Anthocyanin Composition in Cabernet Sauvignon Grape Skins: Effect of Regulated Deficit Irrigation in a Warm Climate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Experimental Site and Vegetal Material
2.3. Experimental Design, Treatments and Berry Sampling
2.4. Extraction of Phenolic Compounds from Grape Berry Skins
2.5. General Analytical Parameters
2.6. HPLC-DAD Analysis of Anthocyanin Composition
2.7. Statistical Analysis
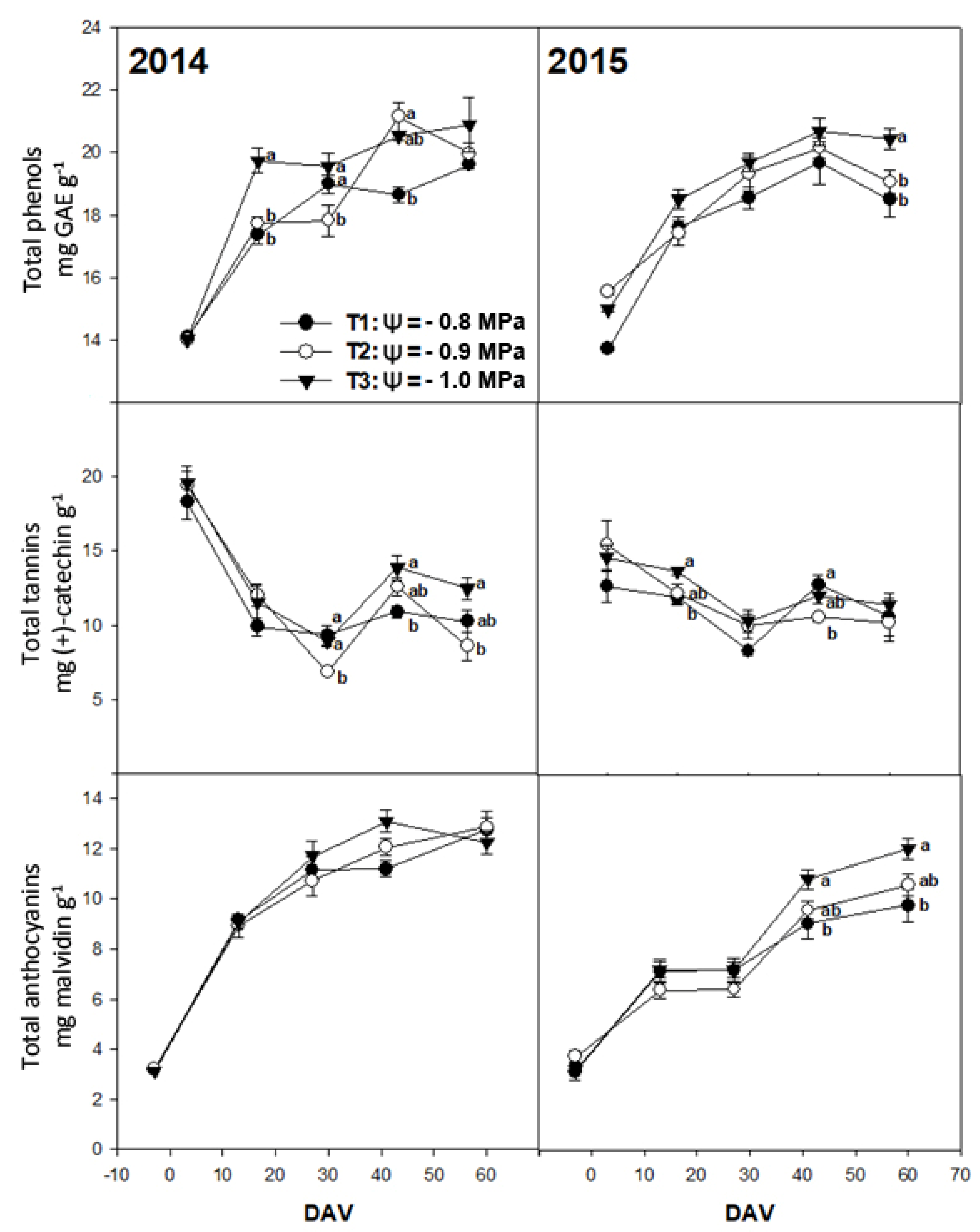
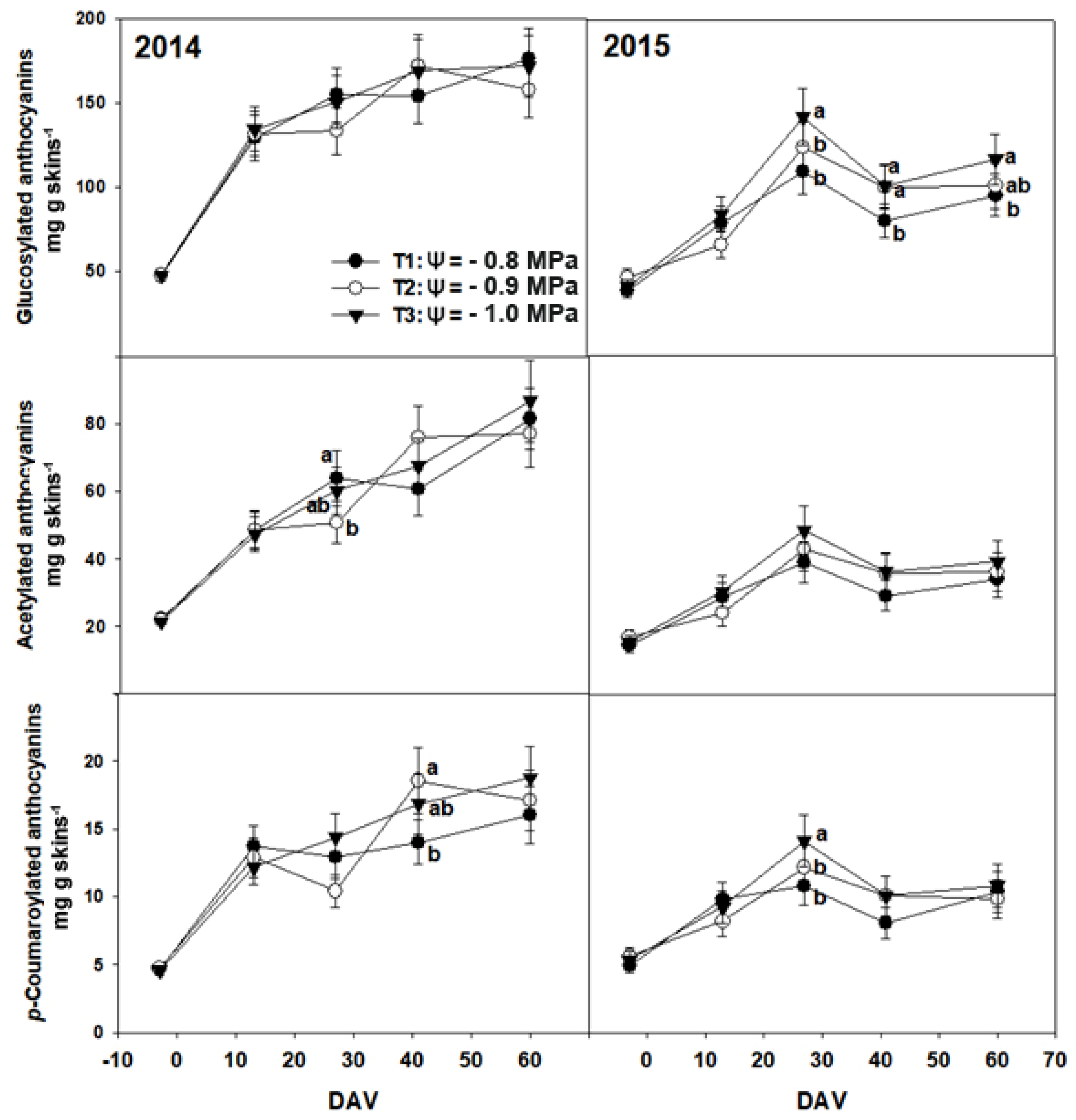
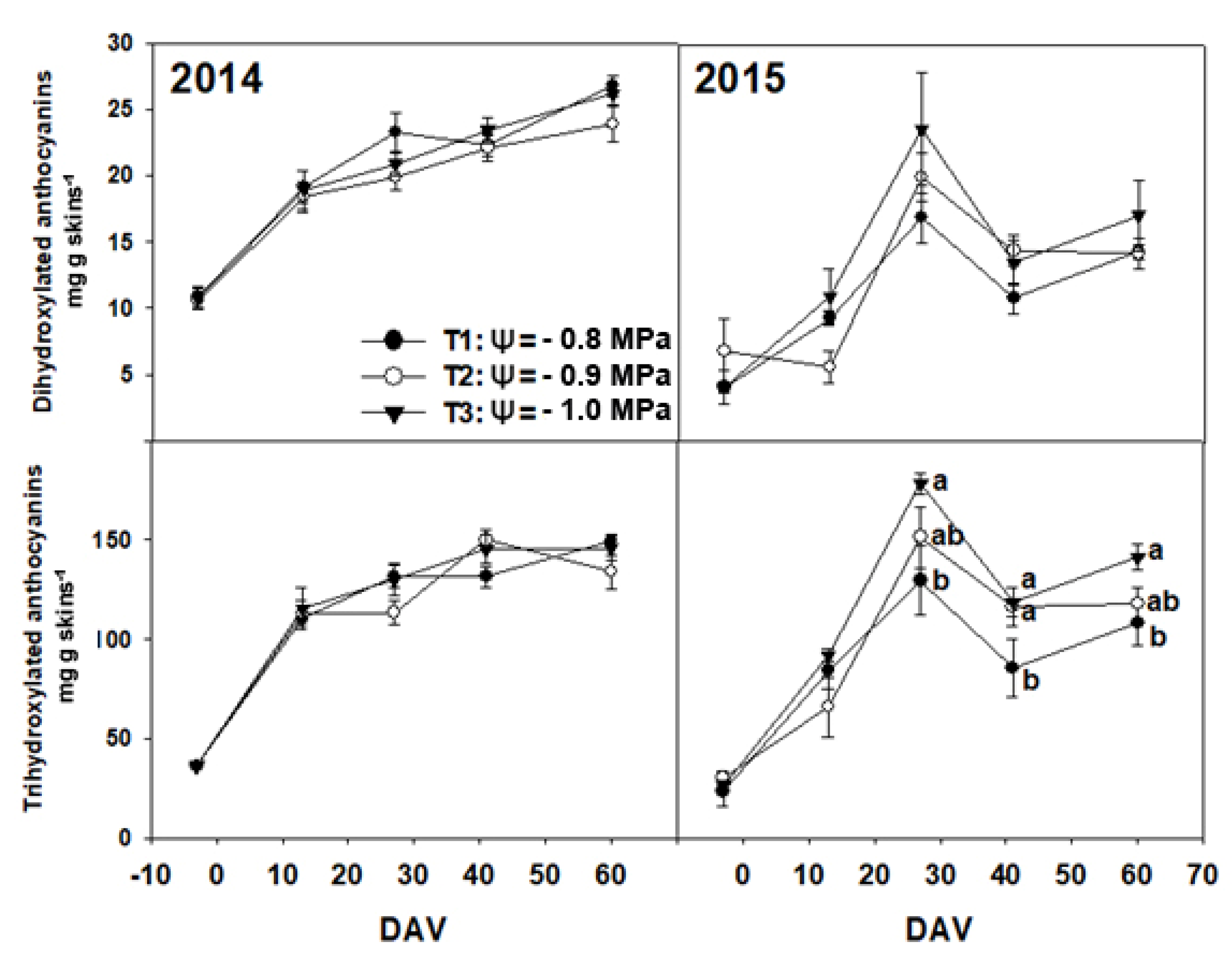
3. Results
3.1. Water Relationships
3.2. Chemical Composition of Grapes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, G.; White, M.; Cooper, O.; Storchmann, K. Climate change and global wine quality. Clim. Change 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Hannah, L.; Roehrdanz, P.R.; Ikegami, M.; Shepard, A.V.; Shaw, M.R.; Tabor, G.; Zhi, L.; Marquet, P.A.; Hijmans, R.J. Climate change, wine, and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, 6907–6912. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.; Matthews, M.; Di Gaspero, G.; Gambetta, G. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef]
- Chaves, M.; Santos, T.; Souza, C.; Ortuño, M.; Rodrigues, M.; Lopes, C.; Maroco, J.; Pereira, J. Deficit irrigation in grapevine improves water-use efficiency while controlling vigour and production quality. Ann. Appl. Biol. 2007, 150, 237–252. [Google Scholar] [CrossRef]
- Bucchetti, B.; Matthews, M.; Falginella, L.; Peterlunger, E.; Castellarin, S. Effect of water deficit on Merlot grape tannins and anthocyanins across four seasons. Sci. Hortic. 2011, 128, 297–305. [Google Scholar] [CrossRef]
- Villangó, S.; Szekeres, A.; Bencsik, O.; Láposi, R.; Pálfi, Z.; Zsófi, Z. The effect of post veraison water deficit on the phenolic composition and concentration of the Kékfrankos (Vitis vinifera L.) berry. Sci. Hortic. 2016, 209, 113–116. [Google Scholar] [CrossRef]
