Application of Rosemary and Eucalyptus Essential Oils on the Preservation of Cucumber Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Plant Material
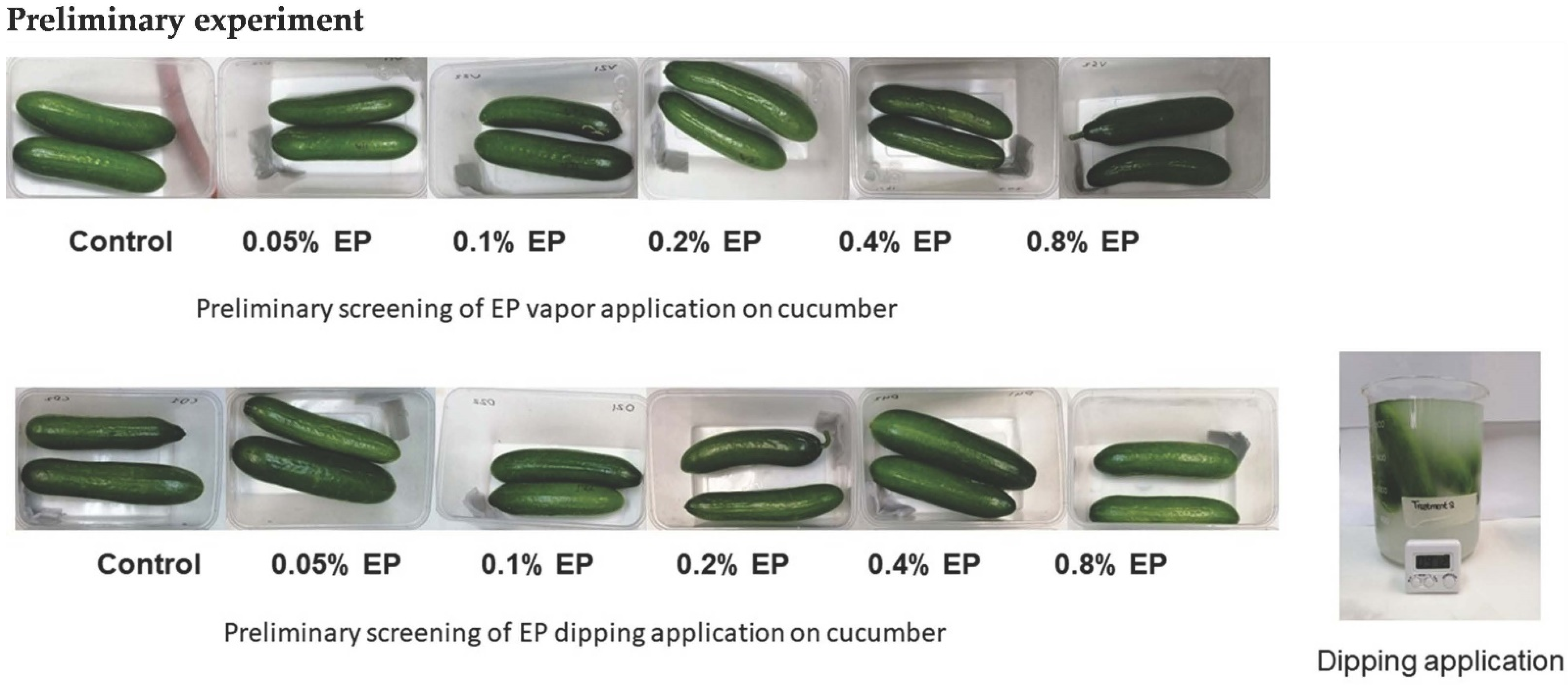
2.2. Preliminary Screening
2.3. Main Experiment
2.3.1. Decay Evaluation
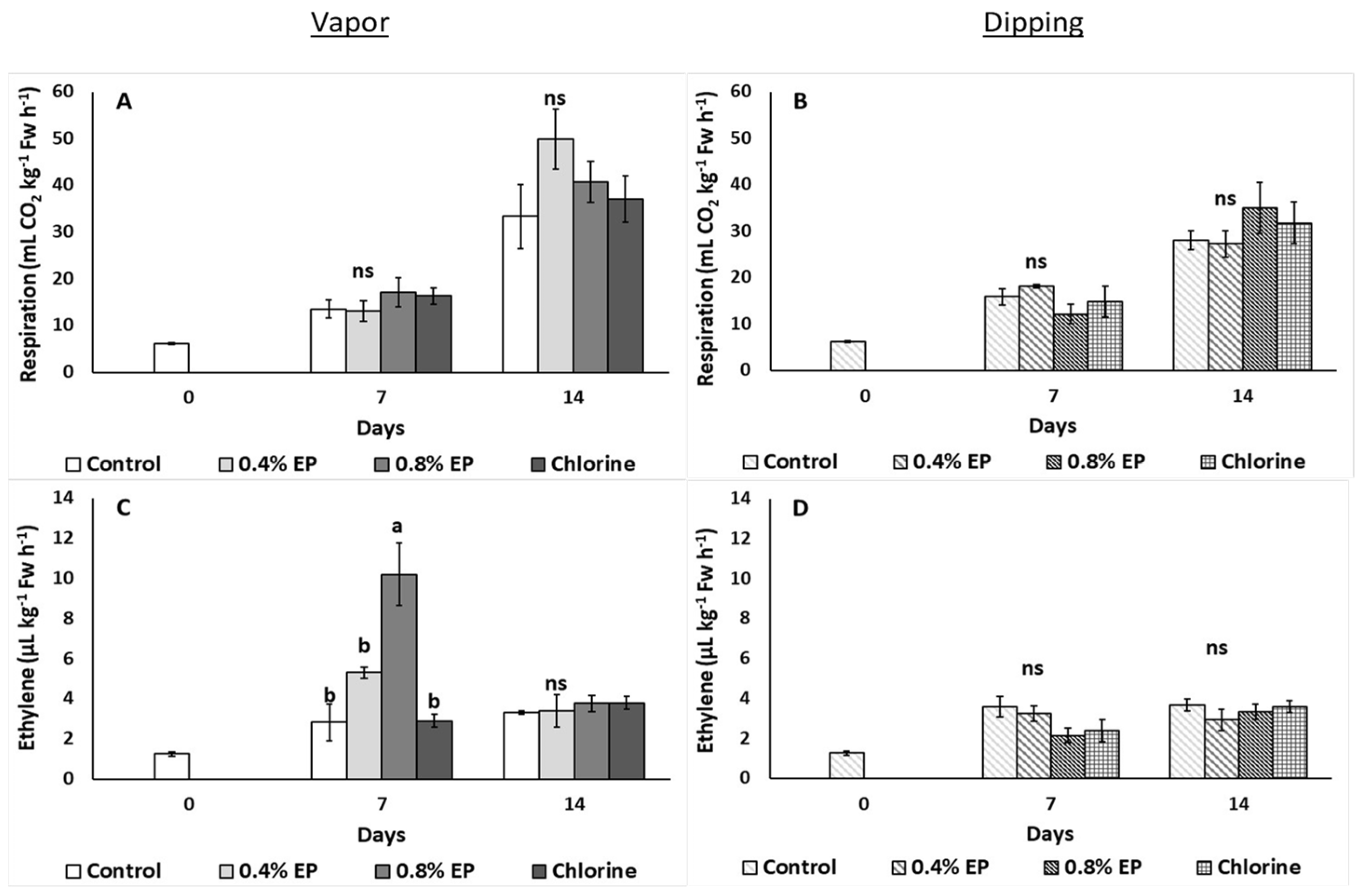
2.3.2. Respiration Rate and Ethylene Emission
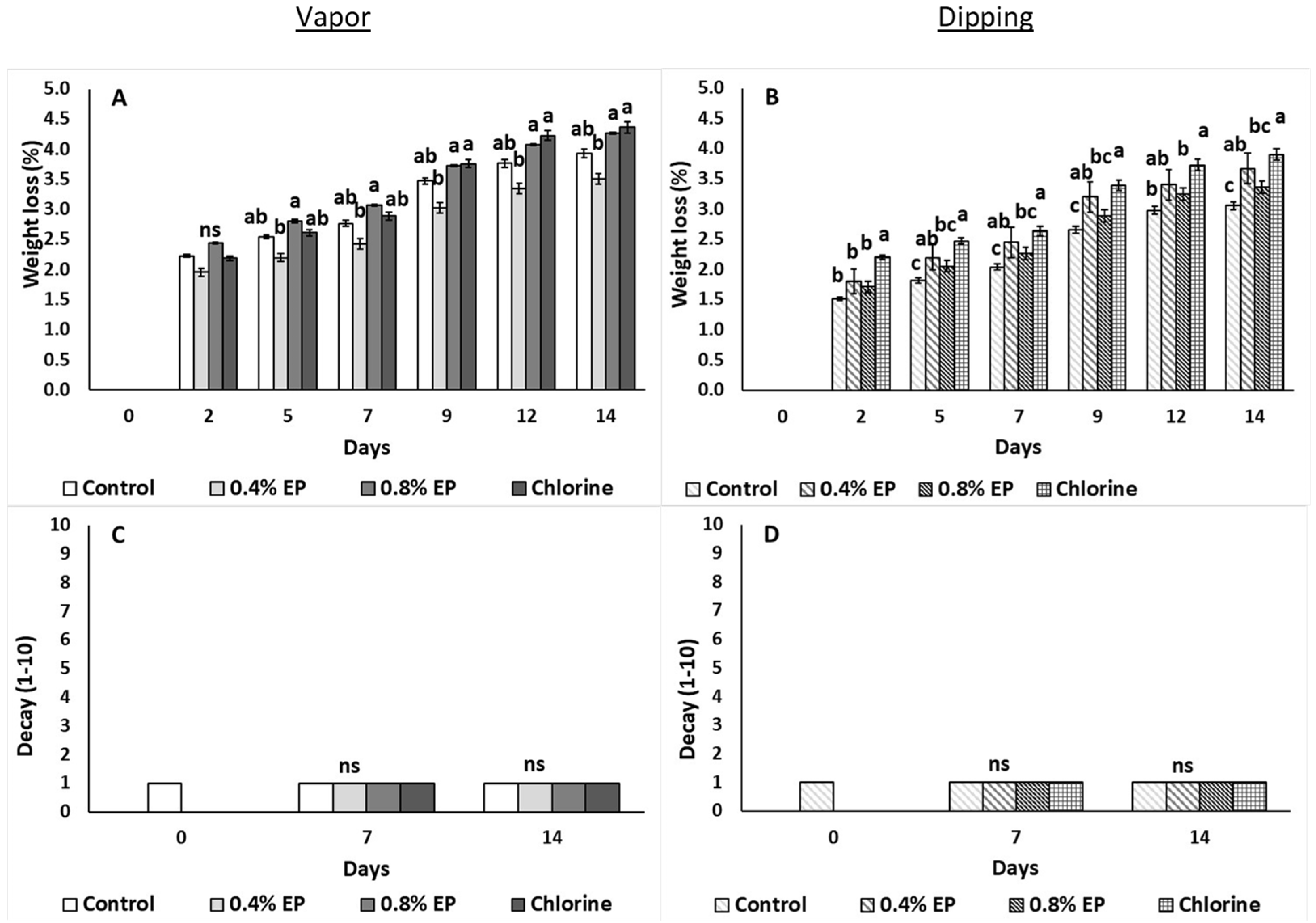
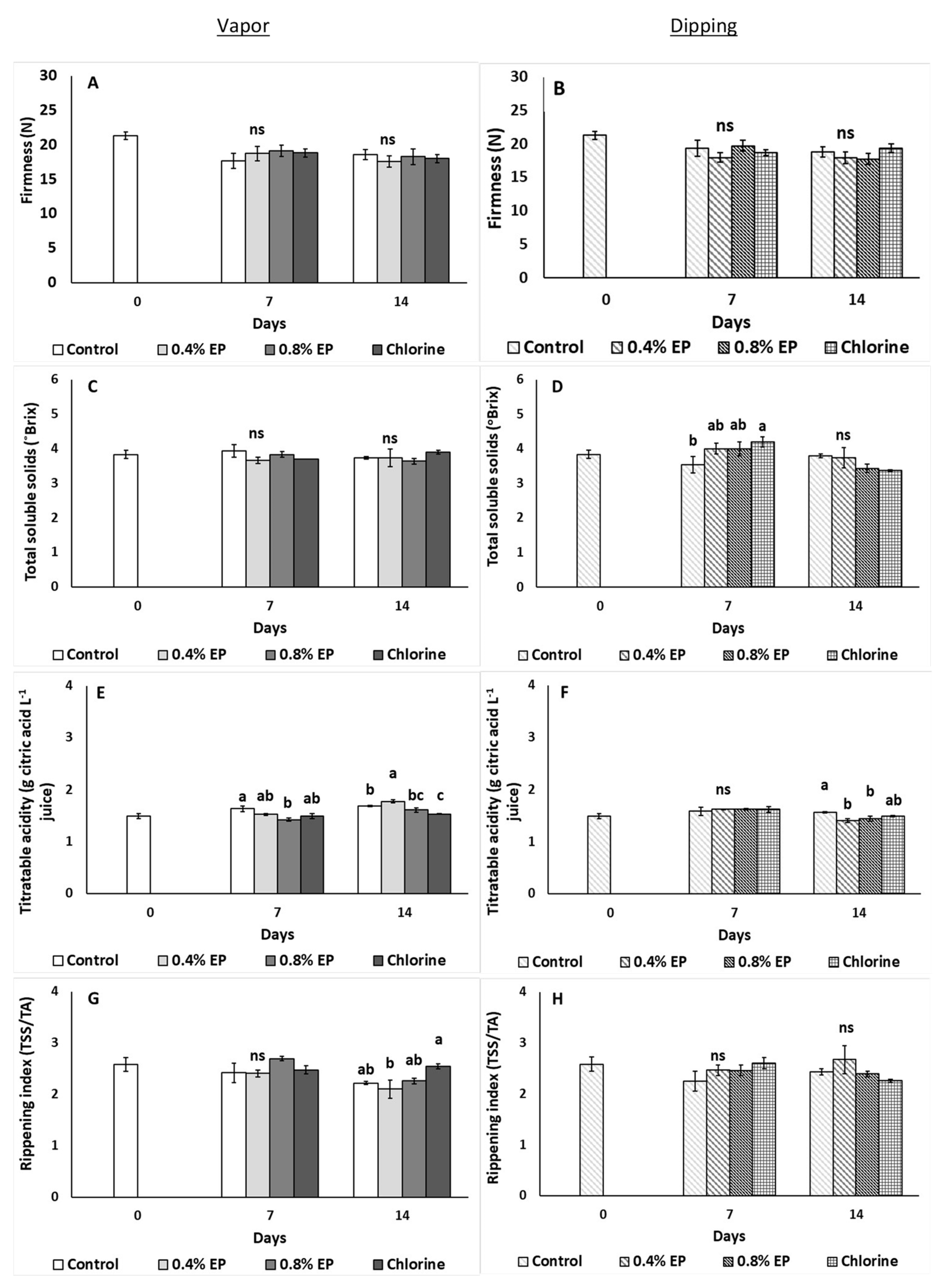
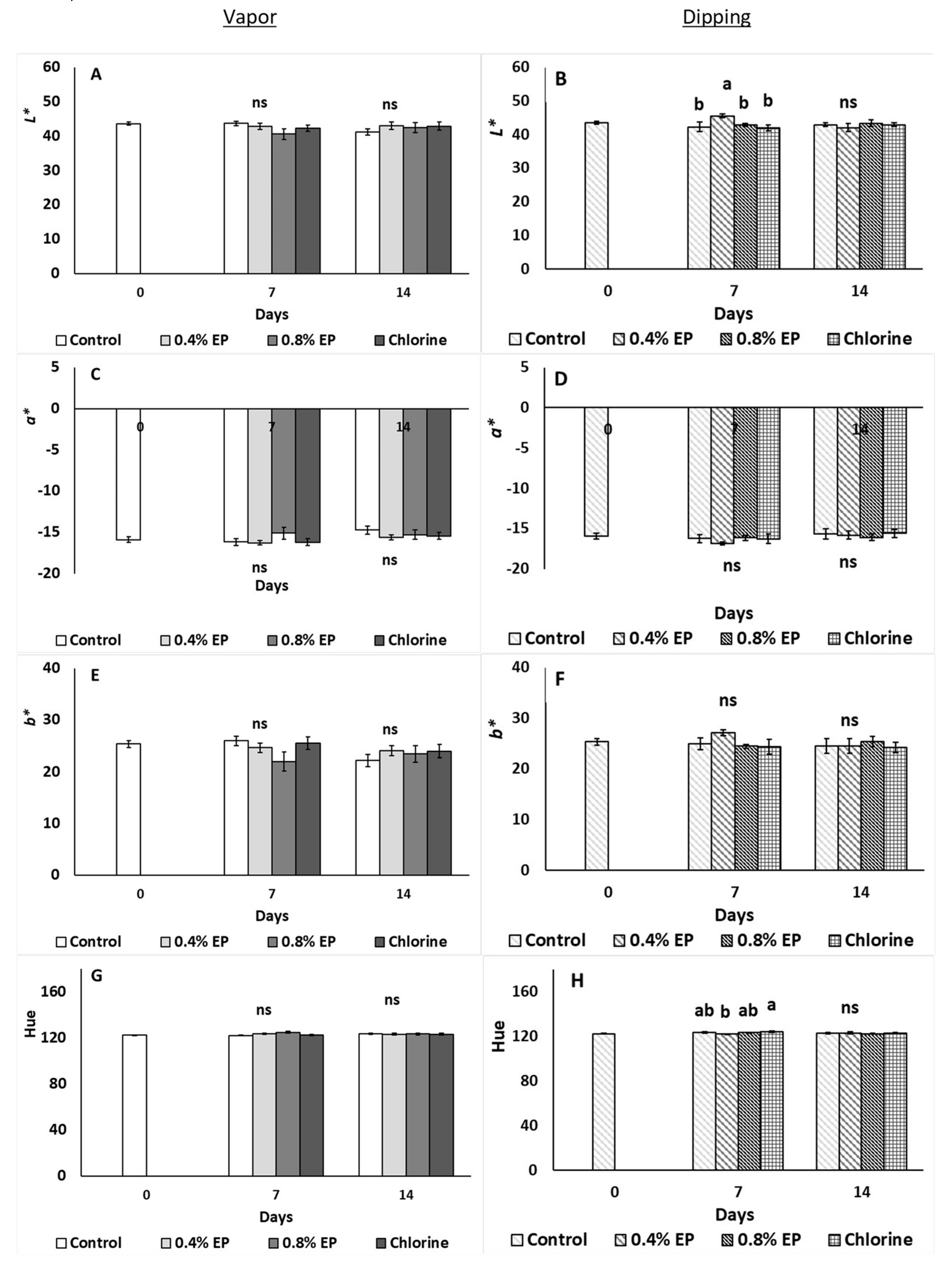
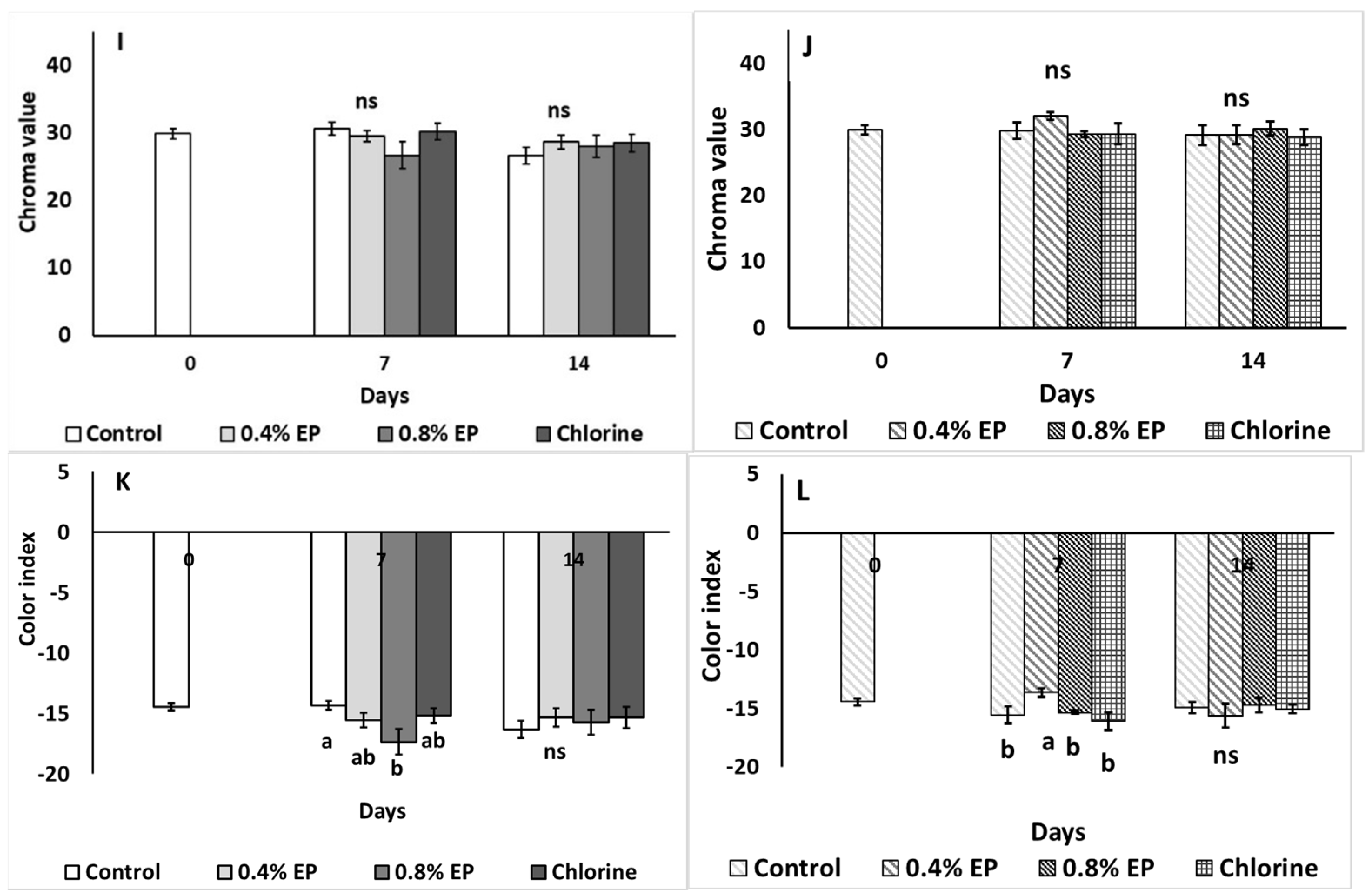
2.3.3. Fruit Weight Loss, Color and Firmness
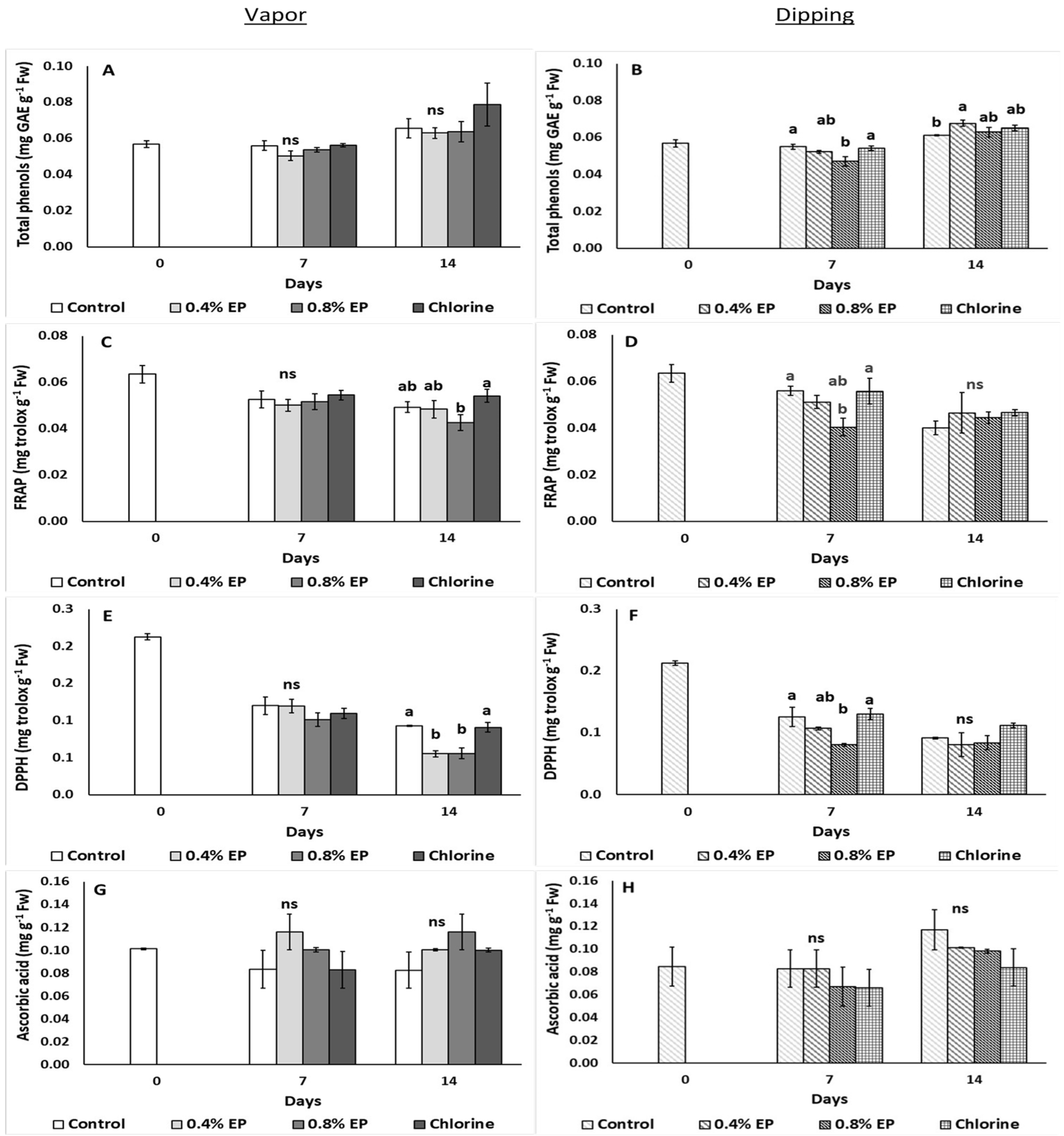
2.3.4. Total Soluble Solids, Titratable Acidity, Ascorbic Acid and Carotenoids
2.3.5. Total Phenolic Content and Antioxidant Activity
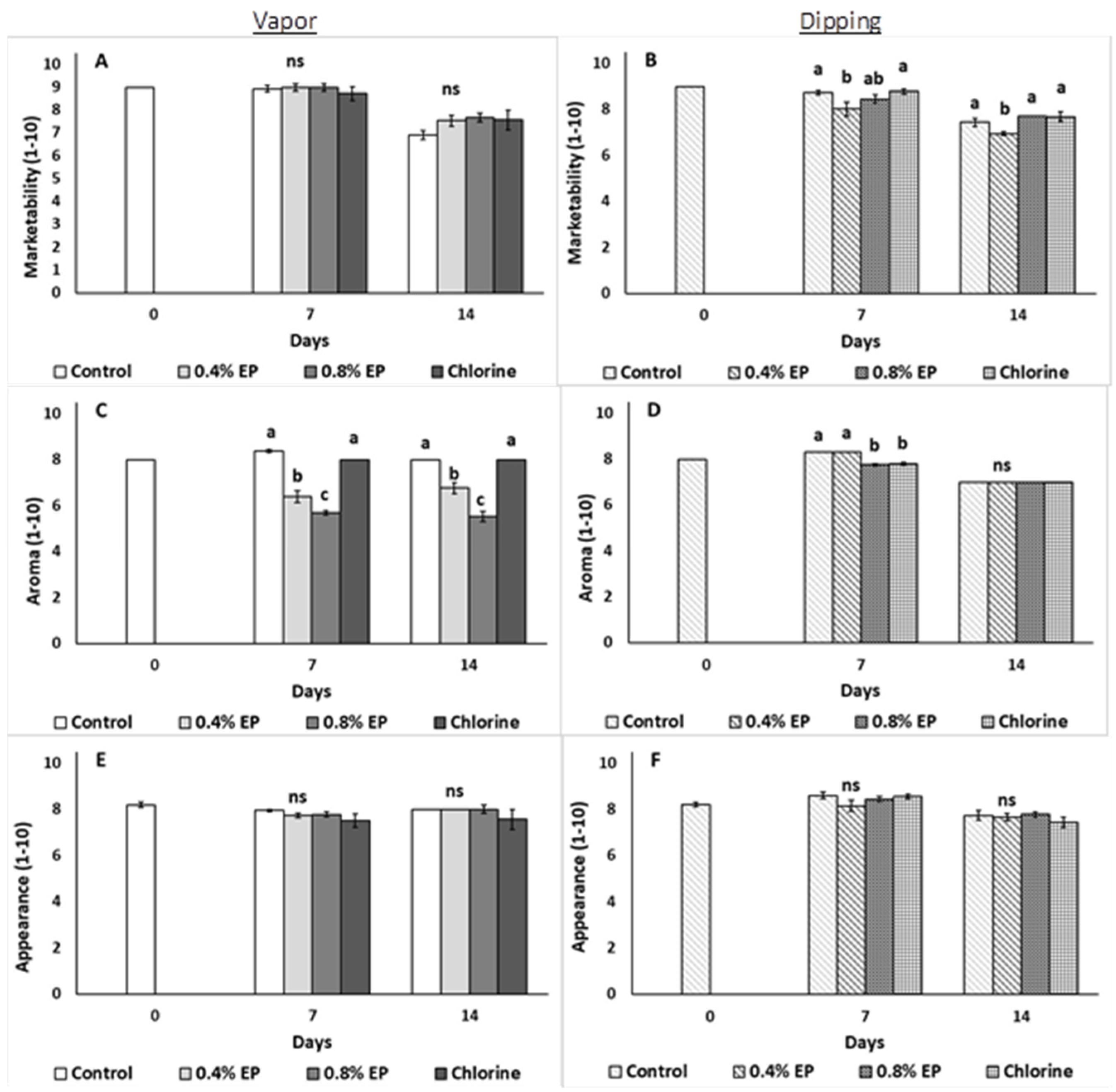
2.3.6. Sensory Evaluation
2.4. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Postharvest Experimental Set Up

References
- Cortbaoui, P.E.; Ngadi, M.O. A New Method to Quantify Postharvest Quality Loss of Cucumber using the Taguchi Approach. Issn 2015, 44, 2224–6088. [Google Scholar]
- Mostafa, Y.S.; Hashem, M.; Alshehri, A.M.; Alamri, S.; Eid, E.M.; Ziedan, E.S.H.E.; Alrumman, S.A. Effective Management of Cucumber Powdery Mildew with Essential Oils. Agriculture 2021, 11, 1177. [Google Scholar] [CrossRef]
- Jahan, S.E.; Hassan, M.K.; Roy, S.; Ahmed, Q.M.; Hasan, G.N.; Muna, A.Y.; Sarkar, M.N. Effects of different postharvest treatments on nutritional quality and shelf life of cucumber. Asian J. Crop. Soil Sci. Plant Nutr. 2020, 2, 51–61. [Google Scholar] [CrossRef]
