Hurdle Approach for Control of Enzymatic Browning and Extension of Shelf Life of Fresh-Cut Leafy Vegetables Using Vacuum Precooling and Modified Atmosphere Packaging: Commercial Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Preparation, Precooling, and Packing
2.3. Shelf Life Evaluation
2.4. Headspace Gas Analysis
2.5. Weight Loss Analysis
2.6. Chlorophyll Analysis
2.7. Phenolics Analysis
2.8. Enzyme Analysis
2.9. Statistical Analysis
3. Results and Discussion
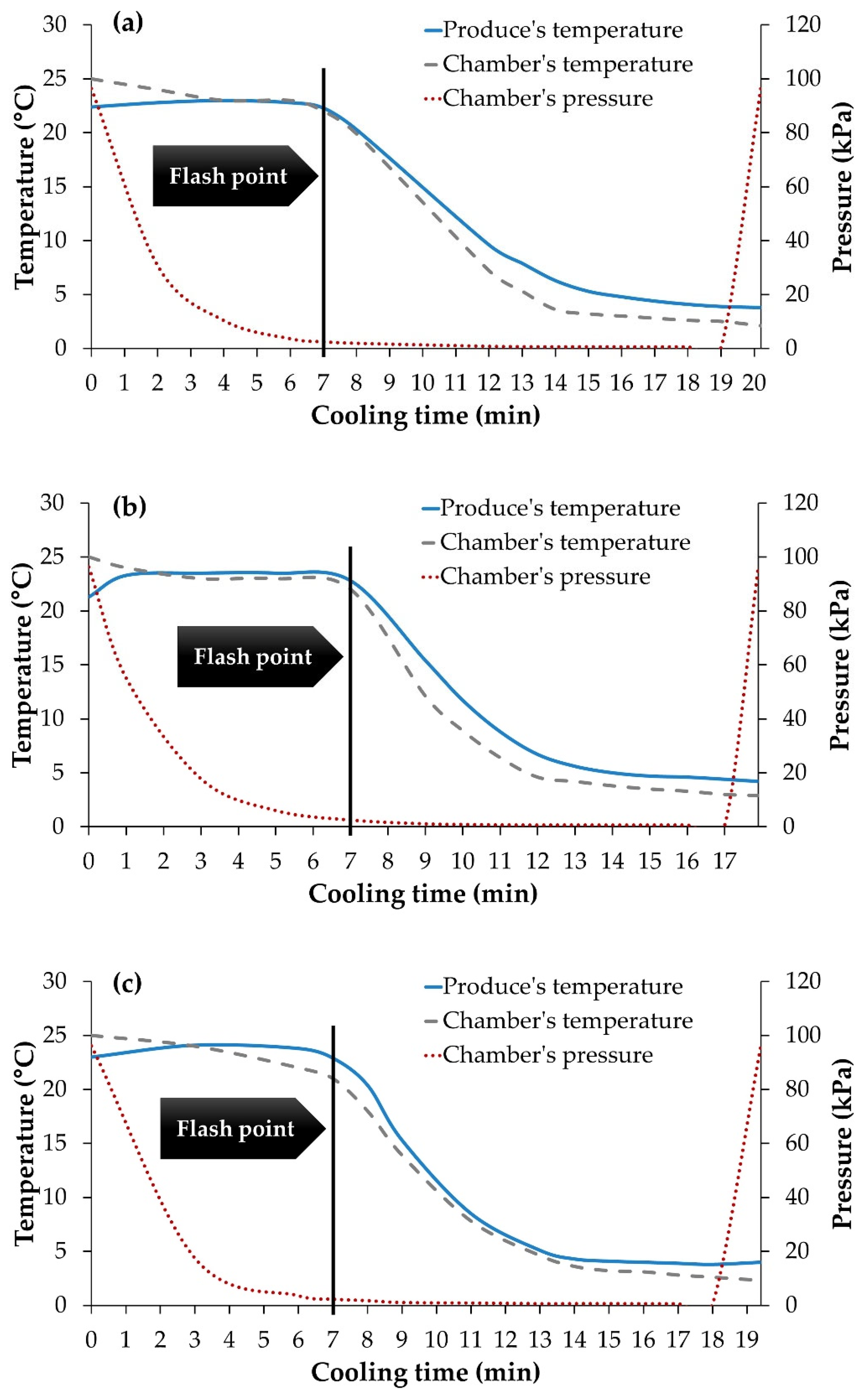
3.1. Changes in Pressure and Temperature during the Vacuum Precooling Process
3.2. Shelf life Evaluation
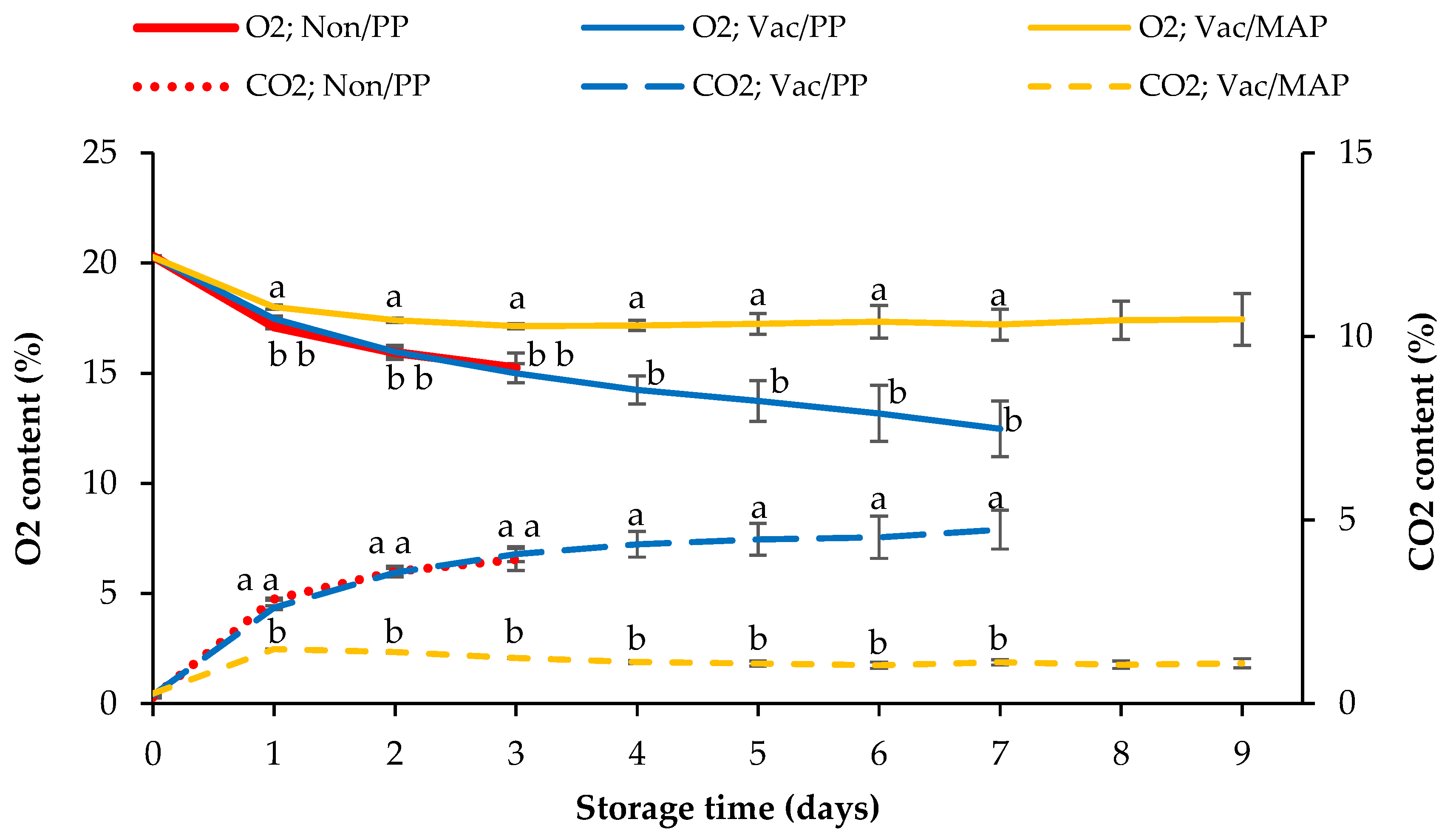
3.3. Headspace Gas Content
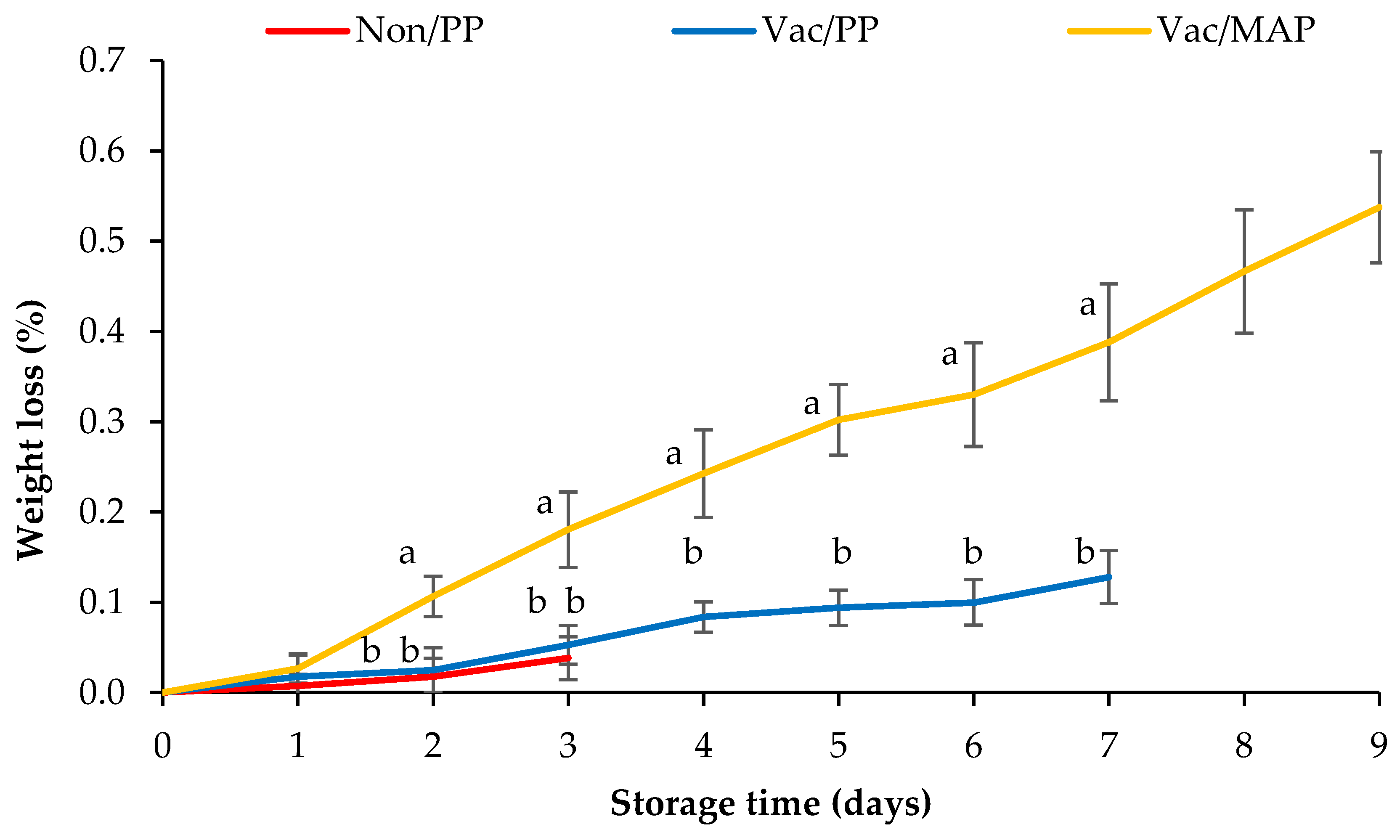
3.4. Weight Loss Percentage
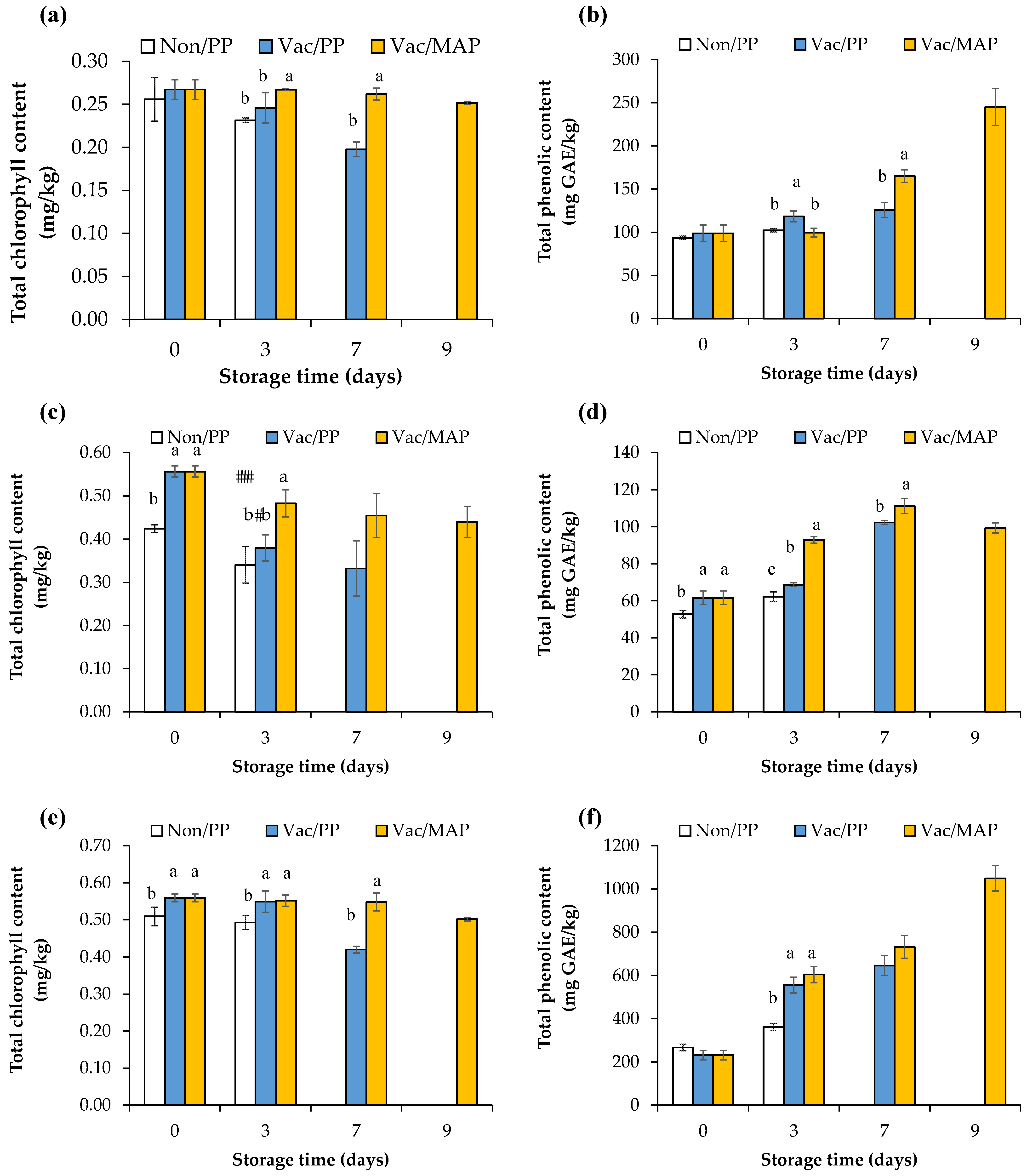
3.5. Chlorophyll Content
3.6. Total Phenolic Content
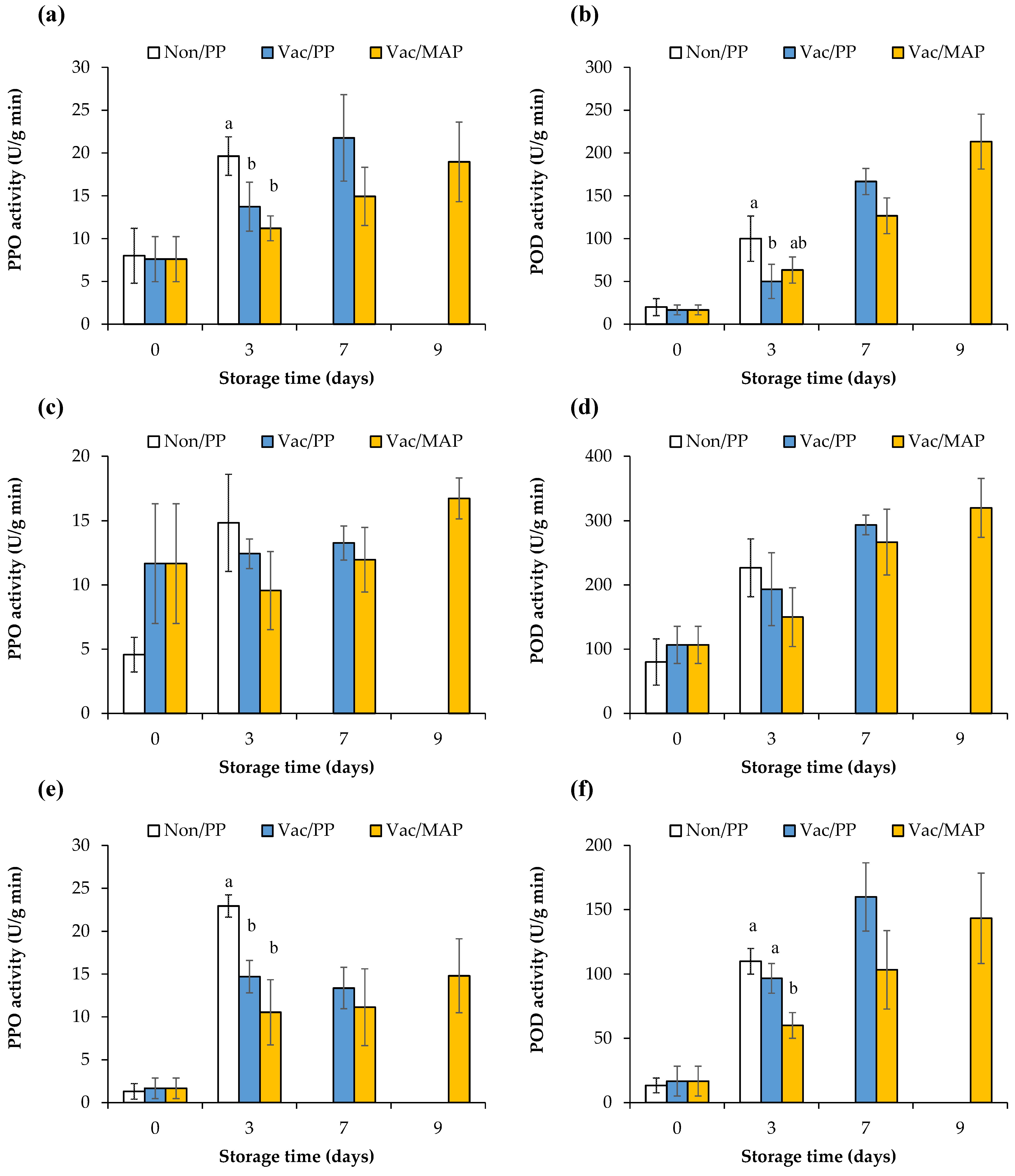
3.7. Enzyme Activity
