Emerging Technologies for Prolonging Fresh-Cut Fruits’ Quality and Safety during Storage
Abstract
:1. Introduction
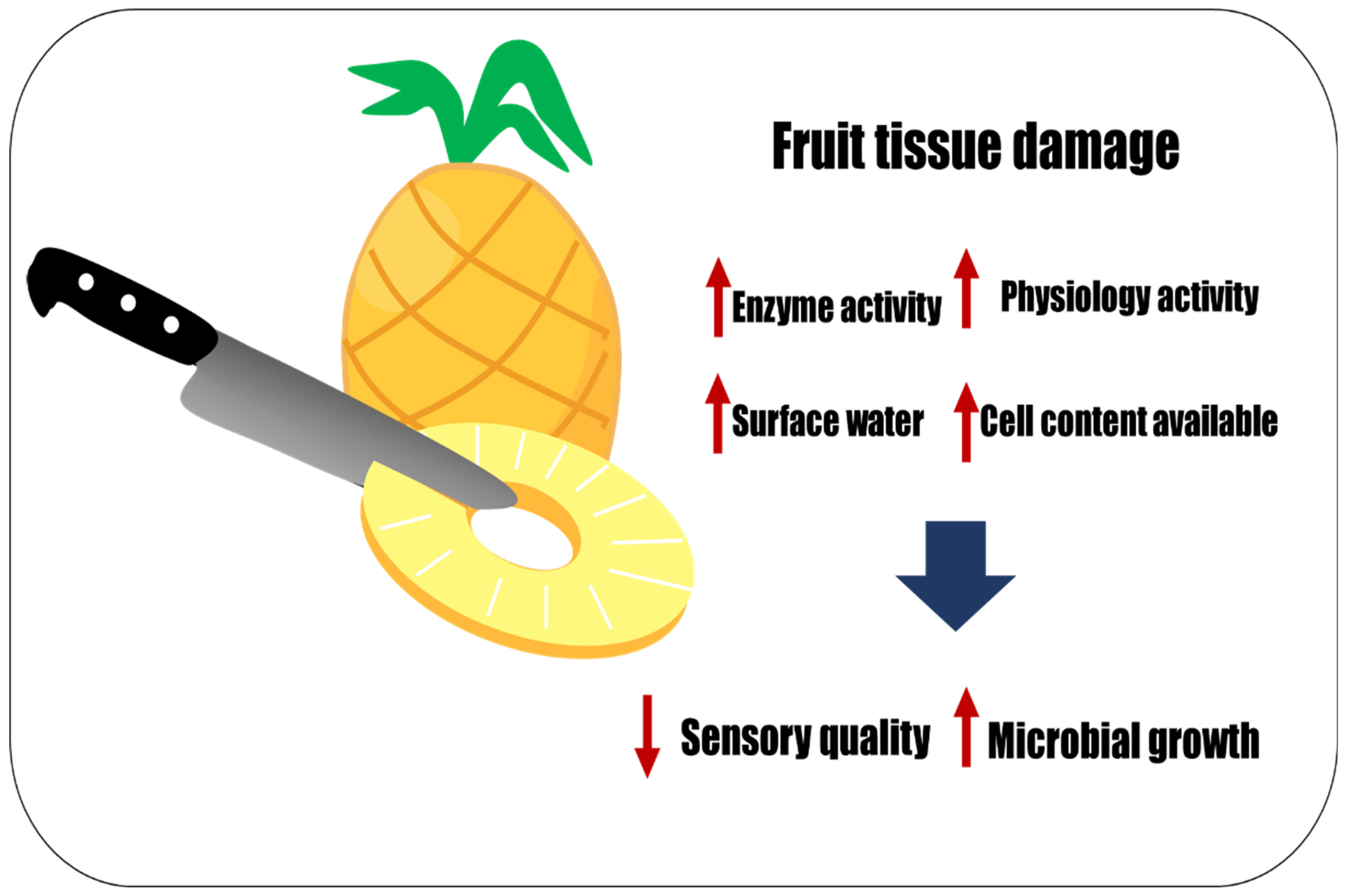
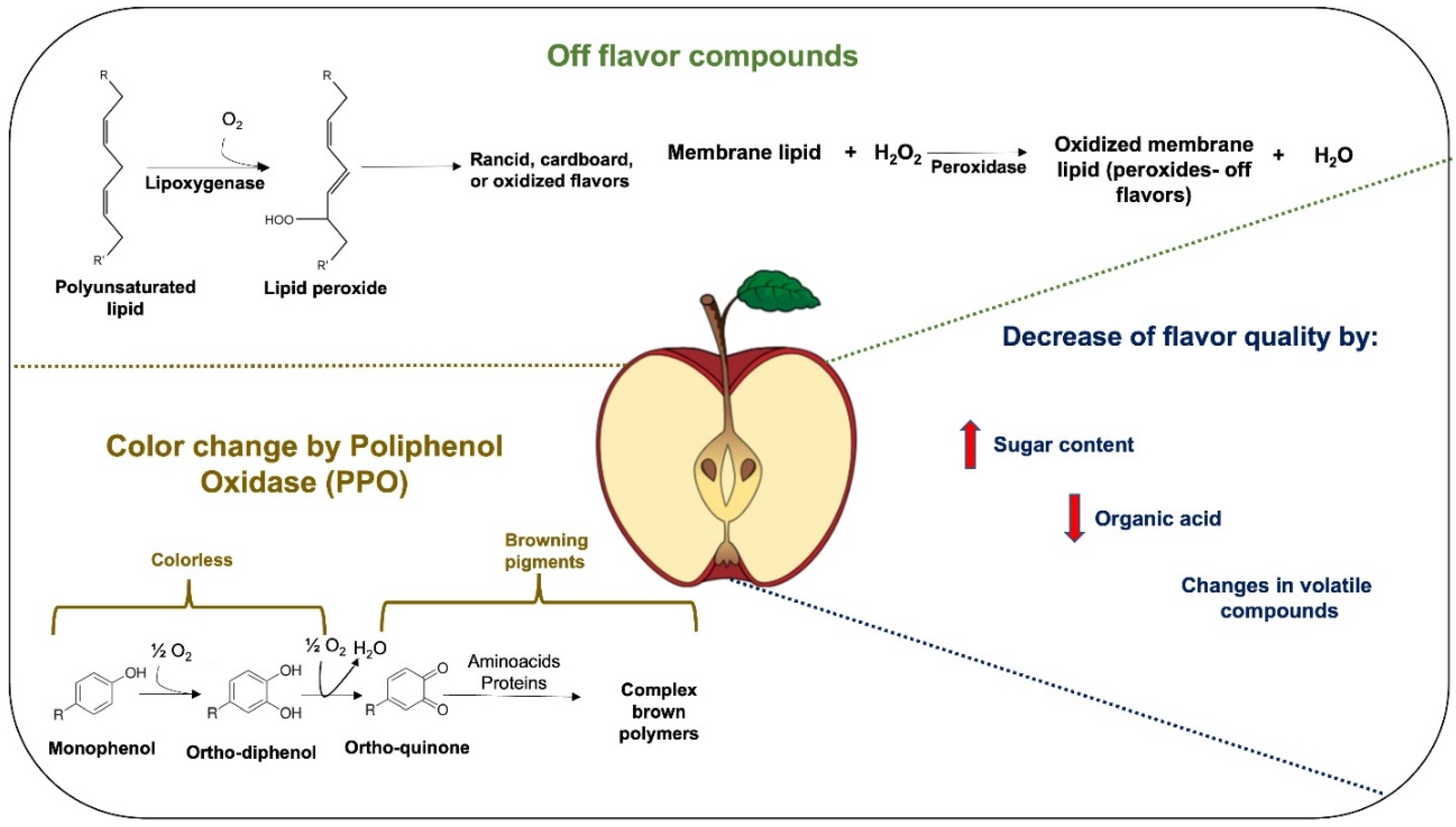
2. Fresh-Cut Fruit Processing Impacts Physicochemical, Sensory, and Microbial Quality
3. Emerging Technologies to Preserve the Shelf Lives of Fresh-Cut Fruits
3.1. Active Packaging
3.1.1. Edible Films and Coatings
| Fresh-Cut Fruit | Edible Film/Coating Material | Treatment Application | Storage Condition | Results | Reference |
|---|---|---|---|---|---|
| Apple | Sodium alginate + Tween-80 + glycerol + thymol-ethanol solution | The container was covered | 5 days at 4 °C |
| [67] |
| Apple | Whey protein concentrate + apple pomace extract | Immersion | 12 days at 5 °C |
| [68] |
| Apple | Sodium alginate + carboxymethyl cellulose + glycerol + calcium chloride + citric acid + shallot waste extracts | Wrapped films | 12 days at 4 °C |
| [69] |
| Apple | Chitosan + ascorbic acid | Immersion | 14 days at 5 °C |
| [70] |
| Apple | Pectin + whey protein + sweet orange essential oil or lemon essential oil | Immersion | 7 days at 4 °C |
| [71] |
| Apple | Chitosan + gelatin + tannic acid | Cover for polyethylene terephthalate packages box | 10 days at 4 °C |
| [72] |
| Kiwifruit | Aloe vera gel + hydroxypropyl methylcellulose + lemon essential oil | Spraying | 10 days at 4 °C |
| [73] |
| Mango | Carrageenan + beeswax | Immersion | 6 days at 6 °C |
| [66] |
| Mango | Citric, ascorbic + potassium sorbate acid + aloe vera | Immersion | 6 days at 7 °C |
| [74] |
| Melon | Citral nanoemulsions + chitosan or carboxymethyl cellulose | Immersion | 7 °C for 14 days |
| [75] |
| Orange | Sodium alginate + cocoa | Immersion | 9 days at 6 °C |
| [32] |
| Papaya | Alginate + oregano essential oil | Immersion | 12 days at 4 °C |
| [76] |
| Papaya | Starch + stearic acid + aloe vera | Immersion | 12 days at 10 °C |
| [77] |
| Pear | Whey protein | Immersion | 4 °C for 28 days |
| [43] |
| Pineapple | Sodium alginate + citral nanoemulsion | Immersion | 4 °C for 12 days |
| [78] |
| Strawberry | Alginate + calcium chloride | Immersion | 15 days at 4 °C |
| [79] |
3.1.2. Modified Atmosphere Packaging
| Bioactive Compounds | MAP | Exposure Time | Result | Fresh-Cut Fruit | References |
|---|---|---|---|---|---|
| Vitamin C | 20% CO2, in air | 10 days | 124 mg/L | Apple (braeburn) | [91] |
| Vitamin C | 7 Kpa CO2 | 28 days | 5.9 mg/100 g | Apple (golden delicious) | [100] |
| Hydroxybenzoic acid | 2.5 O2 + 7 Kpa CO2 | 21 days | 10.1 mg/kg | Strawberry | [101] |
| p-Coumaric acid | 7 Kpa CO2 | 21 days | 7.8 mg/kg | Strawberry | [101] |
| Ellagic acid | 7 Kpa CO2 | 21 days | 73.8 mg/kg | Strawberry | [101] |
| Myricetin | 7 Kpa CO2 | 5 days | 5.2 mg/kg | Strawberry | [101] |
| Quercetin | 7 Kpa CO2 | 21 days | 33.5 mg/kg | Strawberry | [101] |
| Kaempferol | 7 Kpa CO2 | 21 days | 4.0 mg/kg | Strawberry | [101] |
| Vitamin C | 7 Kpa CO2 | 21 days | 400 mg/kg | Strawberry | [101] |
3.2. Natural Preservatives
3.2.1. Antioxidants
3.2.2. Antimicrobials
3.3. Physical Treatments
3.3.1. UV-C Radiation
3.3.2. High Hydrostatic Pressure
3.3.3. Ozone
4. Futures Trends
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Giannakourou, M.C.; Tsironi, T.N. Application of processing and packaging hurdles for fresh-cut fruits and vegetables preservation. Foods 2021, 10, 830. [Google Scholar] [CrossRef]
- Botondi, R.; Barone, M.; Grasso, C. A review into the effectiveness of ozone technology for improving the safety and preserving the quality of fresh-cut fruits and vegetables. Foods 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- FDA. Draft Guidance for Industry: Guide to Minimize Food Safety Hazards of Fresh-Cut Produce. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/draft-guidance-industry-guide-minimize-food-safety-hazards-fresh-cut-produce#:~:text=In%20this%20guidance%2C%20%E2%80%9Cfresh%2D,without%20additional%20processing%20(such%20as (accessed on 12 May 2022).
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Putnik, P.; Bursać Kovačević, D.; Herceg, K.; Levaj, B. Influence of cultivar, anti-browning solutions, packaging gasses, and advanced technology on browning in fresh-cut apples during storage. J. Food Process Eng. 2017, 40, e12400. [Google Scholar] [CrossRef]
- Siddiq, M.; Harte, J.; Beaudry, R.; Dolan, K.; Singh, S.; Saha, K. Physicochemical properties of whole fruit and sensory quality of fresh-cut apples pre-treated with 1-Methylcyclopropene (1-MCP). Int. J. Food Prop. 2014, 17, 1081–1092. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Soliva-Fortuny, R.; Martín-Belloso, O. Control of pathogenic and spoilage microorganisms in fresh-cut fruits and fruit juices by traditional and alternative natural antimicrobials. Compr. Rev. Food Sci. Food Saf. 2009, 8, 157–180. [Google Scholar] [CrossRef]
- Putnik, P.; Roohinejad, S.; Greiner, R.; Granato, D.; Bekhit, A.E.-D.A.; Kovačević, D.B. Prediction and modeling of microbial growth in minimally processed fresh-cut apples packaged in a modified atmosphere: A review. Food Control 2017, 80, 411–419. [Google Scholar] [CrossRef]
