Leaf Volatiles and Relevant Gene Expression as the Specific Characteristics in Citrus depressa Accession Discrimination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Leaf Volatile Organic Compounds (VOCs) Analysis
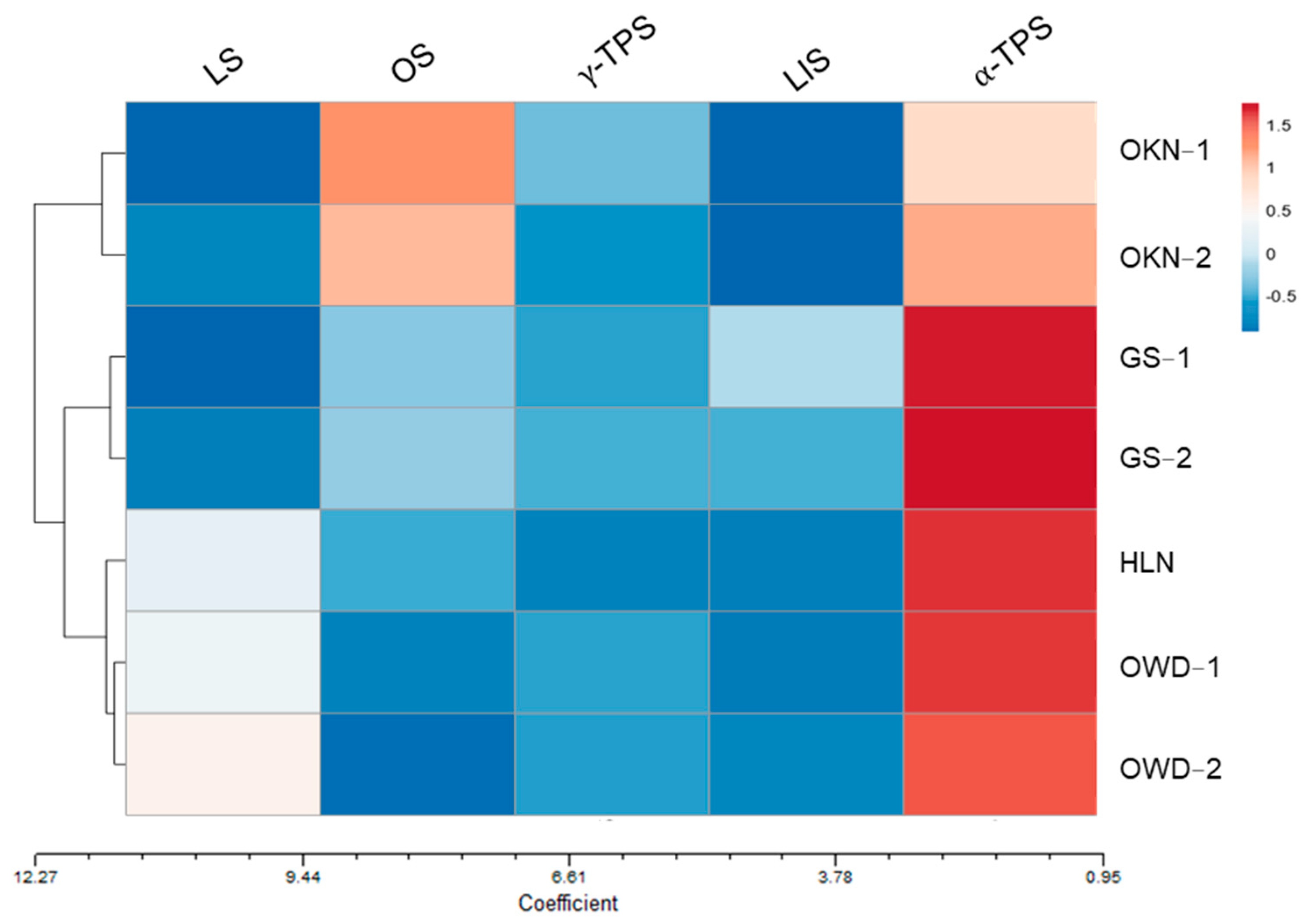
2.3. Selection of VOC-Relevant Genes
2.4. Relative Gene Expression with RT-qPCR Analysis
2.5. Statistical and Multivariate Analysis
3. Results
3.1. Diversity in Leaf Characteristics of Citrus Depressa Accessions
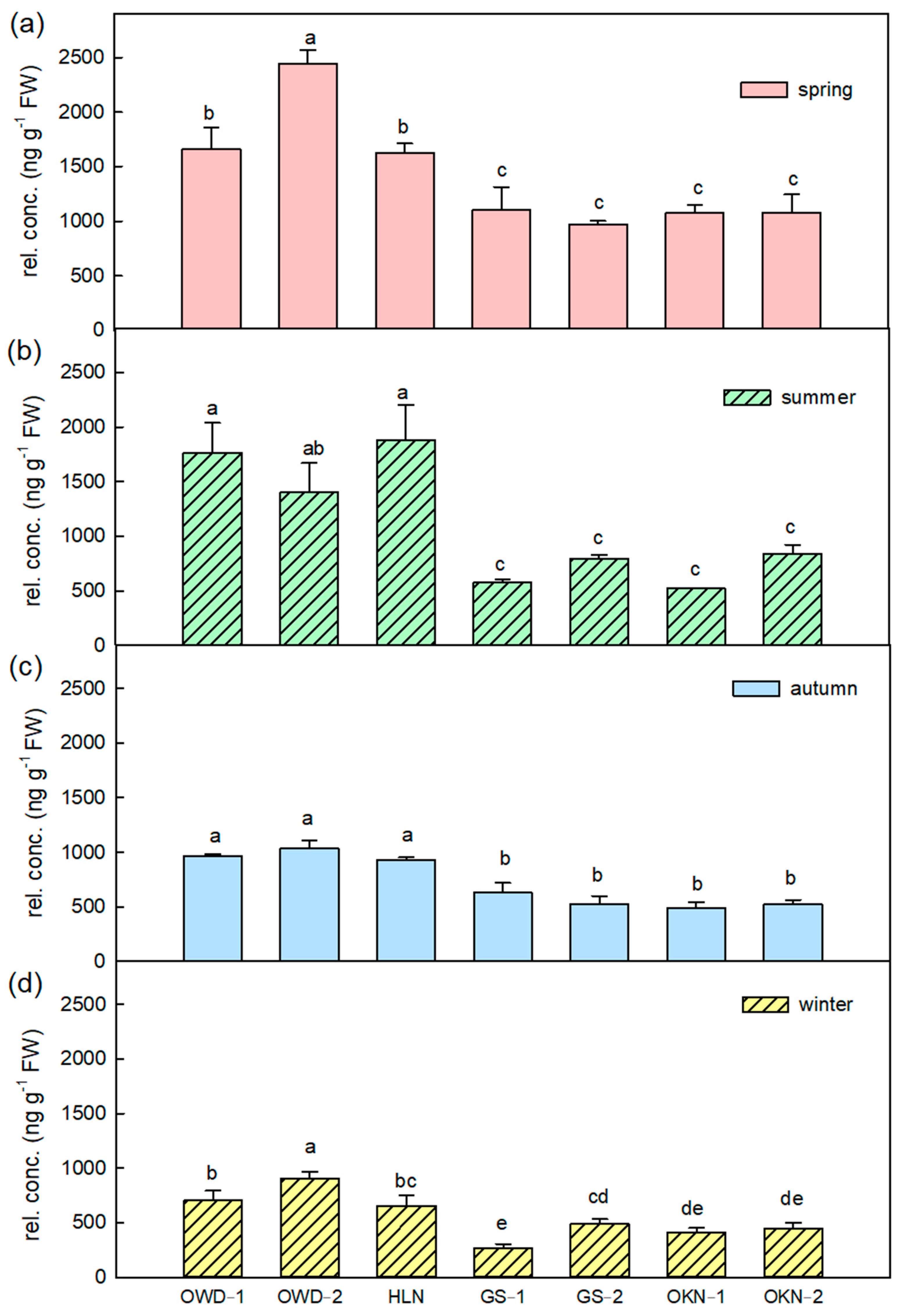
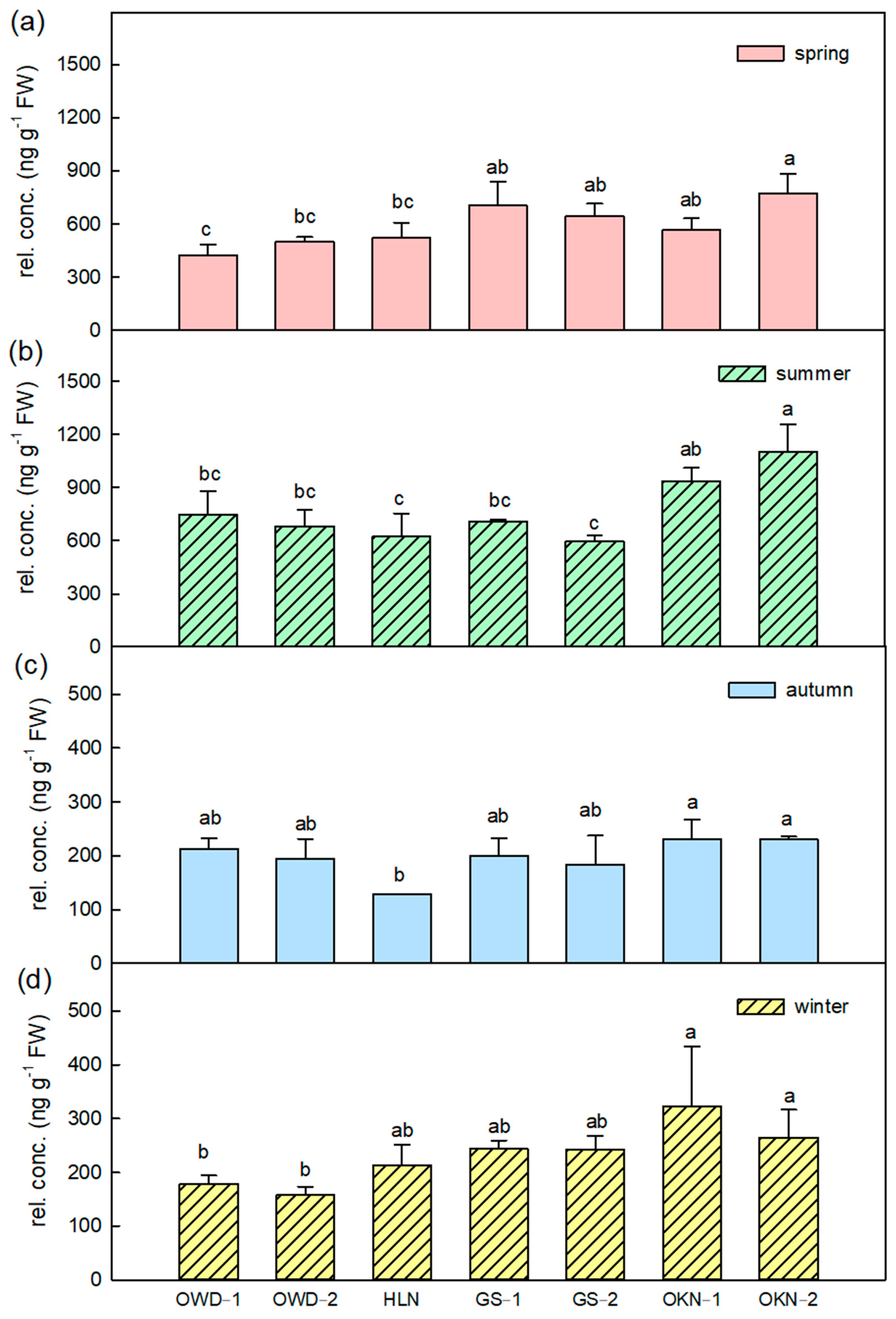
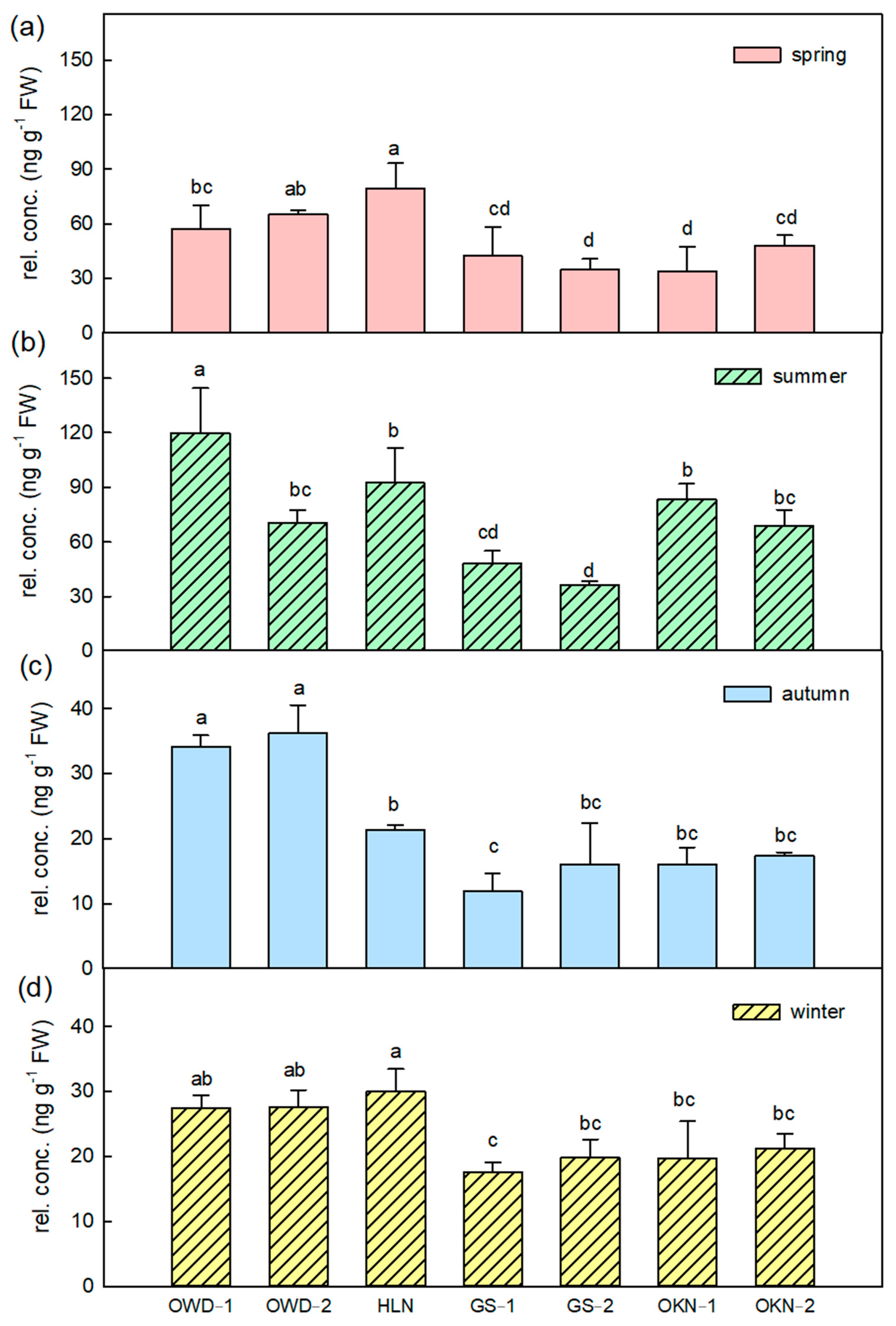
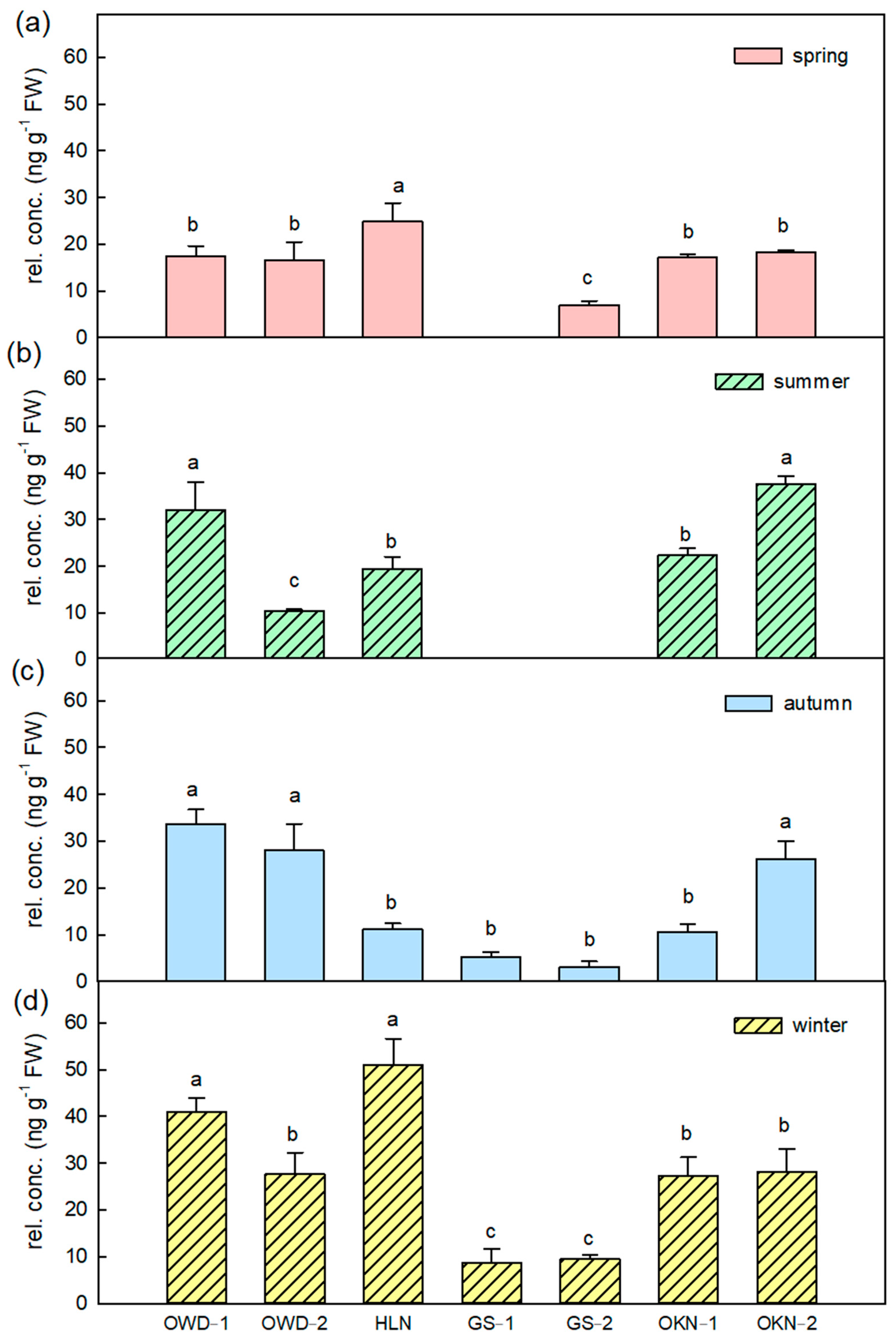
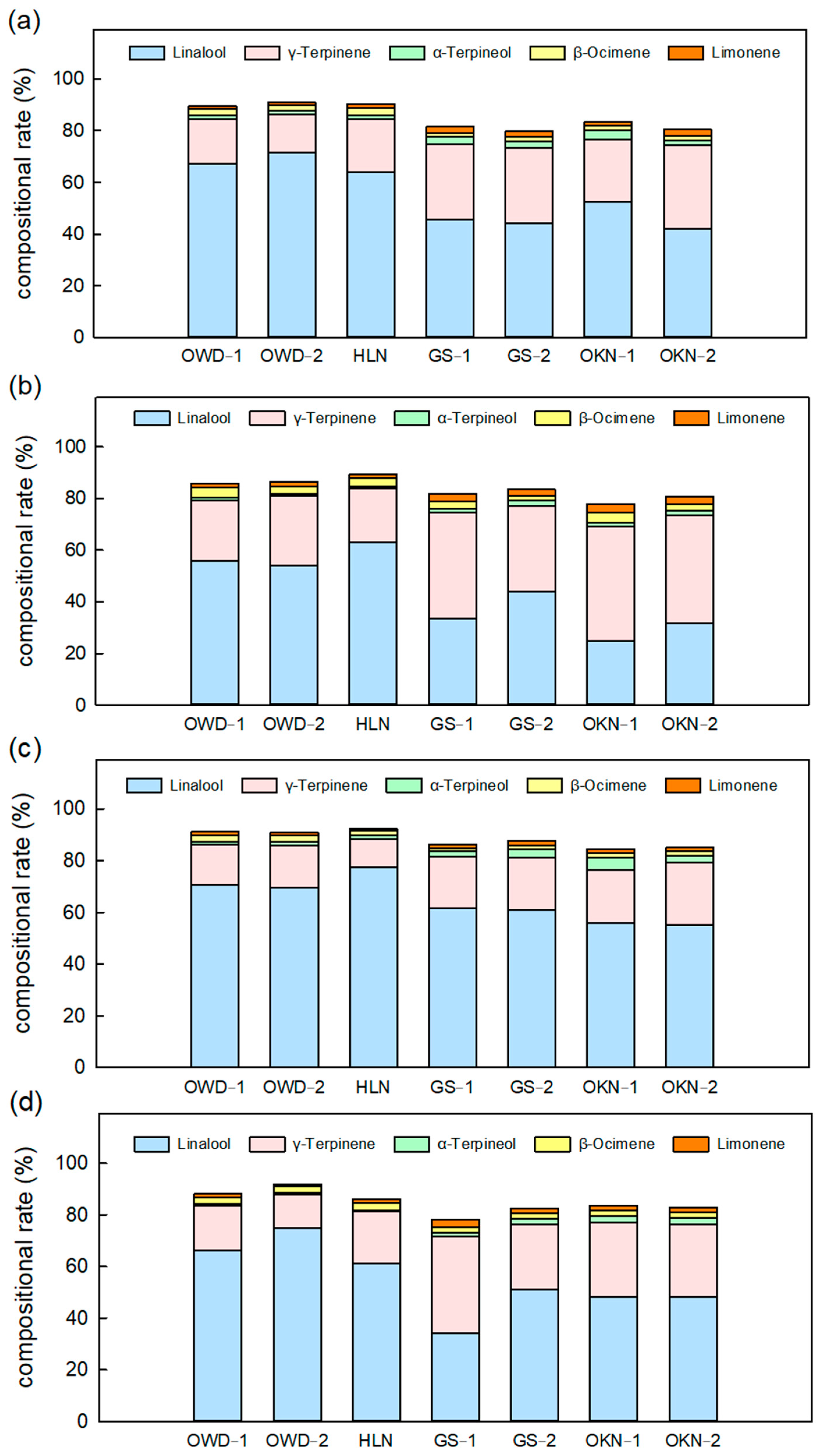
3.2. Featured Volatile Compositions of Citrus Depressa Fresh Leaf and Its Seasonal Variation
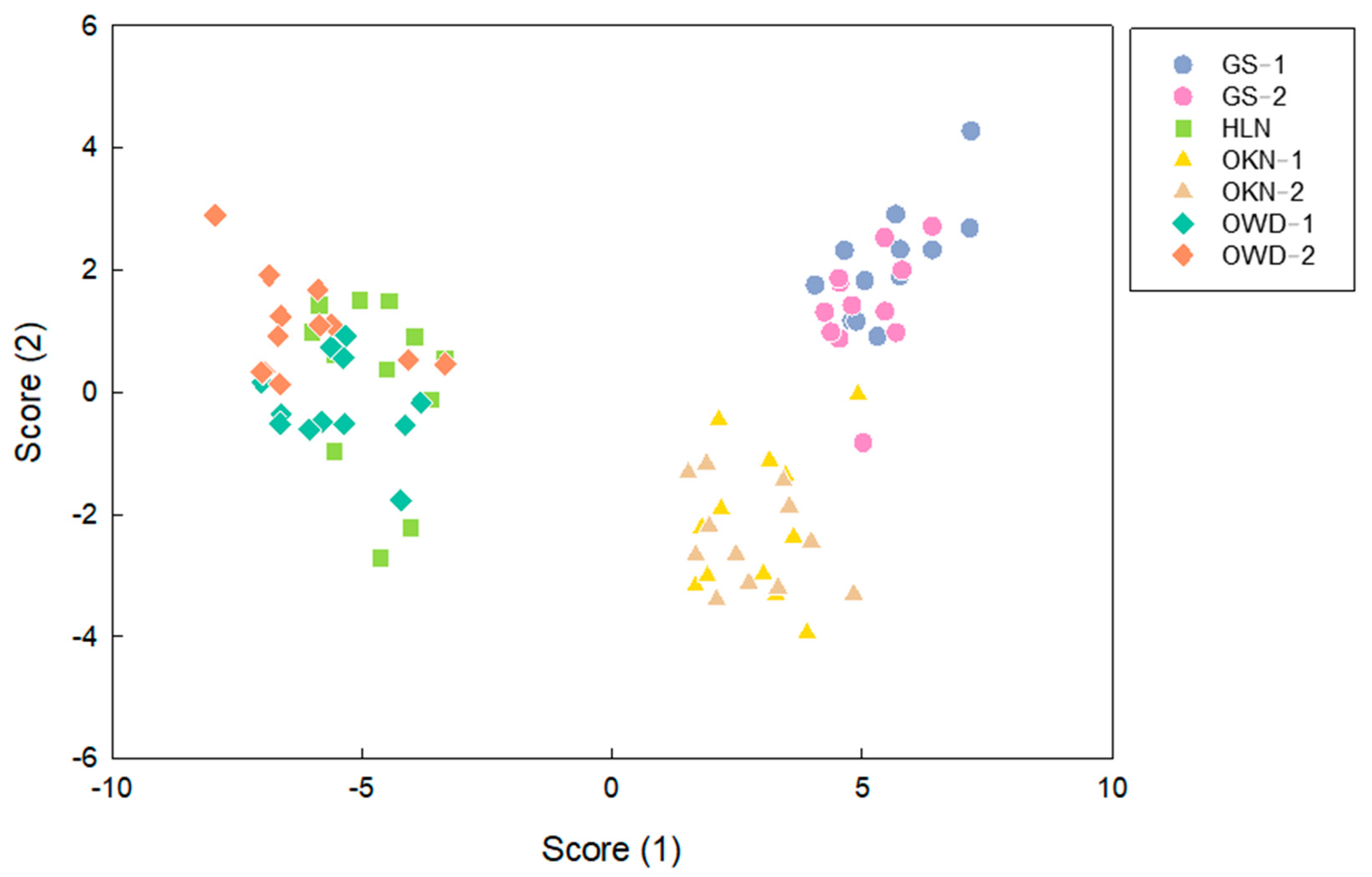
3.3. Linear Discriminant Analysis (LDA) by Fresh Leaf VOCs and Clustering Analysis by Relevant Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanaka, T. Wild citri of the Japanese territories. Bul. Sci. Fak. Terk. Kjusu. Imp. Univ. 1926, 2, 51–58. [Google Scholar]
- Chang, C.E.; Hartley, T.G. Citrus. In Flora of Taiwan; Flora of Taiwan Editorial Committee, Ed.; Epoch Pub. Co.: Taipei, Taiwan, 1993; Volume 3, pp. 513–517. [Google Scholar]