- Kang, H.M.; Park, K.W.; Saltveit, M.E. Elevated growing temperatures during the day improve the postharvest chilling tolerance of greenhouse-grown cucumber (Cucumis sativus) fruit. Postharvest Biol. Technol. 2002, 24, 49–57. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Chrysargyris, A.; Sivakumar, D.; Loulakakis, K. Vapour or dipping applications of methyl jasmonate, vinegar and sage oil for pepper fruit sanitation towards grey mould. Postharvest Biol. Technol. 2016, 118, 120–127. [Google Scholar] [CrossRef]
- Xylia, P.; Botsaris, G.; Chrysargyris, A.; Skandamis, P.; Tzortzakis, N. Variation of microbial load and biochemical activity of ready-to-eat salads in Cyprus as affected by vegetable type, season, and producer. Food Microbiol. 2019, 83, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Xylia, P.; Clark, A.; Chrysargyris, A.; Romanazzi, G.; Tzortzakis, N. Quality and safety attributes on shredded carrots by using Origanum majorana and ascorbic acid. Postharvest Biol. Technol. 2019, 155, 120–129. [Google Scholar] [CrossRef]
- Imaizumi, T.; Yamauchi, M.; Sekiya, M.; Shimonishi, Y.; Tanaka, F. Responses of phytonutrients and tissue condition in persimmon and cucumber to postharvest UV-C irradiation. Postharvest Biol. Technol. 2018, 145, 33–40. [Google Scholar] [CrossRef]