- Weisman, R.J.; Heinrich, A.; Letkiewicz, F.; Messner, M.; Studer, K.; Wang, L.; Regli, S. Estimating national exposures and potential bladder cancer cases associated with chlorination DBPs in US drinking water. Environ. Health Perspect. 2022, 130, 087002. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, B.; Wu, S.; Siddiqui, M.W. Incorporating essential oils or compounds derived thereof into edible coatings: Effect on quality and shelf life of fresh/fresh-cut produce. Trends Food Sci. Technol. 2021, 108, 245–257. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, W.; Chen, Y.; Peng, Y. Browning inhibition of plant extracts on fresh-cut fruits and vegetables—A review. J. Food Process. Preserv. 2022, 46, e16532. [Google Scholar] [CrossRef]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as edible films and coatings: Characteristics and influence on fruit and vegetable quality—A review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Farcuh, M.; Copes, B.; Le-Navenec, G.; Marroquin, J.; Cantu, D.; Bradford, K.J.; Guinard, J.-X.; Van Deynze, A. Sensory, physicochemical and volatile compound analysis of short and long shelf-life melon (Cucumis melo L.) genotypes at harvest and after postharvest storage. Food Chem. X 2020, 8, 100107. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.T.; Liang, Y.Q.; Chai, W.M.; Wei, Q.M.; Yu, Z.Y.; Wang, L.J. Effect of ascorbic acid on tyrosinase and its anti-browning activity in fresh-cut Fuji apple. J. Food Biochem. 2021, 45, e13995. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kasote, D.M.; Jayaprakasha, G.K.; Avila, C.A.; Crosby, K.M.; Patil, B.S. Effect of production system and inhibitory potential of aroma volatiles on polyphenol oxidase and peroxidase activity in tomatoes. J. Sci. Food Agric. 2021, 101, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ren, L.; Li, M.; Qian, J.; Fan, J.; Du, B. Effects of clove essential oil and eugenol on quality and browning control of fresh-cut lettuce. Food Chem. 2017, 214, 432–439. [Google Scholar] [CrossRef]
- Zhou, X.; Xiao, Y.; Meng, X.; Liu, B. Full inhibition of Whangkeumbae pear polyphenol oxidase enzymatic browning reaction by L-cysteine. Food Chem. 2018, 266, 1–8. [Google Scholar] [CrossRef]
- De Barcelos Costa, H.C.; Siguemoto, É.S.; Cavalcante, T.A.B.B.; de Oliveira Silva, D.; Vieira, L.G.M.; Gut, J.A.W. Effect of microwave-assisted processing on polyphenol oxidase and peroxidase inactivation kinetics of açai-berry (Euterpe oleracea) pulp. Food Chem. 2021, 341, 128287. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Wang, Q.; Lu, T.; Ma, H.; Chen, X. The effects of ultrasonication on the phytochemicals, antioxidant, and polyphenol oxidase and peroxidase activities in coffee leaves. Food Chem. 2022, 373, 131480. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, A.L.T.; Leite, T.S.; Cristianini, M. High isostatic pressure and thermal processing of açaí fruit (Euterpe oleracea Martius): Effect on pulp color and inactivation of peroxidase and polyphenol oxidase. Food Res. Int. 2018, 105, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Engmann, F.N.; Ma, Y.; Zhang, H.; Yu, L.; Deng, N. The application of response surface methodology in studying the effect of heat and high hydrostatic pressure on anthocyanins, polyphenol oxidase, and peroxidase of mulberry (Morus nigra) juice. J. Sci. Food Agric. 2014, 94, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
- Istiqamah, A.; Lioe, H.N.; Adawiyah, D.R. Umami compounds present in low molecular umami fractions of asam sunti–A fermented fruit of Averrhoa bilimbi L. Food Chem. 2019, 270, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Meng, K.; Hou, Y.; Han, Y.; Ban, Q.; He, Y.; Suo, J.; Rao, J. Exploring the functions of 9-lipoxygenase (DkLOX3) in ultrastructural changes and hormonal stress response during persimmon fruit storage. Int. J. Mol. Sci. 2017, 18, 589. [Google Scholar] [CrossRef] [PubMed]
- Defilippi, B.G.; Ejsmentewicz, T.; Covarrubias, M.P.; Gudenschwager, O.; Campos-Vargas, R. Changes in cell wall pectins and their relation to postharvest mesocarp softening of “Hass” avocados (Persea americana Mill.). Plant Physiol. Biochem. 2018, 128, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Hernández, R.M.; González-Aguilar, G.A.; Tiznado-Hernández, M.E. Utilization of physicochemical variables developed from changes in sensory attributes and consumer acceptability to predict the shelf life of fresh-cut mango fruit. J. Food Sci. Technol. 2015, 52, 63–77. [Google Scholar] [CrossRef]
- Jayathunge, K.; Gunawardhana, D.; Illeperuma, D.; Chandrajith, U.; Thilakarathne, B.; Fernando, M.; Palipane, K. Physico-chemical and sensory quality of fresh cut papaya (Carica papaya) packaged in micro-perforated polyvinyl chloride containers. J. Food Sci. Technol. 2014, 51, 3918–3925. [Google Scholar] [CrossRef]
- Sanchís, E.; Mateos, M.; Pérez-Gago, M.B. Physicochemical, sensory, and nutritional quality of fresh-cut “Rojo Brillante” persimmon affected by maturity stage and antibrowning agents. Food Sci. Technol. Int. 2016, 22, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Kitzberger, C.S.G.; da Silva, C.M.; dos Santos Scholz, M.B.; Ferreira, M.I.F.; Bauchrowitz, I.M.; Eilert, J.B.; dos Santos Neto, J. Physicochemical and sensory characteristics of plums accesses (Prunus salicina). AIMS AgriFood 2017, 2, 101–112. [Google Scholar]
- Leguizamon-Delgado, M.A.; Duque-Cifuentes, A.L.; Quintero-Castaño, V.D. Physico-chemical and sensory evaluation of a mango-based fruit bar. Dyna 2019, 86, 276–283. [Google Scholar] [CrossRef]
- Rana, S.S.; Pradhan, R.C.; Mishra, S. Image analysis to quantify the browning in fresh cut tender jackfruit slices. Food Chem. 2019, 278, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Tang, S.; Fang, X.; Wang, Z.; Jiang, Y.; Guo, X.; Zhu, J.; Zhang, Y. The Effect of Lactiplantibacillus plantarum BX62 alone or in combination with chitosan on the qualitative characteristics of fresh-cut apples during cold storage. Microorganisms 2021, 9, 2404. [Google Scholar] [CrossRef] [PubMed]
- Glicerina, V.; Siroli, L.; Betoret, E.; Canali, G.; Dalla Rosa, M.; Lanciotti, R.; Romani, S. Characterization and evaluation of the influence of an alginate, cocoa and a bilayer alginate–cocoa coating on the quality of fresh-cut oranges during storage. J. Sci. Food Agric. 2022, 102, 4454–4461. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, B.; Deshi, V.; Ozturk, B.; Siddiqui, M.W. Fresh-cut fruits and vegetables: Quality issues and safety concerns. In Fresh-Cut Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–15. [Google Scholar]
- Zhao, P.; Ndayambaje, J.P.; Liu, X.; Xia, X. Microbial spoilage of fruits: A review on causes and prevention methods. Food Rev. Int. 2020, 1–22. [Google Scholar] [CrossRef]
