Abstract
Tomato, which is regarded as an important worldwide crop, is susceptible to gray mold caused by Botrytis cinerea. Selenium and methyl jasmonate can act as antifungal agents against pathogenic infections. To clarify the effect of selenium and methyl jasmonate on the fungal pathogen, the spore germination and mycelial growth of B. cinerea were investigated in vitro using the growth rate method. Additionally, the electrical conductivity, soluble protein content, malondialdehyde content and oxalic acid secretion of B. cinerea mycelium were also determined to further explore the antifungal mechanism of selenium and methyl jasmonate. The results showed that selenium application significantly increased cell membrane permeability and malondialdehyde content, and methyl jasmonate treatment decreased the soluble protein content in mycelium of B. cinerea. Furthermore, supplementation of the medium with both selenium and methyl jasmonate effectively inhibited spore germination and colony growth of B. cinerea by compromising membrane integrity, and significantly reduced soluble protein content and the oxalic acid secretion of hypha. The resulting incidence of postharvest tomato gray mold with the combination of selenium and methyl jasmonate was 34.7%, which was approximately half of that of the control. To sum up, the combined use of selenium and methyl jasmonate inhibited the normal physiological activity and pathogenicity of B. cinerea, which suggests that selenium and methyl jasmonate have the potential for controlling gray mold disease caused by B. cinerea in postharvest fruits and vegetables. These findings may offer a promising and eco-friendly strategy to control gray mold disease in postharvest fruits and vegetables.
1. Introduction
Tomato belongs to the solanum crops and is loved by people because of its rich nutritional value and great taste. Tomato fruits are thin-skinned and juicy, but susceptible to pathogenic fungi, causing a variety of diseases such as gray mold. Botrytis cinerea (B. cinerea), the pathogen of gray mold, can resist adverse environments by producing spore and sclerotia, infecting the host and causing diseases in the field and during the postharvest storage period of fruits and vegetables, rendering the crop commercially worthless [1,2]. The global economic losses caused by B. cinerea reach 10 to 100 billion USD every year [3]. At present, the prevention and control of gray mold mainly depends on chemical agents [4]. However, extensive application of chemical fungicides may endanger the safety of agricultural products and induce problems such as increased pathogen resistance, environmental pollution, and ecological imbalance, which will not meet the requirements for sustainable development of modern agriculture [5]. Therefore, it is of great significance to develop ecological and effective gray mold control techniques in multiple ways that will be suitable for eco-friendly agricultural development.
Selenium (Se) is an essential trace element for the human body and a beneficial element for plant growth. It has been shown that Se causes negative effects at high concentrations (>5 mg·kg−1) in higher plants but exerts beneficial effects at low concentrations (1 μmol·L−1), such as helping to improve crop quality and yield [6,7,8,9], enhancing antioxidant capacity in crops [10], and likewise alleviating oxidative stress [11]. Additionally, exogenous, which could inhibit sclerotia formation, spore germination, germ tube elongation, and mycelial spread to reduce the infective ability of fungal pathogens [12,13,14]. It has been reported that Se-containing biocomposites can substantially inhibit the activity of potato ring rot causative agent [15]. Additionally, Se nanoparticles (Se NPs) generated beneficial effects by decreasing the activity of Colletotrichum capsici on chili [16], as well as Colletotrichum coccodes and Penicillium digitatum [17]. Se can also inhibit pathogenic fungi such as B. cinerea [18], Sclerotinia sclerotiorum [19], Fusarium graminearum [20] and Phanerochaete chrysosporium [21] in vitro.
Methyl jasmonate (MeJA) is a kind of growth regulator that widely presents in plants. Because of its non-toxic, non-hazardous, and high safety properties, it is often used as an alternative to chemical fungicides for the storage and preservation of fruits and vegetables in recent years [22]. Experiments found that MeJA in vitro can reduce the diameter of lesions, increase the activity of defense-related enzymes, and enhance the disease resistance of fruits [23,24]. It has been illustrated that the application of MeJA at appropriate concentrations in tomatoes [25], blueberries [26], citrus [27] and rosa sterilis fruits [28] is beneficial for preventing postharvest decay and improving fruit quality.
The results of preliminary experiments in our group indicated that in vitro exogenous Se (5.0 mg·L−1) addition, as well as MeJA at a trace concentration (0.4 mM), could noticeably enhance the capability of tomato fruits to resist gray mold disease. However, no information was known regarding the effect of Se in combination with MeJA on B. cinerea, as well as the mechanisms involved. In this study, experiments in vitro were designed using Se and MeJA to directly inhibit B. cinerea and to investigate the control of gray mold disease in tomatoes after harvesting. Furthermore, the underlying mechanism of the inhibitory effect with Se and MeJA treatments on B. cinerea was also assessed. These results are helpful for providing new ideas and methods for controlling tomato gray mold efficiently without environmental risks.