- Tzortzakis, N.G. Maintaining postharvest quality of fresh produce with volatile compounds. Innov. Food Sci. Emerg. Technol. 2007, 8, 111–116. [Google Scholar] [CrossRef]
- Kawhena, T.G.; Tsige, A.A.; Opara, U.L.; Fawole, O.A. Application of gum arabic and methyl cellulose coatings enriched with thyme oil to maintain quality and extend shelf life of “acco” pomegranate arils. Plants 2020, 9, 1690. [Google Scholar] [CrossRef]
- Panahirad, S.; Naghshiband-Hassani, R.; Bergin, S.; Katam, R.; Mahna, N. Improvement of postharvest quality of plum (Prunus domestica L.) using polysaccharide-based edible coatings. Plants 2020, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.F.R.; Palta, J.P. Lysophosphatidylethanolamine, a natural phospholipid, may retard senescence and improve the shelf life of banana fruit. HortScience 2010, 45, S66. [Google Scholar]
- Tzortzakis, N.; Singleton, I.; Barnes, J. Deployment of low-level ozone-enrichment for the preservation of chilled fresh produce. Postharvest Biol. Technol. 2007, 43, 261–270. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Scollard, J.; Francis, G.A.; O’Beirne, D. Some conventional and latent anti-listerial effects of essential oils, herbs, carrot and cabbage in fresh-cut vegetable systems. Postharvest Biol. Technol. 2013, 77, 87–93. [Google Scholar] [CrossRef]