- Feng, S.; Wang, Y. Citrus phytochemicals and their potential effects on the prevention and treatment of obesity: Review and progress of the past 10 years. J. Food Bioact. 2018, 4, 99–106. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Inafuku-Teramoto, S.; Suwa, R.; Fukuzawa, Y.; Kawamitsu, Y. Polymethoxyflavones, synephrine and volatile constitution of peels of citrus fruit grown in Okinawa. J. Jpn. Soc. Hortic. Sci. 2011, 80, 214–224. [Google Scholar] [CrossRef]
- Asikin, Y.; Maeda, G.; Tamaki, H.; Mizu, M.; Oku, H.; Wada, K. Cultivation line and fruit ripening discriminations of Shiikuwasha (Citrus depressa Hayata) peel oils using aroma compositional, electronic nose, and antioxidant analyses. Int. Food Res. J. 2015, 67, 102–110. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Wei, C.C.; Huang, J.; Pan, M.H.; Shahidi, F.; Ho, C.T. Anti-inflammatory effects of polymethoxyflavones from citrus peels: A review. J. Food Bioact. 2018, 3, 76–86. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, S.; Ho, C.T.; Huang, Q. Citrus polymethoxyflavones as regulators of metabolic homoeostasis: Recent advances for possible mechanisms. Trends Food Sci. Technol. 2021, 110, 743–753. [Google Scholar] [CrossRef]
- Swingle, W.T.; Reece, P.C. History, world distribution, botany and varieties. In The Citrus Industry, 2nd ed.; Reuther, E.W., Webber, H.J., Batchelor, L.D., Eds.; University of California Press: Berkeley, CA, USA, 1967; Volume 1, pp. 190–430. [Google Scholar]