- Zhang, H.; Yamamoto, E.; Murphy, J.; Locas, A. Microbiological safety of ready-to-eat fresh-cut fruits and vegetables sold on the Canadian retail market. Int. J. Food Microbiol. 2020, 335, 108855. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Solà, J.; Valero, A.; Abadias, M.; Nicolau-Lapeña, I.; Viñas, I. Evaluation of water-assisted UV-C light and its additive effect with peracetic acid for the inactivation of Listeria monocytogenes, Salmonella enterica and murine norovirus on whole and fresh-cut strawberries during shelf-life. J. Sci. Food Agric. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate outbreaks of foodborne illness in the United States associated with fresh produce from 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef]
- Hasan, S.K.; Ferrentino, G.; Scampicchio, M. Nanoemulsion as advanced edible coatings to preserve the quality of fresh-cut fruits and vegetables: A review. Int. J. Food Sci. Technol. 2020, 55, 1–10. [Google Scholar] [CrossRef]
- Hodges, D.M.; Toivonen, P.M. Quality of fresh-cut fruits and vegetables as affected by exposure to abiotic stress. Postharvest Biol. Technol. 2008, 48, 155–162. [Google Scholar] [CrossRef]
- Dea, S.; Brecht, J.K.; Nunes, M.C.N.; Baldwin, E.A. Occurrence of chilling injury in fresh-cut ‘Kent’mangoes. Postharvest Biol. Technol. 2010, 57, 61–71. [Google Scholar] [CrossRef]
- Marrero, A.; Kader, A.A. Optimal temperature and modified atmosphere for keeping quality of fresh-cut pineapples. Postharvest Biol. Technol. 2006, 39, 163–168. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, C.; Zhai, X.; Zhang, R.; Zhang, S.; Sun, C.; Wang, W.; Hou, H. Effect of lipids with different physical state on the physicochemical properties of starch/gelatin edible films prepared by extrusion blowing. Int. J. Biol. Macromol. 2021, 185, 1005–1014. [Google Scholar] [CrossRef]
- Galus, S.; Mikus, M.; Ciurzyńska, A.; Domian, E.; Kowalska, J.; Marzec, A.; Kowalska, H. The effect of whey protein-based edible coatings incorporated with lemon and lemongrass essential oils on the quality attributes of fresh-cut pears during storage. Coatings 2021, 11, 745. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, Y.; Xie, J.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W.; Li, S.; Liu, Y. Optimization, characterization and evaluation of papaya polysaccharide-corn starch film for fresh cut apples. Int. J. Biol. Macromol. 2021, 166, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Flores-Martínez, N.; Valdez-Fragoso, A.; Jiménez-Islas, H.; Pérez-Pérez, M. Physical, barrier, mechanical and microstructural properties of Aloe vera-gelatin-glycerol edible films incorporated with Pimenta dioica L. Merrill essential oil. Rev. Mex. Ing. Quim. 2017, 16, 109–119. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible films and coatings as food-quality preservers: An overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Pashova, S.; Radev, R.; Dimitrov, G.; Ivanov, J. Edible coatings in food industry related to circular economy. Calitatea 2018, 19, 111–117. [Google Scholar]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the art of antimicrobial edible coatings for food packaging applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial edible films in food packaging: Current scenario and recent nanotechnological advancements-a review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Radev, R.; Pashova, S. Application of edible films and coatings for fresh fruit and vegetables. Qual. Access Success 2020, 21, 108–112. [Google Scholar]
- Hashemi, S.M.B.; Jafarpour, D. Bioactive edible film based on Konjac glucomannan and probiotic Lactobacillus plantarum strains: Physicochemical properties and shelf life of fresh-cut kiwis. J. Food Sci. 2021, 86, 513–522. [Google Scholar] [CrossRef]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Restrepo, A.E.; Rojas, J.D.; García, O.R.; Sánchez, L.T.; Pinzón, M.I.; Villa, C.C. Mechanical, barrier, and color properties of banana starch edible films incorporated with nanoemulsions of lemongrass (Cymbopogon citratus) and rosemary (Rosmarinus officinalis) essential oils. Food Sci. Technol. Int. 2018, 24, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Zhang, Y.; Wang, J.; Sun, Y.; Gao, X.; Xiong, G.; Liang, J. Preparation and antioxidant activity of sodium alginate and carboxymethyl cellulose edible films with epigallocatechin gallate. Int. J. Biol. Macromol. 2019, 134, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Biliaderis, C.G. Edible films and coatings with pectin. Pectin Technol. Physiol. Prop. 2020, 99–123. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Tyagi, S.; Gupta, R.K.; Tyagi, Y.K. Natural gums of plant origin as edible coatings for food industry applications. Crit. Rev. Biotechnol. 2017, 37, 959–973. [Google Scholar] [CrossRef]
- Viana, R.M.; Sá, N.M.; Barros, M.O.; de Fátima Borges, M.; Azeredo, H.M. Nanofibrillated bacterial cellulose and pectin edible films added with fruit purees. Carbohydr. Polym. 2018, 196, 27–32. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Baldwin, E.A. Surface treatments and edible coatings in food preservation. In Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2020; pp. 507–528. [Google Scholar]
- Feng, Z.; Wu, G.; Liu, C.; Li, D.; Jiang, B.; Zhang, X. Edible coating based on whey protein isolate nanofibrils for antioxidation and inhibition of product browning. Food Hydrocoll. 2018, 79, 179–188. [Google Scholar] [CrossRef]
- Shendurse, A.; Gopikrishna, G.; Patel, A.; Pandya, A. Milk protein based edible films and coatings–preparation, properties and food applications. J. Nutr. Health Food Eng. 2018, 8, 219–226. [Google Scholar] [CrossRef]