- Cáceres-Mella, A.; Ribalta-Pizarro, C.; Villalobos-González, L.; Cuneo, I.; Pastenes, C. Controlled water deficit modifies the phenolic composition and sensory properties in Cabernet Sauvignon wines. Sci. Hortic. 2018, 237, 105–111. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.; Páez-Hernández, M.; Rodríguez, J.; Galán-Vidal, C. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Cheynier, V.; Dueñas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.M.; Sarni-Manchado, P.; Fulcrand, H. Structure and properties of wine pigments and tannins. Am. J. Enol. Vitic. 2006, 57, 298–305. [Google Scholar]
- Downey, M.; Harvey, J.; Robinson, S. The effect of bunch shading of berry development and flavonoid accumulation in Shiraz grapes. Aust. J. Grape Wine Res. 2004, 10, 55–73. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yakamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cascales, I.; Fernández-Fernández, J.; López-Roca, J.M.; Gómez-Plaza, E. The maceration process during winemaking. Extraction of anthocyanins from grape skins into wine. Eur. Food Res. Technol. 2005, 221, 163–167. [Google Scholar] [CrossRef]
- Romero, P.; Navarro, J.; Botía Ordaz, P. Towards a sustainable viticulture: The combination of deficit irrigation strategies and agroecological practices in Mediterranean vineyards. A review and update. Agric. Water Manag. 2022, 259, 107216. [Google Scholar] [CrossRef]
- Zarrouk, O.; Francisco, R.; Pinto-Marijuan, M.; Brossa, R.; Santos, R.; Pinheiro, C.; Costa, J.; Lopes, C.; Chaves, M. Impact of irrigation regime on berry development and flavonoids composition in Aragonez (Syn. Tempranillo) grapevine. Agric. Water Manag. 2012, 114, 18–29. [Google Scholar] [CrossRef]
- Talaverano, I.; Ubeda, C.; Cáceres-Mella, A.; Valdés, M.E.; Pastenes, C.; Peña-Neira, A. Water stress and ripeness effects on the volatile composition of Cabernet Sauvignon wines. J. Sci. Food Agric. 2018, 98, 1140–1152. [Google Scholar] [CrossRef]