- Tanaka, T. Fundamental discussion of Citrus classification. Studia Citrol. 1977, 14, 1–6. [Google Scholar]
- Nicolosi, E.; Deng, Z.N.; Gentile, A.; La Malfa, S.; Continella, G.; Tribulato, E. Citrus phylogeny and genetic origin of important species as investigated by molecular markers. Theor. Appl. Genet. 2000, 100, 1155–1166. [Google Scholar] [CrossRef]
- Kalita, B.; Roy, A.; Annamalai, A.; Lakshmi, P.T.V. A molecular perspective on the taxonomy and journey of Citrus domestication. Perspect. Plant Ecol. Evol. Syst. 2021, 53, 125644. [Google Scholar] [CrossRef]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M.; Koizumi, M.; Ito, C.; Furukawa, H. Quantitative study of flavonoids in leaves of Citrus plants. J. Agric. Food Chem. 2000, 48, 3865–3871. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, T.; Nito, N. Metabolic fingerprinting of citrus cultivars and related genera using HPLC and multivariate analysis. J. Plant Stud. 2018, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Deterre, S.C.; McCollum, G.; Leclair, C.; Manthey, J.A.; Bai, J.; Baldwin, E.A.; Plotto, A. Effect of Poncirus trifoliata on the chemical composition of fruits in pedigrees of Citrus scion hybrids. Sci. Hortic. 2021, 277, 109816. [Google Scholar] [CrossRef]