- Sharma, P.; Shehin, V.; Kaur, N.; Vyas, P. Application of edible coatings on fresh and minimally processed vegetables: A review. Int. J. Veg. Sci. 2019, 25, 295–314. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of edible coating with essential oil in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 2467–2480. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.S.; Anjum, M.A.; Naz, S.; Ali, S.; Hussain, S.; Azam, M.; Sardar, H.; Khaliq, G.; Canan, İ.; Ejaz, S. Incorporation of ascorbic acid in chitosan-based edible coating improves postharvest quality and storability of strawberry fruits. Int. J. Biol. Macromol. 2021, 189, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Afifah, N.; Ratnawati, L.; Darmajana, D. Evaluation of Plasticizer Addition in Composite Edible Coating on Quality of Fresh-Cut Mangoes during Storage. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 2nd International Conference on Natural Products and Bioresource Sciences—2018, Tangerang, Indonesia, 1–2 November 2018; IOP Publishing: Bristol, UK, 2019; p. 012029. [Google Scholar]
- Chen, J.; Wu, A.; Yang, M.; Ge, Y.; Pristijono, P.; Li, J.; Xu, B.; Mi, H. Characterization of sodium alginate-based films incorporated with thymol for fresh-cut apple packaging. Food Control 2021, 126, 108063. [Google Scholar] [CrossRef]
- Hammad, K.; Elsayed, N.; Elkashef, H. Development of a whey protein concentrate/apple pomace extract edible coating for shelf life extension of fresh-cut apple. Int. Food Res. J. 2021, 28, 377–385. [Google Scholar] [CrossRef]
- Thivya, P.; Bhosale, Y.; Anandakumar, S.; Hema, V.; Sinija, V. Development of active packaging film from sodium alginate/carboxymethyl cellulose containing shallot waste extracts for anti-browning of fresh-cut produce. Int. J. Biol. Macromol. 2021, 188, 790–799. [Google Scholar] [CrossRef]
- Özdemir, K.S.; Gökmen, V. Effect of chitosan-ascorbic acid coatings on the refrigerated storage stability of fresh-cut apples. Coatings 2019, 9, 503. [Google Scholar] [CrossRef]
- Sumonsiri, N.; Danpongprasert, W.; Thaidech, K. Comparison of sweet orange (Citrus sinensis) and lemon (Citrus limonum) essential oils on qualities of fresh-cut apples during storage. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2020, 21, 47–57. [Google Scholar]
- Zhang, C.; Yang, Z.; Shi, J.; Zou, X.; Zhai, X.; Huang, X.; Li, Z.; Holmes, M.; Daglia, M.; Xiao, J. Physical properties and bioactivities of chitosan/gelatin-based films loaded with tannic acid and its application on the preservation of fresh-cut apples. LWT 2021, 144, 111223. [Google Scholar] [CrossRef]
- Passafiume, R.; Gaglio, R.; Sortino, G.; Farina, V. Effect of three different aloe vera gel-based edible coatings on the quality of fresh-cut “Hayward” kiwifruits. Foods 2020, 9, 939. [Google Scholar] [CrossRef]
- Suriati, L.; Utama, I.; Harsojuwono, B.A.; Gunam, I.B.W. Ecogel incorporated with nano-additives to increase shelf-life of fresh-cut mango. J. Appl. Hortic. 2020, 22, 189–195. [Google Scholar]
- Arnon-Rips, H.; Porat, R.; Poverenov, E. Enhancement of agricultural produce quality and storability using citral-based edible coatings; the valuable effect of nano-emulsification in a solid-state delivery on fresh-cut melons model. Food Chem. 2019, 277, 205–212. [Google Scholar] [CrossRef]
- Tabassum, N.; Khan, M.A. Modified atmosphere packaging of fresh-cut papaya using alginate based edible coating: Quality evaluation and shelf life study. Sci. Hortic. 2020, 259, 108853. [Google Scholar] [CrossRef]
- Kathiresan, S.; Lasekan, O. Effects of glycerol and stearic acid on the performance of chickpea starch-based coatings applied to fresh-cut papaya. CYTA-J. Food. 2019, 17, 365–374. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R.; Vadivel, V. Citral nanoemulsion incorporated edible coating to extend the shelf life of fresh cut pineapples. LWT 2020, 118, 108851. [Google Scholar] [CrossRef]
- Alharaty, G.; Ramaswamy, H.S. The effect of sodium alginate-calcium chloride coating on the quality parameters and shelf life of strawberry cut fruits. J. Compos. Sci. 2020, 4, 123. [Google Scholar] [CrossRef]
- Farina, V.; Passafiume, R.; Tinebra, I.; Palazzolo, E.; Sortino, G. Use of aloe vera gel-based edible coating with natural anti-browning and anti-oxidant additives to improve post-harvest quality of fresh-cut ‘fuji’apple. Agronomy 2020, 10, 515. [Google Scholar] [CrossRef]
- Tapia-Blácido, D.R.; da Silva Ferreira, M.E.; Aguilar, G.J.; Costa, D.J.L. Biodegradable packaging antimicrobial activity. In Processing and Development of Polysaccharide-Based Biopolymers for Packaging Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 207–238. [Google Scholar]
- Zhang, S.; Wei, F.; Han, X. An edible film of sodium alginate/pullulan incorporated with capsaicin. New J. Chem. 2018, 42, 17756–17761. [Google Scholar] [CrossRef]
- Marquez, G.R.; Di Pierro, P.; Mariniello, L.; Esposito, M.; Giosafatto, C.V.; Porta, R. Fresh-cut fruit and vegetable coatings by transglutaminase-crosslinked whey protein/pectin edible films. LWT 2017, 75, 124–130. [Google Scholar] [CrossRef]
- Dhall, R. Advances in edible coatings for fresh fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef]
- Aayush, K.; McClements, D.J.; Sharma, S.; Sharma, R.; Singh, G.P.; Sharma, K.; Oberoi, K. Innovations in the development and application of edible coatings for fresh and minimally processed Apple. Food Control 2022, 141, 109188. [Google Scholar] [CrossRef]
- Nayak, S.L.; Sethi, S.; Sharma, R.; Prajapati, U. Active edible coatings for fresh fruits and vegetables. In Polymers for Agri-Food Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 417–432. [Google Scholar]
- Cozzolino, R.; Cefola, M.; Pace, B.; Malorni, L.; Martignetti, A.; Montemurro, N.; Pellicano, M.P. Quality, sensory and volatile profiles of fresh-cut big top nectarines cold stored in air or modified atmosphere packaging. Int. J. Food Sci. Technol. 2018, 53, 1736–1743. [Google Scholar] [CrossRef]
- Iglesias, M.; Abadias, M.; Anguera, M.; Viñas, I. Efficacy of Pseudomonas graminis CPA-7 against Salmonella spp. and Listeria monocytogenes on fresh-cut pear and setting up of the conditions for its commercial application. Food Microbiol. 2018, 70, 103–112. [Google Scholar] [CrossRef]
- United Nations. Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/sustainable-consumption-production/ (accessed on 23 March 2022).