- Stavropoulou, A.; Loulakakis, K.; Magan, N.; Tzortzakis, N. Origanum dictamnus oil vapour suppresses the development of grey mould in eggplant fruit in vitro. Biomed Res. Int. 2014, 2014, 562679. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Pires, C.; Ramos, C.; Batista, I.; Saraiva, J.A.; Nunes, M.L. Characterization of fish protein films incorporated with essential oils of clove, garlic and origanum: Physical, antioxidant and antibacterial properties. LWT—Food Sci. Technol. 2014, 59, 533–539. [Google Scholar] [CrossRef]
- Istúriz-Zapata, M.A.; Hernández-López, M.; Correa-Pacheco, Z.N.; Barrera-Necha, L.L. Quality of cold-stored cucumber as affected by nanostructured coatings of chitosan with cinnamon essential oil and cinnamaldehyde. Lwt 2020, 123, 109089. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, C.; Li, C.; Lin, L. Essential Oils-Based Antibacterial Agent Against Escherichia coli O157:H7 Biofilm on Cucumber. J. Food Process. Preserv. 2017, 41, e13140. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Xylia, P.; Chrysargyris, A.; Tzortzakis, N.; Tzortzakis, Ν. The combined and single effect of marjoram essential oil, ascorbic acid, and chitosan on fresh-cut lettuce preservation. Foods 2021, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, A.A. Effectiveness of Acetic Acid, Essential Oils and Trichoderma spp. in Controlling Gray Mold Disease on Cucumber. Alex. J. Agric. Sci. 2018, 63, 275–281. [Google Scholar] [CrossRef][Green Version]