2. Materials and Methods
2.1. Fungal Pathogen
B. cinerea was provided by the College of Horticulture and Forestry, Huazhong Agricultural University, and its strain number is B05.10. The spore suspensions were prepared as follows. The sclerotia of the tested strain were sterilized in 75% ethanol for 5 min, and then rinsed three times with sterile distilled water and dried. After that, the nucleus was cut with a sterile blade and placed on the medium with the cut side down. The upside-down petri dish was placed in a constant temperature incubator at 23 ± 1 °C for activation and incubation. Afterwards, fungi plug with the same radius as the nucleus was punched (the diameter of the punched hole was 5 mm) and transferred onto new potato dextrose agar (PDA) plates at 23 ± 1 °C for 3 days. After 10 days incubation, sterile distilled water was added and the spore suspension to be used was obtained through filtration. A hemocytometer was used to calculate the number of spores and the spore concentration was diluted to 1.0 × 106 spores/mL before conducting subsequent experiments.
2.2. Experimental Design
Healthy cherry tomato fruits from the same cultivar were purchased from the market. Tomato fruits with similar appearance and without physical injuries or infections were selected and surface-disinfected with 2% (v/v) sodium hypochlorite, then rinsed with sterile water and air-dried to prepare for the subsequent experiment. In the study, the concentrations of Se and MeJA selected were based on the results of our previous study by Li et al. [29], as well as the preliminary experiments in our group. The experiments in vitro were designed with four treatments as follows: (1) Control (CK); (2) 5.0 mg/L Se (Se); (3) 0.4 mM MeJA (MeJA); (4) 5.0 mg/L Se + 0.4 mM MeJA (Se + MeJA).
2.3. Determination of Mycelial Growth of B. cinerea
The effect of Se and MeJA on mycelial growth of B. cinerea was assessed according to Droby et al. [30]. A plug of mycelial agar was obtained from the edge of the three-day-old fungi cultures using a 5-mm diameter sterilized perforator and transferred to the center of a PDA plate with Se and MeJA treatment (CK, Se, MeJA, or Se + MeJA). Radial mycelial growth of B. cinerea was determined by measuring the colony in two perpendicular directions after incubation at 23 ± 1 °C for 72 h. For determination of mycelial biomass, a petri dish covered with cellophane was used. The method of inoculating and incubating mycelial plugs was the same as that used for radial growth determination described above. After that, the mycelium was scraped and weighed. In vitro tests were performed twice, each with four replicates.
2.4. Determination of Inhibition of Spore Germination
The inhibitory effect of Se and MeJA on the spore germination of B. cinerea was determined according to Ji et al. [31]. A spore suspension of B. cinerea was cultured in potato dextrose broth (PDB) medium supplemented with Se and MeJA treatment at 23 ± 1 °C on a rotary shaker at 180 rpm. After 4 h of incubation, the level of germinated spores was estimated microscopically, as represented by the percentage of the total number of evaluated spores.
The spore germination rate was calculated according to the following formula:
where R is the rate (%), A is the number of spores germinating, and B is the number of spores surveyed. At least 100 spores were examined for each treatment with four replicates.
R = (A/B) × 100%
2.5. Assay of Cell Membrane Permeability
Cell membrane permeability of B. cinerea was assessed in a PDB plate according to Shao et al. [32]. For each sample, 2 mL of spore suspension of B. cinerea was added to a petri dish containing 100 mL PDB medium and incubated on a rotary shaker for 48 h at 23 ± 1 °C. Then, mycelium was harvested after the culture was filtered. For determination of the initial electrical conductivity (ms), 1 g of mycelium was added to a solution supplemented with Se and MeJA treatments. Then, the conductivity of each sample was measured after incubation for 15, 30, 60, 90, 150, and 210 min, respectively.
2.6. Assay of Soluble Protein Content
The soluble protein content of B. cinerea mycelium was determined according to the Coomassie brilliant G-250 method in Bradford [33]. Spores of B. cinerea were cultured in PDB media containing Se and MeJA. Amounts of 0.5 g of mycelium were extracted using a Buchner funnel after 4 and 8 h. Samples were transferred to an ice bath for cooling and dissolved in phosphate buffer (pH 7.8). After centrifugation for 20 min, the supernatant was collected and dyed with Coomassie brilliant blue solution for 5 min. Absorption was detected with an ultraviolet ray spectrophotometer (UV-5200) at the wavelength of 595 nm. Sterile distilled water was used as the control group. In vitro tests were performed twice, each with four replicates.
The soluble protein content was calculated according to the following formula:
where C is the soluble protein content of samples (μg/g); X is the value of the standard curve obtained from the absorbance of the sample, which represents the quantity of protein in the extract of the sample to be tested (μg); VT is the total volume of extraction solution (mL); W is the fresh quantity of samples (g); Vs. is defined as the sample volume for determination (mL).
C = (X × VT)/(W × VS)
2.7. Determination of Malondialdehyde Content
The determination of malondialdehyde (MDA) was performed using a Solarbio MDA content test kit according to the recommendation of the manufacturer. In brief, the content of MDA was measured by means of visible spectrophotometry. After MDA was reacted with thiobarbituric acid (TBA), samples were placed in an ice bath for cooling and centrifuged for 10 min at 10,000× g. The supernatant was used to measure the absorption using a spectrophotometer (UV-5200) at the wavelengths of 450, 532 and 600 nm.
The determination of MDA content was calculated according to the formula:
where C is the MDA concentration of the samples (nmol/g); E is the optical density of the solution at wavelengths of 450, 532 and 600 nm; W is the weight of the samples (g).