- Matar, C.; Guillard, V.; Gauche, K.; Costa, S.; Gontard, N.; Guilbert, S.; Gaucel, S. Consumer behaviour in the prediction of postharvest losses reduction for fresh strawberries packed in modified atmosphere packaging. Postharvest Biol. Technol. 2020, 163, 111119. [Google Scholar] [CrossRef]
- Rux, G.; Bohne, K.; Huyskens-Keil, S.; Ulrichs, C.; Hassenberg, K.; Herppich, W. Effects of modified atmosphere and sugar immersion on physiology and quality of fresh-cut ‘Braeburn’ apples. Food Packag. Shelf Life 2021, 29, 100726. [Google Scholar] [CrossRef]
- Iturralde-García, R.D.; García-Regueiro, J.A.; Castañé, C.; Riudavets, J. Sorption of carbon dioxide by chickpeas packaged in modified atmospheres. J. Stored Prod. Res. 2019, 83, 54–60. [Google Scholar] [CrossRef]
- Dorostkar, M.; Moradinezhad, F.; Ansarifar, E. Influence of active modified atmosphere packaging pre-treatment on shelf life and quality attributes of cold stored apricot fruit. Int. J. Fruit Sci. 2022, 22, 402–413. [Google Scholar] [CrossRef]
- Gorny, J.R. A summary of CA and MA requirements and recommendations for fresh-cut (minimally processed) fruits and vegetables. Acta Hortic. 2003, 600, 609–614. [Google Scholar] [CrossRef]
- Ballard, C.R.; Junior, M.R.M. Health benefits of flavonoids. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 185–201. [Google Scholar]
- Wang, J.; Li, P.; Gong, B.; Li, S.; Ma, H. Phenol metabolism and preservation of fresh in-hull walnut stored in modified atmosphere packaging. J. Sci. Food Agric. 2017, 97, 5335–5342. [Google Scholar] [CrossRef] [PubMed]
- Agar, I.; Streif, J.; Bangerth, F. Effect of high CO2 and controlled atmosphere (CA) on the ascorbic and dehydroascorbic acid content of some berry fruits. Postharvest Biol. Technol. 1997, 11, 47–55. [Google Scholar] [CrossRef]
- Sun, T.; Tadmor, Y.; Li, L. Pathways for carotenoid biosynthesis, degradation, and storage. In Plant and Food Carotenoids; Springer: Berlin/Heidelberg, Germany, 2020; pp. 3–23. [Google Scholar]
- Mapelli-Brahm, P.; Barba, F.J.; Remize, F.; Garcia, C.; Fessard, A.; Khaneghah, A.M.; Sant’Ana, A.S.; Lorenzo, J.M.; Montesano, D.; Meléndez-Martínez, A.J. The impact of fermentation processes on the production, retention and bioavailability of carotenoids: An overview. Trends Food Sci. Technol. 2020, 99, 389–401. [Google Scholar] [CrossRef]
- Soliva-Fortuny, R.C.; Elez-Martínez, P.; Martín-Belloso, O. Microbiological and biochemical stability of fresh-cut apples preserved by modified atmosphere packaging. Innov. Food Sci. Emerg. Technol. 2004, 5, 215–224. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Changes in bioactive composition of fresh-cut strawberries stored under superatmospheric oxygen, low-oxygen or passive atmospheres. J. Food Compos. Anal. 2010, 23, 37–43. [Google Scholar] [CrossRef]
- Smith, A.; Waldron, K.; Maness, N.; Perkins-Veazie, P. Vegetable texture: Measurement and structural implications. In Postharvest Phyusiology and Pathology of Vegetables, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 2003; pp. 297–330. [Google Scholar]
- Cliff, M.A.; Toivonen, P.M.; Forney, C.F.; Lu, C. Quality of fresh-cut apple slices stored in solid and micro-perforated film packages having contrasting O2 headspace atmospheres. Postharvest Biol. Technol. 2010, 58, 254–261. [Google Scholar] [CrossRef]
- Rux, G.; Efe, E.; Ulrichs, C.; Huyskens-Keil, S.; Hassenberg, K.; Herppich, W.B. Effects of pre-processing short-term hot-water treatments on quality and shelf life of fresh-cut apple slices. Foods 2019, 8, 653. [Google Scholar] [CrossRef]
- Siddiq, R.; Auras, R.; Siddiq, M.; Dolan, K.D.; Harte, B. Effect of modified atmosphere packaging (MAP) and NatureSeal® treatment on the physico-chemical, microbiological, and sensory quality of fresh-cut d’Anjou pears. Food Packag. Shelf Life 2020, 23, 100454. [Google Scholar] [CrossRef]
- Feliziani, E.; Romanazzi, G. Postharvest decay of strawberry fruit: Etiology, epidemiology, and disease management. J. Berry Res. 2016, 6, 47–63. [Google Scholar] [CrossRef]
- Mitcham, E.J. Strawberry. In The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks; Agriculture Handbook; Gross, K.C., Wang, C.Y., Saltveit, M., Eds.; United States Department of Agriculture, Agricultural Research Service: Davis, CA, USA, 2016; pp. 559–561. [Google Scholar]
- Nakata, Y.; Izumi, H. Microbiological and quality responses of strawberry fruit to high CO2 controlled atmosphere and modified atmosphere storage. HortScience 2020, 55, 386–391. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Dias, C.; Fonseca, A.; Amaro, A.L.; Vilas-Boas, A.A.; Oliveira, A.; Santos, S.A.; Silvestre, A.J.; Rocha, S.M.; Isidoro, N.; Pintado, M. Natural-based antioxidant extracts as potential mitigators of fruit browning. Antioxidants 2020, 9, 715. [Google Scholar] [CrossRef]
- Vijayalaxmi, S.; Jayalakshmi, S.; Sreeramulu, K. Polyphenols from different agricultural residues: Extraction, identification and their antioxidant properties. J. Food Sci. Technol. 2015, 52, 2761–2769. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Mercado, A.T.; Ayala-Zavala, J.F.; Cruz-Valenzuela, M.R.; Gonzalez-Aguilar, G.A.; Nazzaro, F.; Fratianni, F.; Miranda, M.R.A.d.; Silva-Espinoza, B.A. Using sensory evaluation to determine the highest acceptable concentration of mango seed extract as antibacterial and antioxidant agent in fresh-cut mango. Foods 2018, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Arzuaga, M.; Salsi, M.S.; Piagentini, A.M. Storage quality of fresh-cut apples treated with yerba mate (Ilex paraguariensis). J. Food Sci. Technol. 2021, 58, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Arzuaga, M.; Piagentini, A.M. New antioxidant treatment with yerba mate (Ilex paraguariensis) infusion for fresh-cut apples: Modeling, optimization, and acceptability. Food Sci. Technol. Int. 2018, 24, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Akbari, E.; Gholami, M.; Ghobadi, C. Shelf-life and quality attributes in fresh-cut pear cv. Shahmive treated with different kinds of antioxidants. J. Food Sci. Technol. 2019, 56, 3998–4008. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, F.L.; Essa, M.M.; Arivazhagan, G.; Guizani, N.; Hyuk, S. Evaluation of food protective property of five natural products using fresh-cut apple slice model. Pak. J. Biol. Sci. 2012, 15, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Falagán, N.; Artés, F.; Aguayo, E. Natural additives to preserve quality and improve nutritional value of fresh-cut nectarine. Food Sci. Technol. Int. 2016, 22, 429–439. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, W.; Murtaza, A.; Iqbal, A.; Li, J.; Zhang, J.; Li, J.; Kong, M.; Xu, X.; Pan, S. Eugenol treatment delays the flesh browning of fresh-cut water chestnut (Eleocharis tuberosa) through regulating the metabolisms of phenolics and reactive oxygen species. Food Chem. X 2022, 14, 100307. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Huber, D.J.; Su, Z.; Hu, M.; Gao, Z.; Li, M.; Shi, X.; Zhang, Z. Effect of postharvest spray of apple polyphenols on the quality of fresh-cut red pitaya fruit during shelf life. Food Chem. 2018, 243, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, W.; Liu, S.; Liu, C.; Zheng, L. Effects of melatonin treatment on the enzymatic browning and nutritional quality of fresh-cut pear fruit. Food Chem. 2019, 299, 125116. [Google Scholar] [CrossRef] [PubMed]
