The Alleviation Effects of Biostimulants Application on Lettuce Plants Grown under Deficit Irrigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Biostimulant Treatments and Experimental Design
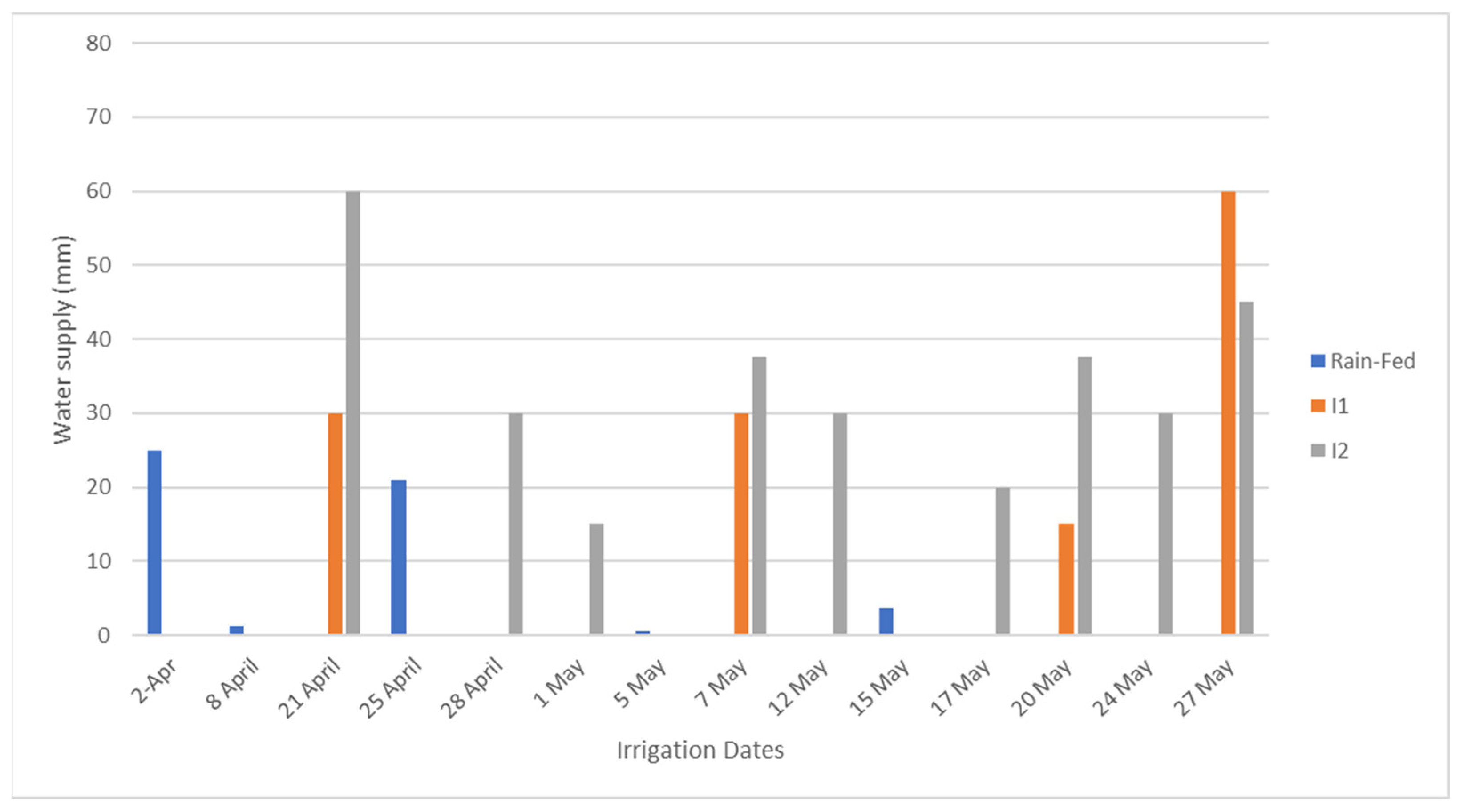
2.2. Irrigation Treatments
2.3. Plant Growth and Crop Perfomance Determination
2.4. Chemical Analyses
2.5. Statistical Analysis
3. Results and Discussion
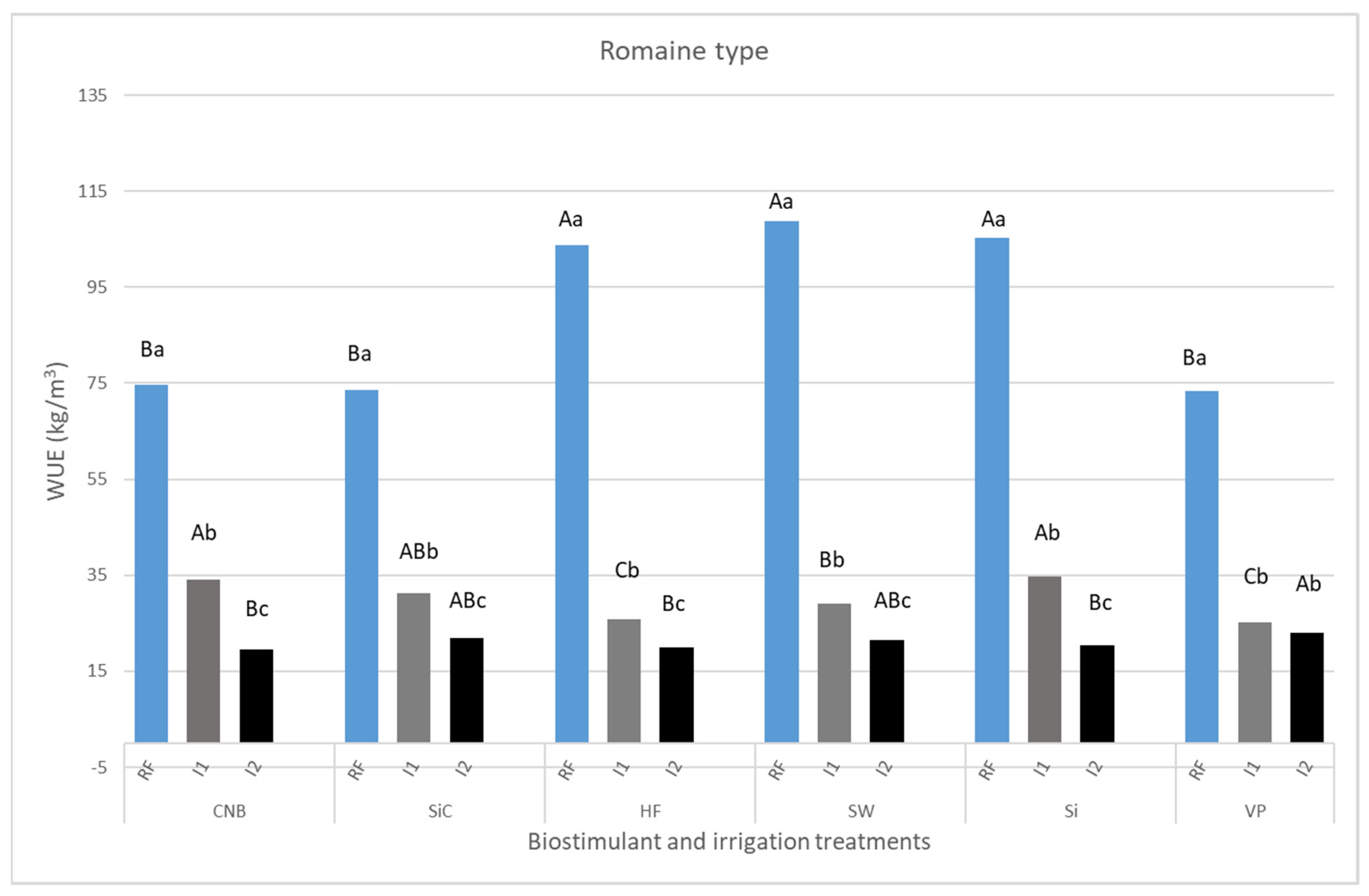
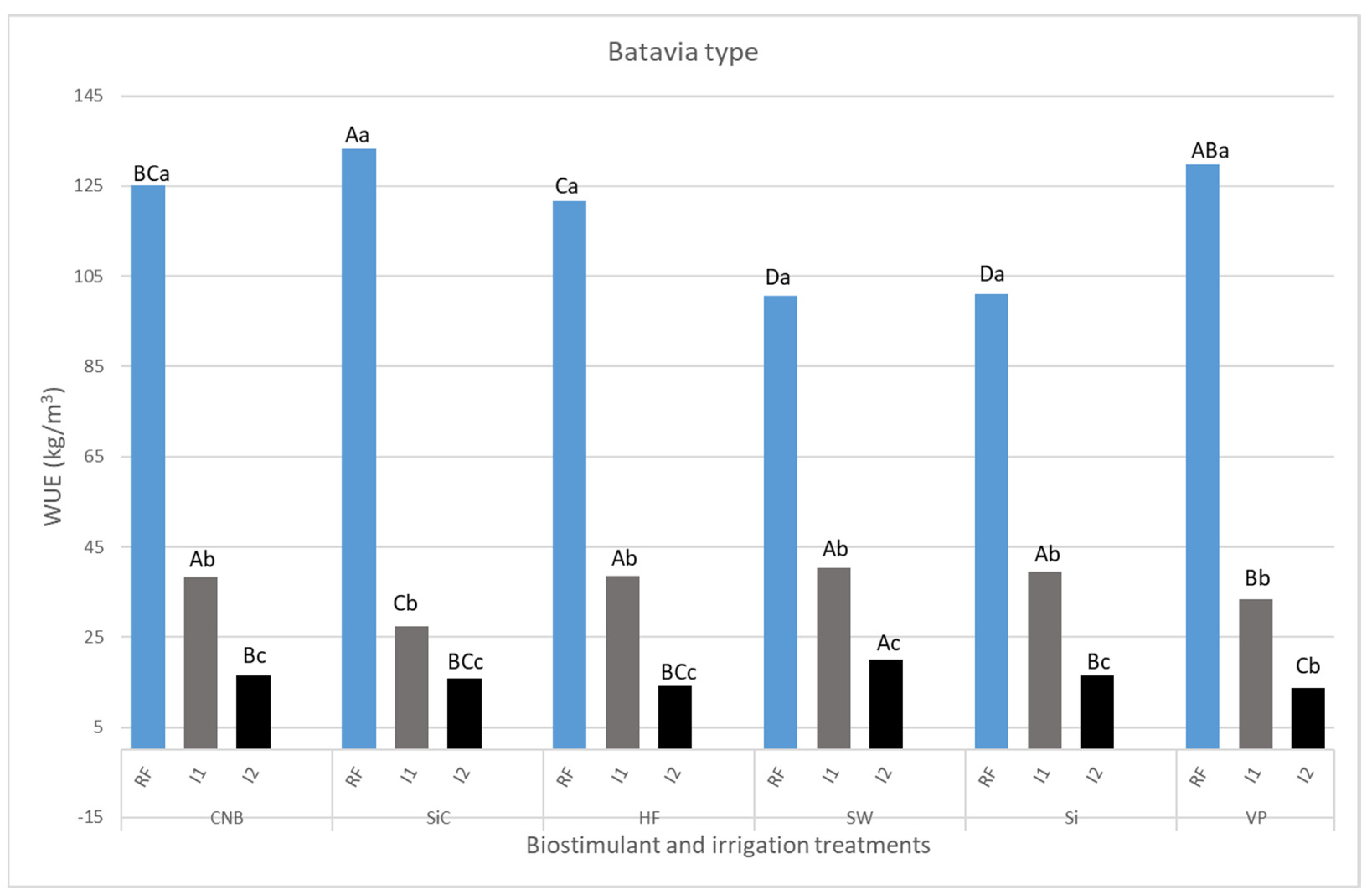
3.1. Plant Biomass and Growth Parameters
3.2. Chemical Composition of Leaves
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pour-Aboughadareh, A.; Omidi, M.; Naghavi, M.R.; Etminan, A.; Mehrabi, A.A.; Poczai, P.; Bayat, H. Effect of water deficit stress on seedling biomass and physio-chemical characteristics in different species of wheat possessing the D genome. Agronomy 2019, 9, 522. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, R.; Chitarra, W.; Antoniou, C.; Ruocco, M.; Fotopoulos, V. Improvement of plant performance under water deficit with the employment of biological and chemical priming agents. J. Agric. Sci. 2018, 156, 680–688. [Google Scholar] [CrossRef]
- Rockström, J.; Williams, J.; Daily, G.; Noble, A.; Matthews, N.; Gordon, L.; Wetterstrand, H.; DeClerck, F.; Shah, M.; Steduto, P.; et al. Sustainable intensification of agriculture for human prosperity and global sustainability. Ambio 2017, 46, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Borsato, E.; Rosa, L.; Marinello, F.; Tarolli, P.; D’Odorico, P. Weak and Strong Sustainability of Irrigation: A Framework for Irrigation Practices Under Limited Water Availability. Front. Sustain. Food Syst. 2020, 4, 17. [Google Scholar] [CrossRef]
- Yu, N.; Zhang, J.; Liu, P.; Zhao, B.; Ren, B. Integrated agronomic practices management improved grain formation and regulated endogenous hormone balance in summer maize (Zea mays L.). J. Integr. Agric. 2020, 19, 1768–1776. [Google Scholar] [CrossRef]
- Kuyah, S.; Sileshi, G.W.; Nkurunziza, L.; Chirinda, N.; Ndayisaba, P.C.; Dimobe, K.; Öborn, I. Innovative agronomic practices for sustainable intensification in sub-Saharan Africa. A review. Agron. Sustain. Dev. 2021, 41, 16. [Google Scholar] [CrossRef]
- Pradipta, A.; Soupios, P.; Kourgialas, N.; Doula, M.; Dokou, Z.; Makkawi, M.; Alfarhan, M.; Tawabini, B.; Kirmizakis, P.; Yassin, M. Remote Sensing, Geophysics, and Modeling to Support Precision Agriculture—Part 2: Irrigation Management. Water 2022, 14, 1157. [Google Scholar] [CrossRef]