- Lota, M.L.; de Rocca Serra, D.; Casanova, J.; Tomi, F. Chemical variability of peel and leaf essential oils of sour orange. Flavour. Fragr. J. 2001, 16, 89–96. [Google Scholar] [CrossRef]
- Lin, S.Y.; Roan, S.F.; Lee, C.L.; Chen, I.Z. Volatile organic components of fresh leaves as indicators of indigenous and cultivated citrus species in Taiwan. Biosci. Biotechnol. Biochem. 2010, 74, 90891. [Google Scholar] [CrossRef] [PubMed]
- Urasaki, N.; Yoshida, K.; Uehara, T.; Inoue, H.; Onda, S.; Kinjyo, H.; Kawano, S. Single nucleotide polymorphism in shiikuwasha (Citrus depressa Hayata) chloroplast DNA, trnL-trnF. J. Trop. Agric. 2005, 49, 246–251. [Google Scholar] [CrossRef]
- Lam, D.T.; Ishikawa, R. Molecular discrimination of landraces of Citrus species in the Okinawa, Japan. Genet. Resour. Crop Evol. 2019, 66, 321–333. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Nagano, Y.; Inafuku-Teramoto, S.; Hashimoto, M.; Mimura, T.; Matsumoto, R.; Yamamoto, M. Characterization of chloroplast matK sequences of Citrus tachibana and Citrus depressa, two indigenous species in Japan. Adv. Hortic. Sci. 2014, 28, 95–99. [Google Scholar]
- Ishikawa, R.; Badenoch, N.; Miyagi, K.; Medoruma, K.; Osada, T.; Onishi, M. Multi-lineages of Shiikuwasha (Citrus depressa Hayata) evaluated by using whole chloroplast genome sequences and its bio-diversity in Okinawa, Japan. Breed. Sci. 2016, 66, 490–498. