Ethylene and Chitosan Affected the Seed Yield Components of Onion Depending More on the Dose than Timing of Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area, Site Description, and Agronomic History of the Experimental Field
2.2. Experimental Device in the Field
- -
- Recommended rate = 100% applied at early application (21 March 2019), hereafter referred as 1st Et100 2nd Et0 3rd Et0. At the time of the application, the crop was at the phenology stage 306 of the BBCH scale [36];
- -
- Rate = 150% compared to the recommended, applied as 100% at early application, as above + additional 50% at mid- or late applications, carried out on 2 April 2019 and 28 April 2019, respectively. In these dates, the phenology stages 402 and 501 of the BBCH scale [36], respectively. These treatments were referred as 1st Et100 2nd Et50 3rd Et0 and 1st Et100 2nd Et0 3rd Et50, respectively;
- -
- Recommended rate = 100% applied at late application (28 April 2019), hereafter referred as 1st Et0 2nd Et0 3rd Et100;
- -
- Control (ethylene never applied), referred as 1st Et0 2nd Et0 3rd Et0.
2.3. Measurements before and after the Application of the Ethylene and Chitosan
2.4. Germination Test
2.5. Computations and Statistical Analyses
3. Results
3.1. Stand Traits and Environmental Conditions during the Growing Season
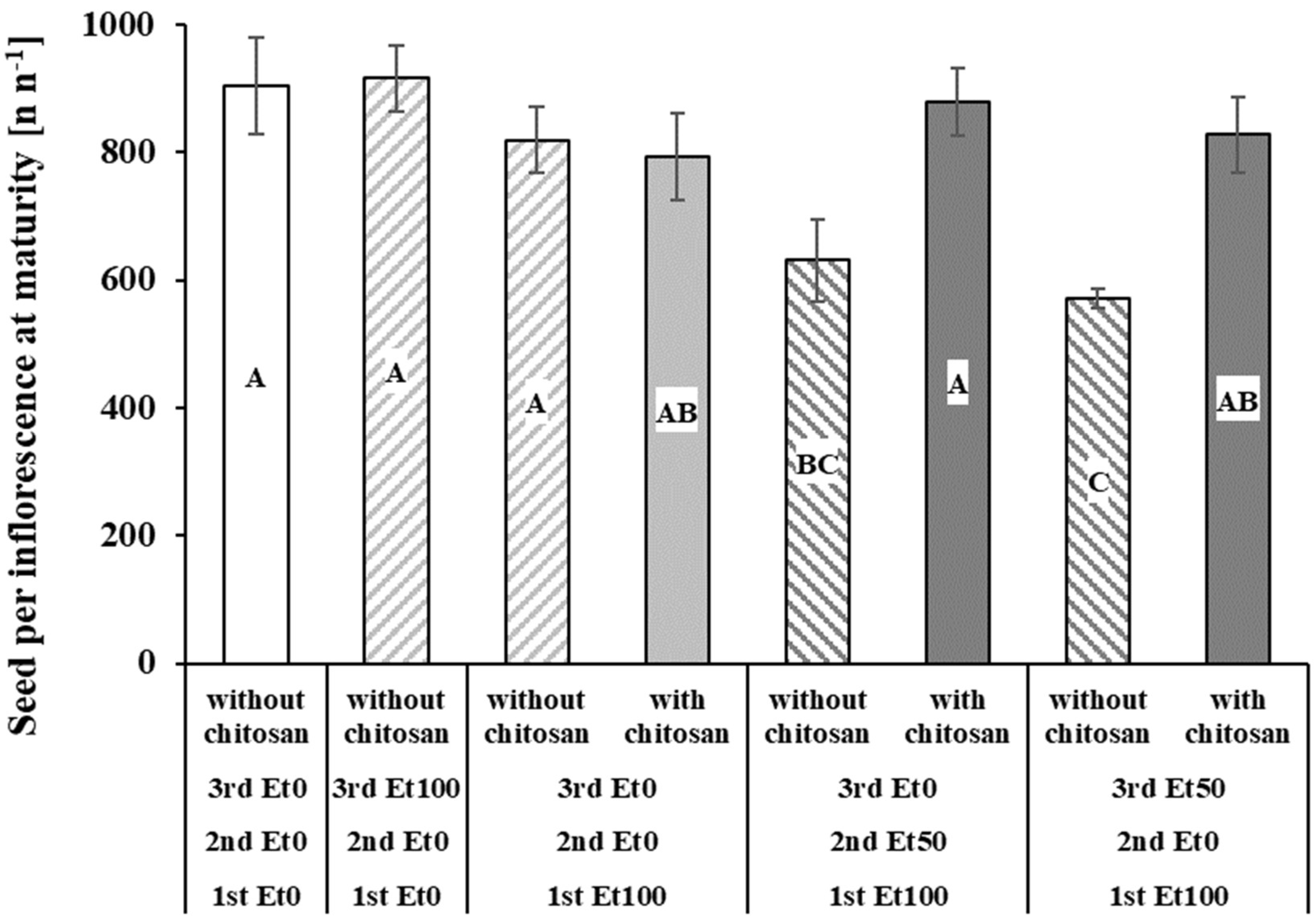
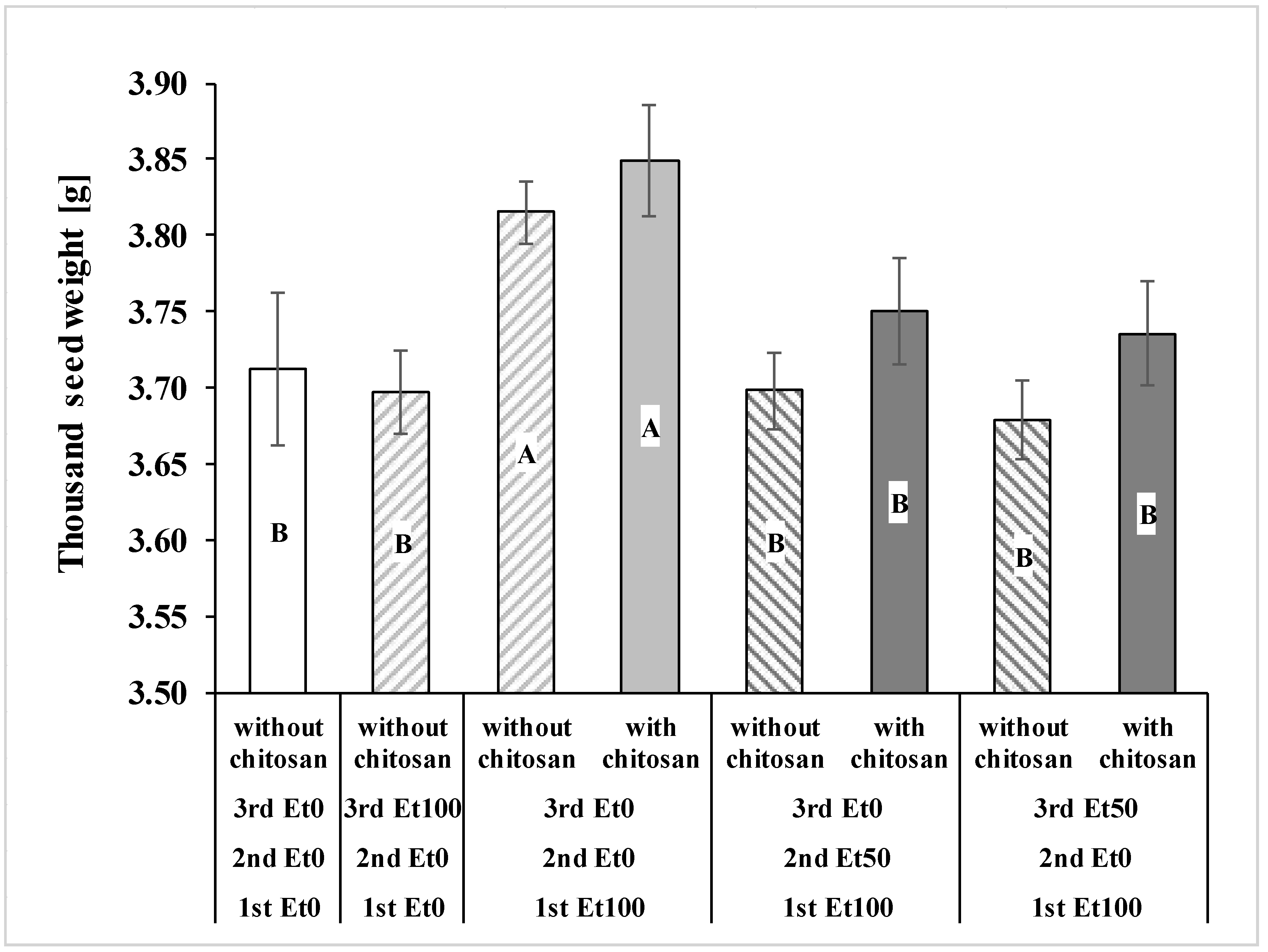
3.2. Grain Yield, Yield Components, and Germination
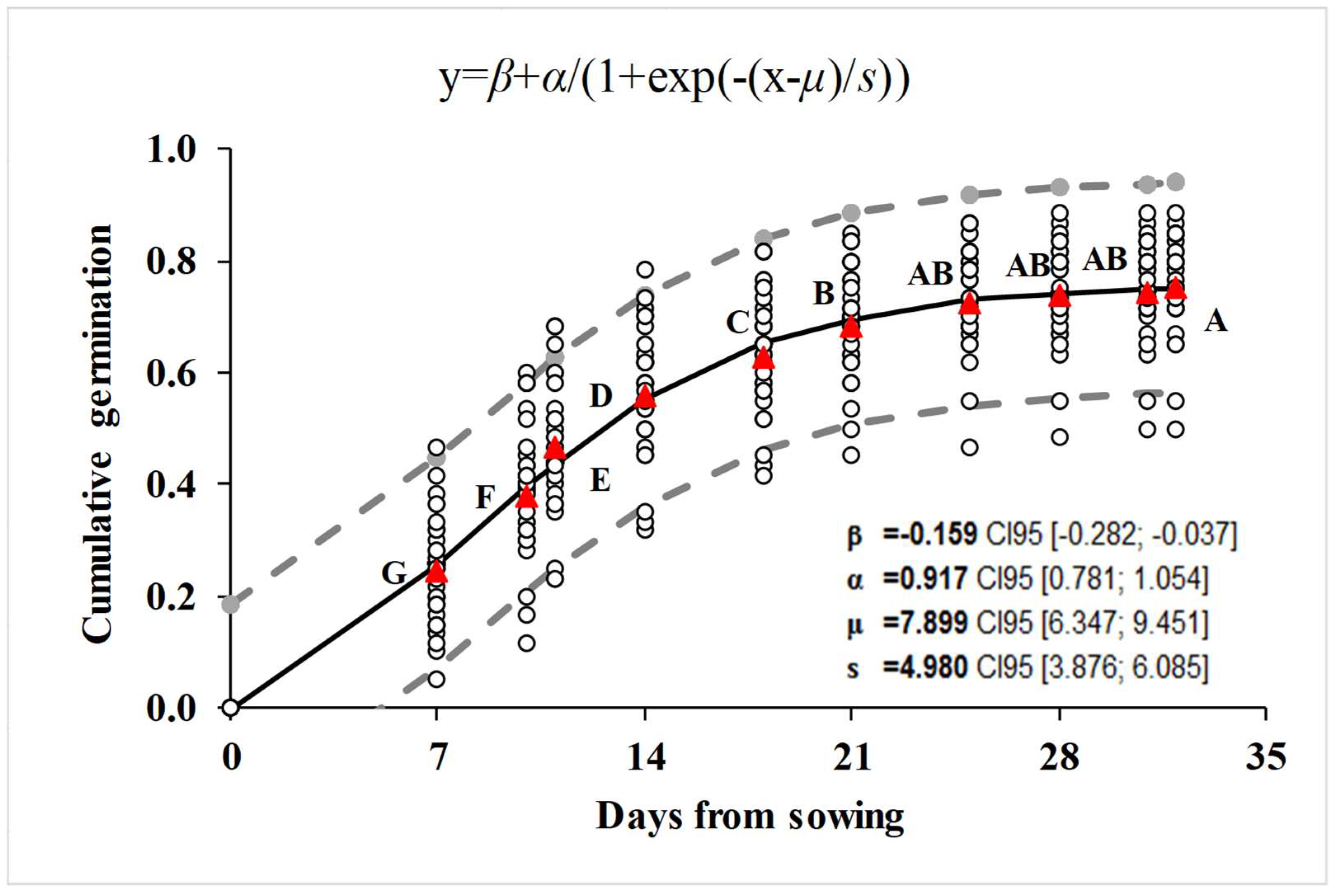
3.3. Cumulative Germination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO FAO/STAT Data. Available online: http://www.fao.org/faostat/en/#home (accessed on 12 July 2022).
- Griffiths, G.; Trueman, L.; Crowther, T.; Thomas, B.; Smith, B. Onions?A global benefit to health. Phyther. Res. 2002, 16, 603–615. [Google Scholar] [CrossRef]
- Megersa, H.G. Propagation Methods of Selected Horticultural Crops by Specialized Organs: Review. J. Hortic. 2017, 4. [Google Scholar] [CrossRef]
- Thirusendura Selvi, D.; Saraswathy, S. Seed viability, seed deterioration and seed quality improvements in stored onion seeds: A review. J. Hortic. Sci. Biotechnol. 2018, 93, 1–7. [Google Scholar] [CrossRef]
- Jones, H.A.; Mann, L.K. Onions and Their Allies. Soil Sci. 1964, 98, 68. [Google Scholar] [CrossRef]
- Rabinowitch, H.D. Onions and Allied Crops; Rabinowitch, H.D., Brewster, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351075169. [Google Scholar]
- Bosch Serra, A.D.; Currah, L. Agronomy of onions. In Allium Crop Science: Recent Advances; CABI: Wallingford, UK, 2002; pp. 187–232. [Google Scholar]
- Di Miceli, G.; Farruggia, D.; Iacuzzi, N.; Bacarella, S.; La Bella, S.; Consentino, B.B. Planting Date and Different N-Fertilization Rates Differently Modulate Agronomic and Economic Traits of a Sicilian Onion Landrace and of a Commercial Variety. Horticulturae 2022, 8, 454. [Google Scholar] [CrossRef]