- Supapvanich, S.; Yimpong, A.; Srisuwanwichan, J. Browning inhibition on fresh-cut apple by the immersion of liquid endosperm from mature coconuts. J. Food Sci. Technol. 2020, 57, 4424–4431. [Google Scholar] [CrossRef]
- Xiao, Y.; Xie, J.; Wu, C.; He, J.; Wang, B. Effects of melatonin treatment on browning alleviation of fresh-cut foods. J. Food Biochem. 2021, 45, e13798. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.M.; Jeganathan, B.; Vasanthan, T.; Roopesh, M. Dipping fresh-cut apples in citric acid before plasma-integrated low-pressure cooling improves Salmonella and polyphenol oxidase inactivation. J. Sci. Food Agric. 2022, 102, 3425–3434. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, T.; Shen, B.; Sun, S.; Song, H.; Chen, D.; Xi, W. Citric acid treatment reduces decay and maintains the postharvest quality of peach (Prunus persica L.) fruit. Food Sci. Nutr. 2019, 7, 3635–3643. [Google Scholar] [CrossRef] [PubMed]
- Dan, Y.; Zhang, S.; Zhong, H.; Yi, H.; Sainz, M.B. Novel compounds that enhance Agrobacterium-mediated plant transformation by mitigating oxidative stress. Plant Cell Rep. 2015, 34, 291–309. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, N.; Wang, J.; Zhang, H.; Li, D.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria× anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef]
- Belgacem, I.; Schena, L.; Teixidó, N.; Romeo, F.; Ballistreri, G.; Abadias, M. Effectiveness of a pomegranate peel extract (PGE) in reducing Listeria monocytogenes in vitro and on fresh-cut pear, apple and melon. Eur. Food Res. Technol. 2020, 246, 1765–1772. [Google Scholar] [CrossRef]
- Ding, P.; Lee, Y. Use of essential oils for prolonging postharvest life of fresh fruits and vegetables. Int. Food Res. J. 2019, 26, 363–366. [Google Scholar]
- Song, Z.; Li, F.; Guan, H.; Xu, Y.; Fu, Q.; Li, D. Combination of nisin and ε-polylysine with chitosan coating inhibits the white blush of fresh-cut carrots. Food Control 2017, 74, 34–44. [Google Scholar] [CrossRef]
- Giacometti, J.; Kovačević, D.B.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Chen, B.; McClements, D.J. Improving the efficacy of essential oils as antimicrobials in foods: Mechanisms of action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef]
- Ahmadi, S.; Fazilati, M.; Nazem, H.; Mousavi, S.M. Green synthesis of magnetic nanoparticles using Satureja hortensis essential oil toward superior antibacterial/fungal and anticancer performance. Biomed Res. Int. 2021, 2021, 8822645. [Google Scholar] [CrossRef] [PubMed]
- Ragno, R.; Papa, R.; Patsilinakos, A.; Vrenna, G.; Garzoli, S.; Tuccio, V.; Fiscarelli, E.; Selan, L.; Artini, M. Essential oils against bacterial isolates from cystic fibrosis patients by means of antimicrobial and unsupervised machine learning approaches. Sci. Rep. 2020, 10, 2653. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Burton, D.; Parra, F.; López, J.; Muñoz, P.; Escobar, H.; Parra, C. Antioxidant and antibacterial capacities of Origanum vulgare l. Essential oil from the arid andean region of chile and its chemical characterization by GC-MS. Metabolites 2020, 10, 414. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Coppo, E.; Barbieri, R.; Barreca, D.; Chebaibi, S.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M.; Daglia, M. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: Mechanisms, synergies and bio-inspired anti-infective materials. Biofouling 2018, 34, 630–656. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial mechanism of oregano essential oil. Ind. Crops Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Edible coatings and antimicrobial nanoemulsions for enhancing shelf life and reducing foodborne pathogens of fruits and vegetables: A review. Sustain. Mater. Technol. 2020, 26, e00215. [Google Scholar] [CrossRef]
- Klangmuang, P.; Sothornvit, R. Active coating from hydroxypropyl methylcellulose-based nanocomposite incorporated with Thai essential oils on mango (cv. Namdokmai Sithong). Food Biosci. 2018, 23, 9–15. [Google Scholar] [CrossRef]
- Radi, M.; Akhavan-Darabi, S.; Akhavan, H.R.; Amiri, S. The use of orange peel essential oil microemulsion and nanoemulsion in pectin-based coating to extend the shelf life of fresh-cut orange. J. Food Process. Preserv. 2018, 42, e13441. [Google Scholar] [CrossRef]
- Tomadoni, B.; Moreira, M.d.R.; Pereda, M.; Ponce, A.G. Gellan-based coatings incorporated with natural antimicrobials in fresh-cut strawberries: Microbiological and sensory evaluation through refrigerated storage. LWT 2018, 97, 384–389. [Google Scholar] [CrossRef]
- Lima, M.d.C.; de Sousa, C.P.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.; De Souza, E. A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb. Pathog. 2019, 130, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic compounds diminish antibiotic resistance of Staphylococcus aureus clinical strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Nithyanand, P.; Vadivel, V. In vitro antibacterial activity of nut by-products against foodborne pathogens and their application in fresh-cut fruit model. J. Food Sci. Technol. 2018, 55, 4304–4310. [Google Scholar] [CrossRef]
- Badr, A.N.; Gromadzka, K.; Shehata, M.G.; Stuper-Szablewska, K.; Drzewiecka, K.; Abdel-Razek, A.G.; Youssef, M.M. Encapsulated Bioactive Ingredients of grape by-products applicate in fresh-cut fruit and juices diminished the ochratoxins. J. Food Process. Preserv. 2021, 45, e15112. [Google Scholar] [CrossRef]
- Falleh, H.; Jemaa, M.B.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential oils and their application in food safety. Front. Sustain. Food Syst. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Han, C.; Zhen, W.; Chen, Q.; Fu, M. UV-C irradiation inhibits surface discoloration and delays quality degradation of fresh-cut stem lettuce. LWT 2021, 147, 111533. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Han, C.; Ji, N.; Jin, P.; Zheng, Y. UV-C treatment maintains quality and enhances antioxidant capacity of fresh-cut strawberries. Postharvest Biol. Technol. 2019, 156, 110945. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Ma, Y.; Zhang, M.; Zhao, Y.; Zhao, X. Effect of UV-C treatment on the quality of fresh-cut lotus (Nelumbo nucifera Gaertn.) root. Food Chem. 2019, 278, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, Y.; Wu, X.; Liu, W.; Jing, X.; Liu, Y.; Yan, J.; Liu, S.; Qin, W. Combination of calcium lactate impregnation with UV-C irradiation maintains quality and improves antioxidant capacity of fresh-cut kiwifruit slices. Food Chem. X 2022, 100329. [Google Scholar] [CrossRef] [PubMed]
- González-Aguilar, G.A.; Villegas-Ochoa, M.A.; Martínez-Téllez, M.; Gardea, A.; Ayala-Zavala, J.F. Improving antioxidant capacity of fresh-cut mangoes treated with UV-C. J. Food Sci. 2007, 72, S197–S202. [Google Scholar] [CrossRef]
- Collado, E.; Venzke Klug, T.; Martínez-Hernández, G.B.; Artés-Hernández, F.; Martínez-Sánchez, A.; Aguayo, E.; Artés, F.; Fernández, J.A.; Gómez, P.A. UV-C pretreatment of fresh-cut faba beans (Vicia faba) for shelf life extension: Effects of domestic microwaving for consumption. Food Sci. Technol. Int. 2020, 26, 140–150. [Google Scholar] [CrossRef]
- Nakajima, S.; Lan, L.; Kanno, S.-i.; Takao, M.; Yamamoto, K.; Eker, A.P.; Yasui, A. UV light-induced DNA damage and tolerance for the survival of nucleotide excision repair-deficient human cells. J. Biol. Chem. 2004, 279, 46674–46677. [Google Scholar] [CrossRef]