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takakura, A.; Tanabe, A.; Teramoto, S.; Kita, M. Diversity of Citrus depressa Hayata (Shiikuwasha) revealed by DNA analysis. Genet. Resour. Crop Evol. 2017, 64, 805–814. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, D.; Tan, C.; Hu, Y.; Sundararajan, B.; Zhou, Z. Profiling of flavonoid and antioxidant activity of fruit tissues from 27 Chinese local citrus cultivars. Plants 2020, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An overview of bioactive flavonoids from Citrus fruits. Appl. Sci. 2021, 12, 29. [Google Scholar] [CrossRef]
- Clery, R.A.; Armendi, A.; Franco, V.; Furrer, S.; Genereux, J.C.; Kahn, T.L.; Koshiro, K. Chemical diversity of citrus leaf essential oils. Chem. Biodivers. 2022, 19, e202100963. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, V.; Costantino, G.; Paoli, M.; Paymal, N.; Quinton, C.; Ollitrault, P.; Luro, F. Intercultivar diversity of sour orange (Citrus aurantium L.) based on genetic markers, phenotypic characteristics, aromatic compounds and sensorial analysis. Agronomy 2021, 11, 1084. [Google Scholar] [CrossRef]
- Petretto, G.L.; Di Pietro, M.E.; Piroddi, M.; Pintore, G.; Mannu, A. Classification of pummelo (Citrus grandis) extracts through UV-VIS-based chemical fingerprint. Beverages 2022, 8, 34. [Google Scholar] [CrossRef]
