Abstract
The current agriculture is facing various challenges to produce enough food to satisfy the need of the human population consumption without having a negative impact on the environment, human health and ecosystems. The exploitation of bioinoculants has been a crucial alternative for green agriculture. Bioinoculants have two great benefits: to promote plant growth by making essential nutrients available to crops and, to increase the tolerance to biotic and abiotic stresses by inducing a long-lasting defense. Certain members of genus Trichoderma have been recognized as biocontrol agents, biofertilizers and stress alleviators for the plants. The use of Trichoderma spp. has also been extended to protect and stimulate growth of horticultural crops. Elucidating the plant signaling events triggered by Trichoderma is of high importance in order to understand the molecular basis involving plant protection against stresses. In this review, the signaling elements of the plants from Trichoderma perception through late defensive responses is discussed. Enhanced understanding how Trichoderma spp. activate defense will lead to improvement in the use of species of this genus to increase crop production with the consequent benefits for human health and care for the environment.
1. Introduction
Stress in plants can be defined as any external condition that limits the photosynthetic rate and reduces the energy conversion ability of a plant to biomass, affecting its growth, development or productivity [1,2]. Plant stress can be classified as abiotic or biotic. The abiotic stress refers to any environmental factor that negatively affects the plant growth and development. Abiotic stress (e.g., extreme temperatures, drought, salinity, radiation and toxic metals) causes serious losses of major crop plants around the world [3]. On the other hand, the presence of plant pathogenic living organisms, especially viruses, fungi, bacteria, nematodes, and herbivores are the causes of plants biotic stress [3].
Plants attempt to adapt and resist the stresses by adjusting their metabolism, signal transduction, gene expression, etc.; however, the plant survival under these stress conditions will depend on the intensity, frequency and exposure time [2].
Population growth as well as current climate change and the crop losses caused by emergence of plant pathogenic microorganisms are challenges that require immediate action to ensure food security and safety in coming years. It has been estimated that agricultural food production needs to increase by about 70% by 2050 to feed an expected world population of 9.1 billion of people [4].
Human food production must focus particularly on sustainable agriculture by the means of ecological practices that maximize food productivity and minimize negative consequences on the environment [5]. In recent years, bio-priming agents are receiving large attention as a promising approach to mitigate the environmental and disease threats in agriculture [6,7,8,9,10].
Bio-priming has been recognized as a low-cost and eco-friendly technology that promotes growth and induces stress tolerance to achieve desired crop yield [11]. Bio-priming consists in the use of beneficial microorganisms [e.g., plant-growth-promoting bacteria (PGPB), fungi, etc.] or materials of biological origin (e.g., humus, chitosan, etc.). These materials can be used in the seeds or the whole plants to promote growth or to improve stress responses. Among these microorganism are included fungi, especially arbuscular mycorrhizal and Trichoderma spp. [12].
Trichoderma is mostly an asexual genus of filamentous fungi (the teleomorphic forms are Hypocrea) that usually are among the most common saprophytic microorganisms living in the rhizosphere [13]. Trichoderma genus contains 375 species that have been described by molecular phylogenetic analysis based on DNA sequencing data [14]. The drastic increase in the number of Trichoderma species has several explanations that are related to the technologies and applications used for identification [14].
Although Trichoderma was isolated for the first time in 1794 from soil and decomposing organic matter [15], it was not until the early 20th century that some Trichoderma species were found to have importance for biofuel industries and plant protection against pathogens by the use of mycoparasitism and/or antibiosis mechanisms [14,16,17]. In the years to follow, many strains of Trichoderma have been described as biocontrol agents [18]. Among Trichoderma species commercially available for agricultural use are T. harzianum, T. virens, T. viride, T. asperellum and T. atroviride [19].
The mechanism by which Trichodema spp. function as biocontrol agents is complex, and the mentioned biocontrol effect varies with the specie of Trichoderma and host plant involved in the interaction [18]. Clearly, environmental conditions (e.g., temperature, pH, salinity and nutrient availability) also influence the biocontrol mechanism [19].
Trichoderma spp. are considered as opportunistic and avirulent plant symbionts [20]. During interaction with host plants, Trichoderma spp. secrete several classes of chemical molecules (e.g., proteins, peptides, oligosaccharides and antibiotics) [10,21]. Some of these compounds may act as hormones that stimulate plant growth and development, or can also act as elicitors, activating defense responses in the host plant [22].
The activation of defense induced by Trichoderma spp. not only reduces plant diseases. It has also been proved that Trichoderma spp. application to the plant increases the tolerance to abiotic stress, such as drought [23,24,25], low temperatures [24,26], salinity [27,28], and can be used to reduce the presence of toxic metals [29,30]. This wide range of beneficial traits to their hosts is due to bio-priming, and is attributed to the induction of long plant basal resistance that improves the defensive capacity of the plants for subsequent stresses [31]. The application of bio-priming agents prepares the plant for a faster and more effectively response against future stresses [32].
Due to the ability of Trichoderma spp. to rapidly produce spores and antibiotic compounds, these fungi have been used for the massive production of commercial formulations that can be stored by months maintaining the beneficial effect for the crop [33]. The most widely used Trichoderma spp. products are formulated in a wettable powder or granules [19]. Ninety percent of various Trichoderma strains are applied to crops, within many horticulture species (e.g., Poaceae, Solanaceae and Cucurbitaceae) specially for the control of plant diseases due to the antagonistic characteristic against phytopathogens (see [34] for review).
The long-lasting dialogue established between plants and Trichoderma is one of the major gaps in the understanding of how this relationship works. In this review, we will focus on the plant signal elements underlying the priming function of Trichoderma spp. that may trigger plant adaptation to stress conditions.
2. Defense Responses at Early Stages of Plant–Trichoderma Interaction
Little is known about the plant host mechanisms that connect the perception of Trichoderma root colonization to the downstream signaling pathways leading to activation of defense and developmental responses [35]. It is assumed that plant defense triggered by Trichoderma spp. is initiated by the perception of microbial-associated molecular patterns (MAMPs) by pattern recognition receptors (PPRs), which are localized on the surface of plant cells [36]. This first phase defense induction is called MAMP-triggered immunity (MTI) [37]. MTI activated by Trichoderma spp. includes defense responses such as oxidative burst, callose deposition, Ca2+ and reactive oxygen species (ROS) signaling as well as the induction of phytoalexins and other secondary metabolites because, at that point, the plant does not recognize that it is a friendly attack [35,38,39].
2.1. Heterotrimeric G Proteins in Trichoderma Recognition
G proteins are membrane-associated, heterotrimeric, and composed of subunits α, β and γ. When GDP is bound, the subunit α associates with the βγ dimer to form an inactive heterotrimer that binds to a G-protein-coupled receptor (GPCR) [40]. When a GPCR detects an extracellular signal, α subunit decreases the GDP affinity and the leaving GDP is replaced with GTP. Once GTP is bound, the α subunit is activated and dissociated both from the GPCR and from βγ dimer [40]. Following activation, both the GTP-bound α subunit and the free βγ complex can bind to downstream effector molecules and mediate a variety of responses in the target cell, including adaptations to environmental and biotic stresses [41,42]. There is one report about the involvement of plant G-proteins after inoculation with Trichoderma. Pea roots inoculated only with T. asperellum showed a transcript accumulation of the Gα1 subunit of the heterotrimeric G protein [43]. This suggests G-proteins play an important role in the Trichoderma recognition by the plant and suggests that the Gα1 subunit (in its active form), could activate downstream signaling elements. Among the roles of Gα1 signaling, activation of plant plasma membrane Ca2+ channels and ROS accumulation have also been widely reported [44,45,46] (Figure 1).
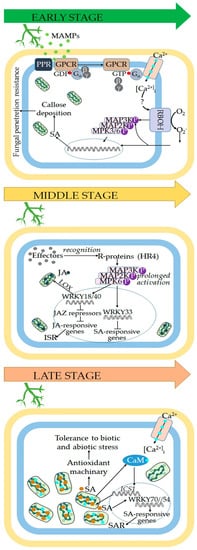
Figure 1.
Mechanism of plant responses according to the time of interaction with Trichoderma. The model is divided into three stages. The earlier stage comprises the first hours of interaction, wherein the plant is avoiding fungal root colonization due to SA phytohormone and consequently the callose deposition. This first stage is initiated by the recognition of MAMPs secreted by Trichoderma, which can trigger early defense responses mediated by Ca2+ and reactive oxygen species and by a rapid but transient activation of MAPK cascades through G heterotrimeric proteins. In the second stage, Trichoderma effectors are recognized by R-proteins to promote JA signaling by sustained MAPK activation, and to suppress SA signaling. Consequently, it is established a beneficial interaction. In the later stage, a second peak in the amount of SA is observed, which may induce antioxidative enzyme activities to reduce the oxidative damage to biomolecules and cells.
2.2. Calcium Mediated Signalling in Trichoderma Bio-Priming
Calcium is a second messenger by which plants modulate signaling pathways to respond to a particular stress. The increase in intracellular calcium concentrations ([Ca2+]i) is one of the earliest signaling events when plants are challenged with biotic and abiotic stimulus [47,48]. Changes in [Ca2+]i are commonly found during interaction between plants and beneficial microorganisms. This is the case for metabolites secreted by T. atroviride which increase [Ca2+]i and defense responses in the first minutes after the treatment in soybean cells [49]. Also, the elicitor HYTOL1 (a hydrophobin abundantly secreted by T. longibrachiatum strain MK1 [50]) may be involved in adhesion of fungal hyphae to the root surface [51], inducing a transient increase of cytosolic Ca2+ in Lotus japonicus cells [52]. These results indicate that the induction of intracellular Ca2+ changes represents an early step during Trichoderma–plant interaction that primes defense mechanisms (Figure 1).
2.3. Early ROS Accumulation
One of the earliest responses during the plant defense strategy is a fast and transient production of intracellular ROS [53]. Plasma membrane NADPH oxidases, known as respiratory burst oxidase homologues (RBOHs), are one of the many sources of ROS that have been implicated in several essential processes in plants [54]. Growing lines of evidence from plants suggest the involvement of NADPH oxidase-generated oxidative burst in extracellular signaling to regulate a wide range of physiological functions in plants [55,56].
A networking between cytosolic concentrations of Ca2+ and RBOH-mediated ROS production has been shown in several studies [57,58,59]. Plant–Trichoderma systems have also demonstrated that these fungi or their metabolites can trigger transient increases in ROS and calcium levels in the first minutes of interaction, activating enhanced immune defense [49,60]. Additionally, tight connections of NADPH oxidases and mitogen-activated protein kinases (MAPKs) are recognized to regulate various biological processes, wherein NADPH oxidase-originated oxidative burst can act upstream to activate the MAPKs cascade [61]. It has been demonstrated that association of T. viride Tv-1511 and peppermint plants produces the activation of a MAPK cascade via NADPH oxidase [61]. All these findings suggest that NADPH oxidase-dependent ROS production plays vital roles in the root colonization (Figure 1).