- Le Roux, B.; van der Laan, M.; Gush, M.B.; Bristow, K.L. Comparing the usefulness and applicability of different water footprint methodologies for sustainable water management in agriculture. Irrig. Drain. 2018, 67, 790–799. [Google Scholar] [CrossRef]
- Liu, Y.; Song, W. Modelling crop yield, water consumption, and water use efficiency for sustainable agroecosystem management. J. Clean. Prod. 2020, 253, 119940. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Fila, G.; Zeinalipour, N.; Badeck, F.W.; Delshad, M.; Ghashghaie, J. Application of water-saving treatments reveals different adaptation strategies in three Iranian melon genotypes. Sci. Hortic. 2019, 256, 108518. [Google Scholar] [CrossRef]
- Hidalgo-Santiago, L.; Navarro-León, E.; López-Moreno, F.J.; Arjó, G.; González, L.M.; Ruiz, J.M.; Blasco, B. The application of the silicon-based biostimulant Codasil® offset water deficit of lettuce plants. Sci. Hortic. 2021, 285, 110177. [Google Scholar] [CrossRef]
- Singh, M.; Singh, P.; Singh, S.; Saini, R.K.; Angadi, S.V. A global meta-analysis of yield and water productivity responses of vegetables to deficit irrigation. Sci. Rep. 2021, 11, 22095. [Google Scholar] [CrossRef]
- Dalal, A.; Bourstein, R.; Haish, N.; Shenhar, I.; Wallach, R.; Moshelion, M. A High-Throughput Physiological Functional Phenotyping System for Time- and Cost-Effective Screening of Potential Biostimulants. bioRxiv 2019. [Google Scholar] [CrossRef]
- Bhupenchandra, I.; Chongtham, S.K.; Devi, E.L.; Ramesh, R.; Choudhary, A.K.; Salam, M.D.; Sahoo, M.R.; Bhutia, T.L.; Devi, S.H.; Thounaojam, A.S. Role of biostimulants in mitigating the effects of climate change on crop performance. Front. Plant Sci. 2022, 13, 967665. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F. Facing climate change: Application of microbial biostimulants to mitigate stress in horticultural crops. Agronomy 2020, 10, 794. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants Application: A Low Input Cropping Management Tool for Sustainable Farming of Vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef]
- Petropoulos, S.A. Practical applications of plant biostimulants in greenhouse vegetable crop production. Agronomy 2020, 10, 1569. [Google Scholar] [CrossRef]
