An Optimized Protocol for Indirect Organogenesis from Root Explants of Agapanthus praecox subsp. orientalis ‘Big Blue’
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material Collection
2.2. Sterilization and Germination
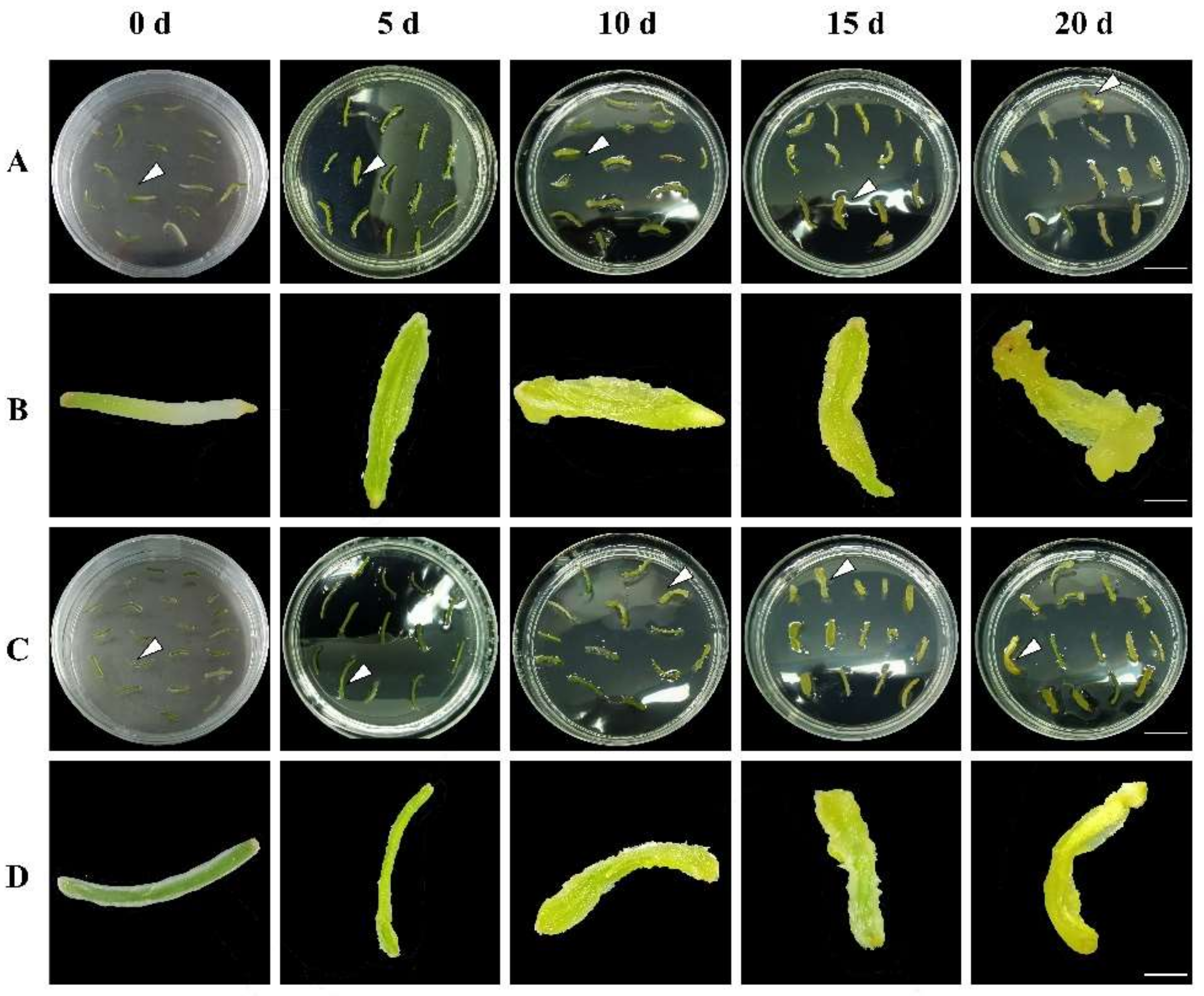
2.3. Callus Induction from Root Explants
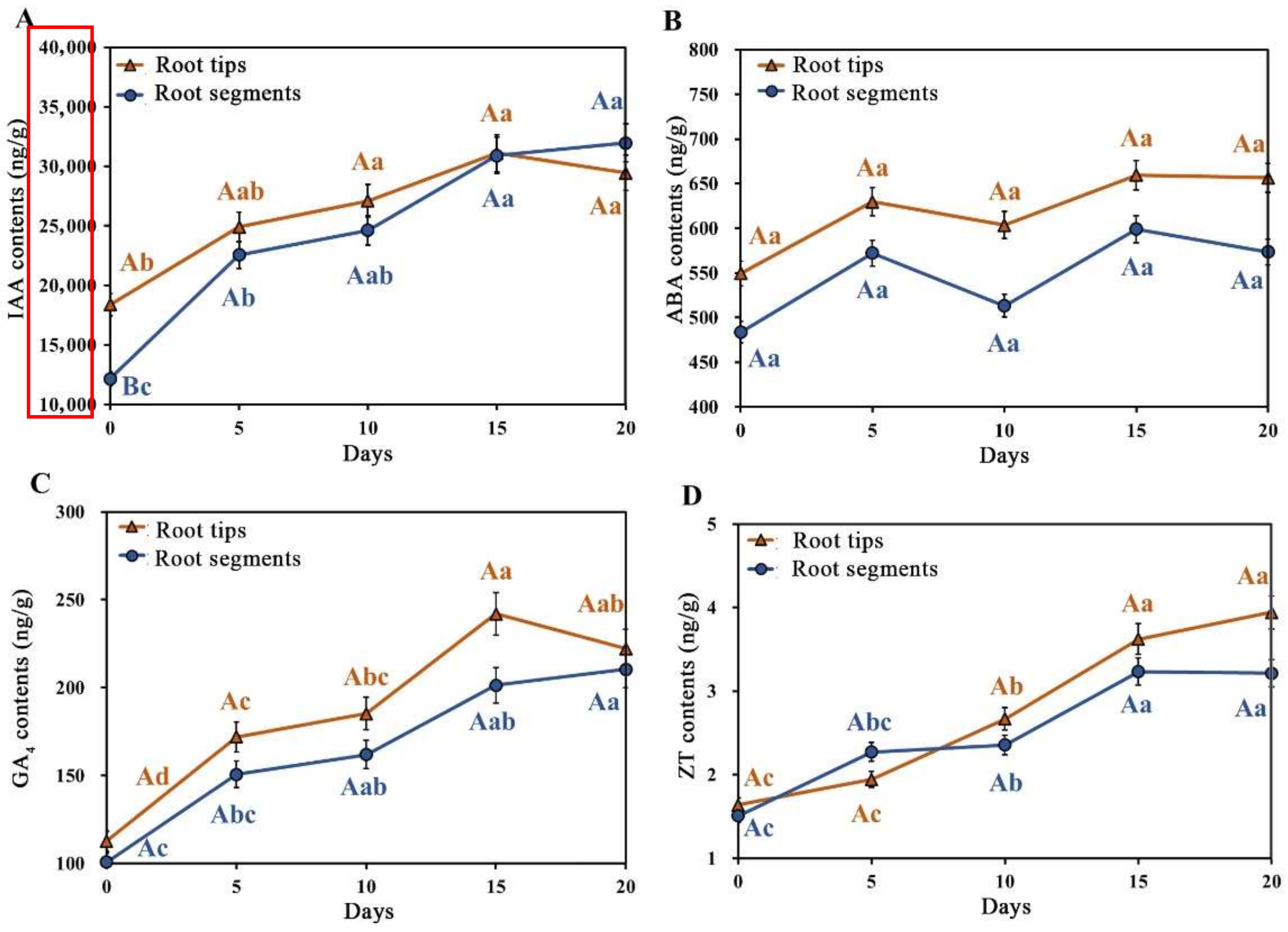
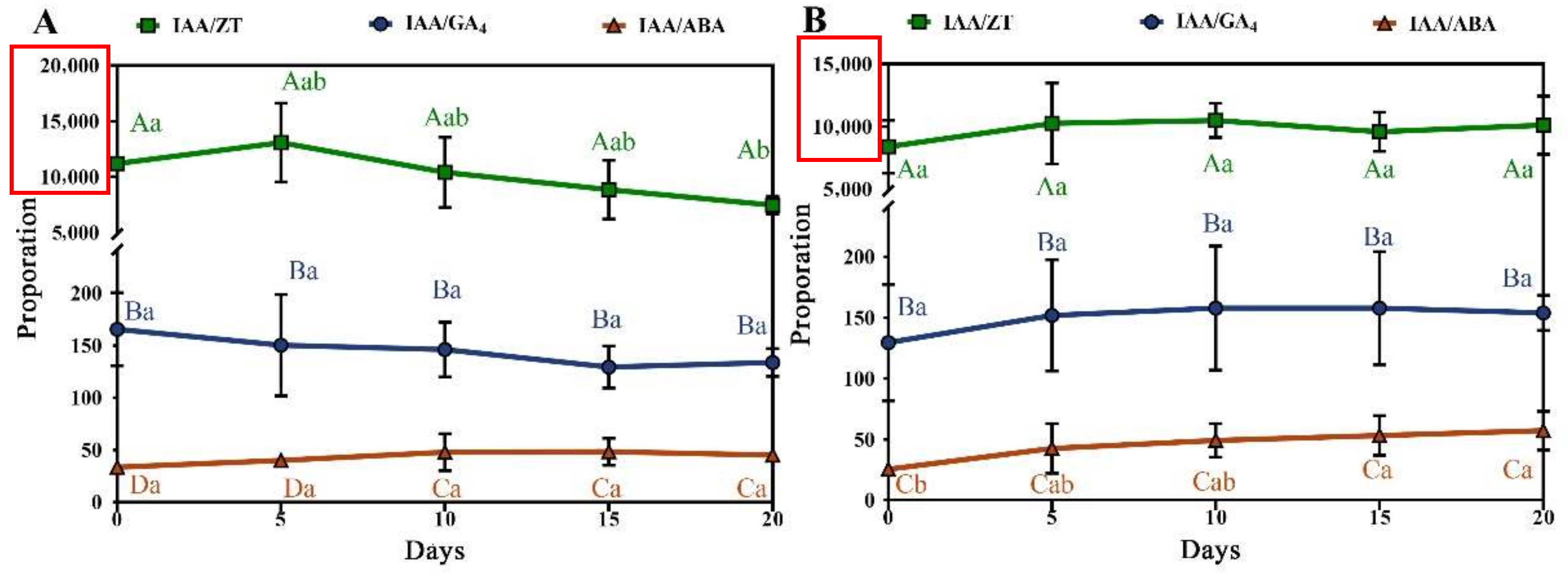
2.4. Analysis of Endogenous Hormone Content during Callus Induction
2.5. Callus Proliferation
2.6. Adventitious Buds Induction and Regeneration
2.7. Culture Conditions
2.8. Experimental Design and Statistical Analysis
3. Results
3.1. Effects of Three PGRs on Callus Induction Rate and Morphogenesis
3.2. Optimization of Three PGRs Combination on Callus Induction from Root Tips
3.3. Role of Four Endogenous Hormones during Callus Induction
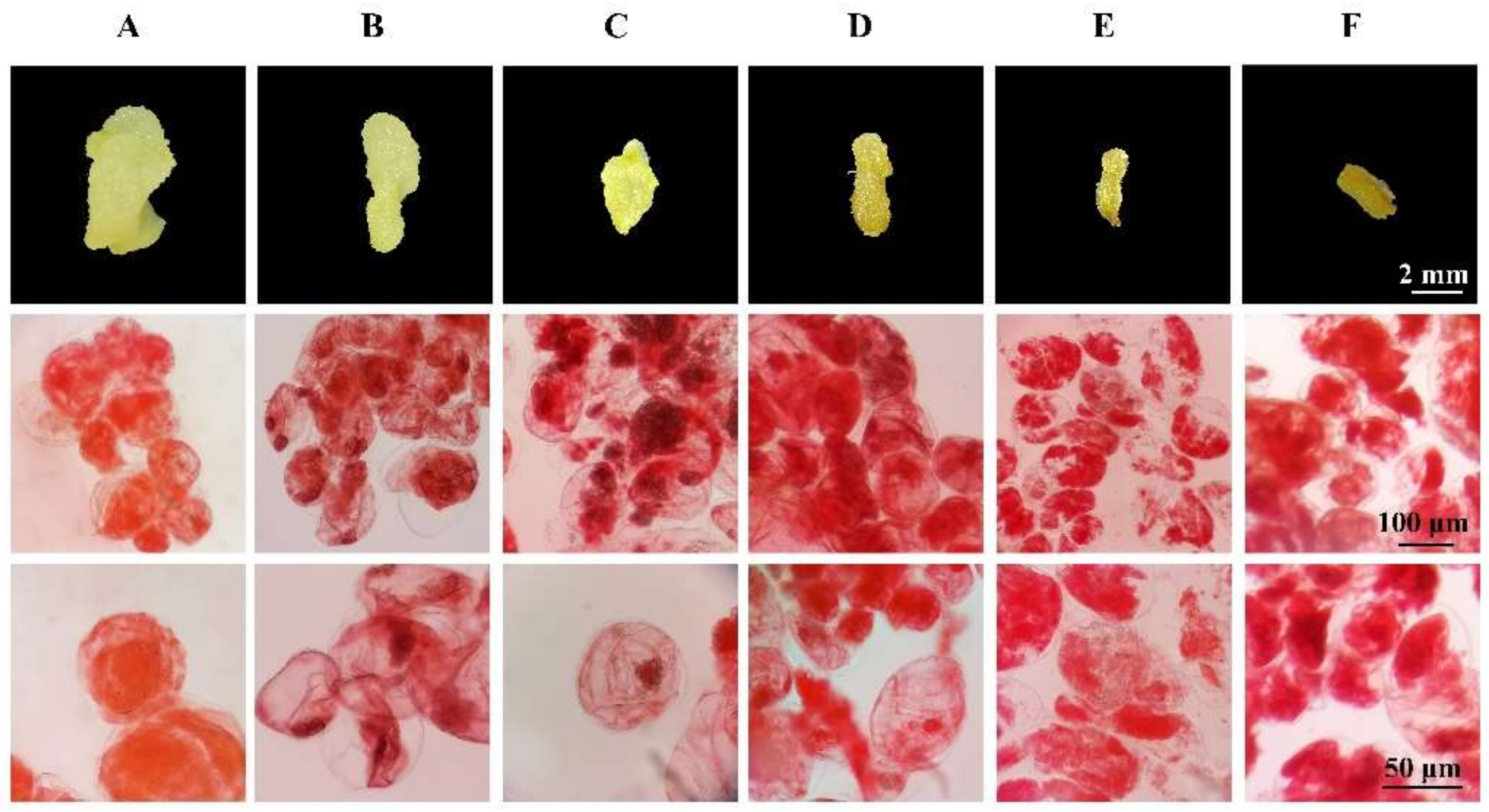
3.4. Effects of Three PGRs on Callus Proliferation Rate and State
3.5. Hardening and Transplanting of Seedlings Dedifferentiated from Callus
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, X. Micropropagation In Vitro of Agapanthus praecox ssp. Orientalis ‘Big Blue’; Shanghai Jiao Tong University: Shanghai, China, 2009; pp. 31–45. [Google Scholar]
- Zurita, F.; Belmont, M.A.; De Anda, J.; White, J.R. Seeking a way to promote the use of constructed wetlands for domestic wastewater treatment in developing countries. Water Sci. Technol. 2011, 63, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Calheiros, C.; Bessa, V.S.; Mesquita, R.; Brix, H.; Rangel, A.; Castro, P. Constructed wetland with a polyculture of ornamental plants for wastewater treatment at a rural tourism facility. Ecol. Eng. 2015, 79, 1–7. [Google Scholar] [CrossRef]