- Kęsik, T.; Błażewicz-woźniak, M. Growth and Yielding of Onion Under Conservation Tillage. Veg. Crop. Res. Bull. 2009, 70, 111–123. [Google Scholar] [CrossRef]
- Vestberg, M.; Saari, K.; Kukkonen, S.; Hurme, T. Mycotrophy of crops in rotation and soil amendment with peat influence the abundance and effectiveness of indigenous arbuscular mycorrhizal fungi in field soil. Mycorrhiza 2005, 15, 447–458. [Google Scholar] [CrossRef]
- Pathak, C.S. Hybrid Seed Production in Onion. J. New Seeds 1999, 1, 89–108. [Google Scholar] [CrossRef]
- Peluffo, S.; González Idiarte, H.; Borges, A.; Arboleya, J.; Galván, G.A. Onion sets as planting material for seed production of three cultivars in Uruguay. Seed Sci. Technol. 2016, 44, 500–513. [Google Scholar] [CrossRef]
- Rodo, A.B.; Marcos-Filho, J. Onion seed vigor in relation to plant growth and yield. Hortic. Bras. 2003, 21, 220–226. [Google Scholar] [CrossRef]
- Dowker, B.D. Onion Breeding. In Onions and Allied Crops; CRC Press: Boca Raton, FL, USA, 2018; pp. 215–232. ISBN 0870554999. [Google Scholar]
- Yalamalle, V.R.; Arunachalam, T.; Kumari, R.; Ithape, D.M.; Ghosh, S.; Singh, M. Ethephon Reduces Lodging and Enhances Seed Yield and Quality in Onion. Int. J. Bio-Resour. Stress Manag. 2020, 11, 601–606. [Google Scholar] [CrossRef]
- Lazko, V.E.; Yakimova, O. V Planting depth - effective agricultural practice perventing lodging of seedstalks of onion seeds. E3S Web Conf. 2020, 224, 04011. [Google Scholar] [CrossRef]
- Anisuzzaman, M.; Ashrafuzzaman, M.; Ismail, M.R.; Uddin, M.K.; Rahim, M.A. Planting time and mulching effect on onion development and seed production. African J. Biotechnol. 2009, 8, 412–416. [Google Scholar]
- Brown, M.J.; Wright, J.L.; Kohl, R.A. Onion-Seed Yield and Quality as Affected by Irrigation Management 1. Agron. J. 1977, 69, 369–372. [Google Scholar] [CrossRef]
- Ashagrie, T.; Belew, D.; Nebiyu, A. Influence of planting date and bulb size on yield and quality of onion (Allium cepa L.) seed production. Cogent Food Agric. 2021, 7. [Google Scholar] [CrossRef]
- Geetharani, P.; Manivannan, M.I.; Ponnuswamy, A.S. Seed production of onion as influenced by the application of growth regulators and nutrients. Asian J. Hortic. 2008, 3, 301–303. [Google Scholar]
- Saia, S.; Giovino, A. An efficient protocol for Cistus crispus L. (Cistaceae) micropropagation. Folia Hortic. 2020, 31, 1–9. [Google Scholar] [CrossRef]
- Saia, S.; Corrado, G.; Vitaglione, P.; Colla, G.; Bonini, P.; Giordano, M.; Stasio, E.D.; Raimondi, G.; Sacchi, R.; Rouphael, Y. An Endophytic Fungi-Based Biostimulant Modulates Volatile and Non-Volatile Secondary Metabolites and Yield of Greenhouse Basil (Ocimum basilicum L.) through Variable Mechanisms Dependent on Salinity Stress Level. Pathogens 2021, 10, 797. [Google Scholar] [CrossRef]
