Salinity-Induced Changes in the Antioxidant Status of Common Basil Plants (Ocimum basilicum L.) Grown under Controlled Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Analyses
2.2.1. Lipid Peroxidation
2.2.2. Enzyme Extraction
2.2.3. Guaiacol Peroxidase (GPOD)
2.2.4. Free Proline Concentration
2.2.5. Total Polyphenols Content
2.2.6. Antiradical Activity
2.2.7. Reducing Sugars
- A—reducing sugars in the glucose solution taken for the test, taken as glucose, in mg;
- V—volume of extract obtained, in mL (100);
- b—a volume of sugar solution taken for the test in mL;
- g—a mass of the sample taken in g;
- 100—conversion factor in %.
2.3. Analysis of Data
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nurzyńska-Wierdak, R.; Rożek, E.; Borowski, B. Response of different basil cultivars to nitrogen and potassium fertilization: Total and mineral nitrogen content in herb. Acta Sci. Pol. Hortorum Cultus 2011, 10, 217–232. [Google Scholar]
- Bojko, K.; Czajka, J.; Meller, E.; Szymańska, J. The influence of substrate type and the method of cultivation on the yielding of sweet basil (Ocimum basilicum L.). Ann. Univ. Mariae Curie-Skłodowska. Sec. EEE Hortic. 2016, 26, 33–41. [Google Scholar]
- Majkowska-Gadomska, J.; Kulczycka, A.; Dobrowolski, A.; Mikulewicz, E. Yield and nutritional value of basil grown in a greenhouse. Acta Agroph. 2017, 24, 455–464. [Google Scholar]
- Carovic, K.; Liber, Z.; Javornik, B.; Kolak, I.; Satovic, Z. Genetic relationship within basil (Ocimum) as revealed by RAPD and AFLP markers. Acta Hortic. 2007, 760, 171–177. [Google Scholar] [CrossRef]
- Kalisz, A.; Jezdinsky, A.; Pokluda, R.; Sękara, A.; Grabowska, A.; Gil, J. Impacts of chilling on photosynthesis and chlorophyll pigment content in Juvenile basil cultivars. Hortic. Environ. Biotechnol. 2016, 57, 330–339. [Google Scholar] [CrossRef]
- Ziombra, M.; Sas-Golak, I. Influence of cultivation method and date on the yield and content of essential oils in basil herb. Rocz. AR Poznań 2000, 31, 579–583. [Google Scholar]
- Lee, S.J.; Umano, K.; Shibamoto, T.; Lee, K.G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005, 91, 131–137. [Google Scholar] [CrossRef]
- Tarchoune, I.; Sgherri, C.; Izzo, R.; Lachaal, M.; Ouerghi, Z.; Navari-Izzo, F. Antioxidative responses of Ocimum basilicum to sodium chloride or sodium sulphate salinization. Plant Physiol. Biochem. 2010, 48, 772–777. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Bogucka-Kocka, A.; Kowalski, R.; Borowski, B. Changes in the chemical composition of the essential oil of sweet basil (Ocimum basilicum L.) depending on the plant growth stage. Chemia 2012, 23, 216–222. [Google Scholar]
- Nowak, J.S. Air-water properties of horticultural substrates. Zesz. Probl. Post. Nauk Rol. 2005, 504, 175–184. [Google Scholar]
- Jadczak, D.; Bojko, K.; Berova, M.; Kaymakanova, M. Effect of salinity stress on growth and photosynthetic activity of common basil plants (Ocimum basilicum L.). J. Cent. Eur. Agric. 2021, 22, 546–556. [Google Scholar] [CrossRef]
- Stępień, P.; Kłbus, G. Water relations and photosynthesis in Cucumis sativus L. leaves under salt stress. Biol. Plant. 2006, 50, 610–616. [Google Scholar] [CrossRef]
- Ahl, S.A.; Meawad, A.A.; Abou-Zeid, E.N.; Ali, M.S. Response of different basil varieties to soil salinity. Int. Agrophys. 2010, 24, 183–188. [Google Scholar]
- Artiola, J.F.; Walworth, J.L.; Musil, S.A.; Crimmins, M.A. Soil and land pollution. In Environmental and Pollution Science, 3rd ed.; Academic Press: London, UK, 2019; pp. 219–235. [Google Scholar]