- Mor, Y.; Halevy, A.H.; Kofranek, A.M.; Reid, M.S. Post-harvest handling of lily of the Nile flowers. J. Am. Soc. Hortic. Sci. 1984, 109, 494–497. [Google Scholar]
- Chen, X.; Lu, L.; Qian, Y.; Fan, Y. Recent advances in germplasm and landscape application of Agapanthus spp. Chin. Landsc. Archit. 2016, 32, 99–105. [Google Scholar]
- Snoeijer, W. Agapanthus: A Revision of the Genus; Timber Press: Portland, OR, USA, 2004. [Google Scholar]
- Supaibulwatana, K.; Mii, M. Organogenesis and somatic embryogenesis from young flower buds of Agapanthus africanus Hoffmanns. Plant Biotechnol. 1997, 14, 23–28. [Google Scholar] [CrossRef]
- Suzuki, S.; Nakano, M. Agrobacterium-mediated transformation in Liliaceous ornamental plants. Jpn. Agric. Res. Q. 2002, 36, 119–127. [Google Scholar] [CrossRef]
- Nakano, M.; Tanaka, S.; Oota, M.; Ookawa, E.; Suzuki, S.; Saito, H. Regeneration of diploid and tetraploid plants from calluse-derived protoplast of Agapanthus praecox ssp. orientalis (Leighton) Leighton. Plant Cell Tissue Organ Cult. 2003, 72, 63–69. [Google Scholar] [CrossRef]
- Ma, K. Establishment of Regeneration System Cultured In Vitro of Agapanthus praecox ssp. Orientalis ‘Big Blue’; Northeast Forestry University: Harbin, China, 2007; pp. 24–26. [Google Scholar]