C = 5 × (6.45 × (E532 − E600) − 1.29 × E450) ÷ W
2.8. Determination of Oxalic Acid Content
The determination of oxalic acid content was performed according to Duan et al. [34]. An amount of 2 mL of spore suspension was added to PDB medium with Se and MeJA treatments and incubated for 48 h in a rotary shaker. Then, the culture was centrifuged at 10,000 rpm for 15 min to obtain the supernatant. Fe3+ standard solution (2 mL), KCL–HCL buffer (20 mL), sulfosalicylic acid (1.2 mL) and supernatant to be measured (0.4 mL) were added to a 25 mL volumetric flask in sequence. The mixture was then diluted to graduation line with double-distilled water, and finally, it was shaken. After 30 min at 25 °C for reaction, the absorbance value was measured at 510 nm.
2.9. Determination of the Inhibition Effect of Gray Mold Disease on Tomato Fruit
The inhibition of gray mold disease using the combination of Se and MeJA (Se + MeJA) on postharvest tomato fruit was determined according to the method in Soylu et al. and Ji et al. [1,31]. The center of the cherry tomato was pierced using a sterile needle to form a hole of 2 mm wide and 2 mm deep, and inoculated with 5 μL spore suspension of B. cinerea (1.0 × 105 spores/mL) at each wound. After drying, each wound was injected with 10 μL of mixed solution, which consisted of 10 mg/L Se and 0.8 mM MeJA. Sterile distilled water (10 μL) was used as the control (CK). The treated fruits were then kept at 23 ± 1 °C for 4 days. The disease development was assessed by the size of lesions and the incidence of gray mold. Each treatment consisted of six replicates.
2.10. Statistical Analysis
All data analysis was processed with SPSS software version 23.0 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was carried out to analyze the results and the method of multiple comparison was performed with Duncan’s test. Differences at p < 0.05 were considered significant.
3. Results
3.1. Effect of Se and MeJA on Mycelial Growth of B. cinerea
As indicated in Figure 1, the mycelial growth of B. cinerea treated with Se, MeJA and Se + MeJA was significantly inhibited (p < 0.05). Moreover, the combined inhibitory effect of Se and MeJA was the greatest among all treatments. When B. cinerea was incubated for 72 h with the combination of Se and MeJA, the inhibition rates of the diameter and biomass of the colonies compared to the control were 31.5% and 80.0%, respectively, in which the pathogenic mycelial diameter was 49.4 mm and the biomass was 0.08 g.
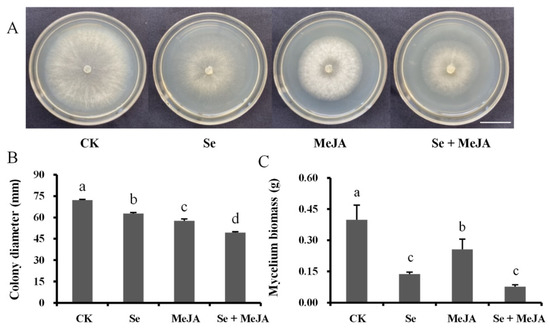
Figure 1.
Effect of Se, MeJA and Se + MeJA on colony growth (A), colony diameter (n = 4) (B) and mycelium biomass (n = 4) (C) of Botrytis cinerea. CK: Control group; Se: 5.0 mg/L Se; MeJA: 0.4 mM MeJA; Se + MeJA: 5.0 mg/L Se with 0.4 mM MeJA. Columns followed by different lowercase letters are statistically different according to Duncan’s multiple range test (p < 0.05). Bars = 22.5 mm.
3.2. Effect of Se and MeJA on Spore Germination of B. cinerea
The inhibitory effect of Se and MeJA on B. cinerea spore germination is presented in Figure 2. Overall, the spore germination rate in the treatment groups with Se and MeJA was significantly lower than that in the control. After 4 h of culture, 68.3% of spores of B. cinerea were germinated in the control treatment, while the lowest percentage of spore germination, which was only 10.8%, was detected in the Se and MeJA combination treatment. Compared with the control group, the inhibition rate in the combined treatment was increased by 83.5%.
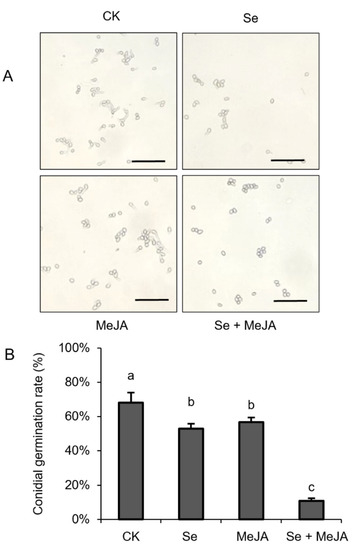
Figure 2.
Effect of Se, MeJA and Se + MeJA on conidial germination. (A) Micrograph of conidial germination; (B) Conidial germination rate (n = 4). CK: Control group; Se: 5.0 mg/L Se; MeJA: 0.4 mM MeJA; Se + MeJA: 5.0 mg/L Se with 0.4 mM MeJA. Values with the same letter are not significantly different at p < 0.05 according to Duncan’s multiple range test. Bars = 40 μm.