- Kaymakanova, M.; Stoeva, N. Physiological reaction of bean plants (Phaseolus vulg. L.) to salt stress. Gen. Appl. Plant Physiol. Spec. 2008, 34, 3–4. [Google Scholar]
- Tarchoune, I.; Degl’Innocenti, E.; Kaddour, R.; Guidi, L.; Lachaâl, M.; Navari-Izzo, F.; Ouerghi, Z. Effects of NaCl or Na2SO4 salinity on plant growth, ion content and photosynthetic activity in Ocimum basilicum L. Acta Physiol. Plant. 2012, 34, 607–615. [Google Scholar] [CrossRef]
- Shannon, M.C. Whole-plant response to salinity. In Plant Environment Interactions; Wilkinson, R.E., Ed.; Marcel Dekker: New York, NY, USA, 1994; pp. 199–244. [Google Scholar]
- Bernstein, N.; Kravchik, M.; Dudai, N. Salinity-induced changes in essential oil, pigments and salts accumulation in sweet basil (Ocimum basilicum) in relation to alterations of morphological development. Ann. Appl. Biol. 2010, 156, 167–177. [Google Scholar] [CrossRef]
- Does Alcohol Make Water Evaporate Faster? Alcoholic Drinks Notes. Available online: www.magicsnewbiewines.com (accessed on 22 July 2022).
- Does Salt Water Evaporate? Techiescientist. Available online: https://www.techiescientist.com/does-salt-water-evaporate (accessed on 22 July 2022).
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Jadczak, D.; Błaszczuk, A.; Rekowska, E. Effect of covering on the content of macroelements in yield of (Ociumum basilicum L.) cultivated for bunch harvest. J. Elem. 2006, 11, 135–141. [Google Scholar]
- Majkowska-Gadomska, J.; Kulczycka, A.; Mikulewicz, E.; Dobrowolski, A. Phytometric features of basil grown in pot with fifferent subsoil volume. Acta Agrophys. 2017, 24, 279–288. [Google Scholar]
- Menezes, R.V.; Azevedo Neto, A.D.; Gheyi, H.G.; Cova, A.M.W.; Silva, H.H.B. Tolerance of basil genotypes to salinity. J. Agric. Sci. 2017, 9, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Meneguzzo, S.; Navam-Izzo, F.; Izzo, R. Antioxidative responses of shoots and roots of wheat to increasing NaCI concentrations. J. Plant Physiol. 1999, 155, 274–280. [Google Scholar] [CrossRef]
- Mittova, V.; Volokita, M.; Guy, M.; Tal, M. Activities of SOD and the ascorbate-glutathione cycle enzymes in subcellular compartments in leaves and roots of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol. Plant. 2000, 110, 42–51. [Google Scholar] [CrossRef]
- Delavari, P.M.; Baghizadeh, A.; Enteshari, S.H.; Kalantari, K.M.; Yazdanpanah, A.; Mousavi, E.A. The effects of salicylic acid on some of the biochemical and morphological characteristic of Ocimum basilicum under salinity stress. Aust. J. Basic Appl. Sci. 2010, 4, 4832–4845. [Google Scholar]
- Golpayegani, A.; Tilebeni, H.G. Effect of biological fertilizers on biochemical and physiological parameters of basil (Ocimum basilicum L.) medicine plant. Am.-Eurasian J. Agric. Environ. Sci. 2011, 11, 445–450. [Google Scholar]
- Amuthavalli, P.; Sivasankaramoorthy, S. Effect of salt stress on the growth and photosynthetic pigments of pigeon pea (Cajanus cajan). J. Appl. Pharm. Sci. 2012, 2, 131–133. [Google Scholar] [CrossRef]
- Hernandez, J.A.; Jiménez, A.; Mullineaux, P.; Sevilia, F. Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ. 2000, 23, 853–862. [Google Scholar] [CrossRef]
- Demiral, T.; Türkan, I. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ. Exp. Bot. 2005, 53, 247–257. [Google Scholar] [CrossRef]
- Mandhania, S.; Madan, S.; Sawhney, V. Antioxidant defense mechanism under salt stress in wheat seedlings. Biol. Plant. 2006, 50, 227–231. [Google Scholar] [CrossRef]
- Khan, M.H.; Panda, S.K. Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiol. Plant. 2008, 30, 81–89. [Google Scholar] [CrossRef]