- Yaacob, J.S.; Syafawati, J. Tissue Culture, Cellular Behaviour and Pigment Analysis of Agapanthus praecox ssp. minimus; University of Malaya: Kuala Lumpur, Malaysia, 2013. [Google Scholar]
- Kang, L. Preliminary report on the experiment of regeneration system of Agpanthus praecox ssp. minimus ‘Storms River’. Contemp. Hortic. 2009, 12, 7–8 + 25. [Google Scholar]
- Hu, Z.; He, Y. Study on tissue culture and plantlet regeneration of Agapanthus praecox ssp. orientalis ‘Big Blue’. North. Hortic. 2011, 10, 118–120. [Google Scholar]
- Suzuki, S.; Oota, M.; Nakano, M. Embryogenic callus induction from leaf explants of the Liliaceous ornamental plant, Agapanthus praecox ssp. orientalis (Leighton) Leighton—Histological study and response to selective agents. Sci. Hortic. 2002, 95, 123–132. [Google Scholar] [CrossRef]
- Suzuki, S.; Supaibulwatana, K.; Mii, M.; Nakano, M. Production of transgenic plants of the Liliaceous ornamental plant Agapanthus praecox ssp. orientalis (Leighton) Leighton via Agrobacterium-mediated transformation of embryogenic calli. Plant Sci. 2001, 161, 89–97. [Google Scholar] [CrossRef]
- Yue, J.; Dong, Y.; Wang, X.; Sun, P.; Wang, S.; Zhang, X.; Zhang, Y. A regeneration system for organogenesis and somatic embryogenesis using leaves of Agapanthus praecox as explants. Chin. Bull. Bot. 2020, 55, 588–595. [Google Scholar]
- Wang, Y.; Fan, X.; Wang, G.; Zhang, D.; Shen, X. Regeneration of Agapanthus praecox ssp. orientalis ‘Big Blue’ via somatic embryogenesis. Propag. Ornam. Plants 2012, 12, 148–154. [Google Scholar]