3.8. The Possibility of Applying Vacuum Precooling and Modified Atmosphere Packaging in the Commercial Fresh-Cut Leafy Vegetables Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rojas-Graü, M.A.; Garner, E.; Martín-Belloso, O. The Fresh-Cut Fruit and Vegetables Industry. In Advances in Fresh-Cut Fruits and Vegetables Processing; Martin-Belloso, O., Soliva-Fortuny, R., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–12. [Google Scholar]
- Massaglia, S.; Merlino, V.M.; Borra, D.; Bargetto, A.; Sottile, F.; Peano, C. Consumer Attitudes and Preference Exploration towards Fresh-Cut Salads Using Best–Worst Scaling and Latent Class Analysis. Foods 2019, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, S.; Tian, C.; Yan, G.; Wang, D. An overview of current status of cold chain in China. Int. J. Refrig. 2018, 88, 483–495. [Google Scholar] [CrossRef]
- Choi, I.-L.; Lee, J.-H.; Choi, D.-H.; Wang, L.-X.; Kang, H.-M. Evaluation of the Storage Characteristics in Maintaining the Overall Quality of Whole and Fresh-Cut Romaine Lettuce during MA Storage. Horticulturae 2021, 7, 461. [Google Scholar] [CrossRef]
- Teng, Z.; Luo, Y.; Bornhorst, E.R.; Zhou, B.; Simko, I.; Trouth, F. Identification of romaine lettuce (Lactuca sativa var. longifolia) Cultivars with reduced browning discoloration for fresh-cut processing. Postharvest Biol. Technol. 2019, 156, 110931. [Google Scholar] [CrossRef]
- Iakimova, E.T.; Woltering, E.J. Nitric oxide prevents wound-induced browning and delays senescence through inhibition of hydrogen peroxide accumulation in fresh-cut lettuce. Innov. Food Sci. Emerg. Technol. 2015, 30, 157–169. [Google Scholar] [CrossRef]
- Oliveira, M.; Abadias, M.; Usall, J.; Torres, R.; Teixidó, N.; Viñas, I. Application of modified atmosphere packaging as a safety approach to fresh-cut fruits and vegetables—A review. Trends Food Sci. Technol. 2015, 46, 13–26. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, G.