2.4. Salicylic Acid Restricts Trichoderma Invasion of Vascular System
The interactions between Trichoderma spp. and plant roots involve recognition, attachment, penetration, colonization and nutrient transfer [62]. It is well known that Trichoderma spp. grow on the outer layer of the roots of the plants [63,64].
During root colonization of Trichoderma spp., salicylic acid (SA) seems to be involved in preventing this fungus from entering the vascular system of the roots as well as in avoiding detrimental effects on plant growth and development of the host plants [65]. SA plays a key role in plant cell wall reinforcement (via callose synthesis) responsible for the limitation of Trichoderma colonization to the outer layers of roots [65]. Endogenous increase in SA levels has been reported in tomato plants inoculated with T. virens and T. harzianum T22 at 24 and 48 hpi, respectively [66,67]. The temporary induction of SA confirms a possible role in avoiding excessive Trichoderma penetration within the roots [65] and underlines the importance of SA in the first steps of the Trichoderma–plant interaction (Figure 1). It has also been shown that T. atroviride and T. cremeum induce changes in the composition of wheat seedlings roots [68]. These species promote lignin deposition and rearrangements of pectins after 14 days of incubation with Trichoderma spp., suggesting that modifications of wheat seedlings roots can be used as a tool against to pathogens [68].
3. Induction of Systemic Plant Defense by Trichoderma spp. Plays Key Role in the Crosstalk between Biotic and Abiotic Stress Responses
After MTI, Trichoderma spp. seem to activate a second layer of defense. In this stage, effectors secreted by fungi species prevent plant recognition and activate the plant systemic resistance to biotic and abiotic stress [36]. The second line of plant defense induction is called effector-triggered immunity (ETI), which is activated by plant resistance protein (R) and it is frequently associated with hypersensitive response (HR) [37]. Despite ETI and PTI involving a similar set of downstream defense responses, including calcium-mediated signaling, activation of MAPK cascades, production of ROS, transcriptional reprogramming, and biosynthesis of antimicrobial compounds [69,70,71,72], the responses during ETI have a longer duration and higher magnitude [73].
Induced resistance is the term used for the induced state of resistance in plants triggered by a biological or chemical inducer. This protects nonexposed plant parts from stresses [74]. Systemic acquired resistance (SAR) and induced systemic resistance (ISR) are two types of induced resistance wherein plant defenses are preconditioned by prior infection or treatment that results in resistance against subsequent challenge by a pathogen or parasite [75]. Plants, in response to virulent, avirulent and nonpathogenic microbes, elicit SAR. For the activation of SAR, the molecule SA and the accumulation of PR proteins are required. In contrast, ISR is triggered by the infection of pathogens, response to insects, herbivores, or upon root colonization by beneficial microbes in the rhizosphere (such as Pseudomonas spp., Bacillus spp. and Trichoderma spp.). Typically ISR is regulated by jasmonic acid and ethylene (JA/ET) [75,76], in some particular cases, ISR can requiere SA accumulation [77].
The first evidence of TISR was published in 1997 by Bigirimana et al. [78], who demonstrated that soil treated with T. harzianum made the leaves of bean plants resistant to diseases that are caused by the fungal pathogens Botrytis cinerea and Colletotrichum lindemuthianum, even though T. harzianum was present only on the roots and not on the foliage. Similar results have been reported for a wide range of host plants with different strains and species of Trichoderma and various classes of plant pathogen including fungi, bacteria, viruses and nematodes [39,78,79,80].
3.1. Trichoderma spp. Induce a Prolonged Activation of Plant MAPK Cascades
Mitogen-activated protein kinase (MAPK) cascades are well conserved signaling proteins in all eukaryotes [81]. Each cascade is minimally constituted of three proteins that are sequentially activated: a MAP kinase kinase kinase (MAPKKK or MAP3K), a MAP kinase kinase (MAPKK or MAP2K) and a MAP kinase (MAPK or MPK) [81,82].
MAPKs are intracellular proteins that can be activated by various stimuli [81]. MAPKs cascades transduce extracellular signals to cellular responses, including the biosynthesis of phytohormones, ROS generation, changes in gene expression, among others [83]. Activation of MAPK cascades is one of the earliest signaling events after plant sensing of PAMPs/MAMPs [84,85,86,87]; however sustained activation of MAPK confers a robust innate immunity [73,88]. Arabidopsis thaliana MPK3 and MPK6, as well as their orthologs in other species, such as tobacco SA-induced protein kinase (SIPK) and wounding-induced protein kinase (WIPK), are involved in plant responses to biotic and abiotic stresses [84,86,87,89,90]. Some studies have found the activation of MPKs associated with plant defense during plant–Trichoderma interactions [35,91,92,93,94,95]. For instance, xylanase, an elicitor from the cell walls of T. virens (TvX), induces the slow and prolonged activation of SIPK in tobacco [91]. Similarly, inoculation with T. atroviride (a specie known to promote root growth by producing auxine-like compounds [94]) in Arabidopsis roots induces the MPK6 activation [95]. Since the modulation of MPK6 is also responsive to auxin-like compounds, it has been suggested that T. atroviride alters root-system architecture modulating MPK6 and auxin action [95]. In addition, the activation of an analog of Arabidopsis MPK6 in peppermint by T. viridae is related with the modulation of essential oil metabolism at the transcriptional level and for enzymatic activation [61]. Interestingly enough, menthol, which is the main terpenoid of peppermint oil, exhibits potential abilities as plant defense potentiator in agriculture and horticulture [92].
Besides activating MPKs through posttranslational modifications, bio-priming with Trichoderma spp. induce expression of plant MPK genes. For example, the inoculation of cucumber (Cucumis sativus) roots with T. asperellum leads to a long-term expression of a Trichoderma-induced MPK (TIPK) gene, which is an ortholog of WIPK and MPK3 [93], while the elicitor HYTOL1 also up-regulates the early and transient expression of MPK3 in L. japonicus [52]. After inoculation in Arabidopsis, T. hamatum induces the expression of MPK3 after 48 h [96] and T. asperelloides of MPK11 at 24 h [35]. It is noteworthy that this last MPK is also responsive to PAMPs/MAMPs [97,98].
It is known that sustained activation of MPK3/6 elicits a massive reprogramming of the defense metabolome, with an accumulation of camalexin and indole glucosinolate derivatives [99]. The activation of both MPKs is also accompanied by many defense-related phytohormones such as SA, JA, and ET [99], suggesting that extended MPK activation could be involved in the modulation of the robustness of the immune signaling during plant–Trichoderma interactions (Figure 1).
3.2. Hormone Signalling Pathways Involved in Systemic Resistance induced by Trichoderma spp.
Plant hormones play a crucial role in the immune signaling networks in response to pathogens and beneficial microbes [100]. Among the most relevant hormones related to the modulation of defense responses are SA, JA, ET and abscisic acid (ABA), however auxin, gibberellic acid (GA), cytokinin (CK), brassinosteroids and peptide hormones could also be implicated in plant defense signaling pathways [101].
Several studies have shown that Trichoderma species induce the production of phytohormones in the host plants such as JA, SA and ET. Since Trichoderma spp. can also produce small amounts of phytohormones such as auxins, GA, SA and ABA [102,103,104,105], it makes difficult to discern the origin of hormones detected in some plant–Trichoderma spp. interactions. The role of specific hormones during plant–Trichoderma interaction seems to be dependent on the experimental condition and organisms involved [66,96,106].
3.2.1. Salicylic Acid
SA plays a key role in plant defense against biotrophic pathogens [107]. The accumulated evidence shows that partial suppression of SA-dependent responses in plants is necessary for the occurrence of the symbiotic association between beneficial microbes and plants [108,109,110,111,112], including Trichoderma spp. [35,113,114]. In this regard, evidence shows a down-regulation of PR-1, a useful marker for the SAR response, in the first hours of various plant–Trichoderma spp. interactions [52,115].
SAR is a long lasting defense modulated by SA. Recently, it has been found that systemic resistance in maize plants primed with T. atroviride at seedling stage is detected until two months later with an increase of SA levels, suggesting SA is a key component of a regulatory network controlling the immunity of silks during systemic resistance [116]. Similarly, Arabidopsis seedlings exposed to T. asperellum Ism T5 volatile for 9 days, stimulate SA accumulation [117]. In recent years, experimental studies have found that application of exogenous SA induces biotic and abiotic stress [118,119,120,121,122], possibly by modulating antioxidative enzyme activities, thereby potentially reducing the damaging levels of ROS [120,123,124]. It is thus possible that alleviation of biotic or abiotic stress observed in plant–Trichoderma systems would involve SA in later stages of the interactions (Figure 1).
3.2.2. Jasmonic Acid
JA is synthesized from the α-linolenic acid of chloroplast membranes by the octadecanoid pathway. JA is a phytohormone involved in diverse physiological processes including plant growth and development [125], and also actively participates in the mediation of plant responses and defenses against herbivore attack, pathogen infection and abiotic stresses, including ozone, ultraviolet radiation, high temperatures, and freezing [125,126,127,128].
Multiple reports have confirmed that Trichoderma spp. can increase the levels of JA in host plants. For instance, during the interaction between tomato plants with T. virens, an increase in endogenous JA levels at 24 hpi has been observed [67]. Similarly to the content of SA, JA significantly increase in T. longibrachiatum H9-inoculated cucumber plants at 96 hpi [114], and in Arabidopsis co-cultivated with both T. virens and T. atroviride 8 days after interaction [129], implying that SA and JA play important roles in regulating the plant response and enhancing plant defense in plants (Figure 1).
3.3. Induction of Plant Defense Gene Expression in Response to Trichoderma spp.
Reprogramming of a cell in response to the perception of an external stimulus involves complex changes in gene expression. The expression of genes appears to be regulated by intracellular signal transduction pathways. For instance, the interaction of plants with a variety of microorganisms results in changes in the level of SA, JA and ET, which are positive regulators of transcription factors (WRKYs), defense genes (PRs), and receptors (R genes) [130].
To link particular pathways with actual defense responses, some molecular tools, such as qPCR, allow the use of the expression of several marker genes as indicators of the activation of specific pathways [9]. Expression studies on defense/stress-related genes suggested that Trichoderma-induced systemic resistance (TISR) might involve both SA- and JA-related pathways.
