Figure S1). Using soil moisture sensors, the FC of field soils was estimated as 100% the morning after early-evening irrigation events (allowing gravity to remove water from macropores).
In both systems, equivalent rates of nitrogen and micronutrients were provided. Initial fertilization was applied as a top dress at 5 g per tree via application of (15-9-12) slow-release fertilizer (Osmocote, The Scotts Co., Marysville, OH, USA). Beginning mid-July, a complete macro and micronutrient fertilizer solution (Peters Professional, 21-7-7, The Scotts Co., Marysville, OH, USA) at 100-ppm N equivalent was injected through a fertigation system to both systems once per week. Leaf chlorosis was observed in containerized plants in mid-July due to the limited buffering capacity of the container media in combination with a basic water source (i.e., 388 mg L−1 CaCO3). Thus, a solution of sulfuric acid was prepared weekly for fertigation events to reduce the pH of the water to 6.0. Weed control was achieved in field-grown liners by a water permeable, woven fabric installed over freshly tilled ‘beds’ prior to planting. A sphere (dia., ~2.5 cm) was removed by flame at 30 cm intervals to facilitate planting. Containers were manually weeded on a weekly basis.
2.3. Sampling
Destructive samples were collected mid-season (15 August 2017) and at the end of the season (15 November 2017). For all scions, 16 trees of each production system (4 per replicate) were harvested at each sample date. The roots of each tree were carefully harvested to avoid fine root loss by gently washing them with deionized water. Roots were stored in 70% Eth at 4 °C until image analysis.
Complete extracted root systems were scanned using a calibrated color optical scanner STD4800 (Epson Seiko Corporation, Nagano, Japan) and analyzed with XLRhizo 2017 software (Regent Instruments Inc., Quebec City, QC, Canada). Detailed root measurements included total root volume (cm3), total root length (cm), total length of ‘fine’ roots (≤0.4 mm in diameter), total length (cm) of ‘non-fine’ roots (>0.4 mm in diameter), total number of root tips, and total (DMC) of root shanks (below the graft union) and roots (g) dried to constant weight in a forced air drying oven at 60 °C. Above-ground tree characteristics included total annual leader length, scion cross-sectional area (SCA), leaf number, total canopy leaf area and individual leaf area measured with a leaf area meter (Li-3000, Licor Inc., Lincoln, NE, USA), and DMC of the shank (below the graft union but above the soil line), scion, and leaves after drying to constant weight.
2.4. Statistical Analysis
Analysis of variance (ANOVA) and Tukey’s HSD test mean separation test was carried out by R statistical package R (v. 3.4.3, R Foundation, Vienna, Austria). At each sample date, the effect of cultivar, system, and their interaction on each response variable was tested to determine the significance.
3. Results
The production system significantly altered all root measures, albeit with differences among cultivars, at both sample dates, i.e., mid-season (15 August) and end-season (15 November), with the exception of root DMC, which was not significantly affected by the production system. However, by the end of the growing period, DMC of roots was significantly higher (~28%) for field-grown liners (Table 1). The effect of cultivar was significant for root DMC at both sampling dates; root systems of ‘Fuji’, ‘Gala’ and ‘Honeycrisp’ field-grown liners plants had 1.2, 1, and 0.9 g more DMC, respectively, than air-pruning container plants (Table 1). The DMC of rootstock shanks was significantly affected by cultivar. There was a significant interaction between system and cultivar for total root tips.

Table 1.
The effect of two production systems (air-pruning containers and field-grown liners) on the growth of below-ground organs of ‘Gala’, ‘Fuji’, and ‘Honeycrisp’ callused benchgrafts on M9 Nic29 rootstock. DMC, dry matter content. Data are means of four replicates; each replicate is the mean of four subsamples, and each subsample was an entire tree. The sample dates of 15 August and 15 November were ~3 and 6 months from planting, respectively.