- Kalisz, A.; Pokluda, R.; Jezdinsky, A.; Sękara, A.; Grabowska, A.; Gil, J.; Neugebauerová, J. Chilling-induced changes in the antioxidant status of basil plants. Acta Physiol. Plant. 2016, 38, 196. [Google Scholar] [CrossRef]
- Khaliq, S.; Ullah, Z.; Rehman, A.; Khaliq, R. Physiological and biochemical basis of salt tolerance in Ocimun basilicum L. J. Med. Plants Stud. 2014, 2, 18–27. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. In Circular. California Agricultural Experiment Station, 2nd ed.; University of Michigan Library: Ann Arbor, MI, USA, 1950; Volume 347, pp. 1–32. [Google Scholar]
- De Vos, C.R.; Schat, H.; Vooijs, R.; Ernst, W.H. Copper-induced damage to the permeability barrier in roots of Silene cucubalus. J. Plant Physiol. 1989, 135, 164–169. [Google Scholar] [CrossRef]
- Mocquot, B.; Vangronsveld, J.; Clijsters, H.; Mench, M. Copper toxicity in young maize (Zea mays L.) plants: Effects on growth, mineral and chlorophyll contents, and enzyme activities. Plant Soil 1996, 182, 287–300. [Google Scholar] [CrossRef]
- Bergmeyer, H.U. Methods of Enzymatic Analysis; Verlag Chemie: Weinheim, Germany, 1974; Volume 2. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Blackwell Scientific Publications: Oxford, UK, 1994; pp. 73–99. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Beta, T.; Naing, S.K.; Nam, S.; Mpofu, A.; Therrien, M. Antioxidant activity in relationship to phenolic content of diverse food barley genotypes. In Antioxidant Measurement and Applications; Shahidi, F., Ho, C., Eds.; American Chemical Society: Washington DC, USA, 2007; pp. 242–254. [Google Scholar]
- Nacheva, L.; Dimitrova, N.; Koleva-Valkova, L.; Tarakanov, I.; Vassilev, A. Effect of led light on the growth of raspberry (Rubus idaeus L.) plant in vitro. Agric. Sci. 2021, 13, 126–140. [Google Scholar]
- Koleva, L.; Petrov, V.; Zlatev, Z. Guide for Exercises in Plant Biochemistry; Academic Publishing House of the Agricultural University: Plovdiv, Bulgaria, 2016; p. 135. [Google Scholar]
- Eyidogan, F.; Tufan, O. Effect of salinity on antioxidant responses of chickpea seedlings. Acta Physiol. Plant. 2007, 29, 485–493. [Google Scholar] [CrossRef]
- Rachoski, M.; Gazquez, A.; Calzadilla, P.; Bezus, R.; Rodriguez, A.; Ruiz, O.; Menendez Santiago Maiale, A. Chlorophyll fluorescence and lipid peroxidation changes in rice somaclonal lines subjected to salt stress. Acta Physiol. Plant. 2015, 37, 37–117. [Google Scholar] [CrossRef]
- Ning, J.F.; Cui, L.H.; Yang, S.H.; Ai, S.Y.; Li, M.J.; Sun, L.L.; Chen, Z.; Wang, R.H.; Zeng, Z.B. Basil ionic responses to seawater stress and the identification of gland salt secretion. J. Anim. Plant Sci. 2015, 25, 131–138. [Google Scholar]
- McCaskill, D.; Croteau, R. Strategies for bioengineering the development and metabolism of glandular tissues in plants. Nat. Biotechnol. 1999, 17, 31–36. [Google Scholar] [CrossRef]
- Choi, Y.E.; Harada, E.; Kim, G.H.; Yoon, E.S.; Sano, H. Distribution of elements on tobacco trichomes and leaves under cadmium and sodium stresses. J. Plant Biol. 2004, 47, 75–82. [Google Scholar] [CrossRef]
- Attia, H.; Ouhibi, C.; Ellili, A.; Msilini, N.; Bouzaïen, G.; Karray, N.; Lachaâl, M. Analysis of salinity effects on basil leaf surface area, photosynthetic activity, and growth. Acta Physiol. Plant. 2011, 33, 823–833. [Google Scholar] [CrossRef]
- Bączek-Kwinta, R.; Serek, B.; Wator, A.; Hura, K. Porównanie aktywności antyoksydacyjnej odmian bazylii mierzonej różnymi metodami. Zesz. Probl. Postępów Nauk. Rol. 2009, 1, 539. Available online: https://www.infona.pl/resource/bwmeta1.element.dl-catalog-66fd27ab-6534-4c25-b0d4-bb11997ec8a3 (accessed on 22 July 2022).