Comparing plants treated with Trichoderma spp. with mock-treated controls, hundreds of genes that are differentially expressed during ISR-prime have been identified [35,66,96] (Table 1, Table 2 and Table 3). The products of the genes are related to defense responses, signal transduction, systemic acquired resistance, antioxidant systems, programmed cell death, etc. [96]. It is difficult to establish a specific time point when early defense responses end, but it has been proposed that 48 hpi would indicate the moment of transition when the plant reprograms its transcriptional machinery mainly towards redox and defense processes, fully accepting that Trichoderma is not an enemy [38,66].
3.3.1. WRKY Transcription Factors
WRKY is a family of transcription factors found exclusively in plants [131]. They bind W-box and/or other cis-elements located in the promoter of their target genes [131]. Most WRKY genes are responsive to pathogens, elicitors, and defense-related phytohormones such as SA or JA, implying a major role for the WRKY gene family in plant immunity [132], but also, the WRKY transcription factors play an important role in the alleviation of abiotic stresses [131,133].
The WRKY proteins regulate the gene expression directly or indirectly by modulating the downstream target genes, by activating or repressing the other genes (encoding transcription factors) or by self-regulating their own expression [131].
Molecular studies have revealed that Arabidopsis plants under interaction with T. atroviride induces the expression of WRKY8, WRKY33, WRKY38, WRKY42 and WRKY60, all of which are considered as positive regulators in JA pathway, while WRKY70 and WRKY54, regulated by the SA pathway, could be activated at later stages of the interaction, when the fungus is fully established in the plant roots [134]. Similarly, the treatment of L. japonicus with hydrophobin HYTOL from T. longibrachiatum, or the inoculation of the common bean (Phaseolus vulgaris L.) with T. velutinum, lead to the expression of WRKY33, but not PR-1 [52,115], suggesting that expression of WRKY33 induced by Trichoderma spp. negatively regulates the SA pathway to evade the plant immunity and to establish a prolonged mutualistic association (Table 1).
On the other hand, the expression of WRKY18, WRKY40 and WRKY60 transcription factors genes in Arabidopsis inoculated with T. asperelloides is observed as early as 9 h. The three WRKY show redundant function in negatively regulating PTI in Arabidopsis [135]. In response to T. asperelloides, these transcription factors negatively regulate the induction of transcript levels of SA marker genes FMO1, PAD3 and CYP71A13, but positively regulate the expression of LOX2 and AOS related to the JA pathway through inhibition of expression of the jasmonate ZIM domain (JAZ) repressors (Figure 1). Because FMO1 negatively regulates root colonization, WRKY18 and WRKY40 could negatively regulate FMO1 to allow a moderate level of colonization [35].
3.3.2. PR Proteins
Pathogenesis-related proteins (PRs) are a structurally diverse group of plant proteins that are induced by various types of pathogens. They are widely distributed in host plants in trace amounts, but are produced in much higher concentration following pathogen attack or stress conditions [136]. PR proteins impede pathogen invasion but also helps in growth and metabolism of the host plants. The PR proteins are grouped according to their properties and functions, and include β-1,3-glucanases, endochitinases, proteinases, proteinase inhibitors, peroxidases, RNases, inhibitors of pathogen hydrolases, and others [137]. Chitinases and β-1,3-glucanases are the major hydrolytic enzymes abundant in plants after fungal pathogen infection [138]. An earlier report showed that cucumber roots induced the activity of peroxidase, β-1,3-glucanase and chitinase, which are apparently of plant origin, 72 h post-inoculation with T. harzianum [13], suggesting that Trichoderma association could reduce disease through activation of both enzymes by hydrolyzing the main constituents of the structural barrier of pathogenic cell wall fungi.
Likewise, induction of PR gene expression is also essential for the development of induced resistance and can require the molecules SA or JA/ET. In Arabidopsis PR-1 that inhibits fungal growth, PR2 also called β-1,3-glucanase and PR-5 are considered to be markers for SAR, while PR-3 (chitinase), PR-4 (chitinase) and PR-12 (plant defensin) are used as markers for JA pathway. Transcriptomic analyses have shown the expression of PR genes in response to Trichoderma spp. (Table 2). The rhizosphere colonization by Trichoderma spp. can support the transcription of some defense-related genes for a relatively long period [139,140]. This effect is particularly strong for those inducible by SA (Table 2), suggesting that the long-term response to Trichoderma in plants may involve SA signaling.

Table 1.
Expression of WRKY genes up-regulated by Trichoderma species.
Table 1.
Expression of WRKY genes up-regulated by Trichoderma species.
| Signaling Pathways Related | Gene | Host Plant (Full Name in the Legend) | Trichoderma Specie or Elicitor | Time after Inoculation | Reference |
|---|---|---|---|---|---|
| JA/abiotic stress | WRKY33 | A. thaliana | T. atroviride | 96–144 h | [134] |
| T. asperelloides T203 | 9–24 h | [35] | |||
| L. japonicus | Hydrophobin HYTOL from T. longibrachiatum | 2 h | [52] | ||
| P. vulgaris | T. velutinum T028 | 45 days | [115] | ||
| S. lycopersicum | T. erinaceum | 24–48 h | [141] | ||
| JA/ET | WRKY8 | A. thaliana | T. atroviride | 24–48 h | [134] |
| T. asperelloides T203 | 24–48 h | [35] | |||
| WRKY38 | A. thaliana | T. atroviride | 96 h | [134] | |
| WRKY42 | 96–144 h | ||||
| WRKY60 | 72–144 h | ||||
| WRKY41 | A. thaliana | T. asperelloides T203 | 9–24 h | [35] | |
| WRKY53 | 24 h | ||||
| WRKY55 | 24 h | ||||
| WRKY18 | 9–24 h | ||||
| WRKY60 | 9–24 h | ||||
| WRKY40 | 9–48 h | ||||
| WRKY1 | V. vinifera | T. harzianum T39 | 4 days | [142] | |
| WRKY-C10 (WRKY transcription factor 6) | V. vinifera | T. harzianum T39 | 4 days | [142] | |
| Negatively regulated by JA/ET. Represses plant basal defense mechanisms | WRKY48 | A. thaliana | T. asperelloides T203 | 9–24 h | [35] |
| SA | WRKY30 | A. thaliana | T. asperelloides T203 | 9 h | [35] |
| WRKY54 | 9 h | ||||
| WRKY15 | 9–24 h | ||||
| WRKY46 | 9–24 h | ||||
| WRKY70 | 48 h | ||||
| WRKY54 | A. thaliana | T. atroviride | 144 h | [134] | |
| WRKY70 * | 144 h | ||||
| Involved in plant defense | WRKY37 | S. lycopersicum | T. erinaceum | 24–48 h | [141] |
WRKY70 is an Arabidopsis gene that is upregulated by two different strains of Trichoderma: T. asperelloides and T. atroviride. Arabidopsis thaliana, Lotus japonicus, Phaseolus vulgaris, Solanum lycopersicum, Vitis vinifera.

Table 2.
Induction of Pathogenesis Related (PR) genes expression by Trichoderma species.
Table 2.
Induction of Pathogenesis Related (PR) genes expression by Trichoderma species.
| Marker for | Gene | Protein Function | Host Plant (Full Name in the Legend) | Trichoderma Specie | Time after Inoculation | References |
|---|---|---|---|---|---|---|
| JA/ET | PR-3 | Chitinase Class 1. Hydrolytic enzymes that disrupt mycelial cell wall Antifungal properties | A. thaliana | T. asperelloides T203 | 24 h | [35] |
| O. sativa | T. harzianum; T.erinaceum; T. atriviride; T. hebeiensis; T. parareesei; T. longibrachiatum; T. resei | NR * | [8] | |||
| S. lycopersicum | T. erinaceum | 24–48 h | [141] | |||
| Acidic endochitinase 3 (Chit3) | Chitinases | V. vinifera | T. harzianum T39 | 4 days | [142] | |
| PR-4 | Basic Chitinases | A. thaliana | T. asperelloides T203 | 24–48 h | [35] | |
| PR-P2 | It is a pathogenesis related 4 (PR4) gene | S. lycopersicum | T. atroviride; T. harzianum | 2 months | [139] | |
| PDF1 | Plant defensin. Membrane permeabilizing functions. | A. thaliana | T. asperelloides T203 | 24 h | [35] | |
| PDF1.2 | 24 h | [35] | ||||
| PDF1.2c | 24 h | [35] | ||||
| PDF1.2 | S. lycopersicum cv. Oogata-fukuju | T. virens | 4–24 h | [67] | ||
| Defensin | O. sativa | T. harzianum; T.erinaceum; T. atriviride; T. hebeiensis; T. parareesei; T. longibrachiatum; T. resei | NR * | [8] | ||
| SA | PR-1 | Antimicrobial function and defense signal amplification. | A. thaliana | T. virens; T. atroviride; | 6–8 days | [129] |
| T. hamatum T382 | 48–72 h | [96] | ||||
| S. lycopersicum | T. atroviride; T. harzianum | 2 months | [139] | |||
| S. lycopersicum cv. Oogata-fukuju | T. virens | 4–24 h | [67] | |||
| PR-2 | Beta-1,3-endoglucanase. Hydrolytic enzymes that disrupt mycelial cell wall | A. thaliana | T. hamatum T382 | 48–72 h | [96] | |
| S. lycopersicum | T. erinaceum | 24–48 h | [141] | |||
| β-1,4-glucanase | Hydrolytic enzyme that disrupts mycelial cell wall | C. sativus | T. asperellum | 48 h | [143] | |
| PR-5 | Osmotins. Membrane permeabilizing proteins. | A. thaliana | T. hamatum T382 | 48–72 h | [96] | |
| T. asperelloides T203 | 24 h | [35] | ||||
| S. lycopersicum | T. hamatum | 5 weeks | [140] | |||
| OSM2 | Trichoderma-induced osmotin 2 | V. vinifera | T. harzianum T39 | 4 days | [142] |
* NR = Not reported. Arabidopsis thaliana, Oryza sativa, Solanum lycopersicum, Vitis vinifera, Cucumis sativus.
3.3.3. Other Defense Gene Markers
The expression of PR genes can be transitory, but strongly potentiates the expression of defense-related proteins when plants are affected with biotic stress. Proteins encoded by resistance genes (R) are found among them. The R proteins recognize effectors from beneficial and pathogenic microorganisms to activate a stronger defense. The HR4 gene that codifies an R protein is induced 96 h after of the Arabidopsis–T. atroviride interaction, suggesting the fungus is activating the recognition system and promoting a beneficial interaction establishment in the plant [130], however, little is known about R genes in beneficial interactions.
Additionally, there is evidence of a link between the accumulation of the phytohormones and changes in the expression of marker genes, which have been identified by analysing their expression patterns after exogenous application of single or combined phytohormone solutions [144]. The evidence has demonstrated that Trichoderma spp. can simultaneously or separately induce ISR and SAR associated with the biosytnthesis of SA, JA and ET according with the induction of the expression of specific resistance marker genes, which are summarized in Table 3.