- Srivastava, N. Biostimulants for plant abiotic stress tolerance. In Biostimulants for Crop Production and Sustainable Agriculture; CABI: Wallingford, UK, 2022. [Google Scholar]
- Rouphael, Y.; Carillo, P.; Garcia-Perez, P.; Cardarelli, M.; Senizza, B.; Miras-Moreno, B.; Colla, G.; Lucini, L. Plant biostimulants from seaweeds or vegetal proteins enhance the salinity tolerance in greenhouse lettuce by modulating plant metabolism in a distinctive manner. Sci. Hortic. 2022, 305, 111368. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Taofiq, O.; Fernandes, Â.; Tzortzakis, N.; Ciric, A.; Sokovic, M.; Barros, L.; Ferreira, I.C.F.R. Bioactive properties of greenhouse-cultivated green beans (Phaseolus vulgaris L.) under biostimulants and water-stress effect. J. Sci. Food Agric. 2019, 99, 6049–6059. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, C. Management of abiotic stress in horticultural crops: Spotlight on biostimulants. Agronomy 2020, 10, 1514. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R. Biostimulants application alleviates water stress effects on yield and chemical composition of greenhouse green bean (Phaseolus vulgaris L.). Agronomy 2020, 10, 181. [Google Scholar] [CrossRef]
- Vetrano, F.; Miceli, C.; Angileri, V.; Frangipane, B.; Moncada, A.; Miceli, A. Effect of bacterial inoculum and fertigation management on nursery and field production of lettuce Plants. Agronomy 2020, 10, 1477. [Google Scholar] [CrossRef]
- Singh, M.; Saini, R.K.; Singh, S.; Sharma, S.P. Potential of Integrating Biochar and Deficit Irrigation Strategies for Sustaining Vegetable Production in Water-limited Regions: A review. HortScience 2019, 54, 1872–1878. [Google Scholar] [CrossRef]
- Mampholo, B.M.; Maboko, M.M.; Soundy, P.; Sivakumar, D. Phytochemicals and overall quality of leafy lettuce (Lactuca sativa L.) varieties grown in closed hydroponic system. J. Food Qual. 2016, 39, 805–815. [Google Scholar] [CrossRef]
- Qin, X.X.; Zhang, M.Y.; Han, Y.Y.; Hao, J.H.; Liu, C.J.; Fan, S.X. Beneficial phytochemicals with anti-tumor potential revealed through metabolic profiling of new red pigmented lettuces (Lactuca sativa L.). Int. J. Mol. Sci. 2018, 19, 1165. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2017, 234, 463–469. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Online Database. Available online: https://www.fao.org/faostat/en/#home (accessed on 24 August 2022).