- Van Leuween, C.; Tregoat, O.; Choné, X.; Bois, B.; Pernet, D.; Gaudillère, J.P. Vine water status is a key factor in grape ripening and vintage quality for red Bordeaux wine. How can it be assessed for vineyard management purposes? J. Int. Sci. Vigne Vin. 2009, 43, 121–134. [Google Scholar] [CrossRef]
- Izquierdo-Hernández, A.; Peña-Neira, A.; López-Solís, R.; Obreque-Slier, E. Low molecular weight phenols, and flavanol fractions in Vitis vinifera L. cv Carménère skins and seeds by differential solvent extraction and high performance liquid chromatography. Anal. Lett. 2015, 49, 1127–1142. [Google Scholar] [CrossRef]
- OIV (International Organization of Vine and Wine). Compendium of International Methods of Wine and Must Analysis; OIV: Paris, France, 2012. [Google Scholar]
- Glories, Y. La coleur des vins rouges. 2eme Partier. Mesure, origine et interpretation. Connaiss. Vigne Vin. 1984, 18, 253–271. [Google Scholar]
- Sarneckis, C.; Dambergs, R.; Jones, P.; Mercurio, M.; Herderich, M.; Smith, P. Quantification of condensed tannins by precipitation with methylcellulose: Development and validation of an optimized tool for grape and wine analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, J.; Stonestreet, E. Le dosage des anthocyanies dans le vin rouge. Bull. Soc. Chim. Fr. 1965, 9, 2649–2652. [Google Scholar]
- Fanzone, M.; Peña-Neira, A.; Gil, M.; Jofré, V.; Assof, M.; Zamora, F. Impact of phenolic and polysaccharidic composition on commercial value of Argentinean Malbec and Cabernet Sauvignon wines. Food Res. Int. 2012, 45, 402–414. [Google Scholar] [CrossRef]
- Tonietto, J.; Carbonneau, A. A multicriteria climatic classification system for grape-growing regions worldwide. Agric. For. Meteorol. 2004, 124, 81–97. [Google Scholar] [CrossRef]