Table 3.
Expression of gene markers positively regulated by Trichoderma species.
Table 3.
Expression of gene markers positively regulated by Trichoderma species.
| Marker for | Gene | Protein Function | Host Plant (Full Name in the Legend) | Trichoderma Specie | Time after Inoculation | Reference |
|---|---|---|---|---|---|---|
| JA/ET | Lox1 | Lipoxygenase enzyme involved in JA synthesis | C. sativus | T. asperellum | 24 h | [61] |
| A. thaliana | T. harzanium | 72 h | [65] | |||
| A. thaliana | T. asperelloides T203 | 24 h | [35] | |||
| S. lycopersicum | T. parareesei | 6 days | [145] | |||
| Lox2 | A. thaliana | T. virens, T. atroviride | 8 days | [129] | ||
| Lox3 | A. thaliana | T. asperelloides T203 | 24 h | [35] | ||
| Lox4 | A. thaliana | T. asperelloides T203 | 24 h | [35] | ||
| LoxA | S. lycopersicum. | T. atroviride, T. harzianum | 2 months | [139] | ||
| HPL | Hydroperoxide lyase | C. sativus | T. asperellum | 24–48 h | [146] | |
| hGS | Homoglutathione synthetase related with oxidative stress | P. vulgaris | T. velutinum T028 | 45 days | [115] | |
| ET | CTR1 | Ethylene signal-associated serine/threonine protein kinase | C. sativus | T. asperellum | 24 h | [143] |
| ETR1 | ||||||
| EIN2 | Key component in ethylene signaling | A. thaliana | T. asperelloides T203 | 48 h | [35] | |
| EIN4 | ||||||
| ERF-A2 | Ethylene-responsive transcription factor | S. lycopersicum | T. parareesei, T. asperellum, T. harzianum | 4 weeks | [147] | |
| CH5b | Endochitinase precursor related to ethylene signaling | P. vulgaris | T. velutinum T028 | 45 days | [115] | |
| SA | PAL1 | Phenylalanine and histidene ammonia-lyase. Enzyme involved in the production of antimicrobial compounds | C. sativus | T. asperellum | 24 h | [143,146] |
| A. thaliana | T. asperelloides T203 | 9–24 h | [35] | |||
| O. sativa | T. harzianum, T.erinaceum, T. atriviride, T. hebeiensis, T. parareesei, T. longibrachiatum, T. resei | NR * | [8] | |||
| PAL2 | A. thaliana | T. asperelloides T203 | 24 h | [35] | ||
| ICS1 | Isochorismate synthase is involved in SA biosynthesis | A. thaliana | T. harzanium | 72 h | [65] | |
| Cals | Callose synthase, involved in callose biosynthesis | A. thaliana | T. harzanium | 72 h | [65] |
* NR = Not reported. Cucumis sativus, Arabidopsis thaliana, Solanum lycopersicum, Phaseolus vulgaris, Oryza sativa.
3.4. Induction of Antioxidant Enzyme Activity Is Modulated by Trichoderma spp.
As noted before, one of the common responses under stress conditions is the generation of ROS. Overproduction of ROS could result in damage to macromolecules such as lipids, proteins and DNA, via oxidation, and in severe cases, leads to cell death. So it is crucial to overcome these effects either by enhancing the intrinsic antioxidant defense or by repairing the damage [148].
Stress-induced ROS accumulation can be counteracted by plant antioxidative defense that consist of enzymatic or nonenzymatic systems. Superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), peroxidases (POD) and glutathione peroxidase (GPX) are the main enzymatic scavengers of superoxide (O2−) and hydrogen peroxide (H2O2) [149], while glutathione (GSH) and ascorbic acid (ASA) are the major non-enzymatic antioxidants that, among other vital functions, maintain cellular redox homeostasis [150]. Keeping ASA and GSH in reduced form is critical for redox homeostasis and cellular vitality [151]. The activity of the enzymes that regenerate these molecules is correlated with resistance to abiotic stresses. These enzymes include glutathione reductase (GR) (which regenerates oxidized GSH), monodehydroascorbate reductase (MDHAR) and dehydroascorbate reductase (DHAR), which regenerate ASA from monodehydroascorbate (MDHA) and dehydroascorbate (DHA) [149].
Recent literature has revealed that Trichoderma spp. reduce the negative effects of plants stressed with biotic and abiotic stimuli through the modulation of the ROS by inducing antioxidant enzymes [24,152,153]. For instance, in the presence of T. harzianum T22, the ratios of reduced to oxidized forms of the molecules for ascorbate and glutathione, and the activity of SOD, APX, MDHAR, DHAR and GR in tomato seedlings are higher than non-inoculated plants. This indicates that T. harzianum T22 enhances systems of ROS scavenging and redox maintenance [151]. Also, T. erinaceum bioprimed tomato plants increase the activities of SOD and CAT compared to a control, and T. hamatum enhances the activity of enzymes CAT, POD, APX, GR and SOD in Ocharenus baccatus [154]. Similarly, the inoculation of maize and rice seeds as well as wheat seedlings with T. harzianum or its metabolites extracts increases SOD and CAT antioxidant enzymes activity [155,156]. This demonstrates that the pre-treatment of biocontrol Trichoderma results in increased activities of the antioxidant enzymatic pool [141].
Trichoderma strains also increase the activity of antioxidative defense through enhanced expression of genes encoding the component enzymes [148]. Transcriptional reprogramming of the oxidative stress response may also influence Trichoderma spp. bio-priming to overcome oxidative damage in stressed plants. Some examples of overexpression of antioxidant-related genes induced by Trichoderma spp. are summarized in Table 4.

Table 4.
Induction of expression of plant antioxidant genes by Trichoderma species.
Table 4.
Induction of expression of plant antioxidant genes by Trichoderma species.
| Gene | Host Plant (Full Name in the Legend) | Trichoderma Specie or Elicitor | Time after Inoculation | Reference |
|---|---|---|---|---|
| CAT | C. sativus | T. asperelloides T203 | 24 h | [35] |
| CAT | O. sativa | T. harzianum; T.erinaceum; T. atriviride; T. hebeiensis; T. parareesei; T. longibrachiatum; T. resei | * NR | [8] |
| CAT | T. aestivum cv.’Yongliang 4 | T. longibrachiatum T6 | * NR | [157] |
| GPX | S. lycopersicum | T. erinaceum | 24–48 h | [141] |
| POD | T. aestivum cv.’Yongliang 4 | T. longibrachiatum T6 | * NR | [157] |
| POD | O. sativa | T. harzianum; T.erinaceum; T. atriviride; T. hebeiensis; T. parareesei; T. longibrachiatum; T. resei | * NR | [8] |
| SOD | O. sativa | T. harzianum; T.erinaceum; T. atriviride; T. hebeiensis; T. parareesei; T. longibrachiatum; T. resei | * NR | [8] |
| SOD | S. lycopersicum | T. erinaceum | 24–48 h | [141] |
| SOD | T. aestivum cv.’Yongliang 4 | T. longibrachiatum T6 | * NR | [157] |
| SOD (Mn) | C. sativus | T. asperelloides T203 | 24 h | [35] |
| SOD (Cu) | C. sativus | T. asperelloides T203 | 24 h | [35] |
* NR = Not reported. Cucumis sativus, Oryza sativa, Triticum aestivum, Solanum lycopersicum.
SA has been widely recognized as a promoter of antioxidant defense, including CAT, SOD, and APX, as well as non-enzymatic antioxidants, to alleviate oxidative stress in plants [124,158,159,160], so the late increase of endogenous SA observed in bioprimed plants with Trichoderma spp., might be responsible for the antioxidant enzymatic mechanism pathway improving the performance of plants under stress conditions (Figure 1). Thus, growing evidence suggests that application of strains of Trichoderma spp. may be an ecological strategy to help plants to recover from biotic and abiotic stress-induced oxidative damage to continue the metabolic and physiological activities in a better way.
3.5. Effects of Trichoderma on Chloroplasts
Chloroplasts are key organelles of the higher plants in which photosynthesis takes place. The chloroplasts are also the major production site of defense molecules including hormones (such as SA, JA, ABA) and secondary messengers like Ca2+ and ROS [161].
The effect of Trichoderma interaction on chloroplast has been poorly explored. Recently, it was observed that T. asperellum and T. harzianum consortium at 108 CFU/mL concentration increased the number and size of chloroplasts in spongy parenchyma of Passiflora caerulea after 60 days [162]. These chloroplasts also showed a reduction of starch grains, which could be related to starch degradation and the translocation of monosaccharides from chloroplasts to the rest of the cell and/or to the phloem [162] (Figure 1).
Additionally, it has been proved that some Trichoderma strains enhance photosynthetic capacity compared to uninoculated controls (see [163] for review) by increasing the photosynthetic pigment content or the expression of genes regulating the biosynthesis of chlorophyll, proteins of the light-harvesting complex, or components of the Calvin cycle [164]. Chloroplasts are considered as sensors and regulators of plant responses to biotic and abiotic stresses [165]. When plants are exposed to stress, they usually lose their photosynthetic capability by an overproduction of ROS formed during excitation of chlorophyll in photosynthesis, causing an oxidative stress in chloroplasts [166]. However, it has been shown that plants inoculated with certain strains of Trichoderma and then challenged by a stress overcome the reduction of photosynthetic capability [26,39,148,167]. This might be due to the protection against ROS levels described previously, but also to the increase in the content of carotenoids detected in the interaction of some plants with Trichoderma spp. [164,168,169,170,171], since carotenoid pigments act as antioxidants that quench singlet oxygen and trap peroxyl radicals [172].
Since chloroplasts produce ROS during cellular stress and ROS act as promoters of programmed cell death (PCD), Trichoderma spp. may be preventing cell death in plants subsequently exposed to stress. Moscatiello et al. [52] demonstrated that despite the fact that HYTOL induced the expression of defense genes, it did not affect cell viability and ultrastructure of L. japonicus cells after treatment. However, other studies have reported some markers of PCD (e.g., caspase 3-like caspase protease activity and by chromatin condensation) in soybean and tobacco cells treated with metabolite mixtures from T. atroviride or xylanase, respectively [49,173].
4. Conclusions and Future Perspectives
The negative consequences of climate change on living organisms and the environment are already forcing us to search for alternative ways of reducing these catastrophic events. Eco-friendly practices for food production have been highlighted to achieve sustainability. In horticultural crops, plant biostimulants have been proposed as agronomic tools to mitigate environmental/abiotic stress effects. However, since our knowledge about the mechanism involved during plant–biostimulant interaction is currently limited, more research is needed to understand exactly what is taking place during interactions. The elucidation of the mechanisms of action will allow us to develop new methods that involve beneficial microorganisms with better performance for the solution of agricultural problems.