- Saia, S.; Colla, G.; Raimondi, G.; Di Stasio, E.; Cardarelli, M.; Bonini, P.; Vitaglione, P.; De Pascale, S.; Rouphael, Y. An endophytic fungi-based biostimulant modulated lettuce yield, physiological and functional quality responses to both moderate and severe water limitation. Sci. Hortic. 2019, 256, 108595. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic Biostimulatory Action: Designing the Next Generation of Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Taofiq, O.; Fernandes, Â.; Tzortzakis, N.; Ciric, A.; Sokovic, M.; Barros, L.; Ferreira, I.C.F.R. Bioactive properties of greenhouse-cultivated green beans (Phaseolus vulgaris L.) under biostimulants and water-stress effect. J. Sci. Food Agric. 2019, 99, 6049–6059. [Google Scholar] [CrossRef]
- Sharma, K.; Rok Lee, Y.; Park, S.W.; Nile, S.H. Importance of growth hormones and temperature for physiological regulation of dormancy and sprouting in onions. Food Rev. Int. 2016, 32, 233–255. [Google Scholar] [CrossRef]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E. Ethylene in Plant Biology, 2nd ed.; Academic Press: Cambridge, MA, USA, 1992; ISBN 978-0-08-091628-6. [Google Scholar]
- Beltrano, J.; Ronco, M.G.; Montaldi, E.R. Drought Stress Syndrome in Wheat Is Provoked by Ethylene Evolution Imbalance and Reversed by Rewatering, Aminoethoxyvinylglycine, or Sodium Benzoate. J. Plant Growth Regul. 1999, 18, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, F.; Bögemann, G.M.; Visser, E.J.W.; Lambers, H.; Voesenek, L.A.C.J. Ethylene Emission and Responsiveness to Applied Ethylene Vary among Poa Species That Inherently Differ in Leaf Elongation Rates. Plant Physiol. 2002, 129, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Davies, W.J. Ozone suppresses soil drying- and abscisic acid (ABA)-induced stomatal closure via an ethylene-dependent mechanism. Plant. Cell Environ. 2009, 32, 949–959. [Google Scholar] [CrossRef]
- Cools, K.; Chope, G.A.; Hammond, J.P.; Thompson, A.J.; Terry, L.A. Ethylene and 1-Methylcyclopropene Differentially Regulate Gene Expression during Onion Sprout Suppression. Plant Physiol. 2011, 156, 1639–1652. [Google Scholar] [CrossRef]
- Singh, S.; Singh, K.; Singh, S. Effect of hormones on growth and yield characters of seed crop of kharif onion (Allium cep L.). Indian J. Plant Physiol. 1995, XXXVIII, 193–196. [Google Scholar]
- Rajestary, R.; Landi, L.; Romanazzi, G. Chitosan and postharvest decay of fresh fruit: Meta-analysis of disease control and antimicrobial and eliciting activities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 563–582. [Google Scholar] [CrossRef]