- Garreaud, R.; Boisier, J.; Rondanelli, R.; Montecinos, A.; Sepúlveda, H.; Veloso-Aguila, D. The Central Chile mega drought (2010–2018): A climate dynamics perspective. Int. J. Climatol. 2020, 40, 421–439. [Google Scholar] [CrossRef]
- Kizildeniz, T.; Mekni, I.; Santesteban, H.; Pascual, I.; Morales, F.; Irigoyen, J.J. Effects of climate change including elevated CO2 concentration, temperature and water deficit on growth, water status, and yield quality of grapevine (Vitis vinifera L.) cultivars. Agric. Water Manag. 2015, 159, 155–164. [Google Scholar] [CrossRef]
- Patakas, A.; Noitsakis, B. Mechanisms involved in diurnal changes of osmotic potential in grapevines under drought conditions. J. Plant Physiol. 1999, 154, 767–774. [Google Scholar] [CrossRef]
- Lovisolo, C.; Hartung, W.; Schubert, A. Whole-plant hydraulic conductance and root-to-shoot flow of abscisic acid are independently affected by water stress in grapevines. Funct. Plant Biol. 2002, 29, 1349–1356. [Google Scholar] [CrossRef]
- Choné, X.; Van Leeuwen, C.; Dubourdieu, D.; Gaudillere, J.P. Stem water potential is a sensitive indicator of grapevine water status. Ann. Bot. 2001, 87, 477–483. [Google Scholar] [CrossRef]
- Escalona, J.; Flexas, J.; Medrano, H. Stomatal and non-stomatal limitations of photosynthesis under water stress in field-grown grapevines. Aust. J. Plant Physiol. 1999, 26, 421–433. [Google Scholar] [CrossRef]
- Chaves, M.; Zarrouk, O.; Francisco, R.; Costa, J.; Santos, T.; Regalado, A.; Rodrigues, M.; Lopes, C. Grapevine under deficit irrigation: Hints from physiological and molecular data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef]
- Thomas, T.; Matthews, M.; Shackel, K. Direct in situ measurement of cell turgor in grape (Vitis vinifera L.) berries during development and in response to plant water deficits. Plant Cell Environ. 2006, 29, 993–1001. [Google Scholar] [CrossRef]