- Maghoumi, M.; Gomez, P.A.; Artés-Hernández, F.; Mostofi, Y.; Zamani, Z.; Artés, F. Hot water, UV-C and superatmospheric oxygen packaging as hurdle techniques for maintaining overall quality of fresh-cut pomegranate arils. J. Sci. Food Agric. 2013, 93, 1162–1168. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; González-Reza, R.M.; Cornejo-Villegas, M.A.; Leyva-Gómez, G.; Urbán-Morlán, Z. Effects of UV-C and edible nano-coating as a combined strategy to preserve fresh-cut cucumber. Polymers 2021, 13, 3705. [Google Scholar] [CrossRef]
- Martín-Vertedor, D.; Rodrigues, N.; Marx, Í.M.; Veloso, A.C.; Peres, A.M.; Pereira, J.A. Impact of thermal sterilization on the physicochemical-sensory characteristics of Californian-style black olives and its assessment using an electronic tongue. Food Control 2020, 117, 107369. [Google Scholar] [CrossRef]
- Nissim, Y.; Shloberg, M.; Biton, I.; Many, Y.; Doron-Faigenboim, A.; Zemach, H.; Hovav, R.; Kerem, Z.; Avidan, B.; Ben-Ari, G. High temperature environment reduces olive oil yield and quality. PLoS ONE 2020, 15, e0231956. [Google Scholar] [CrossRef]
- De la Peña-Armada, R.; Villanueva-Suárez, M.J.; Molina-García, A.D.; Rupérez, P.; Mateos-Aparicio, I. Novel rich-in-soluble dietary fiber apple ingredient obtained from the synergistic effect of high hydrostatic pressure aided by Celluclast®. LWT 2021, 146, 111421. [Google Scholar] [CrossRef]
- Arshadi, M.; Attard, T.M.; Lukasik, R.M.; Brncic, M.; da Costa Lopes, A.M.; Finell, M.; Geladi, P.; Gerschenson, L.N.; Gogus, F.; Herrero, M. Pre-treatment and extraction techniques for recovery of added value compounds from wastes throughout the agri-food chain. Green Chem. 2016, 18, 6160–6204. [Google Scholar] [CrossRef]
- Huang, H.-W.; Hsu, C.-P.; Wang, C.-Y. Healthy expectations of high hydrostatic pressure treatment in food processing industry. J. Food Drug Anal. 2020, 28, 1–13. [Google Scholar] [CrossRef]
- Naderi, N.; House, J.D.; Pouliot, Y.; Doyen, A. Effects of high hydrostatic pressure processing on hen egg compounds and egg products. Compr. Rev. Food Sci. Food Saf. 2017, 16, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K. Food Processing by High Hydrostatic Pressure; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Serment-Moreno, V.; Jacobo-Velázquez, D.A.; Torres, J.A.; Welti-Chanes, J. Microstructural and physiological changes in plant cell induced by pressure: Their role on the availability and pressure-temperature stability of phytochemicals. Food Eng. Rev. 2017, 9, 314–334. [Google Scholar] [CrossRef]
- Escobedo-Avellaneda, Z.; Pérez-Simón, I.; Lavilla-Martín, M.; Baranda-González, A.; Welti-Chanes, J. Enzymatic and phytochemical stabilization of orange–strawberry–banana beverages by high hydrostatic pressure and mild heat. Food Sci. Technol. Int. 2017, 23, 185–193. [Google Scholar] [CrossRef]
- Vázquez-Gutiérrez, J.L.; Hernando, I.; Quiles, A. Changes in tannin solubility and microstructure of high hydrostatic pressure–treated persimmon cubes during storage at 4 C. Eur. Food Res. Technol. 2013, 237, 9–17. [Google Scholar] [CrossRef]
- Woolf, A.B.; Wibisono, R.; Farr, J.; Hallett, I.; Richter, L.; Oey, I.; Wohlers, M.; Zhou, J.; Fletcher, G.C.; Requejo-Jackman, C. Effect of high pressure processing on avocado slices. Innov. Food Sci. Emerg. Technol. 2013, 18, 65–73. [Google Scholar] [CrossRef]
- Denoya, G.I.; Polenta, G.A.; Apóstolo, N.M.; Budde, C.O.; Sancho, A.M.; Vaudagna, S.R. Optimization of high hydrostatic pressure processing for the preservation of minimally processed peach pieces. Innov. Food Sci. Emerg. Technol. 2016, 33, 84–93. [Google Scholar] [CrossRef]
- Vázquez-Gutiérrez, J.L.; Quiles, A.; Vonasek, E.; Jernstedt, J.A.; Hernando, I.; Nitin, N.; Barrett, D.M. High hydrostatic pressure as a method to preserve fresh-cut Hachiya persimmons: A structural approach. Food Sci. Technol. Int. 2016, 22, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Pintado, C.; Nogales, S.; Fernández-León, A.M.; Delgado-Adámez, J.; Hernández, T.; Lozano, M.; Cañada-Cañada, F.; Ramírez, R. Effect of hydrostatic high pressure processing on nectarine halves pretreated with ascorbic acid and calcium during refrigerated storage. LWT-Food Sci. Technol. 2013, 54, 278–284. [Google Scholar] [CrossRef]
- Hu, X.; Ma, T.; Ao, L.; Kang, H.; Hu, X.; Song, Y.; Liao, X. Effect of high hydrostatic pressure processing on textural properties and microstructural characterization of fresh-cut pumpkin (Cucurbita pepo). J. Food Process Eng. 2020, 43, e13379. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kaushik, N.; Rao, P.S.; Mishra, H. High-pressure inactivation of enzymes: A review on its recent applications on fruit purees and juices. Compr. Rev. Food Sci. Food Saf. 2014, 13, 578–596. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-L.; Liu, W.; Zhao, J.; Yuan, C.; Song, Y.; Chen, D.; Ni, Y.-Y.; Li, Q.-H. The effect of high hydrostatic pressure on the microbiological quality and physical–chemical characteristics of Pumpkin (Cucurbita maxima Duch.) during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2014, 21, 24–34. [Google Scholar] [CrossRef]
- Dong, P.; Kong, M.; Yao, J.; Zhang, Y.; Liao, X.; Hu, X.; Zhang, Y. The effect of high hydrostatic pressure on the microbiological quality and physicochemical properties of lotus root during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2013, 19, 79–84. [Google Scholar] [CrossRef]
- Botondi, R.; Moscetti, R.; Massantini, R. A comparative study on the effectiveness of ozonated water and peracetic acid in the storability of packaged fresh-cut melon. J. Food Sci. Technol. 2016, 53, 2352–2360. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.A.; Silva, C.L.; Brandao, T.R. A review on ozone-based treatments for fruit and vegetables preservation. Food Eng. Rev. 2013, 5, 77–106. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Zhang, Y.; Jiang, A.; Hu, W. Aqueous ozone treatment inhibited degradation of cellwall polysaccharides in fresh-cut apple during cold storage. Innov. Food Sci. Emerg. Technol. 2021, 67, 102550. [Google Scholar] [CrossRef]
- Ummat, V.; Singh, A.; Sidhu, G. Effect of aqueous ozone on quality and shelf life of shredded green bell pepper (Capsicum annuum). J. Food Process. Preserv. 2018, 42, 1–14. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Jiang, A.; Zhao, Q.; Liu, S.; Hu, W. Effects of ozonated water on microbial growth, quality retention and pesticide residue removal of fresh-cut onions. Ozone Sci. Eng. 2020, 42, 399–407. [Google Scholar] [CrossRef]
- Alwi, N.A.; Ali, A. Reduction of Escherichia coli O157, Listeria monocytogenes and Salmonella enterica sv. Typhimurium populations on fresh-cut bell pepper using gaseous ozone. Food Control 2014, 46, 304–311. [Google Scholar] [CrossRef]
- Kim, J.-G.; Yousef, A.E.; Khadre, M.A. Ozone and Its Current and Future Application in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Liu, C.; Ma, T.; Hu, W.; Tian, M.; Sun, L. Effects of aqueous ozone treatments on microbial load reduction and shelf life extension of fresh-cut apple. Int. J. Food Sci. Technol. 2016, 51, 1099–1109. [Google Scholar] [CrossRef]
- Chitravathi, K.; Chauhan, O.; Raju, P.; Madhukar, N. Efficacy of aqueous ozone and chlorine in combination with passive modified atmosphere packaging on the postharvest shelf-life extension of green chillies (Capsicum annuum L.). Food Bioproc Technol. 2015, 8, 1386–1392. [Google Scholar] [CrossRef]
- Sripong, K.; Uthairatanakij, A.; Jitareerat, P. Impact of gaseous ozone on microbial contamination and quality of fresh-cut durian. Sci. Hortic. 2022, 294, 110799. [Google Scholar] [CrossRef]