- Czékus, Z.; Iqbal, N.; Pollák, B.; Martics, A.; Ördög, A.; Poór, P. Role of ethylene and light in chitosan-induced local and systemic defence responses of tomato plants. J. Plant Physiol. 2021, 263, 153461. [Google Scholar] [CrossRef]
- Romanazzi, G.; Orçonneau, Y.; Moumni, M.; Davillerd, Y.; Marchand, P.A. Basic Substances, a Sustainable Tool to Complement and Eventually Replace Synthetic Pesticides in the Management of Pre and Postharvest Diseases: Reviewed Instructions for Users. Molecules 2022, 27, 3484. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants; Julius Kühn-Institut (JKI): Quedlinburg, Germany, 2018; ISBN 978-3-95547-071-5. [Google Scholar]
- ISTA. International Rules for Seed Testing, 2017th ed.; ISTA, Ed.; The International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 2017; Volume 2017. [Google Scholar]
- Saia, S.; Aissa, E.; Luziatelli, F.; Ruzzi, M.; Colla, G.; Ficca, A.G.; Cardarelli, M.; Rouphael, Y. Growth-promoting bacteria and arbuscular mycorrhizal fungi differentially benefit tomato and corn depending upon the supplied form of phosphorus. Mycorrhiza 2020, 30, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Suardi, A.; Saia, S.; Stefanoni, W.; Gunnarsson, C.; Sundberg, M.; Pari, L. Admixing Chaff with Straw Increased the Residues Collected without Compromising Machinery Efficiencies. Energies 2020, 13, 1766. [Google Scholar] [CrossRef]
- Suardi, A.; Saia, S.; Alfano, V.; Rezaei, N.; Cetera, P.; Bergonzoli, S.; Pari, L. Pruning harvesting with modular towed chipper: Little effect of the machine setting and configuration on performance despite strong impact on wood chip quality. PLoS ONE 2021, 16, e0261810. [Google Scholar] [CrossRef]
- Bergonzoli, S.; Brambilla, M.; Romano, E.; Saia, S.; Cetera, P.; Cutini, M.; Toscano, P.; Bisaglia, C.; Pari, L. Feeding Emitters for Microirrigation with a Digestate Liquid Fraction up to 25 % Dilution Did Not Reduce Their Performance. Agronomy 2020, 10, 1150. [Google Scholar] [CrossRef]
- Giovino, A.; Mammano, M.M.; Gugliuzza, G.; Saia, S. GERMINATION PATTERN OF CHAMAEROPS HUMILIS SEED AFTER SHORT-TIME STORAGE. Acta Hortic. 2015, 1099, 433–437. [Google Scholar] [CrossRef]
- Giovino, A.; Scibetta, S.; Saia, S.; Ruffoni, B. Influence of seed treatment on germination pattern of Chamaerops humilis. Acta Hortic. 2015, 1099, 427–432. [Google Scholar] [CrossRef]
- Williams, I.H.; Free, J.B. The Pollination of Onion (Allium cepa L.) to Produce Hybrid Seed. J. Appl. Ecol. 1974, 11, 409. [Google Scholar] [CrossRef]
- Devi, S.; Gulati, R.; Tehri, K.; Poonia, A. The pollination biology of onion (Allium cepa L.)-A Review. Agric. Rev. 2015, 36, 1. [Google Scholar] [CrossRef]