- Pastenes, C.; Villalobos, L.; Rios, N.; Reyes, F.; Turgeon, R.; Franck, N. Carbon partitioning in berries in water stressed grapevines: The role of active transport in leaves and fruits. Environ. Exp. Bot. 2014, 107, 154–166. [Google Scholar] [CrossRef]
- Villalobos-González, L.; Peña-Neira, A.; Ibáñez, F.; Pastenes, C. Long-term effects of abscisic acid (ABA) on the grape Berry phenylpropanoid pathway: Gene expression and metabolite content. Plant Physiol. Biochem. 2016, 105, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-H.; Yang, B.-H.; Wang, X.-W.; Li, J.-N.; Li, S.; Yang, X.; Ren, R.-H.; Fang, Y.-L.; Xu, T.-F.; Zhang, Z.-W.; et al. ABA signaling plays a key role in regulated deficit irrigation-driven anthocyanins accumulation in Cabernet Sauvignon grape berries. Environ. Exp. Bot. 2022, 181, 104290. [Google Scholar] [CrossRef]
- Hanlin, R.; Downey, M. Condensed tannin accumulation and composition in skin of Shiraz and Cabernet Sauvignon grapes during berry development. Am. J. Enol. Vitic. 2009, 60, 13–23. [Google Scholar]
- Bogs, J.; Downey, M.; Harvey, J.; Ashton, A.; Tanner, G.; Robinson, S. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol. 2005, 139, 652–663. [Google Scholar] [CrossRef]
- Matus, J.; Loyola, R.; Vega, A.; Peña-Neira, A.; Bordeau, E.; Arce-Johnson, P.; Alcalde, J.A. Post-veraison sunlight exposure induces MYB-mediated transcriptional regulation of anthocyanin and flavonol synthesis in berry skins of Vitis vinifera. J. Exp. Bot. 2009, 60, 853–867. [Google Scholar] [CrossRef]
- Cáceres-Mella, A.; Talaverano, M.I.; Villalobos-González, L.; Ribalta-Pizarro, C.; Pastenes, C. Controlled water deficit during ripening affects proanthocyanidin synthesis, concentration and composition in Cabernet Sauvignon grape skins. Plant Physiol. Biochem. 2017, 117, 34–41. [Google Scholar] [CrossRef]