Trichoderma spp. induce multiple beneficial effects on plants by reducing the severity of diseases, but also by alleviating abiotic stress-induced damage in plants. These promising results are opening the door for sustainable agriculture to exploit the potential of Trichoderma in a safe way for crop plants, agroecosystems, and humans.
Further research into the molecular bases of dialogue in plant–Trichoderma interactions should predict the impact of certain species of this genus on crops or cultivars performance to ensure their effective use.
Author Contributions
Conceptualization, M.S.-G.; investigation, J.P.-T., E.A.-T. and M.I.R.-S.; writing—original draft preparation, M.S.-G. and J.P.-T.; writing—review and editing, M.S.-G., M.I.-P., S.R.P.-R. and A.M.-N.; supervision, M.S.-G. and A.M.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Abscisic acid (ABA); allene oxide synthase (AOS); ascorbate peroxidase (APX); catalase (CAT); cytochrome P450 family 71 polypeptide (CYP71A13); dehydroascorbate reductase (DHAR); ethylene (ET); effector-triggered immunity (ETI); flavin monoxygenase 1 (FMO1); gibberellic acid (GA); guanosine diphosphate (GDP); G-protein-coupled receptor (GPCR); glutathione peroxidase (GPX); glutathione reductase (GR); glutathione (GSH); heterotrimeric G-protein α (Gα1); hypersensitive response (HR); hydrophobin secreted by T. longibrachiatum strain MK1 (HYTOL1); induced systemic resistance (ISR); jasmonic acid (JA); jasmonate ZIM domain (JAZ); lipoxygenase 2(LOX2); microbe-associated molecular patterns (MAMPs); mitogen-activated protein kinases (MAPKs); monodehydroascorbate reductase (MDHAR); MAMP-triggered immunity (MTI); nicotinamide adenine dinucleotide phosphate (NADPH); phytoalexin deficient3 (PAD3); pathogen-associated molecular patterns (PAMPs); programmed cell death (PCD); plant-growth-promoting bacteria (PGPB); peroxidases (POD); pattern recognition receptors (PPRs); pathogenesis-related protein (PR); pattern-triggered immunity (PTI); resistance proteins (R); respiratory burst oxidase homologues (RBOHs); reactive oxygen species (ROS); salicylic acid (SA); systemic acquired resistance (SAR); tobacco SA-induced protein kinase (SIPK); superoxide dismutase (SOD); Trichoderma-induced MPK (TIPK); Trichoderma-induced systemic resistance (TISR); wounding-induced protein kinase (WIPK); transcription factors with the domain WRKYs (WRKY).
References
- Verma, S.; Nizam, S.; Verma, P. Biotic and abiotic stress signaling in plants. In Stress Signaling in Plants: Genomics and Proteomics Perspectives; Sarwat, M., Ahmad, A., Abdin, M.Z., Eds.; Springer Science: New York, NY, USA, 2013; pp. 25–49. [Google Scholar]
- Umar, O.B.; Ranti, L.A.; Abdulbaki, A.S.; Bola, A.L.; Abdulhamid, A.K.; Biola, M.R.; Victor, K.O. Stresses in plants: Biotic and abiotic. In Current Trends in Wheat Research (Internet); Mahmood-ur-Rahman, A., Ed.; InterchOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and abiotic stresses in plants. In Abiotic and Biotic Stress in Plants (Internet); de Oliveira, A.B., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Raney, T.; Steinfeld, H.; Skoet, J. The State of Food and Agriculture 2009: Livestock in the Balance; FAO: Rome, Italy, 2009; Available online: https://www.fao.org/publications/card/en/c/3aa4f41c-4316-5ddd-a656-22a00ef5d414/ (accessed on 22 September 2022).
- FAO. Sustainable Agriculture; FAO: Rome, Italy, 2016; Available online: https://www.fao.org/family-farming/detail/en/c/423952/ (accessed on 22 September 2022).
- Fadiji, A.E.; Santoyo, G.; Yadav, A.N.; Babalola, O.O. Efforts towards overcoming drought stress in crops: Revisiting the mechanisms employed by plant growth-promoting bacteria. Front. Microbiol. 2022, 13, 962427. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Tugizimana, F.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A.; Mhlongo, M.I. Untargeted metabolite profiling to elucidate rhizosphere and leaf metabolome changes of wheat cultivars (Triticum aestivum L.) treated with the plant growth-promoting rhizobacteria (T22) and Bacillus subtilis. Front. Microbiol. 2022, 13, 971836. [Google Scholar] [CrossRef]
- Swain, H.; Adak, T.; Mukherjee, A.K.; Sarangi, S.; Samal, P.; Khandual, A.; Jena, R.; Bhattacharyya, P.; Naik, S.K.; Mehetre, S.T.; et al. Seed biopriming with Trichoderma strains isolated from tree bark improves plant growth, antioxidative defense system in rice and enhance straw degradation capacity. Front. Microbiol. 2021, 12, 633881. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Matsi, T.; Kamou, N.; Avdouli, D.; Mellidou, I.; Karamanoli, K. Decoding the potential of a new Pseudomonas putida strain for inducing drought tolerance of tomato (Solanum lycopersicum) plants through seed biopriming. J. Plant Physiol. 2022, 271, 153658. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Chakraborti, S.; Bera, K.; Sadhukhan, S.; Dutta, P. Bio-priming of seeds: Plant stress management and its underlying cellular, biochemical and molecular mechanisms. Plant Stress 2022, 3, 100052. [Google Scholar] [CrossRef]
- Harman, G.E.; Uphoff, N. Symbiotic root-endophytic soil microbes improve crop productivity and provide environmental benefits. Scientifica 2019, 2019, 9106395. [Google Scholar] [CrossRef]
- Yedidia, I.; Benhamou, N.; Kapulnik, Y.; Chet, I. Induction and accumulation of PR proteins activity during early stages of root colonization by the mycoparasite Trichoderma harzianum strain T-203. Plant Physiol. Biochem. 2000, 38, 863–873. [Google Scholar] [CrossRef]
- Cai, F.; Druzhinina, I.S. In honor of John Bissett: Authoritative guidelines on molecular identification of Trichoderma. Fungal Divers. 2021, 107, 1–69. [Google Scholar] [CrossRef]
- Persoon, C.H. Disposita methodical fungorum. Romers Neues Mag Bot. 1794, 1, 81–128. [Google Scholar]
- Weindling, R. Trichoderma lignorum as a parasite of other soil fungi. Phytopathology 1932, 22, 837–845. [Google Scholar]
- Weindling, R. Studies on a lethal principle effective in the parasitic action of Trichoderma lignorum on Rhizoctonia solani and other soil fungi. Phytopathology 1934, 24, 1153–1179. [Google Scholar]
- Howell, C.R. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Woo, S.L.; Scala, M.; Ruocco, M.; Lorito, M. The molecular biology of the interactions between Trichoderma spp., phytopathogenic fungi, and plants. Phytopathology 2006, 96, 181–185. [Google Scholar] [CrossRef]
- Mona, S.A.; Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Soliman, D.W.K.; Wirth, S.; Egamberdieva, D. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agric. 2017, 16, 1751–1757. [Google Scholar] [CrossRef]
- Cornejo-Ríos, K.; Osorno-Suárez, M.d.P.; Hernández-León, S.; Reyes-Santamaría, M.I.; Juárez-Díaz, J.A.; Pérez-España, V.H.; Peláez-Acero, A.; Madariaga-Navarrete, A.; Saucedo-García, M. Impact of Trichoderma asperellum on chilling and drought stress in tomato (Solanum lycopersicum). Horticulturae 2021, 7, 385. [Google Scholar] [CrossRef]
- Bashyal, B.M.; Parmar, P.; Zaidi, N.W.; Aggarwal, R. Molecular programming of drought-challenged Trichoderma harzianum-bioprimed rice (Oryza sativa L.). Front. Microbiol. 2021, 12, 655165. [Google Scholar] [CrossRef]
- Ghorbanpour, A.; Salimi, A.; Ghanbary, M.A.T.; Pirdashti, H.; Dehestani, A. The effect of Trichoderma harzianum in mitigating low temperature stress in tomato (Solanum lycopersicum L.) plants. Sci. Hortic. 2018, 230, 134–141. [Google Scholar] [CrossRef]
- Sánchez-Montesinos, B.; Diánez, F.; Moreno-Gavira, A.; Gea, F.J.; Santos, M. Plant growth promotion and biocontrol of Pythium ultimum by saline tolerant Trichoderma isolates under salinity stress. Int. J. Environ. Res. Public Health 2019, 16, 2053. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Baki, G.K.; Mostafa, D. The potentiality of Trichoderma harzianum in alleviation the adverse effects of salinity in faba bean plants. Acta Biol. Hung. 2014, 65, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.; Rashk-E-Eram; Naik, S.K.; Choudhary, J.S.; Kumar, S. Heavy metals scavenging potential of Trichoderma asperellum and Hypocrea nigricans isolated from acid soil of Jharkhand. Indian J. Microbiol. 2019, 59, 27–38. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Hao, Y.; Liu, C.; Pan, L.; Zhu, Z. Tolerance mechanism of Trichoderma asperellum to Pb2+: Response changes of related active ingredients under Pb2+ stress. RSC Adv. 2020, 10, 5202–5211. [Google Scholar] [CrossRef] [PubMed]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef] [PubMed]
- Llorens, E.; González-Hernández, A.I.; Scalschi, L.; Fernández-Crespo, E.; Camañes, G.; Vicedo, B.; García-Agustín, P. Chapter 1—Priming mediated stress and cross-stress tolerance in plants: Concepts and opportunities. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Hossain, M.A., Liu, F., Burritt, D.J., Fujita, M., Huang, B., Eds.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Harman, G.E.; Kubicek, C.P. Trichoderma and Gliocladium. In Enzymes, Biological Control and Commercial Applications; Harman, G.E., Ed.; Kubicek, Crc Pr: London, UK, 1998. [Google Scholar] [CrossRef]
- Szczałba, M.; Kopta, T.; Gąstoł, M.; Sękara, A. Comprehensive insight into arbuscular mycorrhizal fungi, Trichoderma spp. and plant multilevel interactions with emphasis on biostimulation of horticultural crops. J. Appl. Microbiol. 2019, 127, 630–647. [Google Scholar] [CrossRef] [PubMed]
- Brotman, Y.; Landau, U.; Cuadros-Inostroza, Á.; Takayuki, T.; Fernie, A.R.; Chet, I.; Viterbo, A.; Willmitzer, L. Trichoderma-plant root colonization: Escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog. 2013, 9, e1003221. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiol. Read Engl. 2012, 158, 17–25. [Google Scholar] [CrossRef]