- Mansour, M.M.F. Nitrogen containing compounds and adaptation of plants to salinity stress. Biol. Plant. 2000, 43, 491–500. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pitchel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Sairam, R.K.; Tyagi, A. Physiology and molecular biology of salinity stress tolerance in plants. Curr. Sci. 2004, 86, 407–421. [Google Scholar]
- Hassanein, R.A.; Bassuony, F.M.; Baraka, D.M.; Khalil, R.R. Physiological effects of nicotinamide and ascorbic acid on Zea mays plant grown under salinity stress. 1-changes in growth, some relevant metabolic activities and oxidative defense systems. Res. J. Agric. Biol. Sci. 2009, 5, 72–81. [Google Scholar]
- Hatano, T.; Ediamatsu, R.; Mori, A.; Fujita, Y.; Yasuhara, T.; Yoshida, T.; Okuda, T. Effects of the interaction of tannins with co-existing substances. VI. Effects of tannins and related polyphenols on superoxide anion radical, and on 1,1-diphenylpierylhydrazyl radical. Chem. Pharm. Bull. 1989, 37, 2016–2021. [Google Scholar] [CrossRef]
- Silva, J.C.; Rodrigues, S.; Feas, X.; Estevinho, L.M. Antimicrobial activity, phenolic profile and role in the inflammation of propolis. Food Chem. Toxicol. 2012, 50, 1790–1795. [Google Scholar] [CrossRef]

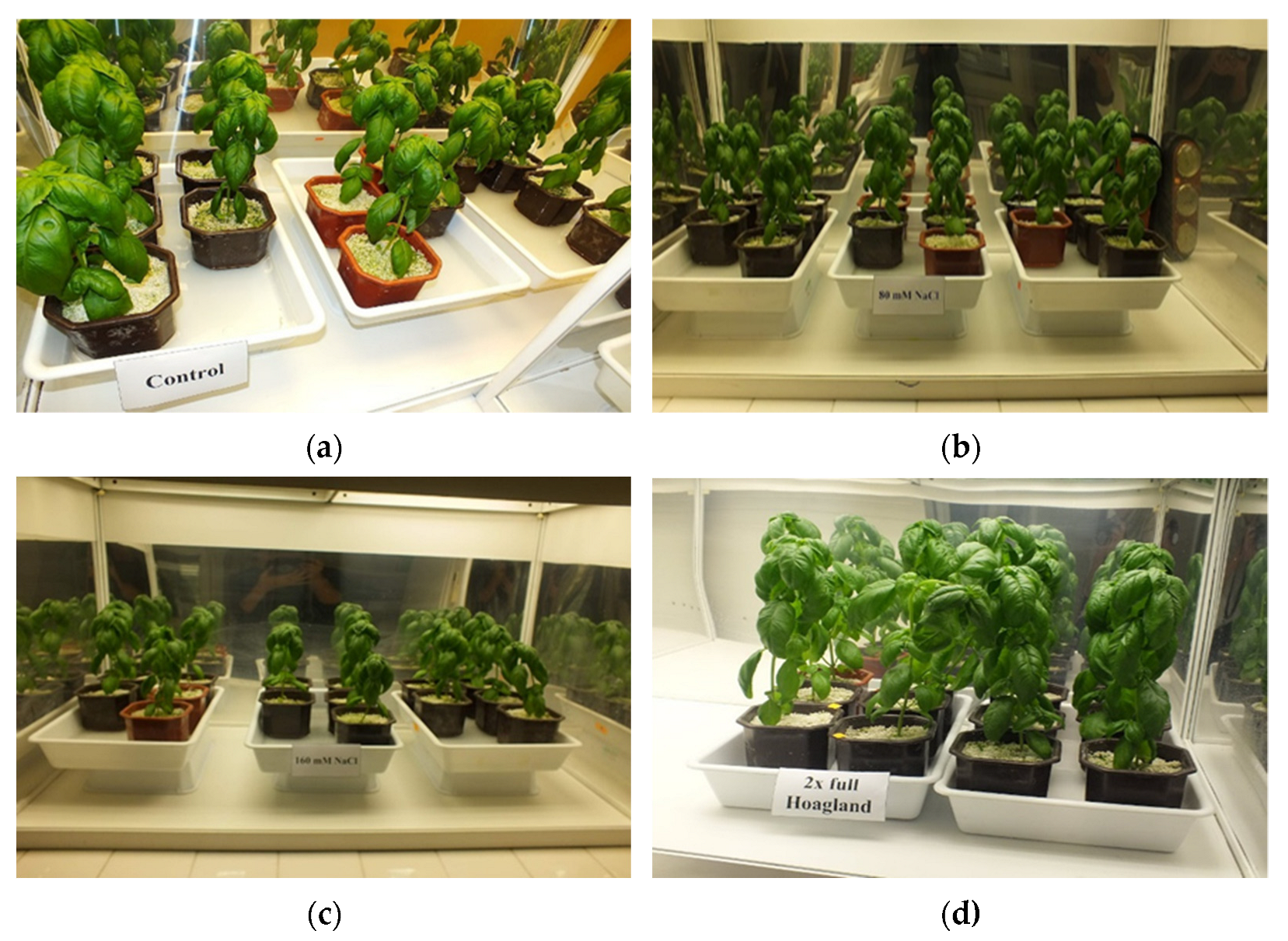
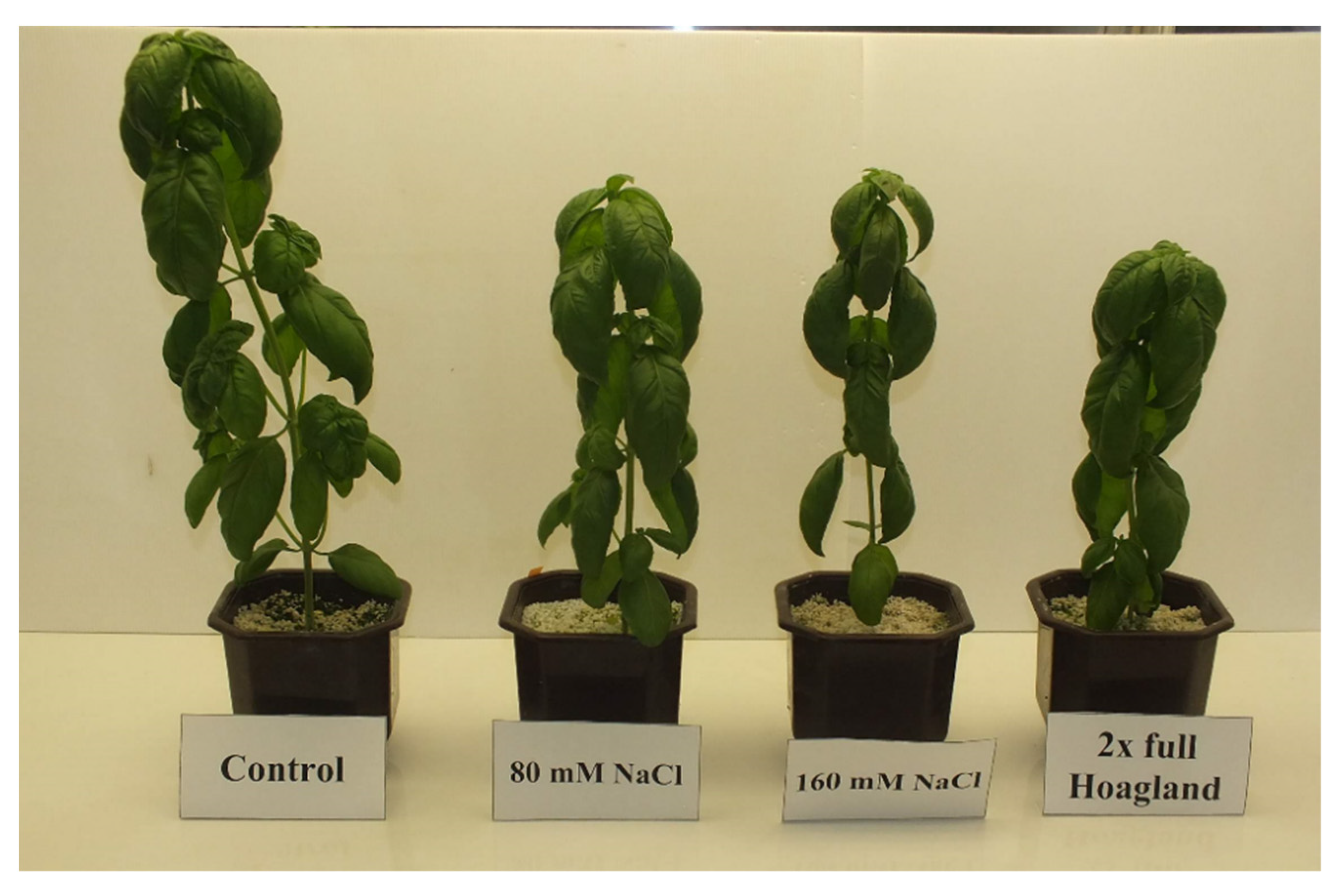