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Yang, Y.; Campbell, J.E. Improving attributional life cycle assessment for decision support: The case of local food in sustainable design. J. Clean. Prod. 2017, 145, 361–366. [Google Scholar] [CrossRef]
- Jiménez-Arias, D.; García-Machado, F.J.; Morales-Sierra, S.; Luis, J.C.; Suarez, E.; Hernández, M.; Valdés, F.; Borges, A.A. Lettuce plants treated with L-pyroglutamic acid increase yield under water deficit stress. Environ. Exp. Bot. 2019, 158, 215–222. [Google Scholar] [CrossRef]
- Chaski, C.; Petropoulos, S.A. The Effects of Biostimulant Application on Growth Parameters of Lettuce Plants Grown under Deficit Irrigation Conditions. Biol. Life Sci. Forum 2022, 16, 4. [Google Scholar] [CrossRef]
- De Pascale, S.; Costa, L.D.; Vallone, S.; Barbieri, C.; Maggio, A. Increasing Water Use Efficiency in Vegetable Crop Production: From Plant to Irrigation Systems Efficiency. Horttechnology 2011, 21, 301–308. [Google Scholar] [CrossRef]
- Bates, L.S. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Alexopoulos, A.A.; Marandos, E.; Assimakopoulou, A.; Vidalis, N.; Petropoulos, S.A.; Karapanos, I.C. Effect of Nutrient Solution pH on the Growth, Yield and Quality of Taraxacum officinale and Reichardia picroides in a Floating Hydroponic System. Agronomy 2021, 11, 1118. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Izzeldin, H.; Lippert, L.F.; Takatori, F.H. An Influence of Water Stress at Different Growth Stages on Yield and Quality of Lettuce Seed. J. Am. Soc. Hortic. Sci. 1980, 105, 68–71. [Google Scholar] [CrossRef]
- Rosental, L.; Still, D.W.; You, Y.; Hayes, R.J.; Simko, I. Mapping and identification of genetic loci affecting earliness of bolting and flowering in lettuce. Theor. Appl. Genet. 2021, 134, 3319–3337. [Google Scholar] [CrossRef]
- Rouphael, Y.; Spíchal, L.; Panzarová, K.; Casa, R.; Colla, G. High-throughput plant phenotyping for developing novel biostimulants: From lab to field or from field to lab? Front. Plant Sci. 2018, 9, 1197. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; Colla, G.; Mori, M. Effect of Vegetal- and Seaweed Extract-Based Biostimulants on Agronomical and Leaf Quality Traits of Plastic Tunnel-Grown Baby Lettuce under Four Regimes of Nitrogen Fertilization. Agronomy 2019, 9, 571. [Google Scholar] [CrossRef]
- Tsouvaltzis, P.; Kasampali, D.S.; Aktsoglou, D.C.; Barbayiannis, N.; Siomos, A.S. Effect of reduced nitrogen and supplemented amino acids nutrient solution on the nutritional quality of baby green and red lettuce grown in a floating system. Agronomy 2020, 10, 922. [Google Scholar] [CrossRef]
- Bulgari, R.; Trivellini, A.; Ferrante, A. Effects of two doses of organic extract-based biostimulant on greenhouse lettuce grown under increasing NaCl concentrations. Front. Plant Sci. 2019, 9, 1870. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Cozzolino, E.; Ottaiano, L.; Petropoulos, S.A.; Nocerino, S.; Pelosi, M.E.; Rouphael, Y.; Mori, M.; Mola, I. Di Effect of Biostimulant Application on Plant Growth, Chlorophylls and Hydrophilic Antioxidant Activity of Spinach (Spinacia oleracea L.) Grown under Saline Stress. Horticulturae 2022, 8, 971. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant-and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Abdipour, M.; Hosseinifarahi, M.; Najafian, S. Effects of Humic Acid and Cow Manure Biochar (CMB) in Culture Medium on Growth and Mineral Concentrations of Basil Plant. Int. J. Hortic. Sci. Technol. 2019, 6, 27–38. [Google Scholar] [CrossRef]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Giordano, M.; El-Nakhel, C.; Cuciniello, A.; Cenvinzo, V.; Colla, G.; Rouphael, Y. Protein Hydrolysate or Plant Extract-based Biostimulants Enhanced Yield and Quality Performances of Greenhouse Perennial Wall Rocket Grown in Different Seasons. Plants 2019, 8, 208. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Monaghan, J.M.; Vickers, L.H.; Grove, I.G.; Beacham, A.M. Deficit irrigation reduces postharvest rib pinking in wholehead Iceberg lettuce, but at the expense of head fresh weight. J. Sci. Food Agric. 2017, 97, 1524–1528. [Google Scholar] [CrossRef]
- Malejane, D.N.; Tinyani, P.; Soundy, P.; Sultanbawa, Y.; Sivakumar, D. Deficit irrigation improves phenolic content and antioxidant activity in leafy lettuce varieties. Food Sci. Nutr. 2018, 6, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Olorunwa, O.J.; Wilson, J.C.; Barickman, T.C. Morphological and Physiological Response of Different Lettuce Genotypes to Salt Stress. Stresses 2021, 1, 285–304. [Google Scholar] [CrossRef]