- Xylia, P.; Chrysargyris, A.; Botsaris, G.; Tzortzakis, N. Potential application of spearmint and lavender essential oils for assuring endive quality and safety. Crop Prot. 2017, 102, 94–103. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Xylia, P.; Chrysargyris, A. Sage essential oil improves the effectiveness of Aloe vera gel on postharvest quality of tomato fruit. Agronomy 2019, 9, 635. [Google Scholar] [CrossRef]
- Poimenidou, S.V.; Bikouli, V.C.; Gardeli, C.; Mitsi, C.; Tarantilis, P.A.; Nychas, G.J.; Skandamis, P.N. Effect of single or combined chemical and natural antimicrobial interventions on Escherichia coli O157: H7, total microbiota and color of packaged spinach and lettuce. Int. J. Food Microbiol. 2016, 220, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Xylia, P.; Chrysargyris, A.; Ahmed, Z.F.R.; Tzortzakis, Ν. Application of Rosemary and Eucalyptus Essential Oils and Their Main Component on the Preservation of Apple and Pear Fruits. Horticulture 2021, 7, 479. [Google Scholar] [CrossRef]
- Santoro, K.; Maghenzani, M.; Chiabrando, V.; Bosio, P.; Gullino, M.L.; Spadaro, D.; Giacalone, G. Thyme and savory essential oil vapor treatments control brown rot and improve the storage quality of peaches and nectarines, but could favor gray mold. Foods 2018, 7, 7. [Google Scholar] [CrossRef]
- de Medeiros Barbosa, I.; da Costa Medeiros, J.A.; de Oliveira, K.Á.R.; Gomes-Neto, N.J.; Tavares, J.F.; Magnani, M.; de Souza, E.L. Efficacy of the combined application of oregano and rosemary essential oils for the control of Escherichia coli, Listeria monocytogenes and Salmonella Enteritidis in leafy vegetables. Food Control 2016, 59, 468–477. [Google Scholar] [CrossRef]