The number of root tips and total root volume were significantly higher for air-pruning containers at the end of the season for ‘Gala’ and ‘Honeycrisp’, but not ‘Fuji’. The total length of roots was also significantly higher in air-pruning containers in all cultivars at both sample periods, though the magnitude of the difference between production systems varied with cultivar; ‘Gala’, ‘Honeycrisp’, and ‘Fuji’ had 4-, 3-, and 2-fold greater total length, respectively, at the end of the season in containers compared with field-grown liners. Similarly, the total length of the fine roots, i.e., ≤0.4 mm diameter, was significantly higher in air-pruning containers for ‘Gala’ and ‘Honeycrisp’.
A distribution analysis of root length by diameter from ≤0.1 mm to 4.9 mm in 0.1 mm increments showed similar root distribution patterns for all cultivars, irrespective of the production system (Figure 1). The total root length of roots with diameters between 0.1 to 0.2 mm was 10–100 times that of other diameter classes. All cultivars had significantly greater root lengths of finer root classes in air-pruning containers compared to the field-grown liners production system (Figure 1). For roots > 1.5 mm diameter, field-grown liners-produced plants had greater total root length than plants in air-pruning containers.
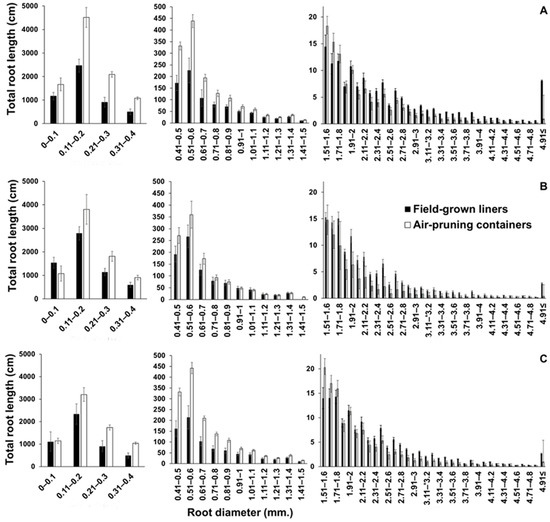
Figure 1.
The effect of two production systems (air-pruning containers and field-grown liners) on the distribution of root length by diameter, in 0.1 mm diameter increments, for (A) ‘Gala’, (B) ‘Fuji’, and (C) ‘Honeycrisp’ trees harvested on 15 November ~6 months from planting as callused benchgrafts. Data are presented for each cultivar in three panels comprising roots of diameters between 0.1 and 0.4 mm (left), between 0.41 and 1.5 mm (center), and between 1.51 and ≥4.91 (right) to account for the different scales of root length for each population of roots. Data are means of four replicates; each replicate is the mean of four subsamples, and each subsample was an entire tree.
By the end-season measurement, the production system and cultivar were significant for all above-ground (scion) growth characteristics, except for the number of leaves per tree, which only differed for cultivar (Table 2). The interaction between system and cultivar was significant for total scion length, scion DMC, canopy leaf area, number of leaves per tree, and single leaf area (Table 2).

Table 2.
The effect of two production systems [air-pruning containers and field-grown liners] on above-ground parameters of ‘Gala’, ‘Fuji’, and ‘Honeycrisp’ callused benchgrafts on M9 Nic29 rootstock. DMC, dry matter content. Data are means of four replicates; each replicate is the mean of four subsamples, and each subsample was an entire tree. The sample dates of 15 August and 15 November were ~3 and 6 months from planting, respectively.
Relative to the cultivar effect, air-pruning containers improved ‘Fuji’ and ‘Honeycrisp’ growth for all above-ground response variables compared to field-grown liners, with the exception of leaf number per plant. ‘Honeycrisp’, the least vigorous cultivar of the three, had the greatest growth benefit from containers for all investigated factors; e.g., containerized ‘Honeycrisp’ plants had ~50% greater canopy leaf area than field-grown liners-produced trees. In contrast, ‘Gala’ was not significantly affected by the production system for any measure.