- Baskaran, P.; Staden, J.V. Rapid in vitro micropropagation of Agapanthus praecox. S. Afr. J. Bot. 2013, 86, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Yaacob, J.S.; Taha, R.M.; Mohajer, S. Organogenesis induction and acclimatization of African blue Lily (Agapanthus praecox ssp. minimus). Int. J. Agric. Biol. 2014, 16, 57–64. [Google Scholar]
- Yaacob, J.S.; Saleh, A.; Elias, H.; Abdullah, S.; Mahmad, N.; Mohamed, N. In vitro regeneration and acclimatization protocols of selected ornamental plants (Agapanthus praecox, Justicia betonica and Celosia cristata). Sains Malays. 2014, 43, 715–722. [Google Scholar]
- Zou, M. Physiological and Biochemical Aspects of Picloram Regulates the Embryonic Potential of Callus in Agapanthus; Shanghai Jiao Tong University: Shanghai, China, 2015. [Google Scholar]
- Ren, N.; Zou, M.; Chen, G.; Shen, X. Difference analysis of redox levels in the callus of Agapanthus praecox ssp. orientalis with different embryonic ability. Mol. Plant Breed. 2022, 20, 1281–1288. [Google Scholar]
- Chen, X.; Lv, X.; Yin, L.; Feng, J.; Zhang, D. Comparison with morphology and germination of Agapanthus praecox seeds under different cultivation region and environment. Acta Agric. Shanghai 2020, 4, 43–47. [Google Scholar]
- Iraqi, D.; Francine, M. Analysis of carbohydrate metabolism enzymes and cellular contents of sugars and proteins during spruce somatic embryogenesis suggests a regulatory role of exogenous sucrose in embryo development. J. Exp. Bot. 2001, 52, 2301–2311. [Google Scholar] [CrossRef] [Green Version]