- Ponce, A.G.; Del Valle, C.; Roula, S.I. Shelf Life of Leafy Vegetables Treated with Natuaral Essential Oils. J. Food Sci. 2004, 69, 50–56. [Google Scholar]
- Werrie, P.Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.L. Phytotoxicity of essential oils: Opportunities and constraints for the development of biopesticides. A review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Charalambous, S.; Xylia, P.; Litskas, V.; Stavrinides, M.; Tzortzakis, N. Assessing the biostimulant effects of a novel plant-based formulation on tomato crop. Sustainability 2020, 12, 8432. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Nikou, A.; Tzortzakis, N. Effectiveness of Aloe vera gel coating for maintaining tomato fruit quality. N. Z. J. Crop Hortic. Sci. 2016, 44, 203–217. [Google Scholar] [CrossRef]
- Bolin, H.R.; Huxsoll, C.C. Effect of Preparation Procedures and Storage Parameters on Quality Retention of Salad-cut Lettuce. J. Food Sci. 1991, 56, 60–62. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Chrysargyris, A.; Panayiotou, C.; Tzortzakis, N. Nitrogen and phosphorus levels affected plant growth, essential oil composition and antioxidant status of lavender plant (Lavandula angustifolia Mill.). Ind. Crops Prod. 2016, 83, 577–586. [Google Scholar] [CrossRef]