3.3. Effect of Se and MeJA on Membrane Permeability of B. cinerea Mycelium
The conductivity of the mycelium, which can reflect the effect of Se and MeJA treatment on mycelial membrane permeability, was monitored when B. cinerea was exposed to Se and MeJA for 15, 30, 45, 60, 90, 150, and 210 min (Figure 3). In brief, the conductivity of the mycelium in Se treatment alone or in combination with MeJA was significantly higher than that in the control group, and the combined effect of Se and MeJA on the conductivity of the mycelium was better than Se or MeJA applied alone after 90 min of incubation. When the incubation time was prolonged, even higher levels of conductivity were observed under the conditions of Se and MeJA in the medium. In addition, mycelial conductivity reached the maximum value (88.73 μs·cm−1) in the treatment with combined Se and MeJA after incubation for 210 min, which was increased by 35.5% compared with the control.
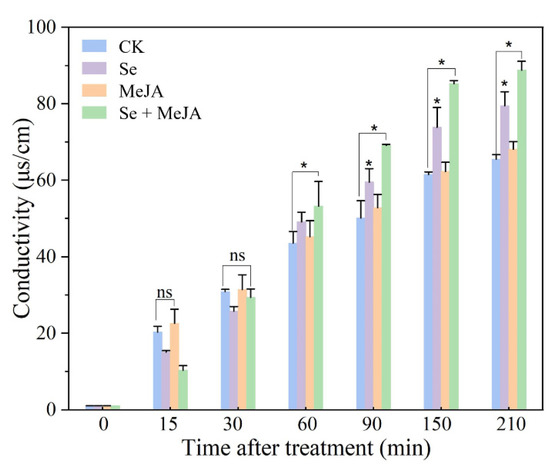
Figure 3.
Effect of Se, MeJA and Se + MeJA on mycelium conductivity (n = 4). CK: Control group; Se: 5.0 mg/L Se; MeJA: 0.4 mM MeJA; Se + MeJA: 5.0 mg/L Se with 0.4 mM MeJA. Data are mean values, and error bars represent SE among four replicates. Values with “ns” are not significantly different and “*” indicates that there is significant difference between the two groups at p < 0.05 according to Duncan’s multiple range test.
3.4. Effect of Se and MeJA on Soluble Protein of B. cinerea Mycelium
The effect of Se and MeJA on B. cinerea mycelium soluble protein content is shown in Figure 4. The R2 value of the corresponding standard curve reached 0.9961. A significant decrease in the soluble protein content of B. cinerea mycelium was detected in the treatments with the application of MeJA separately or in combination with Se, compared with that in the control, while the addition of Se to the PDB medium increased the mycelial soluble protein content slightly after incubation for 4 h. After culturing for 8 h, MeJA applied separately or in combination with Se significantly reduced the mycelial soluble protein content, which was 28.2% and 24.3% lower than that of the control, respectively. However, no significant difference was observed in the treatment adding Se alone when compared with the control.
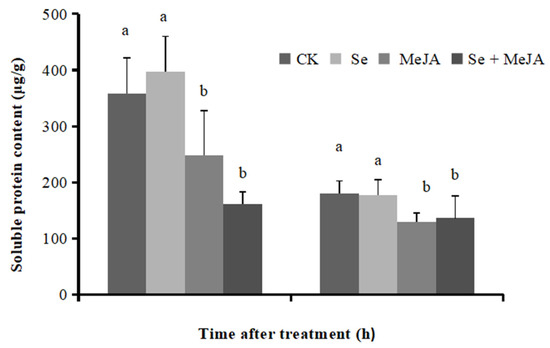
Figure 4.
Effect of Se, MeJA and Se + MeJA on soluble protein content for 4 and 8 h (n = 4). CK: Control group; Se: 5.0 mg/L Se; MeJA: 0.4 mM MeJA; Se + MeJA: 5.0 mg/L Se with 0.4 mM MeJA. Vertical bars indicate standard error. Columns followed by different lowercase letters are statistically different according to Duncan’s multiple range test (p < 0.05).
3.5. Effect of Se and MeJA on MDA Content of B. cinerea Mycelium
The degree of cell membrane damage can be evaluated by the MDA content. Both Se applied separately and in combination with MeJA increased the MDA content of B. cinerea mycelium markedly when compared with the control (Figure 5). The MDA contents of mycelium treated with Se alone or in combination with MeJA were 19.51 nmol·g−1 and 21.52 nmol·g−1, respectively, which increased by 60.9% and 64.6%, respectively, compared with the control. These results indicated that the membrane lipid peroxidation of pathogenic fungi increased, and their membrane systems were destroyed. The application of MeJA alone in the culture medium did not notably affect the mycelium MDA content.
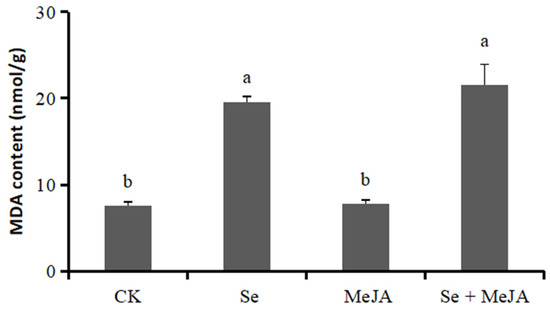
Figure 5.