The proportion of total DMC in roots was significantly lower in plants of air-pruning containers for all cultivars at the end-of-season measures (Figure 2). These data imply greater DMC investment in above-ground organs for plants produced in air-pruning containers compared to those produced in the field.
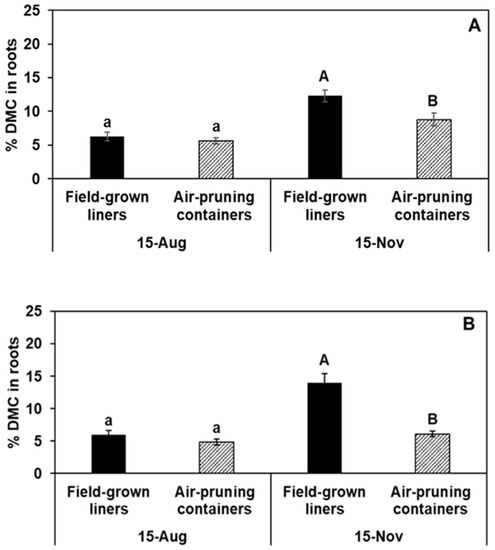
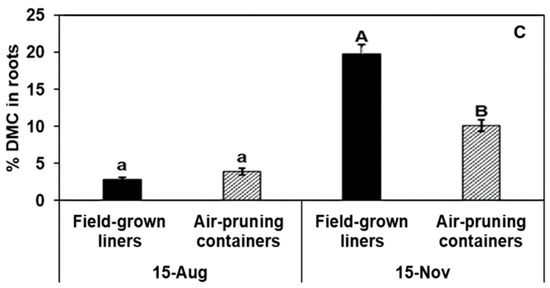
Figure 2.
The effect of two production systems [air-pruning containers and field-grown liners] on the percentage of total plant dry matter content (DMC) in roots of (A) ‘Gala’, (B) ‘Fuji’, and (C) ‘Honeycrisp’ trees harvested on 15 August and 15 November, which was ~3 and 6 months, respectively, from planting of callused benchgrafts. Data are means of four replicates; each replicate is the mean of four subsamples, and each subsample was an entire tree. Bars are ±1 SE.
4. Discussion
In this experiment, air-pruning containers had a marked effect on root production and canopy growth dynamics of first-year apple benchgrafts compared to a field-grown liner production system. Air-pruning containers produced plants with more root tips and proportionately greater fine roots (i.e., roots of ≤0.4mm) but less overall root DMC than the field-grown liner production system. As a result, trees in air-pruning containers allocated proportionately more growth resources to above-ground organs (Figure 2). The general increase in canopy growth of containerized trees was the likely result of greater capacity of fine apple roots to acquire available water and nutrients [23].
Increased fine root production of apple plants in air-pruning containers was also observed for Pinus sylvestris and radiata D. and Platycladus orientalis L. during nursery production [9,10,24]. An increase in the number of root tips in the air-pruning containers is the result of root penetration through the decomposable paper liner into engineered air spaces between the paper liner and the plastic walls of the container. The apical meristems of these roots are then ‘pruned’, causing lateral rooting. These newly generated roots have relatively high growth and respiratory demand [23] and plausibly compete with other sinks for carbohydrate. The effect of this primary growth response may be a relatively low investment in radial root growth compared to field-grown liner-produced plants and thus explain the greater DMC and percentage of larger diameter roots of field-grown liners (Table 1; Figure 1). Though root order data were not collected in this study, the relationship between root diameter and root function of apple roots has been elucidated [20]. The relationship between root diameter and function provides a compelling argument that a 2 mm diameter cutoff for classifying absorptive roots in apple overestimates this population due to inclusion of structural roots, despite its common use in the literature [16,17,18]. Thus, in the present study, only roots with diameters ≤ 0.4 mm were classified as absorptive based on [20]. While fine roots were markedly increased by air-pruning containers compared to field-grown liners, in general, first-year apple plants had relatively high frequencies of small diameter roots regardless of the system (Figure 1), which agrees with a previous study describing root systems of 1-year-old apple trees [25]. Clearly, by the end of the season, some root turnover would have occurred, given the relatively short lifespan of apple roots [16,21]; however, the total population of roots, irrespective of order, was less than three and six months old at the mid- and end-of-season sampling dates. Thus, many of the ‘fine’ roots, irrespective of their order, may have had absorptive capacity, though this capacity has been demonstrated to decrease with root age in several species [23,26].