- Ausubel, F.M. Are innate immune signaling pathways in plants and animals conserved? Nat. Immunol. 2005, 6, 973–979. [Google Scholar] [CrossRef]
- Morán-Diez, M.E.; Martínez de Alba, Á.E.; Rubio, M.B.; Hermosa, R.; Monte, E. Trichoderma and the plant heritable priming responses. J. Fungi 2021, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Neer, E.J. Heterotrimeric G proteins: Organizers of transmembrane signals. Cell 1995, 80, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Urano, D. Genetic and systematic approaches toward G protein-coupled abiotic stress signaling in plants. Front. Plant Sci. 2018, 9, 1378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, P.; Xu, X.; Xie, Q.; Yu, F. Heterotrimeric G protein signalling in plant biotic and abiotic stress response. Plant Biol. 2021, 23, 20–30. [Google Scholar] [CrossRef]
- Patel, J.S.; Sarma, B.K.; Singh, H.B.; Upadhyay, R.S.; Kharwar, R.N.; Ahmed, M. Pseudomonas fluorescens and Trichoderma asperellum enhance expression of Gα subunits of the pea heterotrimeric G-protein during Erysiphe pisi infection. Front. Plant Sci. 2016, 6, 1206. [Google Scholar] [CrossRef]
- Aharon, G.S.; Gelli, A.; Snedden, W.A.; Blumwald, E. Activation of a plant plasma membrane Ca2+ channel by TGα1, a heterotrimeric G protein α-subunit homologue. FEBS Lett. 1998, 424, 17–21. [Google Scholar] [CrossRef]
- Zhu, R.; Dong, X.; Hao, W.; Gao, W.; Zhang, W.; Xia, S.; Liu, T.; Shang, Z. Heterotrimeric G protein-regulated Ca2+ influx and PIN2 asymmetric distribution are involved in Arabidopsis thaliana roots’ avoidance response to extracellular ATP. Front. Plant Sci. 2017, 8, 1522. [Google Scholar] [CrossRef]
- Zhang, W.; Jeon, B.W.; Assmann, S.M. Heterotrimeric G-protein regulation of ROS signalling and calcium currents in Arabidopsis guard cells. J. Exp. Bot. 2011, 62, 2371–2379. [Google Scholar] [CrossRef]
- Aldon, D.; Mbengue, M.; Mazars, C.; Galaud, J.P. Calcium signalling in plant biotic interactions. Int. J. Mol. Sci. 2018, 19, 665. [Google Scholar] [CrossRef]
- Xu, T.; Niu, J.; Jiang, Z. Sensing mechanisms: Calcium signaling mediated abiotic stress in plants. Front. Plant Sci. 2022, 13, 925863. [Google Scholar] [CrossRef]
- Navazio, L.; Baldan, B.; Moscatiello, R.; Zuppini, A.; Woo, S.L.; Mariani, P.; Lorito, M. Calcium-mediated perception and defense responses activated in plant cells by metabolite mixtures secreted by the biocontrol fungus Trichoderma atroviride. BMC Plant Biol. 2007, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, M.; Lanzuise, S.; Lombardi, N.; Woo, S.L.; Vinale, F.; Marra, R.; Varlese, R.; Manganiello, G.; Pascale, A.; Scala, V.; et al. Multiple roles and effects of a novel Trichoderma hydrophobin. Mol. Plant-Microbe Interact. 2015, 28, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Whiteford, J.R.; Spanu, P.D. Hydrophobins and the interactions between fungi and plants. Mol. Plant Pathol. 2002, 3, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Moscatiello, R.; Sello, S.; Ruocco, M.; Barbulova, A.; Cortese, E.; Nigris, S.; Baldan, B.; Chiurazzi, M.; Mariani, P.; Lorito, M.; et al. The hydrophobin HYTLO1 secreted by the biocontrol fungus Trichoderma longibrachiatum triggers a NAADP-mediated calcium signalling pathway in Lotus japonicus. Int. J. Mol. Sci. 2018, 19, 2596. [Google Scholar] [CrossRef]
- Wojtaszek, P. Oxidative burst: An early plant response to pathogen infection. Biochem. J. 1997, 322, 681–692. [Google Scholar] [CrossRef]
- Qu, Y.; Yan, M.; Zhang, Q. Functional regulation of plant NADPH oxidase and its role in signaling. Plant Signal. Behav. 2017, 12, e1356970. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef]
- Marino, D.; Dunand, C.; Puppo, A.; Pauly, N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012, 17, 9–15. [Google Scholar] [CrossRef]
- Kurusu, T.; Kuchitsu, K.; Tada, Y. Plant signaling networks involving Ca2+ and Rboh/Nox-mediated ROS production under salinity stress. Front. Plant Sci. 2015, 6, 427. [Google Scholar] [CrossRef]
- Evans, M.; Choi, W.G.; Gilroy, S.; Morris, R.J. A ROS-assisted calcium wave dependent on the AtRBOHD NADPH oxidase and TPC1 cation channel propagates the systemic response to salt stress. Plant Physiol. 2016, 171, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Mazars, C.; Thuleau, P.; Lamotte, O.; Bourque, S. Cross-talk between ROS and calcium in regulation of nuclear activities. Mol. Plant 2010, 3, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Fan, L.; Fu, K.; Yu, C.; Wang, M.; Xia, H.; Sun, J.; Li, Y.; Chen, J. Cellulase from Trichoderma harzianum interacts with roots and triggers induced systemic resistance to foliar disease in maize. Sci. Rep. 2016, 6, 35543. [Google Scholar] [CrossRef]
- Guo, K.; Sui, Y.; Li, Z.; Huang, Y.; Zhang, H.; Wang, W. Colonization of Trichoderma viride Tv-1511 in peppermint (Mentha × piperita L.) roots promotes essential oil production by triggering ROS-mediated MAPK activation. Plant Physiol. Biochem. 2020, 151, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma research in the genome era. Annu. Rev. Phytopathol. 2013, 51, 105–129. [Google Scholar] [CrossRef]
- Chacón, M.R.; Rodríguez-Galán, O.; Benítez, T.; Sousa, S.; Rey, M.; Llobell, A.; Delgado-Jarana, J. Microscopic and transcriptome analyses of early colonization of tomato roots by Trichoderma harzianum. Int. Microbiol. 2007, 10, 19–27. [Google Scholar]
- Ruano-Rosa, D.; Prieto, P.; Rincón, A.M.; Gómez-Rodríguez, M.V.; Valderrama, R.; Barroso, J.B.; Mercado-Blanco, J. Fate of Trichoderma harzianum in the olive rhizosphere: Time course of the root colonization process and interaction with the fungal pathogen Verticillium dahliae. BioControl 2016, 61, 269–282. [Google Scholar] [CrossRef]
- Alonso-Ramírez, A.; Poveda, J.; Martín, I.; Hermosa, R.; Monte, E.; Nicolás, C. Salicylic acid prevents Trichoderma harzianum from entering the vascular system of roots. Mol. Plant Pathol. 2014, 15, 823–831. [Google Scholar] [CrossRef]
- De Palma, M.; Salzano, M.; Villano, C.; Aversano, R.; Lorito, M.; Ruocco, M.; Docimo, T.; Piccinelli, A.L.; D’Agostino, N.; Tucci, M. Transcriptome reprogramming, epigenetic modifications and alternative splicing orchestrate the tomato root response to the beneficial fungus Trichoderma harzianum. Hortic. Res. 2019, 6, 5. [Google Scholar] [CrossRef]
- Jogaiah, S.; Abdelrahman, M.; Tran, L.S.P.; Ito, S.I. Different mechanisms of Trichoderma virens-mediated resistance in tomato against Fusarium wilt involve the jasmonic and salicylic acid pathways. Mol. Plant Pathol. 2018, 19, 870–882. [Google Scholar] [CrossRef]
- Basińska-Barczak, A.; Błaszczyk, L.; Szentner, K. Plant cell wall changes in common wheat roots as a result of their interaction with beneficial fungi of Trichoderma. Cells 2020, 9, 2319. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-triggered immunity: From pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef]
- Su, J.; Yang, L.; Zhu, Q.; Wu, H.; He, Y.; Liu, Y.; Xu, J.; Jiang, D.; Zhang, S. Active photosynthetic inhibition mediated by MPK3/MPK6 is critical to effector-triggered immunity. PLoS Biol. 2018, 16, e2004122. [Google Scholar] [CrossRef]
- Kuć, J. Induced immunity to plant disease. Bioscience 1982, 32, 854–860. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced systemic resistance (ISR) in plants: Mechanism of action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef]
- Peng, K.C.; Lin, C.C.; Liao, C.F.; Yu, H.C.; Lo, C.T.; Yang, H.H.; Lin, K.-C. Expression of L-amino acid oxidase of Trichoderma harzianum in tobacco confers resistance to Sclerotinia sclerotiorum and Botrytis cinerea. Plant Sci. 2021, 303, 110772. [Google Scholar] [CrossRef]
- Alizadeh, H.; Behboudi, K.; Ahmadzadeh, M.; Javan-Nikkhah, M.; Zamioudis, C.; Pieterse, C.M.J.; Bakker, P.A. Induced systemic resistance in cucumber and Arabidopsis thaliana by the combination of Trichoderma harzianum Tr6 and Pseudomonas sp. Ps14. Biol. Control. 2013, 65, 14–23. [Google Scholar] [CrossRef]
- Bigirimana, J.; Meyer, G.d.; Poppe, J.; Hoefte, M.; Elad, Y. Induction of systemic resistance on bean (Phaseolus vulgaris) by Trichoderma harzianum. Med. Fac. Landbouw. Univ. Gent 1997, 62, 1001–1007. [Google Scholar]
- Martínez-Medina, A.; Fernandez, I.; Lok, G.B.; Pozo, M.J.; Pieterse, C.M.J.; Van Wees, S.C.M. Shifting from priming of salicylic acid- to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol. 2017, 213, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, A.; Fernandez, I.; Sánchez-Guzmán, M.; Jung, S.; Pascual, J.; Pozo, M. Deciphering the hormonal signalling network behind the systemic resistance induced by Trichoderma harzianum in tomato. Front. Plant Sci. 2013, 4, 206. [Google Scholar] [CrossRef] [PubMed]
- MAPK Group. Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 2002, 7, 301–308. [CrossRef] [PubMed]
- Jonak, C.; Ökrész, L.; Bögre, L.; Hirt, H. Complexity, cross talk and integration of plant MAP kinase signalling. Curr. Opin. Plant Biol. 2002, 5, 415–424. [Google Scholar] [CrossRef]
- Taj, G.; Agarwal, P.; Grant, M.; Kumar, A. MAPK machinery in plants. Plant Signal. Behav. 2010, 5, 1370–1378. [Google Scholar] [CrossRef]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.L.; Gomez-Gomez, L.; Boller, T.; Ausubel, F.M.; Sheen, J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef]