| Salt Concentration (A) | The Morphological Part of the Plant (B) | Malondialdehyde | GPOD | Proline |
|---|---|---|---|---|
| Control (½ Hoagland’s solution with 0 mM NaCl) | Leaves | 0.13 ± 0.01 | 0.03 ± 0.01 | 24.97 ± 0.10 |
| Roots | 0.23 ± 0.01 | 1.32 ± 0.34 | 32.50 ± 5.54 | |
| Average for Control | 0.18 ± 0.00 | 0.67 ± 0.18 | 28.74 ± 2.82 | |
| ½ Hoagland’s solution with NaCl (80 mM) | Leaves | 0.17 ± 0.05 | 0.11 ± 0.01 | 38.38 ± 1.49 |
| Roots | 0.25 ± 0.05 | 1.38 ± 0.62 | 68.04 ± 0.06 | |
| Average for ½ Hoagland’s solution with NaCl (80 mM) | 0.21 ± 0.00 | 0.74 ± 0.32 | 53.21 ± 0.72 | |
| ½ Hoagland’s solution with NaCl (160 mM) | Leaves | 0.42 ± 0.04 | 3.76 ± 0.76 | 110.81±0.01 |
| Roots | 0.22 ± 0.01 | 0.10 ± 0.03 | 73.31 ± 3.20 | |
| Average for ½ Hoagland’s solution with NaCl (160 mM) | 0.32 ± 0.02 | 1.93 ± 0.37 | 92.06 ± 1.61 | |
| 4/2 Hoagland’s solution with 0 mM NaCl | Leaves | 0.17 ± 0.01 | 0.06 ± 0.03 | 40.33 ± 0.47 |
| Roots | 0.23 ± 0.13 | 0.88 ± 0.14 | 72.68 ± 4.94 | |
| Average for 4/2 Hoagland’s solution with 0 mM NaCl | 0.20 ± 0.06 | 0.47± 0.08 | 56.51 ± 2.70 | |
| Average for: | Leaves | 0.22 ± 0.04 | 0.99 ± 0.01 | 53.62 ± 0.16 |
| Roots | 0.23 ± 0.02 | 0.92 ± 0.01 | 61.63 ± 3.05 | |
| HSDα=0,05 for: | A | 0.025 | 0.931 | 6.079 |
| B | 0.027 | 0.470 | 3.071 | |
| Interaction | B/A | 0.038 | 0.940 | 6.141 |