Effect of Se, MeJA and Se + MeJA on mycelium MDA content of Botrytis cinerea (n = 4). CK: Control group; Se: 5.0 mg/L Se; MeJA: 0.4 mM MeJA; Se + MeJA: 5.0 mg/L Se with 0.4 mM MeJA. Vertical bars indicate standard error. Columns followed by different lowercase letters are statistically different according to Duncan’s multiple range test (p < 0.05).
3.6. Effect of Se and MeJA on Oxalic Acid Secretion of B. cinerea Mycelium
Oxalic acid can reflect the pathogenicity of B. cinerea. Oxalic acid secretion was significantly reduced after applying Se and MeJA (Figure 6). Compared with the control, both Se treatment and MeJA treatment presented similar decreasing trends of oxalic acid secretion, which was reduced by 69.4% and 65.3%, respectively. Moreover, the minimum value of oxalic acid secretion was 0.1420 mg·mL−1 under the combined stress of Se and MeJA, which was 74.6% lower than that in the control.
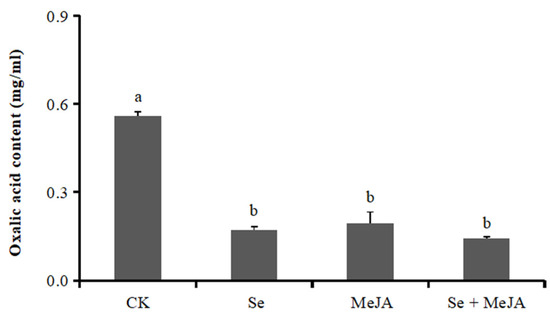
Figure 6.
Effect of Se, MeJA and Se + MeJA on mycelium oxalic acid content of Botrytis cinerea (n = 4). CK: Control group; Se: 5.0 mg/L Se; MeJA: 0.4 mM MeJA; Se + MeJA: 5.0 mg/L Se with 0.4 mM MeJA. Vertical bars indicate standard error. Values with the same letter are not significantly different at p < 0.05 according to Duncan’s multiple range test.
3.7. Effect of Se and MeJA on Gray Mold Disease on Postharvest Tomatoes
As shown in Figure 7, the combination of Se and MeJA was effective in inhibiting the lesion diameter on the tomato fruits, and the incidence was significantly smaller than that of the controls (p < 0.001). After inoculation, each tomato was incubated for four days. The incidence rate with Se and MeJA combination treatment was reduced by 45.7%, compared with 63.9% disease incidence in the control.
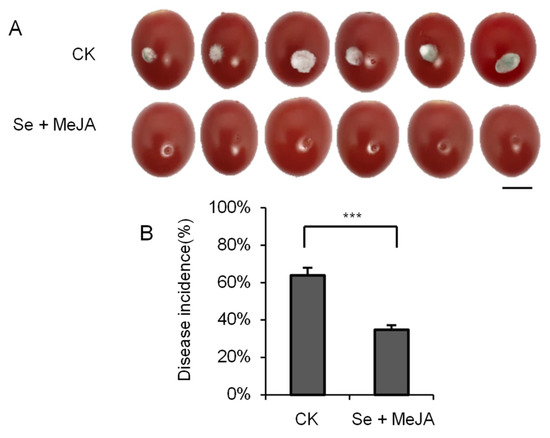
Figure 7.
Effect of Se + MeJA on lesion size (A) and disease incidence (n = 6) (B) of gray mold caused by Botrytis cinerea on tomato fruit after four days of incubation at 23 ± 1 °C. Vertical bars indicate standard error. “***” indicates that there is significant difference between the two groups according to t-test (p < 0.001). Bars = 10 mm.
4. Discussion
Se and MeJA are used as bacteriostats for disease control of various crops because their biochemical properties are safer and more environmentally friendly compared with traditional chemical fungicides. In the current study, we combined Se and MeJA to control postharvest gray mold on tomato fruits, which was based on the good mycelial growth inhibition and environmental safety property that they showed. The results showed that the combined application of Se and MeJA effectively inhibited spore germination and mycelial growth of B. cinerea (Figure 1 and Figure 2), as well as significantly decreasing the lesion diameter and incidence of gray mold disease in tomato fruits (Figure 7), which indicated that the combined application of Se and MeJA had the potential to protect tomato fruits against B. cinerea.
As the cell membrane is the main barrier for organelles to communicate with the outside world, it will accept external stimuli first. It has been reported that exogenous Se causes damage to the membrane system of pathogenic fungi, which results in the inhibition of fungal growth [18,19]. Similarly, a study conducted by Li et al. [35] documented that MeJA treatment increased the conductivity of and leaked intracellular matter from the mycelium of Botryosphaeria dothidea, which disrupted the mycelial cell membrane, resulting in an antifungal effect. In this study, Se treatment increased the membrane permeability and membrane lipid peroxidation of B. cinerea (Figure 3 and Figure 5). Moreover, the inhibitory effect of the treatment combining Se and MeJA was better than that of Se treatment alone; membrane permeability and MDA content were significantly increased, and the soluble protein content of B. cinerea mycelium was noticeably decreased (Figure 3, Figure 4 and Figure 5). Furthermore, the results indicated that the combined application of Se and MeJA damaged the membrane system and increased the amount of soluble protein exudation, leading to cell death.