- Lizama, V.; Pérez-Álvarez, E.; Intrigliolo, D.; Chirivella, C.; Álvarez, I.; García-Esparza, M.J. Effects of the irrigation regimes on grapevine cv. Bobal in a Mediterranean climate: II. Wine, skins, seeds, and grape aromatic composition. Agric. Water Manag. 2021, 256, 107078. [Google Scholar] [CrossRef]
- Yang, B.; He, S.; Liu, Y.; Liu, B.; Ju, Y.; Kang, D.; Sun, X.; Fang, Y. Transcriptomic integrated with metabolomics reveals the effect of regulated deficit irrigation on anthocyanin biosynthesis in Cabernet Sauvignon grape berries. Food Chem. 2020, 314, 126170. [Google Scholar] [CrossRef]
- Calderan, A.; Sivilotti, P.; Braidotti, R.; Mihelcic, A.; Lisjak, K.; Vanzo, A. Managing moderate water deficit increases anthocyanin concentration and proanthocyanidin galloylation in “Refosk” grapes in Northeast Italy. Agric. Water Manag. 2021, 246, 106684. [Google Scholar] [CrossRef]
- Castellarin, S.; Ofeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; Di Gaspero, G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Nuzzo, V.; Tataranni, G.; Manfra, M.; De Nisco, M.; Scopa, A. Berry morphology and composition in irrigated and non-irrigated grapevine (Vitis vinifera L.). J. Plant Physiol. 2012, 169, 1023–1031. [Google Scholar] [CrossRef]
- Bogs, J.; Ebabi, A.; McDavid, D.; Robinson, S. Identification of the flavonoid hydrolases from grapevine and their regulation during fruit development. Plant Physiol. 2006, 140, 179–291. [Google Scholar] [CrossRef] [PubMed]
- Perez-Alvarez, E.P.; Intrigliolo, D.; Vivaldi, G.A.; García-Esparza, M.J.; Lizama, V.; Alvarez, I. effects of the irrigation regimes on grapevine cv. Bobal in a Mediterranean climate: I. Water relations, vine performance and grape composition. Agric. Water Manag. 2021, 248, 106772. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, Y.; Zhang, Y.; Xu, C.; Lu, J.; Wang, Y. The growing season impacts the accumulation and composition of flavonoids in grape skins in two-crop-a-year viticulture. J. Food Sci. Technol. 2017, 54, 2861–2870. [Google Scholar] [CrossRef]