The cold climate and relatively short growing season of Michigan, in combination with limited presence of roots on callused benchgrafts and a late planting date, all contributed to the relatively short seasonal scion extension growth reported. Moreover, the rootstock shanks of benchgrafts for this study were quite long (~20 cm in length); previous research has demonstrated that differences in the vascular anatomy (particularly xylem) between apple rootstocks and scions promote dwarfing [27,28], which can be exacerbated with the increasing length of the rootstock shank [29,30]. Irrespective, when accounting for the ~10 cm of rootstock shank above ground in both production systems, end-of-season tree heights were ~100, 95, and 73 cm for ‘Fuji’, ‘Gala’, and ‘Honeycrisp’ produced in containers, respectively. Field-grown liner trees were significantly shorter (Table 2). While this growth advantage was not associated with a difference in soil moisture availability between systems (Figure S1), we consider that nutrient availability or soil temperature can affect young trees, and these could have differed between the production systems. Nutrient limitations in the field, however, were unlikely due to supplemental fertilization and preexisting nutrient concentrations of native soils, especially relative to N, given the additional N released from the decomposition of organic matter. We expect that fertilized plants in containers were not nutrient limited, given the likelihood for higher nutrient concentrations in closer proximity to roots as a result of the weekly fertigation concomitant with the volumetric containment of roots. Irrespective, the quantitative differences in the number of root tips and root lengths of smaller diameter roots between production systems are evidently a system effect that likely contributed to improved resource acquisition for containerized plants and manifested in greater scion growth.
We observed a significant effect of cultivar on scion growth, which was predominantly associated with the low vigor of ‘Honeycrisp’. Contributing mechanisms to reduced vegetative growth of young, non-fruiting Honeycrisp trees are not clear and may be attributed to anatomical, physiological, and/or biochemical factors. For example, xylem vessel cell size has been associated with scion dwarfing conferred by certain rootstocks [27,28]. Scion vigor may also be associated with concentrations or ratios of growth inhibiting to promoting compounds [31] or nutrient acquisition and transport properties of a given rootstock-scion combination [32,33]. Of the three cultivars evaluated, gas exchange of ‘Honeycrisp’ was similar to Fuji and higher than Gala [34], yet field-grown ‘Honeycrisp’ trees completed their terminal extension growth by mid-season; terminals of container-produced trees only elongated an additional ~20% after 15-August, which was markedly less than ‘Fuji’ or ‘Gala’. The early seasonal cessation of ‘Honeycrisp’ terminal growth is a common observation in the orchard and is hastened by the presence of fruit. Termination of extension growth, concomitant with continued photosynthesis of the canopy, led to increased resource allocation to roots and, ultimately, similar root growth and development to ‘Gala’ and ‘Fuji’ by the end of the season. Cultivars also responded differently to production system. Scion growth of ‘Gala’ trees, for example, was similar between container and field systems, despite having significantly different root systems. The ability of ‘Gala’ to produce similar canopy growth with significantly less root was not observed for the other cultivars and is illustrated by a smaller difference in the percentage of total plant DMC in roots versus shoots when comparing the two production systems (Figure 2). An alternative index is the ratio of total canopy leaf area (cm2) to total root length (cm) (34). More specifically, the total absorptive root length, since presumably plant investment in leaf area is dependent on the capacity of the ephemeral, absorptive roots to meet its evaporative demand. The ratios for each of the cultivars, irrespective of the production system, was 0.13 with the exception of field-produced ‘Gala’, which was two-fold (0.27). These ratios are less than published values [35]; however, the authors could not find similar data for young (≤1-year-old) trees. Fuji differed from other cultivars in having significantly less root DMC in both production systems yet similar canopy growth. These differences illustrate the potential influence of the scion on the root development of a given rootstock (34) and indicate plasticity in the relative distribution of biomass to different organs (e.g., shoots vs. roots) [36,37,38,39].