-B.; Fawole, O.A.; Verboven, P.; Zhang, X.-R.; Wu, D.; Opara, U.L.; Nicolai, B.; Chen, K. Postharvest precooling of fruit and vegetables: A review. Trends Food Sci. Technol. 2020, 100, 278–291. [Google Scholar] [CrossRef]
- Liu, E.; Hu, X.; Liu, S. Theoretical Simulation and Experimental Study on Effect of Vacuum Pre-Cooling for Postharvest Leaf Lettuce. J. Food Nutr. Res. 2014, 2, 443–449. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef]
- Jin, S.; Ding, Z.; Xie, J. Modified Atmospheric Packaging of Fresh-Cut Amaranth (Amaranthus tricolor L.) for Extending Shelf Life. Agriculture 2021, 11, 1016. [Google Scholar] [CrossRef]
- Boonyakiat, D.; Boonprasom, P. Effect of active packaging on quality of Chinese kale. CMU J. Nat. Sci. 2012, 11, 215–221. [Google Scholar]
- Guo, Z.; Liu, H.; Chen, X.; Huang, L.; Fan, J.; Zhou, J.; Chang, X.; Du, B.; Chang, X. Modified-atmosphere packaging maintains the quality of postharvest whole lettuce (Lactuca sativa L. Grand Rapid) by mediating the dynamic equilibrium of the electron transport chain and protecting mitochondrial structure and function. Postharvest Biol. Technol. 2019, 147, 206–213. [Google Scholar] [CrossRef]
- Min, T.; Liu, E.-C.; Xie, J.; Yi, Y.; Wang, L.-M.; Ai, Y.-W.; Wang, H.-X. Effects of Vacuum Packaging on Enzymatic Browning and Ethylene Response Factor (ERF) Gene Expression of Fresh-cut Lotus Root. HortScience 2019, 54, 331–336. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, C.; Zhang, Y.; Niu, D.; Du, J. Effects of aqueous chlorine dioxide treatment on enzymatic browning and shelf-life of fresh-cut asparagus lettuce (Lactuca sativa L.). Postharvest Biol. Technol. 2010, 58, 232–238. [Google Scholar] [CrossRef]
- Maturin, L.; Peeler, J.T. BAM Chapter 3: Aerobic Plate Count. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-3-aerobic-plate-count (accessed on 26 September 2018).