- Tzortzakis, N.G.; Tzanakaki, K.; Economakis, C.D. Effect of origanum oil and vinegar on the maintenance of postharvest quality of tomato. Food Nutr. Sci. 2011, 02, 974–982. [Google Scholar] [CrossRef][Green Version]
- Adams, A.; Adama, A.; Thadius, K.T.; Barau, B. Ginger Essential Oil for Postharvest Quality of Datterino Tomato: Effect of Immersion Duration and Storage Temperature. J. Postharvest Technol. 2018, 06, 109–121. [Google Scholar]
- Rabiei, V.; Shirzadeh, E.; Rabbiangourani, H.; Sharafi, Y. Effect of thyme and lavender essential oils on the qualitative and quantitative traits and storage life of apple “Jonagold” cultivar. J. Med. Plant Res. 2011, 5, 5522–5527. [Google Scholar]
- Hardenburg, R.E.; Watada, A.E.; Wang, C.Y. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks; No. 66; United States Department of Agriculture Research Service: Washington, DC, USA, 1990; p. 130.
- Omoba, O.S.; Onyekwere, U. Postharvest physicochemical properties of cucumber fruits (Cucumber sativus L) treated with chitosan-lemon grass extracts under different storage durations. Afr. J. Biotechnol. 2016, 15, 2758–2766. [Google Scholar]
- Oms-Oliu, G.; Hertog, M.L.A.T.M.; Van de Poel, B.; Ampofo-Asiama, J.; Geeraerd, A.H.; Nicolai, B.M. Metabolic characterization of tomato fruit during preharvest development, ripening, and postharvest shelf-life. Postharvest Biol. Technol. 2011, 62, 7–16. [Google Scholar] [CrossRef]