B. cinerea will secrete toxic proteins, small molecular compounds and so on to help it infect plants during pathogenic processes such as attachment, invasion, and lesion expansion [36]. Oxalic acid serves as an important pathogenic factor of B. cinerea, which can promote the decomposition of the cell wall’s pectin layer and induce cell death [37,38]. In this study, the oxalic acid secretion from B. cinerea treated with Se and MeJA was significantly lower than that in the control (Figure 6), which indicated that Se and MeJA treatment reduced the pathogenicity of B. cinerea. Similar findings were reported for Sclerotinia sclerotiorum, in which the dissolved organic matter in Se-enriched rape straw could significantly reduce oxalic acid secretion from Sclerotinia sclerotiorum [39].
In the current study, it was confirmed that the simultaneous application of Se and MeJA was effective in controlling tomato gray mold. Moreover, the antifungal mechanism was also preliminarily studied. However, the growth of B. cinerea mycelium and the changes in its physicochemical indexes are a complicated process, involving biochemical reactions in cells and the expression levels of pathogenic genes. In addition, changes in antioxidant enzyme activity are another important influencing factor for plant resistance [40,41]. Se and MeJA treatment can regulate the activities of enzymes related to antioxidant and disease resistance in fruits and vegetables, and improve energy metabolism, which is helpful for inhibiting pathogenic fungi [42,43]. For practical application, the underlying mechanisms of the antifungal effect of Se and MeJA treatment in plants should be further investigated.
5. Conclusions
To sum up, the combined application of Se and MeJA increased the permeability of the cell membrane and the degree of membrane lipid peroxidation of B. cinerea, resulting in severe damage to cell membrane integrity and leakage of soluble protein, as well as reducing secretion of oxalic acid, which may act as the mechanism of B. cinerea pathogenicity reduction on tomato fruits. In addition, the Se and MeJA combination effectively inhibited hypha elongation, spore germination and the emergence of tomato gray mold disease. The results provide a new basis for further exploring the mechanism by which Se and MeJA inhibit B. cinerea, and a new way for developing environmentally friendly measures against B. cinerea.
Author Contributions
Conceptualization, X.Y., C.L., C.H. and X.Z.; methodology, X.Y., C.L. and J.X.; formal analysis, J.X.; investigation and resources, K.L., S.C., L.Y. and X.W.; visualization and writing—original draft preparation, J.X. and C.L.; supervision and writing—review and editing, C.H. and X.Z.; funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Opening Project of the Key Laboratory of Testing and Evaluation for Agro-product Safety and Quality, Ministry of Agriculture and Rural Affairs (NK202101), the open funds of the State Key Laboratory of Agricultural Microbiology (AMLKF202009), the Program of Fujian Key Laboratory for Monitoring and Integrated Management of Crop Pests, the Key Laboratory of Se-enriched Product Development and Quality Control, Ministry of Agriculture and Rural Affairs/National–Local Joint Engineering Laboratory of Se-enriched Food Development (Se-2021A01), the Opening Project of Fujian Universities and Colleges Engineering Research Center of Modern Facility Agriculture (G2-KF2007), and the Fundamental Research Funds for the Central Universities (BC2022102).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Soylu, E.M.; Kurt, S.; Soylu, S. In vitro and in vivo antifungal activity of essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int. J. Food Microbiol. 2010, 143, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Vos, C.M.; Yang, Y.; Coninck, B.D.; Cammue, B. Fungal (-like) biocontrol organisms in tomato disease control. Biol. Control 2014, 74, 65–81. [Google Scholar] [CrossRef]