- Yue, J.; Wei, Z.; Li, P.; Wang, Z. Influence on callus induction and physiological characters of callus proliferation in Agapanthus praecox ssp. orientalis by carbon sources. Acta Bot. Boreali Occident. Sin. 2021, 41, 439–449. [Google Scholar]
- Chen, M.; Zhang, J.; Zhou, Y.; Gu, J.; Shen, Q.; Zhang, P. Study on factors influence somatic embryogenesis fromoots of Lilium spp. J. Nucl. Agric. Sci. 2015, 29, 1494–1501. [Google Scholar]
- Tian, H.; Niu, T.; Yu, Q.; Quan, T.; Ding, Z. Auxin gradient is crucial for the maintenance of root distal stem cell identity in Arabidopsis. Plant Signal. Behav. 2013, 8, e26429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Wang, X.; Zhang, J.; Wang, S. Plant tissue culture and its research advances and application in the forage plants. Pratacult Sci. 2011, 28, 1140–1148. [Google Scholar]
- Trifunović-Momčilov, M.; Motyka, V.; Dragićević, I.Č.; Petrić, M.; Jevremović, S.; Malbeck, J.; Holík, J.; Dobrev, P.I.; Subotić, A. Endogenous phytohormones in spontaneously regenerated Centaurium erythraea Rafn. plants grown in vitro. J. Plant Growth Regul. 2016, 35, 543–552. [Google Scholar] [CrossRef]
- Fu, F.; Feng, Z.; Qu, B.; Li, W. Relatianships between induction rate of embryonic callus and endogenous hormones content in Maize. J. Nucl. Agric. Sci. 2006, 1, 10–14. [Google Scholar]