- Ahmed, Z.F.R.; Al Shaibani, F.Y.Y.; Kaur, N.; Maqsood, S.; Schmeda-Hirschmann, G. Improving fruit quality, bioactive compounds, and storage life of date palm (Phoenix dactylifera L., cv. Barhi) using natural elicitors. Horticulturae 2021, 7, 293. [Google Scholar] [CrossRef]
- Ahmed, Z.F.R.; Alnuaimi, A.K.H.; Askri, A.; Tzortzakis, N. Evaluation of Lettuce (Lactuca sativa L.) Production under Hydroponic System: Nutrient Solution Derived from Fish Waste vs. Inorganic Nutrient Solution. Horticulturae 2021, 7, 292. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xylia, P.; Chrysargyris, A.; Shahwar, D.; Ahmed, Z.F.R.; Tzortzakis, N. Application of Rosemary and Eucalyptus Essential Oils on the Preservation of Cucumber Fruit. Horticulturae 2022, 8, 774. https://doi.org/10.3390/horticulturae8090774
Xylia P, Chrysargyris A, Shahwar D, Ahmed ZFR, Tzortzakis N. Application of Rosemary and Eucalyptus Essential Oils on the Preservation of Cucumber Fruit. Horticulturae. 2022; 8(9):774. https://doi.org/10.3390/horticulturae8090774
Chicago/Turabian StyleXylia, Panayiota, Antonios Chrysargyris, Durray Shahwar, Zienab F. R. Ahmed, and Nikolaos Tzortzakis. 2022. "Application of Rosemary and Eucalyptus Essential Oils on the Preservation of Cucumber Fruit" Horticulturae 8, no. 9: 774. https://doi.org/10.3390/horticulturae8090774
APA StyleXylia, P., Chrysargyris, A., Shahwar, D., Ahmed, Z. F. R., & Tzortzakis, N. (2022). Application of Rosemary and Eucalyptus Essential Oils on the Preservation of Cucumber Fruit. Horticulturae, 8(9), 774. https://doi.org/10.3390/horticulturae8090774