- Malécange, M.; Pérez-Garcia, M.D.; Citerne, S.; Sergheraert, R.; Lalande, J.; Teulat, B.; Mounier, E.; Sakr, S.; Lothier, J. Leafamine®, a Free Amino Acid-Rich Biostimulant, Promotes Growth Performance of Deficit-Irrigated Lettuce. Int. J. Mol. Sci. 2022, 23, 7338. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, Q.; Liu, Q.; Zhang, W.; Ding, R. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, O.L.; Calderín, A.; Huelva, R.; Martínez-Balmori, D.; Guridi, F.; Aguiar, N.O.; Olivares, F.L.; Canellas, L.P. Humic substances from vermicompost enhance urban lettuce production. Agron. Sustain. Dev. 2015, 35, 225–232. [Google Scholar] [CrossRef]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous application of amino acids improves the growth and yield of lettuce by enhancing photosynthetic assimilation and nutrient availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef]
- Kopta, T.; Pavlíková, M.; Sȩkara, A.; Pokluda, R.; Maršálek, B. Effect of bacterial-algal biostimulant on the yield and internal quality of Lettuce (Lactuca sativa L.) produced for spring and summer crop. Not. Bot. Hortic. Agrobot. 2018, 46, 615–621. [Google Scholar] [CrossRef]
- Corrado, G.; Vitaglione, P.; Giordano, M.; Raimondi, G.; Napolitano, F.; Di Stasio, E.; Di Mola, I.; Mori, M.; Rouphael, Y. Phytochemical responses to salt stress in red and green baby leaf lettuce (Lactuca sativa L.) varieties grown in a floating hydroponic module. Separations 2021, 8, 175. [Google Scholar] [CrossRef]
- Di Stasio, E.; Rouphael, Y.; Colla, G.; Raimondi, G.; Giordano, M.; Pannico, A.; Pannico, A.; El-Nakhel, C.; de Pascale, S. The influence of Ecklonia maxima seaweed extract on growth, photosynthetic activity and mineral composition of Brassica rapa L. subsp. sylvestris under nutrient stress conditions. Eur. J. Hortic. Sci. 2017, 82, 286–293. [Google Scholar] [CrossRef]
- Rouphael, Y.; De Micco, V.; Arena, C.; Raimondi, G.; Colla, G.; Pascale, S. De Effect of Ecklonia maxima seaweed extract on yield, mineral composition, gas exchange, and leaf anatomy of zucchini squash grown under saline conditions. J. Appl. Phycol. 2017, 29, 459–470. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Anastasiou, M.; Pantelides, I.; Tzortzakis, N. Effects of Ascophyllum nodosum seaweed extracts on lettuce growth, physiology and fresh-cut salad storage under potassium deficiency. J. Sci. Food Agric. 2018, 98, 5861–5872. [Google Scholar] [CrossRef] [PubMed]
- Bonasia, A.; Conversa, G.; Lazzizera, C.; Elia, A. Foliar application of protein hydrolysates on baby-leaf spinach grown at different n levels. Agronomy 2022, 12, 36. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; El-Nakhel, C.; Leone, V.; Mori, M. Effect of seaweed (Ecklonia maxima) extract and legume-derived protein hydrolysate biostimulants on baby leaf lettuce grown on optimal doses of nitrogen under greenhouse conditions. Aust. J. Crop Sci. 2020, 14, 1456–1464. [Google Scholar] [CrossRef]
- Asgharipour, M.R.; Mosapour, H. A foliar application silicon enchances drought tolerance in fennel. J. Anim. Plant Sci. 2016, 26, 1056–1062. [Google Scholar]
- Guimarães, I.T.; De Assis Oliveira, F.; Leal, C.C.P.; De Lima Souza, M.W.; Alves, T.R.C. Foliar application of biofertilizer in semi-hydroponic lettuce fertigated with saline nutrient solution. Comun. Sci. 2020, 11, e3115. [Google Scholar] [CrossRef]
- Kuslu, Y.; Dursun, A.; Sahin, U.; Kiziloglu, F.M.; Turan, M. Short communication. Effect of deficit irrigation on curly lettuce grown under semiarid conditions. Span. J. Agric. Res. 2008, 6, 714–719. [Google Scholar] [CrossRef]
- Saia, S.; Colla, G.; Raimondi, G.; Di Stasio, E.; Cardarelli, M.; Bonini, P.; Vitaglione, P.; De Pascale, S.; Rouphael, Y. An endophytic fungi-based biostimulant modulated lettuce yield, physiological and functional quality responses to both moderate and severe water limitation. Sci. Hortic. 2019, 256, 108595. [Google Scholar] [CrossRef]
- Lin, F.W.; Lin, K.H.; Chang, Y.S.; Lin, K.H.; Wu, C.W. Effects of betaine and chitin on water use efficiency in lettuce (Lactuca sativa var. capitata). HortScience 2020, 55, 89–95. [Google Scholar] [CrossRef]
- Taha, R.S.; Alharby, H.F.; Bamagoos, A.A.; Medani, R.A.; Rady, M.M. Elevating tolerance of drought stress in Ocimum basilicum using pollen grains extract; a natural biostimulant by regulation of plant performance and antioxidant defense system. S. Afr. J. Bot. 2020, 128, 42–53. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Sandhu, D.; Liu, X.; Halvorson, J.J. Spinach (Spinacea oleracea L.) response to salinity: Nutritional value, physiological parameters, antioxidant capacity, and gene expression. Agriculture 2018, 8, 163. [Google Scholar] [CrossRef]
- Pokluda, R.; Sękara, A.; Jezdinský, A.; Kalisz, A.; Neugebauerová, J.; Grabowska, A. The physiological status and stress biomarker concentration of Coriandrum sativum L. plants subjected to chilling are modified by biostimulant application. Biol. Agric. Hortic. 2016, 32, 258–268. [Google Scholar] [CrossRef]