- Penjor, T.; Mimura, T.; Matsumoto, R.; Yamamoto, M.; Nagano, Y. Characterization of limes (Citrus aurantifolia) grown in Bhutan and Indonesia using high-throughput sequencing. Sci. Rep. 2014, 4, 4853. [Google Scholar] [CrossRef]
- Yi, K.U.; Zhin, K.L.; Oh, E.U.; Kim, S.S.; Kim, H.B.; Song, K.J. Phenotypic and genetic characterization of three different types of Dangyooza (Citrus grandis), Korean landrace Citrus. Hortic. Sci. Technol. 2021, 39, 96–105. [Google Scholar] [CrossRef]
- Chi, P.T.L.; Van Hung, P.; Le Thanh, H.; Phi, N.T.L. Valorization of citrus leaves: Chemical composition, antioxidant and antibacterial activities of essential oils. Waste Biomass Valorization 2020, 11, 4849–4857. [Google Scholar] [CrossRef]
- Azam, M.; Jiang, Q.; Zhang, B.; Xu, C.; Chen, K. Citrus leaf volatiles as affected by developmental stage and genetic type. Int. J. Mol. Sci. 2013, 14, 17744–17766. [Google Scholar] [CrossRef]
- Zhu, F.; Xu, J.; Ke, Y.; Huang, S.; Zeng, F.; Luan, T.; Ouyang, G. Applications of in vivo and in vitro solid-phase microextraction techniques in plant analysis: A review. Anal. Chim. Acta 2013, 794, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Oyedeji, A.O.; Okunowo, W.O.; Osuntoki, A.A.; Olabode, T.B.; Ayo-Folorunso, F. Insecticidal and biochemical activity of essential oil from Citrus sinensis peel and constituents on Callosobrunchus maculatus and Sitophilus zeamais. Pestic. Biochem. Physiol. 