The dual advantage of increased canopy growth and absorptive fine root production of containerized trees may not necessarily be sustained following establishment in an orchard since (1) overwinter survival rates of apple roots <0.3 mm in diameter was 3–12% compared with 55–60% for roots 0.5–1 mm in diameter [21], (2) conductance of water between native soils and the soilless media after transplanting presents challenges to maintaining optimal soil moisture content in the rhizosphere, and (3) the higher percentage of coarse roots (or non-fine roots) of field-grown liners may possess potentially greater storage pools of nonstructural carbohydrate to support spring growth emergence than fine roots. Irrespective, planting the paper-lined root system of air-pruning containers eliminates root loss or disturbance compared to considerable root loss from bare root harvest, storage and transportation operations. Whether or not the benefits observed in first-year growth from air-pruning containers result in a growth and/or cropping advantage after transplanting in an orchard is the subject of our continued research.
5. Conclusions
We conclude that air-pruning containers modify the root and canopy growth of apple trees favorably compared to field-grown liners. These changes resulted in greater scion growth during an initial ‘nursery’ period and may facilitate a sustained growth advantage given the limited root disturbance following transplanting.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8090797/s1, Figure S1: Soil moisture content of air pruning containers (blue) and field liners (orange) as percent of filed capacity (see Materials and Methods for methodology and measurement). Data are means of 4 soil moisture sensors (one per replicate). Air pruning containers were instrumented at the start of the experiment. Field liners were instrumented in late July when kc values were increased in order to verify that sufficient water was being delivered to field plots.
Author Contributions
M.E. and T.C.E.: conceptualization; M.E.: formal analysis; T.C.E.: funding acquisition; T.C.E.: methodology; M.E.: investigation; T.C.E.: resources; M.E.: writing—original draft; T.C.E.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [Michigan Apple Committee] grant number [MAC18-002].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Austin Chase and Alex Deahl for the considerable time spent preparing and analyzing roots. We also thank Emily Lavely for her insightful comments to improve the manuscript via an internal review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bravin, E.; Kilchenmann, A.; Leumann, M. Six hypotheses for profitable apple production based on the economic work-package within the ISAFRUIT Project. J. Hort. Sci. Biotechnol. 2009, 84, 164–167. [Google Scholar] [CrossRef]
- DeMarree, A.; Robinson, T.; Hoying, S. Economics and the orchard system decision. Compact. Fruit Tree 2003, 36, 42–49. [Google Scholar]
- Goedegebure, J. Economic aspects of super-intensive apple orchards. Acta Hortic. 1993, 349, 285–294. [Google Scholar] [CrossRef]
- Heijerman, G.; Roelofs, P.; Groot, M. Profitability of the Dutch growing system of ‘Conference’. Acta Hortic. 2015, 1094, 233–238. [Google Scholar] [CrossRef]
- Robinson, T.L. Effects of tree density and tree shape on apple orchard performance. Acta Hortic. 2007, 732, 405–414. [Google Scholar] [CrossRef]
- Robinson, T.L.; Hoying, S.A.; Reginato, G.H. The tall spindle planting system: Principles and performance. Acta Hortic. 2011, 903, 571–579. [Google Scholar] [CrossRef]
- Gallardo, K.; Galinato, S. 2019 Cost Estimates of Establishing, Producing, and Packing ‘Honeycrisp’ Apples in Washington; Washington State University: Pullman, WA, USA, 2020; Available online: http://ses.wsu.edu/wp-content/uploads/2020/11/TB70E.pdf (accessed on 15 July 2022).