- Tournas, V.; Stack, M.E.; Mislivec, P.B.; Koch, H.A.; Bandler, R. BAM Chapter 18: Yeasts, Molds and Mycotoxins. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-18-yeasts-molds-and-mycotoxins (accessed on 26 September 2018).
- ISO11290-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria spp.—Part 1: Detection Method. ISO: Geneva, Switzerland, 2017; pp. 11290–12017. Available online: https://www.iso.org/standard/60313.html (accessed on 26 September 2020).
- Ragaert, P.; Jacxsens, L.; Vandekinderen, I.; Baert, L.; Devlieghere, F. Microbiological and Safety Aspects of Fresh-Cut Fruits and Vegetables. In Advances in Fresh-Cut Fruits and Vegetables Processing; Martin-Belloso, O., Soliva-Fortuny, R., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 53–86. [Google Scholar]
- Waghmare, R.B.; Annapure, U.S. Integrated effect of sodium hypochlorite and modified atmosphere packaging on quality and shelf life of fresh-cut cilantro. Food Packag. Shelf Life 2015, 3, 62–69. [Google Scholar] [CrossRef]
- Witham, F.H.; Blaydes, D.F.; Devlin, R.M. Experiments in Plant Physiology; Van Nostrand Reinhold Company: New York, NY, USA, 1971. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M.; Lester, P. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Kim, D.-H.; Kim, H.-B.; Chung, H.-S.; Moon, K.-D. Browning control of fresh-cut lettuce by phytoncide treatment. Food Chem. 2014, 159, 188–192. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singh, R.P.; Heldman, D.R. Appendices. In Introduction to Food Engineering; Singh, R.P., Heldman, D.R., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 793–849. [Google Scholar]
- Ozturk, H.M.; Ozturk, H.K. Effect of pressure on the vacuum cooling of iceberg lettuce. Int. J. Refrig. 2009, 32, 402–410. [Google Scholar] [CrossRef]
- Song, X.-Y.; Liu, B.-L.; Jaganathan, G.K. Mathematical simulation on the surface temperature variation of fresh-cut leafy vegetable during vacuum cooling. Int. J. Refrig. 2016, 65, 228–237. [Google Scholar] [CrossRef]
- Lorente-Mento, J.M.; Valverde, J.M.; Serrano, M.; Pretel, M.T. Fresh-Cut Salads: Consumer Acceptance and Quality Parameter Evolution during Storage in Domestic Refrigerators. Sustainability 2022, 14, 3473. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Bouletis, A.D.; Papa, E.A.; Gkagtzis, D.C.; Hadjichristodoulou, C.; Papaloucas, C. Microbial and sensory quality of “Lollo verde” lettuce and rocket salad stored under active atmosphere packaging. Anaerobe 2011, 17, 307–309. [Google Scholar] [CrossRef] [PubMed]
- García, C.J.; Gil, M.I.; Tomás-Barberán, F.A. Targeted Metabolomics Analysis and Identification of Biomarkers for Predicting Browning of Fresh-Cut Lettuce. J. Agric. Food Chem. 2019, 67, 5908–5917. [Google Scholar] [CrossRef]
- Calonico, C.; Delfino, V.; Pesavento, G.; Mundo, M.; Lo Nostro, A. Microbiological Quality of Ready-to-eat Salads from Processing Plant to the Consumers. J. Food Nutr. Res. 2019, 7, 427–434. [Google Scholar] [CrossRef]
- He, S.; Zhang, G.; Yu, Y.; Li, R.; Yang, Q. Effects of vacuum cooling on the enzymatic antioxidant system of cherry and inhibition of surface-borne pathogens. Int. J. Refrig. 2013, 36, 2387–2394. [Google Scholar] [CrossRef]