- Sajjad, A.; Saeed, S.; Masood, A. Pollinator community of onion (Allium cepa L.) and its role in crop reproductive success. Pak. J. Zool. 2008, 40, 451–456. [Google Scholar]
- Ewies, M.A.; El-Sahhar, K.F. Observations on the Behaviour of Honeybees on Onion and their Effects on Seed Yield. J. Apic. Res. 1977, 16, 194–196. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Hasan, M.M.; Hasan, M.M.; Moniruzzaman, M.; Kabir Howlander, M.H. Effect of bulb size and plant spacing on seed production of onion (Allium cepa L.). Bangladesh J. Agric. Res. 2012, 37, 405–414. [Google Scholar] [CrossRef]
- El Balla, M.M.A.; Hamid, A.A.; Abdelmageed, A.H.A. Effects of time of water stress on flowering, seed yield and seed quality of common onion (Allium cepa L.) under the arid tropical conditions of Sudan. Agric. Water Manag. 2013, 121, 149–157. [Google Scholar] [CrossRef]
- Woltering, E.J.; Van Doorn, W.G. Role of Ethylene in Senescence of Petals—Morphological and Taxonomical Relationships. J. Exp. Bot. 1988, 39, 1605–1616. [Google Scholar] [CrossRef]
- Iqbal, N.; Trivellini, A.; Masood, A.; Ferrante, A.; Khan, N.A. Current understanding on ethylene signaling in plants: The influence of nutrient availability. Plant Physiol. Biochem. 2013, 73, 128–138. [Google Scholar] [CrossRef]
- Dey, S.; Corina Vlot, A. Ethylene responsive factors in the orchestration of stress responses in monocotyledonous plants. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitch, H.D.; Friedlander, B.; Peters, R. Dwarf Flower Stalk in Onion: Characterization, Genetic Control, and Physiological Response to Ethephon and Gibberellic Acid. J. Am. Soc. Hortic. Sci. 1991, 116, 574–579. [Google Scholar] [CrossRef]
- Ouzounidou, G.; Giannakoula, A.; Asfi, M.; Ilias, I. Differential responses of onion and garlic against plant growth regulators. Pakistan J. Bot. 2011, 43, 2051–2057. [Google Scholar]
- Prajapati, S.; Jamkar, T.; Singh, O.P.; Raypuriya, N.; Mandloi, R.; Jain, P.K. Plant growth regulators in vegetable production: An overview. Plant Arch. 2015, 15, 619–626. [Google Scholar]
- Orsini, R.; Bianchelli, M.; Pacioni, P.; Santilocchi, R. Effetto di Trattamenti brachizzanti sulle caratteristiche agronomico-sanitarie di cipolla da seme (Allium cepa L.). In Aggiornamenti Sulla Peronospora Della Cipolla ed Altre Avversità Delle Colture Ortive da Seme; Romanazzi, G., Mancini, V., Feliziani, E., Rossi, M., Nardi, S., Eds.; Università Politecnica delle Marche, Facoltà di Agraria: Ancona, Italy, 2012. [Google Scholar]
- Saia, S.; Fragasso, M.; De Vita, P.; Beleggia, R. Metabolomics Provides Valuable Insight for the Study of Durum Wheat: A Review. J. Agric. Food Chem. 2019, 67, 3069–3085. [Google Scholar] [CrossRef]