- Balestrini, R.; Brunetti, C.; Chitarra, W.; Nerva, L. Photosynthetic Traits and Nitrogen Uptake in Crops: Which Is the Role of Arbuscular Mycorrhizal Fungi? Plants 2020, 9, 1105. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Choi, S.; Colla, G.; Cardarelli, M.; Kim, H.J. Effects of Plant-Derived Protein Hydrolysates on Yield, Quality, and Nitrogen Use Efficiency of Greenhouse Grown Lettuce and Tomato. Agronomy 2022, 12, 18. [Google Scholar] [CrossRef]
- Abu-Shahba, M.S.; Mansour, M.M.; Mohamed, H.I.; Sofy, M.R. Comparative Cultivation and Biochemical Analysis of Iceberg Lettuce Grown in Sand Soil and Hydroponics with or without Microbubbles and Macrobubbles. J. Soil Sci. Plant Nutr. 2021, 21, 389–403. [Google Scholar] [CrossRef]
- Pereira, C.; Dias, M.I.; Petropoulos, S.A.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Calhelha, R.C.; Ivanov, M.; Stojković, D.; Soković, M.; et al. The effects of biostimulants, biofertilizers and water-stress on nutritional value and chemical composition of two spinach genotypes (Spinacia oleracea L.). Molecules 2019, 24, 4494. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Levizou, E.; Ntatsi, G.; Fernandes, Â.; Petrotos, K.; Akoumianakis, K.; Barros, L.; Ferreira, I. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017, 214, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Ferrante, A. Effect of glutamic acid foliar applications on lettuce under water stress. Physiol. Mol. Biol. Plants 2021, 27, 1059–1072. [Google Scholar] [CrossRef]
- Yavuz, D.; Kılıç, E.; Seymen, M.; Dal, Y.; Kayak, N.; Kal, Ü.; Yavuz, N. The effect of irrigation water salinity on the morph-physiological and biochemical properties of spinach under deficit irrigation conditions. Sci. Hortic. 2022, 304, 111272. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; Connell, S.O. Plant Physiology and Biochemistry Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Luziatelli, F.; Ficca, A.G.; Colla, G.; Svecova, E.; Ruzzi, M. Effects of a protein hydrolysate-based biostimulant and two micronutrient based fertilizers on plant growth and epiphytic bacterial population of lettuce. Acta Hortic. 2016, 1148, 43–48. [Google Scholar] [CrossRef]
- Johnson, M.P.; Havaux, M.; Triantaphylidès, C.; Ksas, B.; Pascal, A.A.; Robert, B.; Davison, P.A.; Ruban, A.V.; Horton, P. Elevated zeaxanthin bound to oligomeric LHCII enhances the resistance of Arabidopsis to photooxidative stress by a lipid-protective, antioxidant mechanism. J. Biol. Chem. 2007, 282, 22605–22618. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Islam, M.T.; Oba, S. Salinity stress accelerates nutrients, dietary fiber, minerals, phytochemicals and antioxidant activity in Amaranthus tricolor leaves. PLoS ONE 2018, 13, e0206388. [Google Scholar] [CrossRef]
- Singh, M.; Tiwari, N. Microbial amelioration of salinity stress in HD 2967 wheat cultivar by up-regulating antioxidant defense. Commun. Integr. Biol. 2021, 14, 136–150. [Google Scholar] [CrossRef] [PubMed]

| Biostimulants | Irrigation | Romaine | Batavia |
|---|---|---|---|
| RF | 28.7 ± 3.1 Aab | 19.1 ± 2.6 Bcd | |
| CNB | I1 | 28.3 ± 3.7 Aab | 23.1 ± 3.1 Ab |
| I2 | 26.9 ± 3.1 Bbc | 25.9 ± 5.8 Aab | |
| RF | 29.3 ± 1.3 Aab | 19.2 ± 3.0 Bcd | |
| SiC | I1 | 24.0 ± 2.2 Bc | 20.5 ± 2.3 Bcd |
| I2 | 24.4 ± 2.5 Bc | 26.8 ± 4.0 Aa | |
| RF | 28.8 ± 2.1 Aab | 22.7 ± 2.5 Bbc | |
| HF | I1 | 28.1 ± 2.6 Aab | 20.1 ± 4.8 Bcd |
| I2 | 26.0 ± 2.8 Bbc | 28.5 ± 4.3 Aa | |
| RF | 27.7 ± 3.0 Abc | 21.2 ± 3.4 Abc | |
| SW | I1 | 26.8 ± 2.4 Abbc | 19.3 ± 1.9 Acd |
| I2 | 25.2 ± 2.7 Bc | 20.9 ± 3.8 Acd | |
| RF | 24.7 ± 1.4 Bc | 17.9 ± 2.5 Bd | |
| Si | I1 | 30.1 ± 3.1 Aa | 22.7 ± 4.0 Abc |
| I2 | 24.9 ± 2.5 Bc | 24.3 ± 3.1 Ab | |
| RF | 27.6 ± 2.9 Abc | 18.8 ± 2.3 Bcd | |
| VP | I1 | 28.1 ± 2.2 Aab | 19.9 ± 3.5 Bcd |
| I2 | 25.7 ± 1.9 Bbc | 23.9 ± 3.6 Ab |
| Biostimulant | Irrigation Treatment | Romaine | Batavia |
|---|---|---|---|
| CNB | RF | 26.7 ± 1.5 Ab | 24.8 ± 1.6 Aa |
| I1 | 28.1 ± 2.1 Aab | 17.4 ± 3.2 Bc | |
| I2 | 19.5 ± 1.6 Bc | 15.5 ± 3.2 Bd | |
| SiC | RF | 31.3 ± 1.2 Aab | 18.4 ± 3.6 Ac |
| I1 | 28.9 ± 1.5 Bab | 17.1 ± 2.3 Ac | |
| I2 | 20.5 ± 1.5 Cc | 17.2 ± 2.6 Ac | |
| HF | RF | 31.2 ± 1.8 Aab | 17.9 ± 3.5 Bc |
| I1 | 25.4 ± 1.0 Bb | 21.3 ± 4.3 Ab | |
| I2 | 24.6 ± 1.0 Bb | 14.5 ± 2.9 Cd | |
| SW | RF | 27.3 ± 1.2 Bab | 24.3 ± 7.0 Aa |
| I1 | 33.3 ± 1.2 Aa | 18.4 ± 2.2 Bab | |
| I2 | 19.0 ± 1.0 Cc | 15.5 ± 2.8 Cd | |
| Si | RF | 29.5 ± 1.5 Aab | 24.0 ± 4.0 Aa |
| I1 | 29.9 ± 1.0 Aab | 15.3 ± 2.9 Bd | |
| I2 | 19.7 ± 1.3 Bc | 15.6 ± 4.5 Bd | |
| VP | RF | 31.9 ± 1.8 Aab | 25.7 ± 4.3 Aa |
| I1 | 26.5 ± 1.3 Bb | 19.8 ± 4.2 Bbc | |
| I2 | 14.9 ± 2.0 Cd | 14.8 ± 2.7 Cd |