- Weiberg, A.; Wang, M.; Lin, F.M.; Zhao, H.W.; Zhang, Z.H.; Kaloshian, I.; Huang, H.D.; Jin, H.L. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Kan, J.A.L.V.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Pietro, A.D.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Acosta, A.; Rodriguez, C. Fungicide resistance of Botrytis cinerea in tomato greenhouses in the Canary Islands and effectiveness of non-chemical treatments against gray mold. World J. Microbiol. Biotechnol. 2014, 30, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Sharma, S.; Kaur, S.; Nayyar, H. Selenium in agriculture: A nutrient or contaminant for crops? Arch. Agron. Soil Sci. 2014, 60, 1593–1624. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Rehman, M.Z.U.; Rinklebe, J.; Tsang, D.C.W.; Tack, F.M.G.; Abbasi, G.H.; Hussain, A.; Igalavithana, A.D.; Lee, B.C.; et al. Effects of selenium on the uptake of toxic trace elements by crop plants: A review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2531–2566. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.B.; Liu, J.; Chen, Y.L.; Zhang, X.J. Exploring the effects of selenium treatment on the nutritional quality of tomato fruit. Food Chem. 2018, 252, 9–15. [Google Scholar] [CrossRef]
- D’Amato, R.; Feudis, M.D.; Hasuoka, P.E.; Regni, L.; Pacheco, P.H.; Onofri, A.; Businelli1, D.; Proietti1, P. The selenium supplementation influences olive tree production and oil stability against oxidation and can alleviate the water deficiency effects. Front. Plant Sci. 2018, 9, 1191. [Google Scholar] [CrossRef]
- Dai, H.; Jia, G. Effects of Se on the growth, tolerance, and antioxidative systems of three alfalfa cultivars. Environ. Sci. Pollut. Res. 2017, 24, 15196–15201. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Daneshvar Hakimi Meybodi, N.; Peijnenburg, W. Mitigation of the effect of drought on growth and yield of pomegranates by foliar spraying of different sizes of selenium nanoparticles. J. Sci. Food Agric. 2021, 101, 5202–5213. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Hu, C.X.; Jia, W.; Cai, M.M.; Zhao, Y.Y.; Tang, Y.N.; Yang, D.D.; Zhou, Y.J.; Sun, X.C.; Zhao, X.H. Selenium reduces the pathogenicity of Sclerotinia sclerotiorum by inhibiting sclerotial formation and germination. Ecotoxicol. Environ. Saf. 2019, 183, 109503. [Google Scholar] [CrossRef] [PubMed]
- Troni, E.; Beccari, G.; D’Amato, R.; Tini, F.; Baldo, D.; Senatore, M.T.; Beone, G.M.; Fontanella, M.C.; Prodi, A.; Businelli, D.; et al. In vitro evaluation of the inhibitory activity of different selenium chemical forms on the growth of a Fusarium proliferatum strain isolated from rice seedlings. Plants 2021, 10, 1725. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.L.; Yin, X.B.; Lin, Z.Q.; Bañuelos, G.S.; Yuan, L.X.; Liu, Y.; Li, M. Inhibitory effect of selenium against Penicillium expansum and its possible mechanisms of action. Curr. Microbiol. 2014, 69, 192–201. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Tsivileva, O.M.; Drevko, Y.B.; Ibragimova, D.N.; Koftin, O.V. Effect of Selenium-containing biocomposites from medicinal mushrooms on the potato ring rot causative agent. Dokl. Biol. Sci. 2018, 479, 67–69. [Google Scholar] [CrossRef]
- Joshi, S.M.; De Britto, S.; Jogaiah, S.; Ito, S.i. Mycogenic selenium nanoparticles as potential new generation broad spectrum antifungal molecules. Biomolecules 2019, 9, 419. [Google Scholar] [CrossRef]
- Fardsadegh, B.; Vaghari, H.; Mohammad-Jafari, R.; Najian, Y.; Jafarizadeh-Malmiri, H. Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract. Green Process. Synth. 2019, 8, 191–198. [Google Scholar] [CrossRef]
- Wu, Z.; Yin, X.; BaUelos, G.S.; Lin, Z.Q.; Zhu, Z.; Liu, Y.; Yuan, L.; Li, M. Effect of selenium on control of postharvest gray mold of tomato fruit and the possible mechanisms involved. Front. Microbiol. 2016, 6, 1441. [Google Scholar] [CrossRef]
- Jia, W.; Hu, C.X.; Ming, J.J.; Zhao, Y.Y.; Xin, J.; Sun, X.C.; Zhao, X.H. Action of selenium against Sclerotinia sclerotiorum: Damaging membrane system and interfering with metabolism. Pestic. Biochem. Physiol. 2018, 150, 10–16. [Google Scholar] [CrossRef]
- Mao, X.; Hua, C.; Yang, L.; Zhang, Y.; Sun, Z.; Li, L.; Li, T. The effects of selenium on wheat fusarium head blight and DON accumulation were selenium compound-dependent. Toxins 2020, 12, 573. [Google Scholar] [CrossRef]
- Espinosa-Ortiz, E.J.; Gonzalez-Gil, G.; Saikaly, P.E.; Hullebusch, E.D.; Lens, P.N.L. Effects of selenium oxyanions on the white-rot fungus Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 2015, 99, 2405–2418. [Google Scholar] [CrossRef] [PubMed]
- Waseernack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.Y.; Zhao, X.Y.; Chen, M.; Fu, Y.Q.; Xiang, M.L.; Chen, J.Y. Effect of exogenous methyl jasmonate treatment on disease resistance of postharvest kiwifruit. Food Chem. 2020, 305, 125483. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.Y.; Chen, X.R.; Xu, W.; Fan, S.S.; Wan, T.; Zhang, J.; Cai, Y.L. Methyl jasmonate induces postharvest disease resistance to decay caused by Alternaria alternata in sweet cherry fruit. Sci. Horicult. 2022, 292, 110624. [Google Scholar] [CrossRef]