| A/B | 0.038 | 1.316 | 8.597 | |
| Salt Concentration (A) | The Morphological Part of the Plant (B) | Total Polyphenols | Reducing Sugars | Antiradical Activity |
|---|---|---|---|---|
| Control (½ Hoagland’s solution with 0 mM NaCl) | Leaves | 487.93 ± 44.68 | 0.54 ± 0.020 | 26.10 ± 1.72 |
| Roots | 504.63 ± 89.21 | 0.46 ± 0.100 | 27.56 ± 5.32 | |
| Average for Control | 496.28 ± 22.27 | 0.50 ± 0.035 | 26.83 ± 3.52 | |
| ½ Hoagland’s solution with NaCl (80 mM) | Leaves | 424.60 ± 40.03 | 0.53 ± 0.010 | 26.34 ± 2.28 |
| Roots | 498.69 ± 84.43 | 0.54 ± 0.350 | 24.87 ± 4.01 | |
| Average for ½ Hoagland’s solution with NaCl (80 mM) | 461.64 ± 62.23 | 0.54 ± 0.045 | 25.60 ± 3.15 | |
| ½ Hoagland’s solution with NaCl (160 mM) | Leaves | 402.51 ± 29.85 | 0.53 ± 0.010 | 20.81 ± 2.38 |
| Roots | 469.99 ± 29.85 | 0.63 ± 0.035 | 21.13 ± 1.50 | |
| Average for ½ Hoagland’s solution with NaCl (160 mM) | 436.25 ± 5.01 | 0.58 ± 0.050 | 20.97± 1.94 | |
| 4/2 Hoagland’s solution with 0 mM NaCl | Leaves | 525.39 ± 59.44 | 0.70 ± 0.200 | 28.12 ± 2.51 |
| Roots | 498.90 ± 12.42 | 0.47 ± 0.080 | 25.12 ± 2.23 | |
| Average for 4/2 Hoagland’s solution with 0 mM NaCl | 462.14 ± 23.51 | 0.59 ± 0.085 | 26.62 ± 2.37 | |
| Average for: | Leaves | 435.11 ± 13.78 | 0.58 ± 0.050 | 25.34 ± 2.22 |