| Biostimulant | Irrigation Treatment | Plant Weight (g) | Number of Leaves | Weight of Leaves (g) | Leaf Area (cm2) | Dry Weight (%) | Specific Leaf Area (m2/kg) |
|---|---|---|---|---|---|---|---|
| RF | 402.7 ± 12.0 Bb | 36 ± 1 Be | 298.5 ± 7.1 Bc | 5905.4 ± 173.6 Bb | 8.3 ± 3.9 Aa | 26.8 ± 1.2 Cd | |
| CNB | I1 | 437.4 ± 10.6 Ab | 42 ± 1.4 Ac | 362.4 ± 6.9 Ab | 6647.6 ± 108.3 Ab | 5.0 ± 0.3 Bc | 36.6 ± 1.5 Ba |
| I2 | 363.1 ± 18.3 Cb | 36.8 ± 1.6 Bc | 284.8 ± 5.9 Bb | 5209.1 ± 134.9 Cb | 3.8 ± 0.8 Cb | 51.1 ± 1.6 Aa | |
| RF | 429.1 ± 12.8 Aa | 43.6 ± 1.3 Bb | 346.6 ± 18.5 Aa | 5997.0 ± 129.7 Ab | 7.4 ± 0.7 Aab | 23.9 ± 2.6 Ce | |
| SiC | I1 | 312.9 ± 11.0 Cd | 44 ± 1.8 Ab | 257.8 ± 13.9 Cd | 4630.9 ± 198.6 Be | 6.9 ± 0.6 Ba | 27.8 ± 2.9 Bc |
| I2 | 348.1 ± 8.1 Bc | 36.2 ± 1.3 Cc | 280.4 ± 14.7 Bb | 4808.8 ± 109.0 Bc | 5.6 ± 0.5 Ca | 32.1 ± 1.9 Ad | |
| RF | 392.1 ± 10.4 Bc | 45.4 ± 1.6 Aa | 322.5 ± 9.2 Bb | 6375.5 ± 120.8 Aa | 6.6 ± 0.6 Ab | 31.0 ± 1.0 Bb | |
| HF | I1 | 438.9 ± 14.2 Ab | 37.6 ± 1.0 Ce | 355.5 ± 12.4 Ab | 6472.7 ± 193.1 Ac | 6.2 ± 0.4 Bb | 30.0 ± 1.6 Bb |
| I2 | 311.5 ± 8.4 Cd | 42 ± 1.8 Ba | 253.0 ± 8.7 Cc | 4813.7 ± 163.3 Bc | 5.5 ± 0.5 Ca | 35.3 ± 2.0 Ac | |
| RF | 323.6 ± 18.8 Cd | 41.2 ± 2.2 Ac | 260.4 ± 12.9 Bd | 5176.5 ± 198.0 Be | 6.9 ± 1.4 Ab | 29.5 ± 1.2 Bc | |
| SW | I1 | 460.5 ± 10.4 Aa | 42.6 ± 1.9 Ac | 379.3 ± 8.0 Aa | 6928.8 ± 147.6 Aa | 6.4 ± 0.7 Bab | 28.8 ± 1.9 Bbc |
| I2 | 440.1 ± 14.4 Ba | 37.2 ± 1.6 Bc | 362.8 ± 7.5 Aa | 6718.7 ± 146.3 Aa | 4.2 ± 0.5 Cb | 44.5 ± 1.9 Ab | |
| RF | 325.4 ± 11.2 Cd | 43.2 ± 1.8 Bb | 267.5 ± 6.4 Bd | 5392.1 ± 118.0 Bd | 8.1 ± 1.7 Aa | 25.8 ± 1.9 Cd | |
| Si | I1 | 451.2 ± 12.8 Aa | 46.8 ± 1.0 Aa | 357.3 ± 7.3 Ab | 6542.8 ± 109.4 Abc | 6.2 ± 0.7 Bb | 30.3 ± 1.8 Bb |
| I2 | 361.3 ± 11.8 Bb | 40.4 ± 1.9 Cb | 283.4 ± 5.2 Bb | 5167.4 ± 124.9 Bb | 5.6 ± 0.7 Ca | 33.1 ± 1.7 Ad | |
| RF | 417.9 ± 19.1 Aab | 41.2 ± 1.6 Ac | 324.9 ± 6.7 Ab | 5679.3 ± 109.1 Ac | 4.5 ± 1.7 Cc | 46.8 ± 2.0 Aa | |
| VP | I1 | 381.3 ± 13.8 Bc | 39.6 ± 1.4 Bd | 297.3 ± 9.9 Bc | 5125.4 ± 152.7 Bd | 6.9 ± 0.4 Aa | 25.4 ± 1.5 Cd |
| I2 | 302.7 ± 14.2 Ce | 37.4 ± 1.10 Cc | 245.6 ± 1.0 Cc | 4495.3 ± 105.8 Cd | 5.2 ± 0.6 Ba | 36.1 ± 1.4 Bc |
| Biostimulant | Irrigation Treatment | Plant Weight (g) | Number of Leaves | Weight of Leaves (g) | Leaf Area (cm2) | Dry Weight (%) | Specific Leaf Area (m2/kg) |
|---|---|---|---|---|---|---|---|
| CNB | RF | 240.2 ± 16.9 Cc | 25.4 ± 1.6 Bb | 172.6 ± 12.2 Cc | 3645 ± 168 Cd | 8.4 ± 1.1 Aa | 25.8 ± 2.2 Cd |
| I1 | 388.1 ± 14.4 Ba | 27.6 ± 1.1 Abc | 318.8 ± 10.7 Ba | 6700 ± 113 Bb | 5.5 ± 0.7 Bab | 39.3 ± 1.2 Bd | |
| I2 | 431 ± 24 Ad | 28.0 ± 0.7 Ab | 340 ± 10 Ac | 7025 ± 70 Ac | 4.3 ± 0.4 Ca | 48.5 ± 2.2 Ac | |
| SiC | RF | 236.9 ± 19.7 Cc | 26.4 ± 1.1 Bb | 183.5 ± 16.8 Cc | 3852 ± 139 Cc | 7.6 ± 1.7 Aa | 29.4 ± 8.2 Cc |
| I1 | 358.0 ± 16.4 Bb | 27.2 ± 1.6 Bcd | 278.4 ± 12.3 Bb | 5695 ± 129 Bc | 5.0 ± 0.5 Bb | 41.6 ± 2.0 Bc | |
| I2 | 482.6 ± 13.8 Ab | 30.8 ± 1.6 Aa | 384.6 ± 11.4 Ab | 7479 ± 64 Ab | 3.8 ± 0.6 Cb | 53.1 ± 1.8 Aa | |
| HF | RF | 333.6 ± 13.1 Bb | 29.6 ± 1.2 Aa | 266.2 ± 10.4 Ba | 5065 ± 166 Bb | 6.0 ± 0.6 Abc | 31.8 ± 2.1 Cb |
| I1 | 296.2 ± 12.9 Cd | 28 ± 1.7 Bab | 231.0 ± 14.7 Cd | 5173 ± 206 Bd | 5.9 ± 1.2 Aa | 39.9 ± 1.7 Bcd | |
| I2 | 442.2 ± 13.9 Acd | 29 ± 2 Aab | 336.7 ± 16.0 Ac | 7045 ± 60 Ac | 4.1 ± 0.5 Bab | 51.3 ± 1.5 Ab | |
| SW | RF | 350.1 ± 14.2 Ba | 28.8 ± 1.9 Ba | 262.8 ± 10.2 Ba | 5499 ± 174 Aa | 5.8 ± 0.6 Ac | 36.2 ± 2.3 Ba |
| I1 | 333.1 ± 11.5 Bc | 28.4 ± 1.4 Ba | 252.8 ± 11.0 Bc | 5548 ± 112 Ac | 5.2 ± 0.9 Bbc | 44.1 ± 3.0 Ab | |
| I2 | 472.9 ± 18.1 Ab | 31.6 ± 1.2 Aa | 385.6 ± 12.7 Ab | 5678 ± 154 Ad | 3.7 ± 0.6 Cb | 45.5 ± 1.5 Ad | |
| Si | RF | 338.5 ± 9.1 Cab | 25.6 ± 0.9 Bb | 204.6 ± 12.5 Cb | 3818 ± 103 Bc | 6.7 ± 0.9 Ab | 28.7 ± 1.4 Bc |
| I1 | 396.2 ± 11.6 Ba | 26.4 ± 1.4 Bd | 315.3 ± 9.6 Ba | 7515 ± 254 Aa | 4.6 ± 0.7 Bc | 52.2 ± 2.0 Aa | |
| I2 | 451.3 ± 6.7 Ac | 28.4 ± 0.6 Ab | 342.9 ± 15.6 Ac | 7678 ± 117 Aa | 4.3 ± 0.8 Bab | 54.3 ± 2.1 Aa | |
| VP | RF | 235.8 ± 11.4 Cc | 26.0 ± 1.9 Cb | 187.5 ± 7.9 Cc | 4012 ± 176 Cc | 7.7 ± 2.0 Aa | 30.1 ± 3.6 Cbc |
| I1 | 287.4 ± 11.9 Bd | 28.4 ± 2.1 Ba | 239.3 ± 6.9 Bd | 5062 ± 83 Bd | 5.8 ± 0.4 Ba | 37.5 ± 1.6 Be | |
| I2 | 507.4 ± 14.6 Aa | 30.4 ± 2 Aa | 413.2 ± 10.6 Aa | 7662 ± 177 Aa | 4.5 ± 0.4 Ca | 41.4 ± 1.6 Ae |
| Biostimulant | Irrigation Treatment | Free Proline mg/g f.w. | Chlorophyll a mg/gr f.w. | Chlorophyll b mg/gr f.w. | Total Chlorophylls mg/gr f.w. | Carotenoids mg/gr f.w. |
|---|---|---|---|---|---|---|
| CNB | RF | 3.42 ± 0.005 Ba | 0.016 ± 0.0002 Cc | 0.008 ± 0.0000 Bb | 0.024 ± 0.0002 Bd | 0.021 ± 0.0004 Bd |
| I1 | 4.28 ± 0.02 Ab | 0.023 ± 0.0005 Bc | 0.011 ± 0.0000 Ab | 0.034 ± 0.0005 Ab | 0.030 ± 0.0002 Aa | |