- Robinson, T.; Hoying, S.; Sazo, M.M.; Marree, A.D.; Dominguez, L. A vision for apple orchard systems of the future. NY Fruit Q. 2013, 21, 11–16. [Google Scholar]
- Rune, G. Slits in container wall improve root structure and stem straightness of out planted Scots pine seedlings. Silva Fenn. 2003, 37, 333–342. [Google Scholar] [CrossRef][Green Version]
- Ortega, U.; Majada, J.; Mena-Petite, A.; Sanchez-Zabala, J.; Rodriguez Iturrizar, N.; Txarterina, K.; Azpitarte, J.; Duñabeitia, M. Field performance of Pinus radiata D. Don produced in nursery with different types of containers. New Forest 2006, 31, 97–112. [Google Scholar] [CrossRef]
- Van Sambeek, J.W.; Godsey, L.D.; Walter, W.D.; Garrett, H.E.; Dwyer, J.P. Field performance of Quercus bicolor established as repeatedly air-root-pruned container and bareroot planting stock. Open J. For. 2016, 6, 163–176. [Google Scholar]
- Pregitzer, K.S.; Laskowski, M.J.; Butron, A.J.; Lessard, V.C.; Zak, D.R. Variation in sugar maple root respiration with root diameter and soil depth. Tree Physiol. 1998, 18, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Rytter, R.-M. The effect of limited availability of N or water on C allocation to fine roots and annual fine root turnover in Alnus incana and Salix viminalis. Tree Physiol. 2013, 33, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Makkonen, K.; Helmisaari, H.S. Assessing fine-root biomass and production in a Scots pine stand—Comparison of soil core and root in-growth core methods. Plant Soil 1999, 210, 43–50. [Google Scholar] [CrossRef]
- Noguchi, K.; Sakata, T.; Mizoguchi, T.; Takahashi, M. Estimating the production and mortality of fine roots in a Japanese cedar (Cryptomeria japonica D. Don) plantation using a minirhizotron technique. J. For. Res. 2005, 10, 435–441. [Google Scholar] [CrossRef]
- An, H.; Luo, F.; Wu, T.; Wang, Y.; Xu, X.; Zhang, X.; Han, Z. Dwarfing effect of apple rootstocks is intimately associated with low number of fine roots. HortScience 2017, 52, 503–512. [Google Scholar] [CrossRef]
- Hooijdonk, B.v.; Woolley, D.; Warrington, I.; Tustin, S. Rootstocks Modify Scion Architecture, Endogenous Hormones, and Root Growth of Newly Grafted ‘Royal Gala’ Apple Trees. J. Am. Soc. Hort. Sci. 2011, 136, 93–102. [Google Scholar] [CrossRef]
- Liu, A.; Abdelfattah, A.; Wasserman, B.; Wisniewski, M.; Droby, M.; Fazio, G.; Mazzola, M.; Wu, X. Contrasting effects of genotype and root size on the fungal and bacterial communities associated with apple rootstocks. Hortic. Res. 2022, 9, uhab013. [Google Scholar] [CrossRef] [PubMed]
- Lavely, E.K.; Chen, W.; Peterson, K.A.; Klodd, A.E.; Volder, A.; Marini, R.P.; Eissenstat, D.M. On characterizing root function in perennial horticultural crops. Am. J. Bot. 1987, 107, 1214–1224. [Google Scholar] [CrossRef]
- Mccormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Erik, A.; Iversen, C.M.; Jackson, R.B. Redefining fine roots improves under-standing of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.E.; Eissenstat, D.M. Beyond the roots of young seedlings: The influence of age and order on fine root physiology. J. Plant Growth Regul. 2002, 21, 324–334. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Wells, C.E.; Yanai, R.D.; Whitbeck, J.L. Building roots in a changing environment: Implications for root longevity. New Phytol. 2000, 147, 33–42. [Google Scholar] [CrossRef]