| 2014 | 2015 | ||||||
|---|---|---|---|---|---|---|---|
| DAV | T1 | T2 | T3 | T1 | T2 | T3 | |
| pH | −3 | 2.57 ± 0.02 | 2.58 ± 0.01 | 2.56 ± 0.02 | 2.83 ± 0.00 | 2.87 ± 0.03 | 2.81 ± 0.01 |
| 13 | 2.84 ± 0.03 b | 3.01 ± 0.01 a | 2.88 ± 0.02 b | 3.05 ± 0.02 | 2.99 ± 0.02 | 2.99 ± 0.03 | |
| 27 | 3.14 ± 0.02 | 3.15 ± 0.01 | 3.13 ± 0.01 | 3.36 ± 0.02 | 3.35 ± 0.02 | 3.31 ± 0.02 | |
| 41 | 3.29 ± 0.03 b | 3.40 ± 0.04 a | 3.44 ± 0.03 a | 3.54 ± 0.01 | 3.54 ± 0.02 | 3.54 ± 0.01 | |
| 60 | 3.50 ± 0.04 | 3.58 ± 0.04 | 3.59 ± 0.04 | 3.69 ± 0.02 ab | 3.73 ± 0.02 a | 3.63 ± 0.01 b | |
| Titratable acidity | −3 | 15.32 ± 0.15 | 15.30 ± 0.15 | 15.45 ± 0.16 | 12.40 ± 0.09 | 11.30 ± 0.23 | 11.75 ± 0.47 |
| (g tartaric acid L−1) | 13 | 7.77 ± 0.19 | 8.13 ± 0.18 | 8.03 ± 0.31 | 5.65 ± 0.18 b | 6.47 ± 0.16 a | 5.97 ± 0.20 ab |
| 27 | 5.30 ± 0.15 | 5.47 ± 0.25 | 5.25 ± 0.24 | 8.92 ± 4.51 | 4.80 ± 0.14 | 4.75 ± 0.13 | |
| 41 | 4.13 ± 0.13 a | 3.72 ± 0.18 b | 3.97 ± 0.16 ab | 3.80 ± 0.09 | 3.72 ± 0.06 | 3.62 ± 0.12 | |
| 60 | 3.42 ± 0.13 | 2.97 ± 0.10 | 3.07 ± 0.15 | 3.45 ± 0.09 | 3.57 ± 0.08 | 3.60 ± 0.11 | |
| Soluble solids (°Brix) | −3 | 12.97 ± 0.25 | 13.00 ± 0.25 | 12.88 ± 0.30 | 12.33 ± 0.05 | 12.00 ± 0.41 | 12.47 ± 0.45 |
| 13 | 16.90 ± 0.30 | 16.07 ± 0.32 | 16.07 ± 0.25 | 17.60 ± 0.30 | 16.07 ± 0.56 | 16.87 ± 0.58 | |
| 27 | 18.97 ± 0.40 | 18.30 ± 0.33 | 19.07 ± 0.15 | 17.80 ± 0.68 b | 19.27 ± 0.71 ab | 20.47 ± 0.50 a | |
| 41 | 20.20 ± 0.28 a | 18.37 ± 0.27 b | 19.83 ± 0.46 a | 21.93 ± 0.30 a | 20.27 ± 0.50 b | 20.63 ± 0.48 ab | |
| 60 | 21.53 ± 0.42 | 21.57 ± 0.41 | 21.13 ± 0.28 | 21.13 ± 0.22 | 21.27 ± 0.37 | 21.27 ± 0.38 | |
| Average berry weight (g) | −3 | 0.98 ± 0.02 | 0.99 ± 0.02 | 0.98 ± 0.02 | 0.85 ± 0.02 b | 0.97 ± 0.04 a | 0.93 ± 0.01 a |
| 13 | 1.09 ± 0.05 | 1.09 ± 0.02 | 1.07 ± 0.03 | 1.07 ± 0.02 | 1.05 ± 0.02 | 1.11 ± 0.01 | |