- Zayed, E.M.M.; Abd Elbar, O.H. Morphogenesis of immature female inflorescences of date palm in vitro. Ann. Agric. Sci. 2015, 60, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Shi, X.; Wu, X.; Wang, X.; Zhou, K. The influence of endogenous hormones on culture capability of different explants in rice. Sci. Agric. Sin. 2004, 12, 1819–1823. [Google Scholar]
- Zhang, Y.; He, g.; Rong, G.; Liu, X.; Ni, S. Changes of endogenous hormones during the culture of callus from Sopatholobus suberechtus. Biotechnol. Bull. 2017, 33, 66–70. [Google Scholar]
- Li, Y. Callus induction and plant regeneration from floral organ of Lilium ‘Manissa’. Chin. J. Trop. Crops 2013, 34, 1507–1512. [Google Scholar]



| Explant Types | Concentration (mg/L) | ||||
|---|---|---|---|---|---|
| PIC 1 | TDZ 2 | 2,4-D 3 | KT 4 | NAA 5 | |
| root tips, root segments | 1.00 | 0.00 | 0.50 | / | / |
| 1.00 | 0.40 | 1.00 | / | / | |
| 1.00 | 0.60 | 2.00 | / | / | |
| 2.00 | 0.00 | 1.00 | / | / | |
| 2.00 | 0.40 | 2.00 | / | / | |
| 2.00 | 0.60 | 0.50 | / | / | |
| 3.00 | 0.00 | 2.00 | / | / | |
| 3.00 | 0.40 | 0.50 | / | / | |
| 3.00 | 0.60 | 1.00 | / | / | |
| root tips | 1.00 | / | / | 0.00 | 0.10 |
| 1.00 | / | / | 1.00 | 0.50 | |
| 1.00 | / | / | 1.50 | 1.00 | |
| 2.00 | / | / | 0.00 | 0.50 | |
| 2.00 | / | / | 1.00 | 1.00 | |
| 2.00 | / | / | 1.50 | 0.10 | |
| 3.00 | / | / | 0.00 | 1.00 | |
| 3.00 | / | / | 1.00 | 0.10 | |
| 3.00 | / | / | 1.50 | 0.50 | |
| PIC Concentration (mg/L) | TDZ Concentration (mg/L) | 2,4-D Concentration (mg/L) | Induction Rate (%) | |||||
|---|---|---|---|---|---|---|---|---|
| RT 1 | RS 2 | RT | RS | RT | RS | RT | RS | |