- Bouma, T.J.; Yanai, R.D.; Elkin, A.D.; Hartmond, U.; Floresalva, D.E.; Eissenstat, D.M. Estimating age-dependent costs and benefits of roots with contrasting life span: Comparing apples and oranges. New Phytol. 2001, 150, 685–695. [Google Scholar] [CrossRef]
- Feng, Z.; Yang, X.; Liang, H.; Kong, Y.; Hui, D.; Zhao, J.; Guo, E.; Fan, B. Improvements in the root morphology, physiology, and anatomy of Platycladus orientalis seedlings from air-root pruning. HortScience 2018, 53, 1750–1756. [Google Scholar] [CrossRef]
- Atucha, A.; Emmett, B.; Bauerle, T.L. Growth rate of fine root systems influences rootstock tolerance to replant disease. Plant Soil 2014, 376, 337–346. [Google Scholar] [CrossRef]
- Volder, A.; Smart, D.R.; Bloom, A.J.; Eissenstat, D.M. Rapid decline in nitrate uptake and respiration with age in fine lateral roots of grape: Implications for root efficiency and competitive effectiveness. New Phytol. 2005, 165, 493–502. [Google Scholar] [CrossRef] [PubMed]
- DeJong, T.M.; Tombesi, S.; Basile, B.; Da Silva, D. Beakbane and Thompson (1939, East Malling) had it right: Scion vigour is physiologically linked to the xylem anatomy of the rootstock. Asp. Appl. Biol. 2013, 119, 51–58. [Google Scholar]
- Tombesi, S.; Johnson, R.S.; Day, K.R.; DeJong, T.M. Interactions between rootstock, inter-stem and scion xylem vessel characteristics of peach trees growing on rootstocks with contrasting size-controlling characteristics. AoB Plants 2010, 2010, plq013. [Google Scholar] [CrossRef]
- Parry, M.S. The effects of budding height on the field performance of two apple cultivars on three rootstocks. J. Hortic. Sci. 1986, 61, 1–7. [Google Scholar] [CrossRef]
- Webster, A.D. Vigour mechanisms in dwarfing rootstocks for temperate fruit trees. Acta Hortic. 2004, 658, 29–41. [Google Scholar] [CrossRef]
- Lordan, J.; Fazio, G.; Francescatto, P.; Robinson, T.L. Effects of apple (Malus x domestica) rootstocks on scion performance and hormone concentration. Sci. Hortic. 2017, 225, 96–105. [Google Scholar] [CrossRef]
- Fazio, G.; Kviklys, A.; Grusak, M.A.; Robinson, T.L. Phenotypic diversity and QTL mapping of absorption and translocation of nutrients by apple rootstocks. Asp. Appl. Biol. 2013, 119, 37–50. [Google Scholar]
- Reighard, G.L.; Bridges, W.; Rauh, B.; Mayer, N.A. Prunus rootstocks influence peach leaf and fruit nutrient content. Acta Hortic. 2013, 984, 117–124. [Google Scholar] [CrossRef]
- Elsysy, M.A.; Mickelbart, M.V.; Hirst, P.M. Effect of fruiting and biennial bearing potential on spur quality and leaf gas exchange in apple. J. Amer. Soc. Hort. Sci. 2019, 144, 31–37. [Google Scholar] [CrossRef]
- Atkinson, D. The distribution and effectiveness of the roots of tree crops. Hort. Rev. 1980, 2, 424–490. [Google Scholar]
- Johnson, I.R. A model of the partitioning of growth between the shoots and roots of vegetative plants. Ann. Bot. 1985, 55, 421–431. [Google Scholar] [CrossRef]
- Robinson, D. Compensatory changes in the partitioning of dry matter in relation to nitrogen uptake and optimal variations in growth. Ann. Bot. 1986, 58, 841–848. [Google Scholar] [CrossRef]
- Johnson, I.R.; Thornley, J.H.M. A model of root: Shoot partitioning with optimal growth. Ann. Bot. 1987, 60, 133–142. [Google Scholar] [CrossRef]
- Van der Werf, A.; Visser, J.; Schieving, F.; Lambers, H. Evidence for Optimal Partitioning of Biomass and Nitrogen at a Range of Nitrogen Availabilities for a Fast- and Slow-Growing Species. Funct. Ecol. 1993, 7, 63–74. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).