| Roots | 443.05 ± 3.05 | 0.53 ± 0.080 | 24.67 ± 3.27 | |
| HSDα=0.05 for: | A | 135.397 | 0.028 | 3.003 |
| B | n.s. | 0.015 | n.s. | |
| Interaction | B/A | n.s. | 0.029 | n.s. |
| A/B | n.s. | 0.040 | n.s. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadczak, D.; Bojko, K.; Kaymakanova, M.; Berova, M. Salinity-Induced Changes in the Antioxidant Status of Common Basil Plants (Ocimum basilicum L.) Grown under Controlled Conditions. Horticulturae 2022, 8, 775. https://doi.org/10.3390/horticulturae8090775
Jadczak D, Bojko K, Kaymakanova M, Berova M. Salinity-Induced Changes in the Antioxidant Status of Common Basil Plants (Ocimum basilicum L.) Grown under Controlled Conditions. Horticulturae. 2022; 8(9):775. https://doi.org/10.3390/horticulturae8090775
Chicago/Turabian StyleJadczak, Dorota, Kamila Bojko, Miroslava Kaymakanova, and Malgozhata Berova. 2022. "Salinity-Induced Changes in the Antioxidant Status of Common Basil Plants (Ocimum basilicum L.) Grown under Controlled Conditions" Horticulturae 8, no. 9: 775. https://doi.org/10.3390/horticulturae8090775
APA StyleJadczak, D., Bojko, K., Kaymakanova, M., & Berova, M. (2022). Salinity-Induced Changes in the Antioxidant Status of Common Basil Plants (Ocimum basilicum L.) Grown under Controlled Conditions. Horticulturae, 8(9), 775. https://doi.org/10.3390/horticulturae8090775