- Zipfel, C.; Kunze, G.; Chinchilla, D.; Caniard, A.; Jones, J.D.G.; Boller, T.; Felix, G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 2006, 125, 749–760. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK Cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Takahashi, F.; Yoshida, R.; Ichimura, K.; Mizoguchi, T.; Seo, S.; Yonezawa, M.; Maruyama, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The mitogen-activated protein kinase cascade MKK3–MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell 2007, 19, 805–818. [Google Scholar] [CrossRef]
- Tsuda, K.; Mine, A.; Bethke, G.; Igarashi, D.; Botanga, C.J.; Tsuda, Y.; Glazebrook, J.; Sato, M.; Katagiri, F. Dual regulation of gene expression mediated by extended MAPK activation and salicylic acid contributes to robust innate immunity in Arabidopsis thaliana. PLoS Genet. 2013, 9, e1004015. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Katou, S.; Seto, H.; Gomi, K.; Ohashi, Y. The mitogen-activated protein kinases WIPK and SIPK regulate the levels of jasmonic and salicylic acids in wounded tobacco plants. Plant J. Cell Mol. Biol. 2007, 49, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jin, H.; Yang, K.Y.; Kim, C.Y.; Baker, B.; Zhang, S. Interaction between two mitogen-activated protein kinases during tobacco defense signaling. Plant J. Cell Mol. Biol. 2003, 34, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yano, A.; Shinshi, H. Slow and prolonged activation of the p47 protein kinase during hypersensitive cell death in a culture of tobacco cells. Plant Physiol. 1999, 119, 1465–1472. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsuzuki, C.; Hachisu, M.; Iwabe, R.; Nakayama, Y.; Nonaga, Y.; Sukegawa, S.; Horito, S.; Arimura, G.-I. An amino acid ester of menthol elicits defense responses in plants. Plant Mol. Biol. 2022, 109, 523–531. [Google Scholar] [CrossRef]
- Shoresh, M.; Gal-On, A.; Leibman, D.; Chet, I. Characterization of a mitogen-activated protein kinase gene from Cucumber required for Trichoderma-conferred plant resistance. Plant Physiol. 2006, 142, 1169–1179. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Alfaro-Cuevas, R.; López-Bucio, J. Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol. Plant Microbe Interact. 2014, 27, 503–514. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; López-Bucio, J.S.; Méndez-Bravo, A.; Macías-Rodríguez, L.; Ramos-Vega, M.; Guevara-García, Á.A.; López-Bucio, J. Mitogen-activated protein kinase 6 and ethylene and auxin signaling pathways are involved in Arabidopsis root-system architecture alterations by Trichoderma atroviride. Mol. Plant Microbe Interact. 2015, 28, 701–710. [Google Scholar] [CrossRef]
- Mathys, J.; De Cremer, K.; Timmermans, P.; Van Kerkhove, S.; Lievens, B.; Vanhaecke, M.; Cammue, B.P.A.; De Coninck, B. Genome-wide characterization of ISR induced in Arabidopsis thaliana by Trichoderma hamatum T382 against infection. Front. Plant Sci. 2012, 3, 108. [Google Scholar] [CrossRef]
- Eschen-Lippold, L.; Bethke, G.; Palm-Forster, M.A.T.; Pecher, P.; Bauer, N.; Glazebrook, J.; Scheel, D.; Lee, J. MPK11—A fourth elicitor-responsive mitogen-activated protein kinase in Arabidopsis thaliana. Plant Signal. Behav. 2012, 7, 1203–1205. [Google Scholar] [CrossRef]
- Bethke, G.; Pecher, P.; Eschen-Lippold, L.; Tsuda, K.; Katagiri, F.; Glazebrook, J.; Scheel, D.; Lee, J. Activation of the Arabidopsis thaliana mitogen-activated protein kinase MPK11 by the flagellin-derived elicitor peptide, flg22. Mol. Plant-Microbe Interact. 2012, 25, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Lassowskat, I.; Böttcher, C.; Eschen-Lippold, L.; Scheel, D.; Lee., J. Sustained mitogen-activated protein kinase activation reprograms defense metabolism and phosphoprotein profile in Arabidopsis thaliana. Front. Plant Sci. 2014, 5, 554. [Google Scholar] [CrossRef] [PubMed]
- Shigenaga, A.M.; Berens, M.L.; Tsuda, K.; Argueso, C.T. Towards engineering of hormonal crosstalk in plant immunity. Curr. Opin. Plant Biol. 2017, 38, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Illescas, M.; Pedrero-Méndez, A.; Pitorini-Bovolini, M.; Hermosa, R.; Monte, E. Phytohormone production profiles in Trichoderma species and their relationship to wheat plant responses to water stress. Pathogens 2021, 10, 991. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Majewska, M.; Hanaka, A.; Tyśkiewicz, K.; Pawlik, A.; Janusz, G. Phytohormones (Auxin, Gibberellin) and ACC deaminase in vitro synthesized by the mycoparasitic Trichoderma DEMTkZ3A0 strain and changes in the level of auxin and plant resistance markers in wheat seedlings inoculated with this strain conidia. Int. J. Mol. Sci. 2019, 20, 4923. [Google Scholar] [CrossRef] [PubMed]
- Kamalov, L.S.; Turgunov, K.K.; Aripova, S.F.; Abdilalimov, O. Gibberillin A-3 from the microscopic fungus Trichoderma harzianum. Chem. Nat. Compd. 2018, 54, 421–422. [Google Scholar] [CrossRef]
- Pérez, E.; Rubio, M.B.; Cardoza, R.E.; Gutiérrez, S.; Bettiol, W.; Monte, E.; Hermosa, R. The importance of chorismate mutase in the biocontrol potential of Trichoderma parareesei. Front. Microbiol. 2015, 6, 1181. [Google Scholar] [CrossRef]
- Vos, C.M.F.; De Cremer, K.; Cammue, B.P.A.; De Coninck, B. The toolbox of Trichoderma spp. in the biocontrol of Botrytis cinerea disease. Mol. Plant Pathol. 2015, 16, 400–412. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- López-Ráez, J.A.; Verhage, A.; Fernández, I.; García, J.M.; Azcón-Aguilar, C.; Flors, V.; Pozo, M.J. Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. J. Exp. Bot. 2010, 61, 2589–2601. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.J.; Azcón-Aguilar, C. Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 2007, 10, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.; Zechmann, B.; Molitor, A.; Trujillo, M.; Petutschnig, E.; Lipka, V.; Kogel, K.-H.; Schäfer, P. Broad-spectrum suppression of innate immunity is required for colonization of Arabidopsis roots by the fungus Piriformospora indica. Plant Physiol. 2011, 156, 726–740. [Google Scholar] [CrossRef] [PubMed]
- Peleg-Grossman, S.; Golani, Y.; Kaye, Y.; Melamed-Book, N.; Levine, A. NPR1 protein regulates pathogenic and symbiotic interactions between Rhizobium and legumes and non-legumes. PLoS ONE 2009, 4, e8399. [Google Scholar] [CrossRef] [PubMed]
- Alfiky, A.; Weisskopf, L. Deciphering Trichoderma–plant–pathogen interactions for better development of biocontrol applications. J. Fungi 2021, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Morán-Diez, E.; Rubio, B.; Domínguez, S.; Hermosa, R.; Monte, E.; Nicolás, C. Transcriptomic response of Arabidopsis thaliana after 24 h incubation with the biocontrol fungus Trichoderma harzianum. J. Plant Physiol. 2012, 169, 614–620. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, Y.; Ge, W.; Jia, Z.; Song, S.; Zhang, L.; Huang, Y. Involvement of jasmonic acid, ethylene and salicylic acid signaling pathways behind the systemic resistance induced by Trichoderma longibrachiatum H9 in cucumber. BMC Genom. 2019, 20, 144. [Google Scholar] [CrossRef]
- Mayo, S.; Cominelli, E.; Sparvoli, F.; González-López, O.; Rodríguez-González, A.; Gutiérrez, S.; Casquero, P.A. Development of a qPCR strategy to select bean genes involved in plant defense response and regulated by the Trichoderma velutinum–Rhizoctonia solani interaction. Front. Plant Sci. 2016, 7, 1109. [Google Scholar] [CrossRef]
- Agostini, R.B.; Postigo, A.; Rius, S.P.; Rech, G.E.; Campos Bermudez, V.A.; Vargas, W.A. Long-lasting primed state in maize plants: Salicylic acid and steroid signaling pathways as key players in the early activation of immune responses in silks. Mol. Plant Microbe Interact. 2019, 32, 95–106. [Google Scholar] [CrossRef]
- Kottb, M.; Gigolashvili, T.; Großkinsky, D.K.; Piechulla, B. Trichoderma volatiles effecting Arabidopsis: From inhibition to protection against phytopathogenic fungi. Front. Microbiol. 2015, 6, 995. [Google Scholar] [CrossRef]
- Senaratna, T.; Touchell, D.; Bunn, E.; Dixon, K. Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul. 2000, 30, 157–161. [Google Scholar] [CrossRef]
- Azooz, M.M.; Youssef, A.M.; Ahmad, P. Evaluation of salicylic acid (SA) application on growth, osmotic solutes and antioxidant enzyme activities on broad bean seedlings grown under diluted seawater. Int. J. Plant Physiol. Biochem. 2011, 3, 253–264. [Google Scholar] [CrossRef]
- Ahmad, P.; Nabi, G.; Ashraf, M. Cadmium-induced oxidative damage in mustard [Brassica juncea (L.) Czern. & Coss.] plants can be alleviated by salicylic acid. South Afr. J. Bot. 2011, 77, 36–44. [Google Scholar] [CrossRef]
- Gunes, A.; Inal, A.; Alpaslan, M.; Eraslan, F.; Bagci, E.G.; Cicek, N. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J. Plant Physiol. 2007, 164, 728–736. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Fatkhutdinova, D.R. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003, 164, 317–322. [Google Scholar] [CrossRef]
- Dat, J.F.; Foyer, C.H.; Scott, I.M. Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol. 1998, 118, 1455–1461. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mohamed, H.I.; Mahmoud, A.; Elkelish, A.; Misra, A.N.; Guy, K.M.; Kamran, M.; Ai, S.; Zhang, M. Salicylic acid stimulates antioxidant defense and osmolyte metabolism to alleviate oxidative stress in watermelons under excess boron. Plants 2020, 9, 724. [Google Scholar] [CrossRef]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Beltrán-Peña, E.; Herrera-Estrella, A.; López-Bucio, J. Trichoderma-induced plant immunity likely involves both hormonal- and camalexin-dependent mechanisms in Arabidopsis thaliana and confers resistance against necrotrophic fungi Botrytis cinerea. Plant Signal. Behav. 2011, 6, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Sáenz-Mata, J.; Jiménez-Bremont, J.F. HR4 gene is induced in the Arabidopsis-Trichoderma atroviride beneficial interaction. Int. J. Mol. Sci. 2012, 13, 9110–9928. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.; Oelmüller, R. WRKY transcription factors: Jack of many trades in plants. Plant Signal. Behav. 2014, 9, e27700. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, C.; Wang, H.; Guo, Z. WRKY transcription factors: Evolution, binding, and action. Phytopathol. Res. 2019, 1, 13. [Google Scholar] [CrossRef]