- De Vita, P.; Taranto, F. Durum wheat (Triticum turgidum ssp. durum) breeding to meet the challenge of climate change. In Advances in Plant Breeding Strategies: Cereals; Springer: Berlin/Heidelberg, Germany, 2019; Volume 5, pp. 471–524. ISBN 9783030231088. [Google Scholar]
- Thomas, T.H.; Rankin, W.E.F. Effect of ethephon on bulbing, bull-necking, yield and sprouting during storage of two onion cultivars (Allium cepa L.). J. Hortic. Sci. 1982, 57, 465–467. [Google Scholar] [CrossRef]
- Tholen, D.; Pons, T.L.; Voesenek, L.A.C.J.; Poorter, H. The role of ethylene perception in the control of photosynthesis. Plant Signal. Behav. 2008, 3, 108–109. [Google Scholar] [CrossRef]
- Völz, R.; Heydlauff, J.; Ripper, D.; von Lyncker, L.; Groß-Hardt, R. Ethylene Signaling Is Required for Synergid Degeneration and the Establishment of a Pollen Tube Block. Dev. Cell 2013, 25, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Galmarini, C.R. The use of genetic, manual and chemical methods to control pollination in vegetable hybrid seed production: A review. Plant Breed. 2017, 136, 287–299. [Google Scholar] [CrossRef]
- Hughes, W.G.; Bennett, M.D.; Bodden, J.J.; Galanopoulou, S. Effects of time of application of Ethrel on male sterility and ear emergence in wheat Triticum aestivum. Ann. Appl. Biol. 1974, 76, 243–252. [Google Scholar] [CrossRef]
- Bittelli, M.; Flury, M.; Campbell, G.S.; Nichols, E.J. Reduction of transpiration through foliar application of chitosan. Agric. For. Meteorol. 2001, 107, 167–175. [Google Scholar] [CrossRef]
- Katiyar, D.; Hemantaranjan, A.; Singh, B. Chitosan as a promising natural compound to enhance potential physiological responses in plant: A review. Indian J. Plant Physiol. 2015, 20, 1–9. [Google Scholar] [CrossRef]
- Abu-Muriefah, S.S. Effect of chitosan on common bean (Phaseolus vulgaris L.) plants grown under water stress conditions. Int. Res. J. Agric. Sci. Soil Sci. 2013, 3, 192–199. [Google Scholar]
- Iriti, M.; Castorina, G.; Vitalini, S.; Mignani, I.; Soave, C.; Fico, G.; Faoro, F. Chitosan-induced ethylene-independent resistance does not reduce crop yield in bean. Biol. Control 2010, 54, 241–247. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Sivakumar, D. Chitosan, a Biopolymer With Triple Action on Postharvest Decay of Fruit and Vegetables: Eliciting, Antimicrobial and Film-Forming Properties. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Spurr, C.J.; Fulton, D.A.; Brown, P.H.; Clark, R.J. Changes in Seed Yield and Quality with Maturity in Onion (Allium cepa L., cv. ’Early Cream Gold’). J. Agron. Crop Sci. 2002, 188, 275–280. [Google Scholar] [CrossRef]
- Macias-Leon, M.A.; Leskovar, D.I. Plant Growth Regulator Effects on Germination and Root Traits of ‘Lambada’ and ‘Don Victor’ Onion Cultivars. HortScience 2017, 52, 1759–1764. [Google Scholar] [CrossRef]