2020, 168, 104643. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.; Felipe, L.D.O.; Bicas, J.L. Production, properties, and applications of α-terpineol. Food Bioprocess Technol. 2020, 13, 1261–1279. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Sharma, N.R. Fragrance stimulation mechanisms of flowers and their regulation under environmental constraints. J. Plant Growth Regul. 2022, 41, 1–23. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Lamine, M.; Rahali, F.Z.; Hamdaoui, G.; Selmi, S.; Mliki, A.; Gargouri, M. Associating chemical analysis to molecular markers for the valorization of Citrus aurantium leaves: A useful starting point for marker-assisted selection. Euphytica 2017, 213, 44. [Google Scholar] [CrossRef]

| Accession 1 | Leaf | Leaf Petiole Wing | ||||
|---|---|---|---|---|---|---|
| Length (mm) | Broadness (mm) | Shape Ratio 3 | Length (mm) | Broadness (mm) | Shape Ratio | |
| OWD-1 | 65.46 ± 4.41 c,2 | 35.43 ± 2.82 c | 1.85 ± 0.13 bc | 9.57 ± 1.17 a | 1.66 ± 0.24 d | 5.89 ± 1.11 a |
| OWD-2 | 64.83 ± 4.85 c | 34.00 ± 1.92 c | 1.91 ± 0.19 bc | 8.84 ± 1.74 ab | 1.49 ± 0.13 d | 5.93 ± 1.08 a |
| HLN | 77.19 ± 10.37 b | 43.54 ± 7.07 a | 1.79 ± 0.19 c | 7.26 ± 1.88 b | 1.70 ± 0.21 cd | 4.24 ± 0.84 b |
| GS-1 | 77.77 ± 8.21 b | 42.81 ± 4.12 a | 1.82 ± 0.13 bc | 9.90 ± 2.31 a | 2.28 ± 0.32 a | 4.37 ± 0.89 b |