- Yeoh, W.K.; Ali, A.; Forney, C.F. Effects of ozone on major antioxidants and microbial populations of fresh-cut papaya. Postharvest Biol. Technol. 2014, 89, 56–58. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Influence of pre-harvest ozone exposure on quality of strawberry fruit under simulated retail conditions. Postharvest Biol. Technol. 2008, 49, 10–18. [Google Scholar] [CrossRef]
- Koh, P.C.; Noranizan, M.A.; Karim, R.; Nur Hanani, Z.A. Sensory quality and flavour of alginate coated and repetitive pulsed light treated fresh-cut cantaloupes (Cucumis melo L. Var. Reticulatus Cv. Glamour) during storage. J. Food Sci. Technol. 2019, 56, 2563–2575. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sido, R.; Huang, R.; Chen, H. Application of water-assisted pulsed light treatment to decontaminate raspberries and blueberries from Salmonella. Int. J. Food Microbiol. 2015, 208, 43–50. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Lopes, M.M.; Silva, E.O.; Laurent, S.; Charles, F.; Urban, L.; de Miranda, M.R.A. The influence of pulsed light exposure mode on quality and bioactive compounds of fresh-cut mangoes. J. Food Sci. Technol. 2017, 54, 2332–2340. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, A.E.D.; Fonseca, K.S.; da Silva Gomes, W.K.; Monteiro da Silva, A.P.; de Oliveira Silva, E.; Puschmann, R. Control of browning of minimally processed mangoes subjected to ultraviolet radiation pulses. J. Food Sci. Technol. 2017, 54, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.R.; Álvarez, M.V.; Martín-Belloso, O.; Soliva-Fortuny, R. Effects of pulsed light treatments and pectin edible coatings on the quality of fresh-cut apples: A hurdle technology approach. J. Sci. Food Agric. 2017, 97, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Avalos-Llano, K.R.; Martín-Belloso, O.; Soliva-Fortuny, R. Effect of pulsed light treatments on quality and antioxidant properties of fresh-cut strawberries. Food Chem. 2018, 264, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.U.; Rathi, P.; Beshai, H.; Sarabha, G.K.; Deen, M.J. Fruit quality monitoring with smart packaging. Sensors 2021, 21, 1509. [Google Scholar] [CrossRef]
- Chaudhary, V.; Punia Bangar, S.; Thakur, N.; Trif, M. Recent advancements in smart biogenic packaging: Reshaping the future of the food packaging industry. Polymers 2022, 14, 829. [Google Scholar] [CrossRef]

| Antioxidant/ Source of Antioxidant | Fresh-Cut Fruit | Treatment Application | Principal Results | References |
|---|---|---|---|---|
| Rosmarinic acid, p-Coumaric acid, Trans-Cinnamic acid, Hydroxyphenyllactic acid, caffeic acid, ascorbic acid, gallic acid, citric acid and BHA | Apple | Immersion (500 µg/mL of each antioxidant) | Reduced the browning, maintained the acidic pH and restricted growth of L. monocytogenes even after 10 days of treatment. | [116] |
| Calcium ascorbate; vanillin or cinnamic acid | Nectarine | Immersion (6% calcium L-ascorbate) | Reduced browning | [117] |
| Mango seed extract | Mango | Immersion (6.25 g/L of the extract) | Preserved fresh-cut fruits, increasing polyphenols, flavonoids and antioxidant capacity. | [112] |
| Apple polyphenols | Red pitaya | Spraying (5 g/L apple polyphenols) | Maintained sensory (retention of color, delay of the softening) and nutritional attributes of fresh-cut red pitaya fruit. | [119] |
| Yerba mate (Ilex paraguariensis) Citric acid Ascorbic acid | Apple | Infusion (1.2% yerba mate + 0.9% citric acid + 1.0% ascorbic acid) | Increased antioxidant capacity and decreased browning. The color, flavor and texture of the apples were kept. | [113,114] |
| Phenolics from juice or extract of pomegranate and kiwifruit | Pear | Immersion (0.3% of antioxidants) | Improved antioxidant capacity and prevented enzymatic browning. | [115] |
| Melatonin | Pear | Soaked with 0, 0.05, 0.1 and 0.5 mM melatonin | Reduced the surface browning, maintained the titratable acidity, enhanced total phenolic content and antioxidant capacity, and delayed the reduction of ascorbic acid. | [120] |
| Extracts of: Elderberry flower (Sambucus L.) Vine (Vitis vinifera L.) leaves and branches Pear (Pyrus communis L. “Rocha”) pulp, peel and pomace Olive (Oleo europaea L.) leaves and branches Apple (Malus domestica L.) peel and pomace Acorn (Quercus L.) bark Bitter Melon (Momordica charantia L.) whole plant Strawberry tree (Arbutus Unedo L.) leaves and branches Potato plant (Solanum tuberosum L.) leaves | Pear | Sprayed (9.5 mg/mL, 5 mg/mL and 16 mg/mL) | Delaying fresh-cut pear browning expansion. Strawberry leaves and branches were the best antioxidant extracts. | [110] |
| Coconut liquid endosperm | Apple | Immersion (100% into the coconut liquid) | Coconut liquid endosperms are feasible natural agent inhibiting browning incidence of fresh-cut fruits during storage. | [121] |
| Melatonin | Apple and Pear | Immersion (0.05, 0.1, and 0.2 mM melatonin) | Reduced surface browning in fresh-cut foods. | [122] |
| Citric acid | Apple | Immersion (5% citric acid) | Inactivation of Salmonella and polyphenol oxidase. | [123] |
| Eugenol | Water chestnut | Immersion (0.4% and 1.5% eugenol) | Eugenol exhibited inhibitory effect on fresh-cut water chestnuts browning. Eugenol could also enhance the enzymatic/non-enzymatic antioxidant capacity and alleviate the ROS damage to membrane | [118] |
| Fresh-Cut Fruit | Treatment | Results | References |
|---|---|---|---|
| Apple | Aqueous ozone 1.4 mg/L At 5 and 10 min |
| [184] |
| Apple | Aqueous ozone 1.4 mg/L at 5 min |
| [179] |
| Papaya | Gaseous ozone 9.2 µL/L at 10, 20 and 30 min |
| [187] |
| Durian | Gaseous ozone 900 mg/L at 3 and 5 min |
| [186] |
| Durian | Gaseous ozone 900 mg/L for 14 days at 4 °C of storage |
| [186] |
| Apple | Aqueous ozone 1.4 µL/L at 5 min for 12 days and 4 °C of storage |
| [184] |
| Papaya | Gaseous ozone 9.2 µL/L for 10 and 30 min directly |
| [187] |
| Bell pepper | Gaseous ozone 9 ppm for 6 h |
| [182] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iturralde-García, R.D.; Cinco-Moroyoqui, F.J.; Martínez-Cruz, O.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Borboa-Flores, J.; Cornejo-Ramírez, Y.I.; Bernal-Mercado, A.T.; Del-Toro-Sánchez, C.L. Emerging Technologies for Prolonging Fresh-Cut Fruits’ Quality and Safety during Storage. Horticulturae 2022, 8, 731. https://doi.org/10.3390/horticulturae8080731
Iturralde-García RD, Cinco-Moroyoqui FJ, Martínez-Cruz O, Ruiz-Cruz S, Wong-Corral FJ, Borboa-Flores J, Cornejo-Ramírez YI, Bernal-Mercado AT, Del-Toro-Sánchez CL. Emerging Technologies for Prolonging Fresh-Cut Fruits’ Quality and Safety during Storage. Horticulturae. 2022; 8(8):731. https://doi.org/10.3390/horticulturae8080731
Chicago/Turabian StyleIturralde-García, Rey David, Francisco Javier Cinco-Moroyoqui, Oliviert Martínez-Cruz, Saúl Ruiz-Cruz, Francisco Javier Wong-Corral, Jesús Borboa-Flores, Yaeel Isbeth Cornejo-Ramírez, Ariadna Thalia Bernal-Mercado, and Carmen Lizette Del-Toro-Sánchez. 2022. "Emerging Technologies for Prolonging Fresh-Cut Fruits’ Quality and Safety during Storage" Horticulturae 8, no. 8: 731. https://doi.org/10.3390/horticulturae8080731
APA StyleIturralde-García, R. D., Cinco-Moroyoqui, F. J., Martínez-Cruz, O., Ruiz-Cruz, S., Wong-Corral, F. J., Borboa-Flores, J., Cornejo-Ramírez, Y. I., Bernal-Mercado, A. T., & Del-Toro-Sánchez, C. L. (2022). Emerging Technologies for Prolonging Fresh-Cut Fruits’ Quality and Safety during Storage. Horticulturae, 8(8), 731. https://doi.org/10.3390/horticulturae8080731