- Whalley, W.R.; Finch-Savage, W.E.; Cope, R.E.; Rowse, H.R.; Bird, N.R.A. The response of carrot (Daucus carota L.) and onion (Allium cepa L.) seedlings to mechanical impedance and water stress at sub-optimal temperatures. Plant, Cell Environ. 1999, 22, 229–242. [Google Scholar] [CrossRef]
- Finch-Davage, W.E.; Phelps, K. Onion (Allium cepa L.) Seedling Emergence Patterns can be Explained by the Influence of Soil Temperature and Water Potential on Seed Germination. J. Exp. Bot. 1993, 44, 407–414. [Google Scholar] [CrossRef]
- Rowse, H.; Finch-Savage, W. Hydrothermal threshold models can describe the germination response of carrot (Daucus carota) and onion (Allium cepa) seed populations across both sub- and supra-optimal temperatures. New Phytol. 2003, 158, 101–108. [Google Scholar] [CrossRef]
- Ellis, R.H.; Butcher, P.D. The Effects of Priming and ‘Natural’ Differences in Quality amongst Onion Seed Lots on the Response of the Rate of Germination to Temperature and the Identification of the Characteristics under Genotypic Control. J. Exp. Bot. 1988, 39, 935–950. [Google Scholar] [CrossRef]
- Daarman, J.; Brocklehurst, P.A.; Drew, R.L.K. Effects of osmotic priming and ageing on onion seed germination. Ann. Appl. Biol. 1986, 108, 639–648. [Google Scholar] [CrossRef]
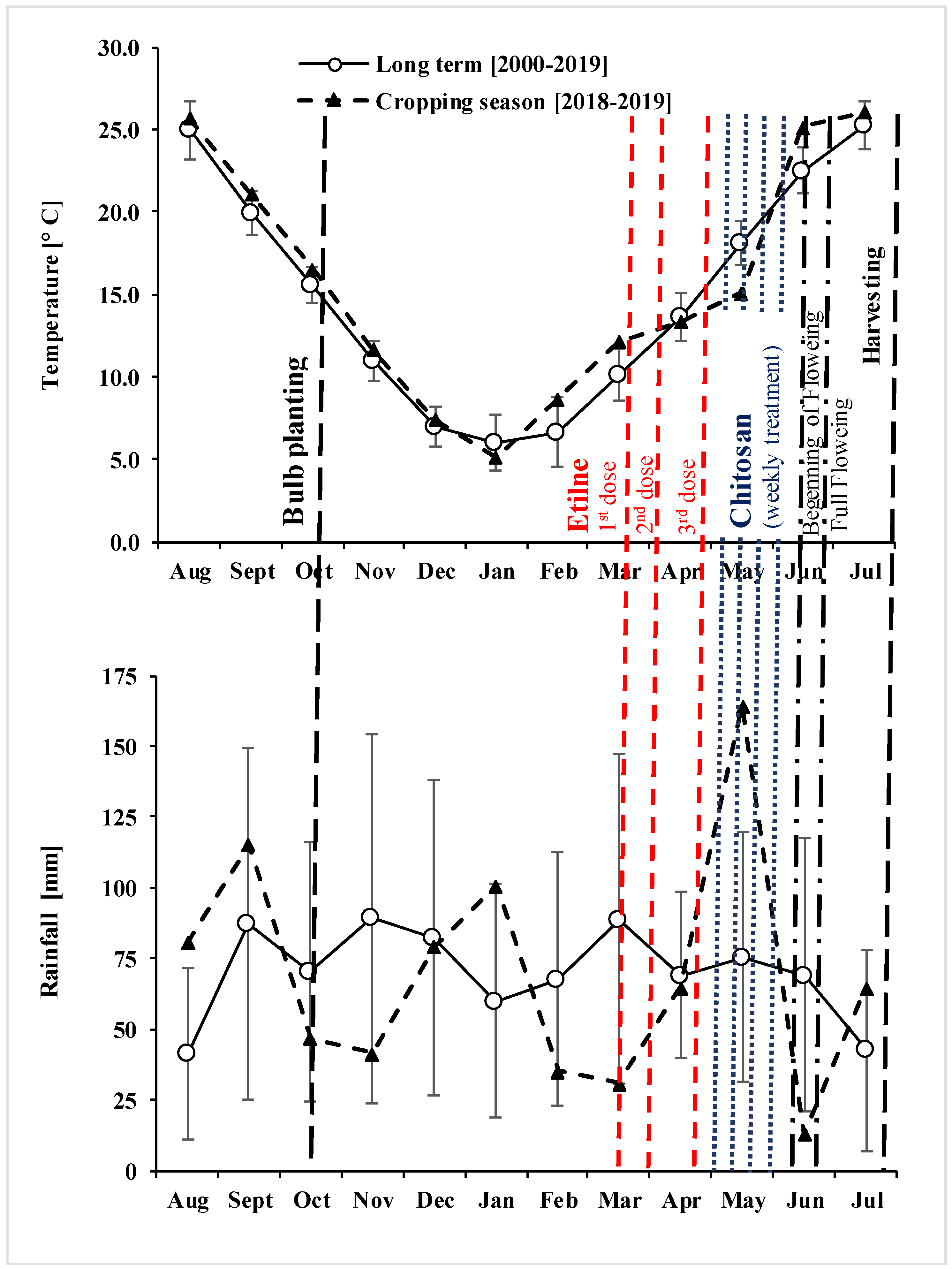

| Date (DD/MM/YYYY) | Management Technique | Active Ingredient/Formulation | Product [kg or L ha−1] | N [kg ha−1] | P2O5 [kg ha−1 | SO3 [kg ha−1] |
|---|---|---|---|---|---|---|
| 26 September 2018 | Herbicide | Glyphosate | 2.0 L | - | - | - |
| 14 October 2018 | Herbicide | Pendimetalin | 1.5 L | - | - | - |
| 17 October 2018 | P fertilization | Single superphosphate | 500 kg | - | 230 | |
| 7 November 2018 | Localised starter fertilization | Gran verde top start 8 N-35 P (including 5% SO3 and 0.8% Zn) | 18.8 kg | 1.5 | 6.6 | - |
| 4 December 2018 | Fungicide | Copper oxychloride | 3.10 kg | - | - | - |
| 7 February 2019 | N fertilization | N [35 N-23 SO3] | 437.5 kg | 153.1 | - | 100.6 |
| 26 February 2019 | Fungicide | Copper oxychloride | 1.5 kg | - | - | - |
| 9 March 2019 | Fungicide | Iprovalicarb + Copper oxychloride | 1.3 kg | - | - | - |
| 19 March 2019 | Fungicide | Mancozeb + Zoxamide | 1.25 kg + 0.63 L | - | - | - |
| 1 April 2019 | N fertilization | N [35 N-23 SO3] | 187.5 | 65.6 | - | 43.1 |
| 19 April 2019 | Fungicide | Metalaxil + Dimetomorf + Pyraclostrobin | 2.5 kg + 2.5 L | - | - | - |
| 3 May 2019 | Fungicide | Chlortalonil + Metalaxyl | 1.88 L | - | - | - |
| 6 May 2019 | N fertilization | Ammonium nitrate 26 N | 187.5 kg | 48.75 | - | - |
| 23 May 2019 | Fungicide | Zoxamide + Cimoxanil | 0.63 L + 0.38 kg | - | - | - |
| 8 June 2019 | Fungicide | Dimetomorf + Pyraclostrobin | 2.5 L | - | - | - |