| GS-2 | 75.04 ± 5.96 b | 40.07 ± 3.43 ab | 1.88 ± 0.13 bc | 10.08 ± 2.45 a | 2.45 ± 0.28 a | 4.18 ± 1.09 b |
| OKN-1 | 85.82 ± 7.41 a | 41.67 ± 5.76 a | 2.00 ± 0.32 b | 9.21 ± 2.25 a | 1.92 ± 0.24 b | 4.86 ± 1.20 b |
| OKN-2 | 81.77 ± 12.95 ab | 37.45 ± 3.21 bc | 2.30 ± 0.31 a | 8.46 ± 1.83 ab | 1.87 ± 0.15 bc | 4.52 ± 0.86 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-Y.; Liao, Y.-Y.; Chen, P.-A. Leaf Volatiles and Relevant Gene Expression as the Specific Characteristics in Citrus depressa Accession Discrimination. Horticulturae 2022, 8, 773. https://doi.org/10.3390/horticulturae8090773
Lin S-Y, Liao Y-Y, Chen P-A. Leaf Volatiles and Relevant Gene Expression as the Specific Characteristics in Citrus depressa Accession Discrimination. Horticulturae. 2022; 8(9):773. https://doi.org/10.3390/horticulturae8090773
Chicago/Turabian StyleLin, Shu-Yen, Yung-Yu Liao, and Po-An Chen. 2022. "Leaf Volatiles and Relevant Gene Expression as the Specific Characteristics in Citrus depressa Accession Discrimination" Horticulturae 8, no. 9: 773. https://doi.org/10.3390/horticulturae8090773
APA StyleLin, S.-Y., Liao, Y.-Y., & Chen, P.-A. (2022). Leaf Volatiles and Relevant Gene Expression as the Specific Characteristics in Citrus depressa Accession Discrimination. Horticulturae, 8(9), 773. https://doi.org/10.3390/horticulturae8090773