- Kongwong, P.; Boonyakiat, D.; Poonlarp, P. Extending the shelf life and qualities of baby cos lettuce using commercial precooling systems. Postharvest Biol. Technol. 2019, 150, 60–70. [Google Scholar] [CrossRef]
- Islam, M.; Lee, Y.; Mele, M.A.; Choi, I.; Jang, D.; Ko, Y.; Kim, Y.; Kang, H. Effect of modified atmosphere packaging on quality and shelf life of baby leaf lettuce. Qual. Assur. Saf. Crop. Foods 2019, 11, 749–756. [Google Scholar] [CrossRef]
- Ranjbaran, M.; Datta, A.K. Pressure-driven infiltration of water and bacteria into plant leaves during vacuum cooling: A mechanistic model. J. Food Eng. 2019, 246, 209–223. [Google Scholar] [CrossRef]
- Wu, S.M.; Shu, F.Y.; Huang, D.F. Effects of packaging materials and types on postharvest nutritional quality of mini Pakchoi Brassica chinensis. Int. J. Agric. Biol. Eng. 2016, 9, 207–213. [Google Scholar] [CrossRef]
- Ding, T.; Liu, F.; Ling, J.G.; Kang, M.L.; Yu, J.F.; Ye, X.Q.; Liu, D.H. Comparison of different cooling methods for extending shelf life of postharvest broccoli. Int. J. Agric. Biol. Eng. 2016, 9, 178–185. [Google Scholar] [CrossRef]
- Luo, F.; Cheng, S.-C.; Cai, J.-H.; Wei, B.-D.; Zhou, X.; Zhou, Q.; Zhao, Y.-B.; Ji, S.-J. Chlorophyll degradation and carotenoid biosynthetic pathways: Gene expression and pigment content in broccoli during yellowing. Food Chem. 2019, 297, 124964. [Google Scholar] [CrossRef]
- Mampholo, M.B.; Sivakumar, D.; Van Rensburg, J. Variation in Bioactive Compounds and Quality Parameters in Different Modified Atmosphere Packaging during Postharvest Storage of Traditional Leafy Vegetables (A maranthus cruentus L and Solanum Retroflexum ). J. Food Qual. 2015, 38, 1–12. [Google Scholar] [CrossRef]
- Altunkaya, A.; Gokmen, V. Effect of various inhibitors on enzymatic browning, antioxidant activity and total phenol content of fresh lettuce (Lactuca sativa). Food Chem. 2008, 107, 1173–1179. [Google Scholar] [CrossRef]
- Mattos, L.M.; Moretti, C.L.; Da Silva, E.Y.Y. Effects of modified atmosphere packaging on quality attributes and physiological responses of fresh-cut crisphead lettuce. CyTA J. Food 2013, 11, 392–397. [Google Scholar] [CrossRef]
- Luna, M.C.; Tudela, J.A.; Tomás-Barberán, F.A.; Gil, M.I. Modified atmosphere (MA) prevents browning of fresh-cut romaine lettuce through multi-target effects related to phenolic metabolism. Postharvest Biol. Technol. 2016, 119, 84–93. [Google Scholar] [CrossRef]
- Zhan, L.; Li, Y.; Hu, J.; Pang, L.; Fan, H. Browning inhibition and quality preservation of fresh-cut romaine lettuce exposed to high intensity light. Innov. Food Sci. Emerg. Technol. 2012, 14, 70–76. [Google Scholar] [CrossRef]


| Storage Time (Days) | Treatment | Visual Evaluation (Score) | |||
|---|---|---|---|---|---|
| Color | Freshness | Browning at the Cutting Edge | Overall Acceptance | ||
| 0 | Non/PP | 9.0 ± 0.0 | 9.0 ± 0.0 | 9.0 ± 0.0 | 9.0 ± 0.0 |
| Vac/PP | 9.0 ± 0.0 | 9.0 ± 0.0 | 9.0 ± 0.0 | 9.0 ± 0.0 | |
| Vac/MAP | 9.0 ± 0.0 | 9.0 ± 0.0 | 9.0 ± 0.0 | 9.0 ± 0.0 | |
| 3 | Non/PP | 8.2 ± 0.8 | 7.2 ± 0.4 b | 6.4 ± 0.5 b | 6.2 ± 0.4 b |
| Vac/PP | 8.4 ± 0.5 | 8.4 ± 0.5 a | 7.8 ± 0.8 a | 7.6 ± 0.5 a | |
| Vac/MAP | 8.2 ± 0.4 | 8.2 ± 0.4 a | 7.8 ± 0.8 a | 7.6 ± 0.5 a | |
| 7 | Non/PP | Not Applicable | Not Applicable | Not Applicable | Not Applicable |