| I2 | 0.50 ± 0.002 Cf | 0.0308 ± 0.0001 Ac | 0.008 ± 0.0004 Bb | 0.039 ± 0.0004 Ab | 0.023 ± 0.0000 Bc | |
| SiC | RF | 2.47 ± 0.01 Ae | 0.023 ± 0.0001 Bb | 0.015 ± 0.0002 Aa | 0.038 ± 0.0001 Bb | 0.028 ± 0.0001 Ab |
| I1 | 1.39 ± 0.003 Cf | 0.035 ± 0.0002 Aa | 0.011 ± 0.0003 Bb | 0.046 ± 0.0001 Aa | 0.032 ± 0.0000 Aa | |
| I2 | 1.65 ± 0.01 Bd | 0.017 ± 0.0001 Ce | 0.005 ± 0.0004 Cc | 0.022 ± 0.0004 Cd | 0.015 ± 0.0001 Bd | |
| HF | RF | 2.74 ± 0.04 Bc | 0.026 ± 0.003 Aab | 0.007 ± 0.0006 Bb | 0.033 ± 0.0030 Bc | 0.024 ± 0.0001 Cc |
| I1 | 2.81 ± 0.007 Bd | 0.027 ± 0.0002 Ab | 0.018 ± 0.0002 Aa | 0.045 ± 0.0004 Aa | 0.029 ± 0.0001 Ba | |
| I2 | 6.29 ± 0.07 Ab | 0.014 ± 0.001 Bf | 0.004 ± 0.0007 Bc | 0.018 ± 0.0011 Ce | 0.039 ± 0.0026 Aa | |
| SW | RF | 2.65 ± 0.009 Ad | 0.013 ± 0.0001 Bd | 0.007 ± 0.0003 Bb | 0.020 ± 0.0002 Bd | 0.015 ± 0.0001 Be |
| I1 | 1.73 ± 0.004 Be | 0.023 ± 0.0004 Ac | 0.012 ± 0.0003 Ab | 0.035 ± 0.0001 Ab | 0.029 ± 0.0001 Aa | |
| I2 | 1.54 ± 0.06 Ce | 0.023 ± 0.0004 Ae | 0.012 ± 0.0003 Aa | 0.035 ± 0.0001 Ac | 0.029 ± 0.0001 Ab | |
| Si | RF | 2.96 ± 0.005 Cb | 0.027 ± 0.0002 Ca | 0.014 ± 0.0004 Aa | 0.042 ± 0.0001 Ba | 0.034 ± 0.0002 Aa |
| I1 | 5.54 ± 0.03 Ba | 0.034 ± 0.0005 Ba | 0.011 ± 0.0016 Ab | 0.045 ± 0.0017 Ba | 0.031 ± 0.0004 Aba | |
| I2 | 8.29 ± 0.03 Aa | 0.040 ± 0.0002 Aa | 0.013 ± 0.0003 Aa | 0.054 ± 0.0004 Aa | 0.028 ± 0.000 Bb | |
| VP | RF | 1.89 ± 0.006 Cf | 0.016 ± 0.0003 Cc | 0.006 ± 0.0003 Ab | 0.021 ± 0.0005 Bd | 0.017 ± 0.0001 B |
| I1 | 3.86 ± 0.01 Ac | 0.028 ± 0.0001 Bb | 0.001 ± 0.0001 Bc | 0.038 ± 0.0001 Ab | 0.024 ± 0.0001 Ab | |
| I2 | 2.04 ± 0.003 Bc | 0.035 ± 0.0036 B A | 0.002 ± 0.0108 Bd | 0.037 ± 0.0260 Abc | 0.022 ± 0.0002 Ac |
| Biostimulant | Irrigation Treatment | Free Proline mg/g f.w. | Chlorophyll a mg/gr f.w. | Chlorophyll b mg/gr f.w. | Total Chlorophylls mg/gr f.w. | Free Proline mg/g f.w. |
|---|---|---|---|---|---|---|
| CNB | RF | 3.02 ± 0.003 Ac | 0.026 ± 0.0004 Bc | 0.007 ± 0.0001 Bc | 0.033 ± 0.0003 Bc | 0.029 ± 0.0002 Bb |
| I1 | 2.69 ± 0.03 Be | 0.025 ± 0.0008 Bd | 0.008 ± 0.0004 Bb | 0.033 ± 0.001 Bd | 0.018 ± 0.0001 Cd | |
| I2 | 2.09 ± 0.006 Cd | 0.037 ± 0.0007 Aa | 0.020 ± 0.0002 Aa | 0.057 ± 0.0005 Aa | 0.042 ± 0.0023 Aa | |
| SiC | RF | 4.17 ± 0.01 Aa | 0.040 ± 0.0003 Aa | 0.019 ± 0.0001 Aa | 0.059 ± 0.0004 Aa | 0.045 ± 0.0001 Aa |
| I1 | 0.71 ± 0.03 Bf | 0.035 ± 0.0009 Bb | 0.008 ± 0.0006 Bb | 0.043 ± 0.001 Bb | 0.012 ± 0.0004 Ce | |
| I2 | 0.63 ± 0.007 Be | 0.015 ± 0.001 Cc | 0.004 ± 0.0003 Cd | 0.019 ± 0.001 Cd | 0.036 ± 0.0001 Bb | |
| HF | RF | 3.72 ± 0.08 Ab | 0.036 ± 0.001 Ab | 0.011 ± 0.0002 Bb | 0.047 ± 0.002 Ab | 0.025 ± 0.0006 Bc |
| I1 | 3.15 ± 0.02 Bc | 0.030 ± 0.0008 Bc | 0.008 ± 0.0002 Bb | 0.037 ± 0.0009 Bc | 0.021 ± 0.0001 Bc | |
| I2 | 2.24 ± 0.01 Cc | 0.031 ± 0.0002 Bb | 0.016 ± 0.0002 Ab | 0.047 ± 0.0001 Ab | 0.032 ± 0.0001 Ac | |
| SW | RF | 2.60 ± 0.006 Bd | 0.019 ± 0.0002 Bd | 0.012 ± 0.0003 Ab | 0.031 ± 0.0002 Bc | 0.025 ± 0.0002 Ac |
| I1 | 2.91 ± 0.003 Ad | 0.020 ± 0.0001 Be | 0.008 ± 0.0002 Ab | 0.028 ± 0.0004 Be | 0.022 ± 0.0001 Ac | |
| I2 | 2.28 ± 0.009 Cc | 0.031 ± 0.002 Ab | 0.009 ± 0.0002 Ac | 0.040 ± 0.002 Ac | 0.025 ± 0.0009 Ad | |
| Si | RF | 2.09 ± 0.02 Ce | 0.012 ± 0.0003 Be | 0.003 ± 0.0002 Bd | 0.015 ± 0.0005 Be | 0.021 ± 0.002 Bd |
| I1 | 5.58 ± 0.18 Aa | 0.059 ± 0.0002 Aa | 0.027 ± 0.0002 Aa | 0.086 ± 0.0004 Aa | 0.055 ± 0.0001 Aa | |
| I2 | 2.58 ± 0.005 Bb | 0.014 ± 0.0001 Bc | 0.007 ± 0.0003 Bc | 0.020 ± 0.0002 Bd | 0.018 ± 0.0001 Be | |
| VP | RF | 2.96 ± 0.02 Cc | 0.021 ± 0.0008 Ad | 0.005 ± 0.0003 Be | 0.026 ± 0.0006 Bd | 0.015 ± 0.0002 Ce |
| I1 | 3.83 ± 0.003 Ab | 0.022 ± 0.0002 Ae | 0.009 ± 0.0002 Ab | 0.031 ± 0.0002 Ad | 0.028 ± 0.0001 Ab | |
| I2 | 3.10 ± 0.02 Ba | 0.009 ± 0.0001 Bd | 0.003 ± 0.0004 Bd | 0.012 ± 0.0005 Ce | 0.023 ± 0.008 Bd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaski, C.; Petropoulos, S.A. The Alleviation Effects of Biostimulants Application on Lettuce Plants Grown under Deficit Irrigation. Horticulturae 2022, 8, 1089. https://doi.org/10.3390/horticulturae8111089
Chaski C, Petropoulos SA. The Alleviation Effects of Biostimulants Application on Lettuce Plants Grown under Deficit Irrigation. Horticulturae. 2022; 8(11):1089. https://doi.org/10.3390/horticulturae8111089
Chicago/Turabian StyleChaski, Christina, and Spyridon A. Petropoulos. 2022. "The Alleviation Effects of Biostimulants Application on Lettuce Plants Grown under Deficit Irrigation" Horticulturae 8, no. 11: 1089. https://doi.org/10.3390/horticulturae8111089
APA StyleChaski, C., & Petropoulos, S. A. (2022). The Alleviation Effects of Biostimulants Application on Lettuce Plants Grown under Deficit Irrigation. Horticulturae, 8(11), 1089. https://doi.org/10.3390/horticulturae8111089