- Zhu, Z.; Tian, S. Resistant responses of tomato fruit treated with exogenous methyl jasmonate to Botrytis cinerea infection. Sci. Hortic. 2012, 142, 38–43. [Google Scholar] [CrossRef]
- Wang, H.B.; Kou, X.H.; Wu, C.; Fan, G.J.; Li, T.T. Methyl jasmonate induces the resistance of postharvest blueberry to gray mold caused by Botrytis cinerea. J. Sci. Food Agric. 2020, 100, 4272–4281. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Fang, W.; Lu, H.; Zhu, R.; Lu, L.; Zheng, X.; Yu, T. Inhibition of green mold disease in mandarins by preventive applications of methyl jasmonate and antagonistic yeast cryptococcus laurentii. Postharvest Biol. Technol. 2014, 88, 72–78. [Google Scholar] [CrossRef]
- Shi, T.; Pan, T.; Guo, M. First isolation and identification of Neopestalotiopsis clavispora causing postharvest rot of Rosa sterilis and its control with methyl jasmonate and calcium chloride. Horticulturae 2022, 8, 190. [Google Scholar] [CrossRef]
- Li, C.Y.; Hu, C.X.; Xie, J.T.; Shi, G.Y.; Wang, X.; Yuan, X.; Li, K.Y.; Chen, S.Q.; Zhao, X.H.; Fan, G.C. Selenium Combined with Methyl Jasmonate to Control Tomato Gray Mold by Optimizing Microbial Community Structure in Plants. J. Fungi 2022, 8, 731. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; El Ghaouth, A.; Wilson, C. Influence of food additives on the control of postharvest of apple and peach and efficacy of the yeast-based biocontrol product aspire. Postharvest Biol. Technol. 2003, 27, 127–135. [Google Scholar] [CrossRef]
- Ji, D.C.; Chen, T.; Ma, D.Y.; Liu, J.L.; Xu, Y.; Tian, S.P. Inhibitory effects of methyl thujate on mycelial growth of Botrytis cinerea and possible mechanisms. Postharvest Biol. Technol. 2018, 142, 46–54. [Google Scholar] [CrossRef]
- Shao, X.; Cheng, S.; Wang, H.; Yu, D.; Mungai, C. The possible mechanism of antifungal action of tea tree oil on Botrytis cinerea. J. Appl. Microbiol. 2013, 114, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Duan, Y.B.; Ge, C.Y.; Liu, S.M.; Chen, C.J.; Zhou, M.G. Effect of phenylpyrrole fungicide fludioxonil on morphological and physiological characteristics of Sclerotinia sclerotiorum. Pestic. Biochem. Physiol. 2013, 106, 61–67. [Google Scholar] [CrossRef]
- Li, X.Q.; Long, Y.H.; Yi, X.H.; Wu, X.M.; Zhao, Z.B.; Fan, R.; Mo, F.X.; Jiang, Y.L.; Huang, Y.X.; Tang, J.W. Mechanism of action of methyl jasmonate against kiwifruit soft rot and its effect on fruit quality. Food Sci. 2019, 40, 239–248. [Google Scholar]
- Wang, M.; Weiberg, A.; Dellota, E.; Yamane, D.; Jin, H. Botrytis small RNA Bc-sir37 suppresses plant defense genes by cross-kingdom RNAi. RNA Biol. 2017, 14, 421–428. [Google Scholar] [CrossRef]
- Han, Y.; Joosten, H.J.; Niu, W.; Zhao, Z.; Mariano, P.S.; Mccalman, M.; Kan, J.; Schaap, P.J.; Dunaway-Mariano, D. Oxaloacetate hydrolase, the C-C bond lyase of oxalate secreting fungi. J. Biol. Chem. 2007, 282, 9581–9590. [Google Scholar] [CrossRef]
- Kumari, S.; Tayal, P.; Sharma, E.; Kapoor, R. Analyses of genetic and pathogenic variability among Botrytis cinerea isolates. Microbiol. Res. 2014, 169, 862–872. [Google Scholar] [CrossRef]
- Jia, W.; Hu, C.X.; Xu, J.Y.; Ming, J.J.; Zhao, Y.Y.; Cai, M.M.; Sun, X.C.; Liu, X.W.; Zhao, X.H. Dissolved organic matter derived from rape straw pretreated with selenium in soil improves the inhibition of Sclerotinia sclerotiorum growth. J. Hazard. Mater. 2019, 369, 601–610. [Google Scholar] [CrossRef]
- Lo’ay A, A., L.; Ismail, H.; Kassem, H.S. Postharvest treatment of ‘Florida Prince’ peaches with a calcium nanoparticle–ascorbic acid mixture during cold storage and its effect on antioxidant enzyme activities. Horticulturae 2021, 7, 499. [Google Scholar]
- Tomilova, O.G.; Kryukova, N.A.; Efimova, M.V.; Kovtun, I.S.; Kolomeichuk, L.V.; Kryukov, V.Y.; Glupov, V.V. Early physiological response of potato plants to entomopathogenic fungi under hydroponic conditions. Horticulturae 2021, 7, 217. [Google Scholar]
- Wang, S.Y.; Shi, X.C.; Liu, F.Q.; Laborda, P. Effects of exogenous methyl jasmonate on quality and preservation of postharvest fruits: A review. Food Chem. 2021, 353, 129482. [Google Scholar] [PubMed]
- Zhu, Z.; Chen, Y.; Shi, G.; Zhang, X.J. Selenium delays tomato fruit ripening by inhibiting ethylene biosynthesis and enhancing the antioxidant defense system. Food Chem. 2017, 219, 179–184. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).