| Vac/PP | 6.4 ± 0.5 | 6.0 ± 0.7 | 5.2 ± 0.4 | 5.2 ± 0.4 b | |
| Vac/MAP | 6.8 ± 0.8 | 6.6 ± 0.5 | 6.0 ± 0.7 | 6.4 ± 0.5 a | |
| 9 | Non/PP | Not Applicable | Not Applicable | Not Applicable | Not Applicable |
| Vac/PP | Not Applicable | Not Applicable | Not Applicable | Not Applicable | |
| Vac/MAP | 5.6 ± 1.1 | 5.2 ± 1.1 | 5.8 ± 0.4 | 5.4 ± 0.9 | |
| Storage Time (Days) | Treatment | Total Aerobic Bacteria (log CFU/g) | Yeast and Molds (log CFU/g) | Listeria monocytogenes (/25 g) |
|---|---|---|---|---|
| 0 | Non/PP | 4.02 ± 0.57 a | 1.00 ± 0.00 | Not detected |
| Vac/PP | 2.56 ± 0.23 b | Not detected | Not detected | |
| Vac/MAP | 2.53 ± 0.20 b | Not detected | Not detected | |
| 4 | Non/PP | 4.99 ± 0.06 a | 1.79 ± 0.10 a | Not detected |
| Vac/PP | 4.22 ± 0.07 b | 1.30 ± 0.30 b | Not detected | |
| Vac/MAP | 4.30 ± 0.10 b | 1.10 ± 0.17 b | Not detected | |
| 8 | Non/PP | Not Applicable | Not Applicable | Not Applicable |
| Vac/PP | 6.19 ± 0.43 a | 2.86 ± 0.62 | Not detected | |
| Vac/MAP | 5.46 ± 0.43 b | 2.34 ± 0.59 | Not detected | |
| 10 | Non/PP | Not Applicable | Not Applicable | Not Applicable |
| Vac/PP | Not Applicable | Not Applicable | Not Applicable | |
| Vac/MAP | 6.26 ± 0.57 | 2.58 ± 0.13 | Not detected |
| Criteria | Specification | Shelf Life (Days) | ||||
|---|---|---|---|---|---|---|
| Non/PP | Vac/PP | Vac/MAP | ||||
| Visual quality | ||||||
| Color (score) | ≥5.00 | 5.8 ± 0.4 c | 7.8 ± 0.4 b | 9.2 ± 0.8 a | ||
| Freshness (score) | ≥5.00 | 5.6 ± 0.5 c | 7.8 ± 0.4 b | 9.0 ± 1.0 a | ||
| Browning at the cutting edge (score) | ≥5.00 | 3.8 ± 0.9 c | 7.2 ± 0.4 b | 9.4 ± 0.5 a | ||
| Overall acceptance (score) | ≥5.00 | 4.4 ± 0.9 c | 7.4 ± 0.5 b | 9.2 ± 0.8 a | ||
| Microorganisms | Target | Tolerance | Unaccepted | |||
| Total aerobic bacteria (CFU/g) | 105 | 106 | 108 | 4.3 ± 0.6 c | 8.3 ± 0.6 b | 10.0 ± 0.0 a |
| Yeast and molds (CFU/g) | 103 | 104 | 105 | 4.0 ± 0.0 c | 7.3 ± 0.6 b | 10.0 ± 0.0 a |
| Listeria monocytogenes (/25 g) | Absent | Absent | 102 | 4.0 ± 0.0 | 8.0 ± 0.0 | 10.0 ± 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanakamol, W.; Kongwong, P.; Chuamuangphan, C.; Bundhurat, D.; Boonyakiat, D.; Poonlarp, P. Hurdle Approach for Control of Enzymatic Browning and Extension of Shelf Life of Fresh-Cut Leafy Vegetables Using Vacuum Precooling and Modified Atmosphere Packaging: Commercial Application. Horticulturae 2022, 8, 745. https://doi.org/10.3390/horticulturae8080745
Wanakamol W, Kongwong P, Chuamuangphan C, Bundhurat D, Boonyakiat D, Poonlarp P. Hurdle Approach for Control of Enzymatic Browning and Extension of Shelf Life of Fresh-Cut Leafy Vegetables Using Vacuum Precooling and Modified Atmosphere Packaging: Commercial Application. Horticulturae. 2022; 8(8):745. https://doi.org/10.3390/horticulturae8080745
Chicago/Turabian StyleWanakamol, Warissara, Pratsanee Kongwong, Chaipichit Chuamuangphan, Damorn Bundhurat, Danai Boonyakiat, and Pichaya Poonlarp. 2022. "Hurdle Approach for Control of Enzymatic Browning and Extension of Shelf Life of Fresh-Cut Leafy Vegetables Using Vacuum Precooling and Modified Atmosphere Packaging: Commercial Application" Horticulturae 8, no. 8: 745. https://doi.org/10.3390/horticulturae8080745