- Bai, Y.; Sunarti, S.; Kissoudis, C.; Visser, R.G.F.; van der Linden, C.G. The role of tomato WRKY genes in plant responses to combined abiotic and biotic stresses. Front. Plant Sci. 2018, 9, 801. [Google Scholar] [CrossRef]
- Sáenz-Mata, J.; Salazar-Badillo, F.B.; Jiménez-Bremont, J.F. Transcriptional regulation of Arabidopsis thaliana WRKY genes under interaction with beneficial fungus Trichoderma atroviride. Acta Physiol. Plant. 2014, 36, 1085–1093. [Google Scholar] [CrossRef]
- Xu, X.; Chen, C.; Fan, B.; Chen, Z. Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 2006, 18, 1310–1326. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier Academic Press: San Diego, CA, USA, 2005. [Google Scholar]
- Van Loon, L.C.; Van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Ebrahim, S.; Kalidindi, U.; Singh, B. Pathogenesis related (PR) proteins in plant defense mechanism. In Science against Microbial Pathogens: Communicatiog Research and Tecnological Advances; Méndez-Vilas, A., Ed.; Formatex Researc Center: Badajoz, Spain, 2011; pp. 1043–1054. [Google Scholar]
- Tucci, M.; Ruocco, M.; De Masi, L.; De Palma, M.; Lorito, M. The beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Mol. Plant Pathol. 2011, 12, 341–354. [Google Scholar] [CrossRef]
- Alfano, G.; Ivey, M.L.L.; Cakir, C.; Bos, J.I.B.; Miller, S.A.; Madden, L.V.; Kamoun, S.; Hoitink, H.A.J. Systemic modulation of gene expression in tomato by Trichoderma hamatum 382. Phytopathology 2007, 97, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Aamir, M.; Kashyap, S.P.; Zehra, A.; Dubey, M.K.; Singh, V.K.; Ansari, W.A.; Upadhyay, R.S.; Singh, S. Trichoderma erinaceum bio-priming modulates the WRKYs defense programming in tomato against the Fusarium oxysporum f. sp. lycopersici (Fol) challenged condition. Front. Plant Sci. 2019, 10, 911. [Google Scholar] [CrossRef]
- Perazzolli, M.; Moretto, M.; Fontana, P.; Ferrarini, A.; Velasco, R.; Moser, C.; Delledonne, M.; Pertot, I. Downy mildew resistance induced by Trichoderma harzianum T39 in susceptible grapevines partially mimics transcriptional changes of resistant genotypes. BMC Genom. 2012, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Yedidia, I.; Chet, I. Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in Cucumber by Trichoderma asperellum T203. Phytopathology 2005, 95, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Hoth, S.; Morgante, M.; Sanchez, J.P.; Hanafey, M.K.; Tingey, S.V.; Chua, N.H. Genome-wide gene expression profiling in Arabidopsis thaliana reveals new targets of abscisic acid and largely impaired gene regulation in the abi1-1 mutant. J. Cell Sci. 2002, 115, 4891–4900. [Google Scholar] [CrossRef]
- Rubio, M.B.; Quijada, N.M.; Pérez, E.; Domínguez, S.; Monte, E.; Hermosa, R. Identifying beneficial qualities of Trichoderma parareesei for plants. Appl. Environ. Microbiol. 2014, 80, 1864–1873. [Google Scholar] [CrossRef]
- Yedidia, I.; Shoresh, M.; Kerem, Z.; Benhamou, N.; Kapulnik, Y. Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in Cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins. Appl. Environ. Microbiol. 2003, 69, 7343–7353. [Google Scholar] [CrossRef]
- Morán-Diez, M.E.; Tranque, E.; Bettiol, W.; Monte, E.; Hermosa, R. Differential response of tomato plants to the application of three Trichoderma species when evaluating the control of Pseudomonas syringae populations. Plants 2020, 9, 626. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the regulation of reactive oxygen species metabolism in plants under abiotic stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef]
- Mastouri, F.; Björkman, T.; Harman, G.E. Trichoderma harzianum enhances antioxidant defense of tomato seedlings and resistance to water deficit. Mol. Plant Microbe Interact. 2012, 25, 1264–1271. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- de Pinto, M.C.; Francis, D.; De Gara, L. The redox state of the ascorbate-dehydroascorbate pair as a specific sensor of cell division in tobacco BY-2 cells. Protoplasma 1999, 209, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Ren, J.J.; Zhao, H.J.; Wang, X.L.; Wang, T.H.; Jin, S.D.; Wang, Z.-H.; Li, C.-Y.; Liu, A.-R.; Lin, X.-M.; et al. Trichoderma harzianum improves defense against Fusarium oxysporum by regulating ROS and RNS metabolism, redox balance, and energy flow in Cucumber roots. Phytopathology 2019, 109, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, Z.; Li, Z.; Wang, Y.; Yang, K. Alleviation of the effects of saline-alkaline stress on maize seedlings by regulation of active oxygen metabolism by Trichoderma asperellum. PLoS ONE 2017, 12, e0179617. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Al Huqail, A.A.; Egamberdieva, D. Alleviation of abiotic salt stress in Ochradenus baccatus (Del.) by Trichoderma hamatum (Bonord.) Bainier. J. Plant Interact. 2014, 9, 857–868. [Google Scholar] [CrossRef]
- Yasmeen, R.; Siddiqui, Z.S. Physiological responses of crop plants against Trichoderma harzianum in saline environment. Acta Bot. Croat. 2017, 76, 154–162. [Google Scholar] [CrossRef]
- Mironenka, J.; Różalska, S.; Bernat, P. Potential of Trichoderma harzianum and its metabolites to protect wheat seedlings against Fusarium culmorum and 2,4-D. Int. J. Mol. Sci. 2021, 22, 13058. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Application of Plant-Growth-Promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Elansary, H.O.; El-Shanhorey, N.A.; Abdel-Hamid, A.M.E.; Ali, H.M.; Elshikh, M.S. Salicylic acid-regulated antioxidant mechanisms and gene expression enhance rosemary performance under saline conditions. Front. Physiol. 2017, 8, 716. [Google Scholar] [CrossRef]
- Saidi, I.; Yousfi, N.; Borgi, M.A. Salicylic acid improves the antioxidant ability against arsenic-induced oxidative stress in sunflower (Helianthus annuus) seedling. J. Plant Nutr. 2017, 40, 2326–2335. [Google Scholar] [CrossRef]
- Torun, H.; Novák, O.; Mikulík, J.; Pěnčík, A.; Strnad, M.; Ayaz, F.A. Timing-dependent effects of salicylic acid treatment on phytohormonal changes, ROS regulation, and antioxidant defense in salinized barley (Hordeum vulgare L.). Sci. Rep. 2020, 10, 13886. [Google Scholar] [CrossRef] [PubMed]
- Serrano, I.; Audran, C.; Rivas, S. Chloroplasts at work during plant innate immunity. J. Exp. Bot. 2016, 67, 3845–3854. [Google Scholar] [CrossRef] [PubMed]
- Şesan, T.E.; Oancea, A.O.; Ştefan, L.M.; Mănoiu, V.S.; Ghiurea, M.; Răut, I.; Constantinescu-Aruxandei, D.; Toma, A.; Savin, S.; Bira, A.F.; et al. Effects of foliar treatment with a Trichoderma plant biostimulant consortium on Passiflora caerulea L. Yield and quality. Microorg. 2020, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Doni, F.; Khadka, R.B.; Uphoff, N. Endophytic strains of Trichoderma increase plants’ photosynthetic capability. J. Appl. Microbiol. 2021, 130, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Andrzejak, R.; Janowska, B. Flowering, nutritional status, and content of chloroplast pigments in leaves of Gladiolus hybridus L. ‘Advances Red’ after application of Trichoderma spp. Sustainability 2022, 14, 4576. [Google Scholar] [CrossRef]
- Song, Y.; Feng, L.; Alyafei, M.A.M.; Jaleel, A.; Ren, M. Function of chloroplasts in plant stress responses. Int. J. Mol. Sci. 2021, 22, 13464. [Google Scholar] [CrossRef]
- Li, M.; Kim, C. Chloroplast ROS and stress signaling. Plant Commun. 2021, 3, 100264. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Y.-F.; Liu, Z.-H.; Li, Z.-T.; Yang, K.-J. Trichoderma asperellum alleviates the effects of saline-alkaline stress on maize seedlings via the regulation of photosynthesis and nitrogen metabolism. Plant Growth Regul. 2018, 85, 363–374. [Google Scholar] [CrossRef]
- Metwally, R.A.; Al-Amri, S. Individual and interactive role of Trichoderma viride and arbuscular mycorrhizal fungi on growth and pigment content of onion plants. Lett. Appl. Microbiol. 2020, 70, 79–86. [Google Scholar] [CrossRef]
- Abdel-Fattah, G.M.; Shabana, Y.M.; Ismail, A.E.; Rashad, Y.M. Trichoderma harzianum: A biocontrol agent against Bipolaris oryzae. Mycopathologia 2007, 164, 81–89. [Google Scholar] [CrossRef]
- da Silva, L.R.; Valadares-Inglis, M.C.; Peixoto, G.H.; de Luccas, B.E.; Muniz, P.H.; Magalhães, D.M.; Moraes, M.C.B.; de Mello, S.C.M. Volatile organic compounds emitted by Trichoderma azevedoi promote the growth of lettuce plants and delay the symptoms of white mold. Biol. Control. 2021, 152, 104447. [Google Scholar] [CrossRef]
- Hosseinzeynali, A.; Abbaszadeh Dahaji, P.; Alaei, H.; Hosseinifard, J.; Akhgar, A. Effect of Trichoderma on growth and nutrition of Pistachio trees under common garden condition. J. Soil Biol. 2021, 8, 115–129. [Google Scholar]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Yano, A.; Suzuki, K.; Uchimiya, H.; Shinshi, H. Induction of hypersensitive cell death by a fungal protein in cultures of tobacco cells. Mol. Plant-Microbe Interact. 1998, 11, 115–123. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).