| 27 | 1.11 ± 0.03 | 1.14 ± 0.03 | 1.11 ± 0.02 | 1.20 ± 0.03 | 1.19 ± 0.03 | 1.21 ± 0.03 | |
| 41 | 1.10 ± 0.03 | 1.12 ± 0.04 | 1.16 ± 0.02 | 1.13 ± 0.02 | 1.12 ± 0.02 | 1.15 ± 0.02 | |
| 60 | 1.14 ± 0.03 | 1.11 ± 0.03 | 1.09 ± 0.05 | 1.11 ± 0.03 | 1.09 ± 0.05 | 1.07 ± 0.02 | |
| Skin weight/total weight | −3 | 0.10 ± 0.04 | 0.10 ± 0.04 | 0.09 ± 0.04 | 0.15 ± 0.02 | 0.12 ± 0.00 | 0.10 ± 0.00 |
| relationship | 13 | 0.11 ± 0.04 a | 0.10 ± 0.04 ab | 0.10 ± 0.04 b | 0.11 ± 0.00 a | 0.10 ± 0.01 b | 0.09 ± 0.00 b |
| 27 | 0.11 ± 0.04 ab | 0.12 ± 0.05 a | 0.10 ± 0.04 b | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.09 ± 0.00 | |
| 41 | 0.11 ± 0.04 b | 0.11 ± 0.04 b | 0.12 ± 0.05 a | 0.12 ± 0.00 | 0.10 ± 0.00 | 0.11 ± 0.01 | |
| 60 | 0.11 ± 0.04 ab | 0.10 ± 0.04 b | 0.12 ± 0.05 a | 0.12 ± 0.00 | 0.12 ± 0.00 | 0.13 ± 0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aris, G.; Cuneo, I.F.; Pastenes, C.; Cáceres-Mella, A. Anthocyanin Composition in Cabernet Sauvignon Grape Skins: Effect of Regulated Deficit Irrigation in a Warm Climate. Horticulturae 2022, 8, 796. https://doi.org/10.3390/horticulturae8090796
Aris G, Cuneo IF, Pastenes C, Cáceres-Mella A. Anthocyanin Composition in Cabernet Sauvignon Grape Skins: Effect of Regulated Deficit Irrigation in a Warm Climate. Horticulturae. 2022; 8(9):796. https://doi.org/10.3390/horticulturae8090796
Chicago/Turabian StyleAris, Gonzalo, Italo F. Cuneo, Claudio Pastenes, and Alejandro Cáceres-Mella. 2022. "Anthocyanin Composition in Cabernet Sauvignon Grape Skins: Effect of Regulated Deficit Irrigation in a Warm Climate" Horticulturae 8, no. 9: 796. https://doi.org/10.3390/horticulturae8090796
APA StyleAris, G., Cuneo, I. F., Pastenes, C., & Cáceres-Mella, A. (2022). Anthocyanin Composition in Cabernet Sauvignon Grape Skins: Effect of Regulated Deficit Irrigation in a Warm Climate. Horticulturae, 8(9), 796. https://doi.org/10.3390/horticulturae8090796