| 1.00 | 0.00 | 0.50 | 76.60 | 25.57 | ||||
| 1.00 | 0.40 | 1.00 | 33.33 | 11.45 | ||||
| 1.00 | 0.60 | 2.00 | 26.19 | 23.98 | ||||
| 2.00 | 0.00 | 1.00 | 48.53 | 26.40 | ||||
| 2.00 | 0.40 | 2.00 | 20.83 | 25.94 | ||||
| 2.00 | 0.60 | 0.50 | 54.02 | 11.56 | ||||
| 3.00 | 0.00 | 2.00 | 25.49 | 36.03 | ||||
| 3.00 | 0.40 | 0.50 | 39.29 | 23.04 | ||||
| 3.00 | 0.60 | 1.00 | 38.10 | 16.92 | ||||
| k1 3 | 45.38 a * | 17.93 b | 50.21 a | 32.77 a | 56.64 a | 19.77 a | ||
| k2 4 | 41.13 a | 21.82 a | 31.15 b | 5.96 b | 39.99 b | 16.48 a | ||
| k3 5 | 34.29 a | 6.88 c | 39.43 ab | 7.89 b | 24.17 b | 10.37 b | ||
| R 6 | 11.09 | 14.94 | 19.06 | 26.81 | 32.46 | 9.40 | ||
| PIC Concentration (mg/L) | KT Concentration (mg/L) | NAA Concentration (mg/L) | Induction Rate (%) | |
|---|---|---|---|---|
| 1.00 | 0.00 | 0.10 | 88.89 | |
| 1.00 | 1.00 | 0.50 | 61.11 | |
| 1.00 | 1.50 | 1.00 | 83.33 | |
| 2.00 | 0.00 | 0.50 | 72.22 | |
| 2.00 | 1.00 | 1.00 | 66.67 | |
| 2.00 | 1.50 | 0.10 | 100.00 | |
| 3.00 | 0.00 | 1.00 | 88.89 | |
| 3.00 | 1.00 | 0.10 | 88.89 | |
| 3.00 | 1.50 | 0.50 | 65.08 | |
| k1 1 | 77.78 a | 72.22 a | 92.59 a | |
| k2 2 | 79.63 a | 82.80 a | 66.14 b | |
| k3 3 | 80.95 a | 83.33 a | 79.63 ab | |
| R 4 | 3.17 | 11.11 | 26.46 |
| PIC Concentration (mg/L) | 6-BA 1 Concentration (mg/L) | NAA Concentration (mg/L) | Proliferation Rate (%) | |
|---|---|---|---|---|
| 1.00 | 0.00 | 0.10 | 31.69 | |
| 1.00 | 0.40 | 0.20 | 43.45 | |
| 1.00 | 1.00 | 0.40 | 72.74 | |
| 1.50 | 0.00 | 0.20 | 35.43 | |