| 26 June 2019 | Fungicide | Thiophanate-methyl | 1.25 L | - | - | - |
| Effect | Treat | Chit (Treat) | |
|---|---|---|---|
| Num DF | 4 | 3 | |
| Seed Yield [g m−2] | Den DF | 24 | 24 |
| F | 5.59 | 7.76 | |
| p | 0.0025 | 0.0009 | |
| Inflorescence at full blooming [n m−2] | Den DF | 20.12 | 20.17 |
| F | 0.57 | 0.19 | |
| p | 0.6873 | 0.9012 | |
| Seeds per Inflorescence at seed maturity | Den DF | 20.15 | 20.24 |
| F | 6.47 | 9.27 | |
| p | 0.0016 | 0.0005 | |
| Thousand seed weight [g] | Den DF | 20.39 | 20.66 |
| F | 5.69 | 1.04 | |
| p | 0.0031 | 0.3938 | |
| Final germination [%] | Den DF | 21.75 | 22.03 |
| F | 1.2 | 0.54 | |
| p | 0.3406 | 0.6578 |
| Effect | Num DF | Den DF | F | p |
|---|---|---|---|---|
| Time | 9 | 60.35 | 243.52 | <0.0001 |
| Treat | 4 | 20.31 | 0.63 | 0.6449 |
| Chit(Treat) | 3 | 20.31 | 0.6 | 0.6225 |
| Treat × Time | 36 | 99.8 | 0.55 | 0.9784 |
| Chit × Time (Treat) | 27 | 93.43 | 0.56 | 0.9556 |
| Function Fitted * | r2 Coef Det | DF Adj r2 | Fit Std Err | F-Statistic | |
|---|---|---|---|---|---|
| y = β + α/(1 + exp(−(x − μ)/s)) | 0.86 | 0.86 | 0.09 | 727.49 | |
| Coefficient | Value | Standard error | t-Value | lower 95% Confidence Limits | upper 95% Confidence Limits |
| b | −0.16 | 0.06 | −2.59 | −0.28 | −0.04 |
| α | 0.92 | 0.07 | 13.29 | 0.78 | 1.05 |
| μ | 7.90 | 0.78 | 10.08 | 6.35 | 9.45 |
| s | 4.98 | 0.56 | 8.93 | 3.88 | 6.08 |
| Source | Sum of Squares | df | Mean Square | F-Statistic | |
| Regression | 19.46 | 3 | 6.49 | 727.49 | |
| Error | 3.10 | 348 | 0.01 | ||
| Total | 22.57 | 351 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vecchiotti, D.; Angeletti, F.G.S.; Romanazzi, G.; Mariotti, M.; Saia, S. Ethylene and Chitosan Affected the Seed Yield Components of Onion Depending More on the Dose than Timing of Application. Horticulturae 2022, 8, 781. https://doi.org/10.3390/horticulturae8090781
Vecchiotti D, Angeletti FGS, Romanazzi G, Mariotti M, Saia S. Ethylene and Chitosan Affected the Seed Yield Components of Onion Depending More on the Dose than Timing of Application. Horticulturae. 2022; 8(9):781. https://doi.org/10.3390/horticulturae8090781
Chicago/Turabian StyleVecchiotti, Daniele, Francesco G. S. Angeletti, Gianfranco Romanazzi, Marco Mariotti, and Sergio Saia. 2022. "Ethylene and Chitosan Affected the Seed Yield Components of Onion Depending More on the Dose than Timing of Application" Horticulturae 8, no. 9: 781. https://doi.org/10.3390/horticulturae8090781
APA StyleVecchiotti, D., Angeletti, F. G. S., Romanazzi, G., Mariotti, M., & Saia, S. (2022). Ethylene and Chitosan Affected the Seed Yield Components of Onion Depending More on the Dose than Timing of Application. Horticulturae, 8(9), 781. https://doi.org/10.3390/horticulturae8090781