| 1.50 | 0.40 | 0.40 | 23.13 | |
| 1.50 | 1.00 | 0.10 | 31.92 | |
| 2.00 | 0.00 | 0.40 | 14.87 | |
| 2.00 | 0.40 | 0.10 | 43.60 | |
| 2.00 | 1.00 | 0.20 | 31.69 | |
| k1 2 | 0.493 a | 0.273 b | 0.357 a | |
| k2 3 | 0.302 b | 0.367 ab | 0.363 a | |
| k3 4 | 0.295 b | 0.449 a | 0.369 a | |
| R 5 | 0.197 | 0.176 | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Q.; Guo, X.; Zhang, Y.; Li, Q.; Chen, G.; Sun, H.; Wang, W.; Shen, X. An Optimized Protocol for Indirect Organogenesis from Root Explants of Agapanthus praecox subsp. orientalis ‘Big Blue’. Horticulturae 2022, 8, 715. https://doi.org/10.3390/horticulturae8080715
Tang Q, Guo X, Zhang Y, Li Q, Chen G, Sun H, Wang W, Shen X. An Optimized Protocol for Indirect Organogenesis from Root Explants of Agapanthus praecox subsp. orientalis ‘Big Blue’. Horticulturae. 2022; 8(8):715. https://doi.org/10.3390/horticulturae8080715
Chicago/Turabian StyleTang, Qianwen, Xiangxin Guo, Yuanshan Zhang, Qingyun Li, Guanqun Chen, Huale Sun, Weiming Wang, and Xiaohui Shen. 2022. "An Optimized Protocol for Indirect Organogenesis from Root Explants of Agapanthus praecox subsp. orientalis ‘Big Blue’" Horticulturae 8, no. 8: 715. https://doi.org/10.3390/horticulturae8080715
APA StyleTang, Q., Guo, X., Zhang, Y., Li, Q., Chen, G., Sun, H., Wang, W., & Shen, X. (2022). An Optimized Protocol for Indirect Organogenesis from Root Explants of Agapanthus praecox subsp. orientalis ‘Big Blue’. Horticulturae, 8(8), 715. https://doi.org/10.3390/horticulturae8080715





