Phytochemicals and Antioxidant Activities of Conventionally Propagated Nodal Segment and In Vitro-Induced Callus of Bougainvillea glabra Choisy Using Different Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Callus Induction
2.1.1. Plant Materials and Sterilization
Chemicals and Reagents
2.1.2. Preparation of Basal Medium, Aseptic Condition and Glassware
2.1.3. Callus Induction as Affected by Cytokinin and Auxin
2.1.4. Culture Maintenance
2.1.5. Fresh and Dry Weight of Callus
2.2. Quantification of Phenolic Content and Antioxidant Activities of B. glabra Nodal Segments and In Vitro-Induced Calli
2.2.1. Planting Materials and Preparation of Extract
2.2.2. Total Phenolic Acids Content
2.2.3. Total Flavonoids Content
2.2.4. DPPH Free Radical Scavenging Activity
2.2.5. ABTS Scavenging Activity
2.2.6. Iron (II) Chelating Activity
2.3. Experimental Design and Statistical Analysis
3. Results
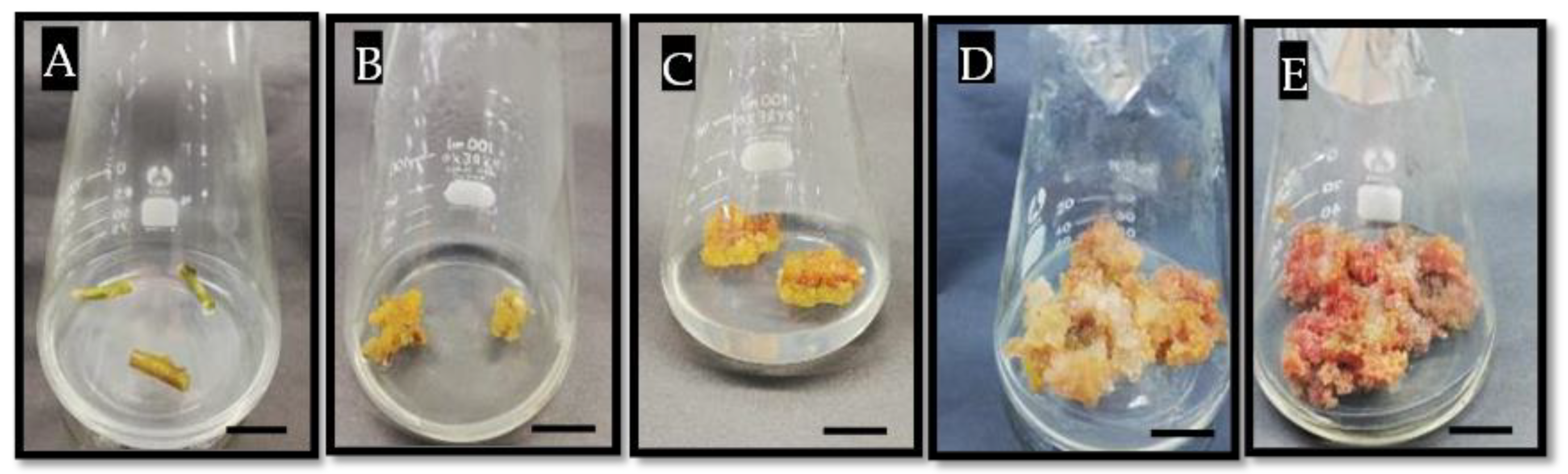
3.1. In Vitro Callus Induction of Bougainvillea glabra via Nodal Segment
3.1.1. The Main Effect of 2,4-D, BAP and Light Regimes on Callus Induction of B. glabra
3.1.2. Synergistic Effect of Cytokinin, Auxins, and Light Regime on Callus Induction
3.2. Quantification of Phenolics Contents and Antioxidant Activities of In Vitro-Induced Calli and Conventionally Propagated Plant of Bougainvillea glabra
3.2.1. Total Phenolic Acid and Total Flavonoid Content
3.2.2. Antioxidant Activities
| Source of Sample | Type of Solvent | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DPPH (mg TE/g DW) | ABTS (mg TE/g DW) | |||||||||
| Aqueous | Ethanol | Acetone | Hexane | Mean | Aqueous | Ethanol | Acetone | Hexane | Mean | |
| Node | 7.64 ± 0.01 a | 2.98 ± 0.11 b | 1.01 ± 0.01 e | 0.24 ± 0.08 gh | 2.97 ± 0.68 A | 1.51 ± 0 a | 0.72 ± 0.01 d | 0.58 ± 0.01 ef | 0.46 ± 0.02 gh | 0.82 ± 0.12 A |
| Callus induced in light | 2.12 ± 0.03 c | 1.37 ± 0.06 d | 0.89 ± 0.05 f | 0.19 ± 0.08 hi | 1.14 ± 0.56 B | 1.12 ± 0 b | 0.68 ± 0.01 de | 0.54 ± 0.03 fg | 0.40 ± 0.01 j | 0.69 ± 0.08 B |
| Callus induced in dark | 0.80 ± 0.18 f | 0.59 ± 0.07 g | 0.40 ± 0.06 gh | 0.14 ± 0.02 ij | 0.48 ± 0.44 C | 0.98 ± 0 c | 0.40 ± 0.01 hi | 0.30 ± 0.01 ij | 0.16 ± 0.01 jk | 0.46 ± 0.09 C |
| Mean | 3.52 ± 0.64 A | 1.64 ± 0.46 B | 0.76 ± 0.31 C | 0.19 ± 0.33 D | 1.20 ± 0.08 A | 0.60 ± 0.05 B | 0.48 ± 0.04 C | 0.34 ± 0.05 D | ||
| Source of Sample | Type of Solvent | ||||
|---|---|---|---|---|---|
| Iron (II) Chelating Activity (%) | |||||
| Aqueous | Ethanol | Acetone | Hexane | Mean | |
| Node | 29.64 ± 0.77 b | 20.08 ± 1.31 de | 6.64 ± 2.18 h | 17.02 ± 1.24 ef | 18.35 ± 2.55 B |
| Callus induced in light | 26.87 ± 2.45 bc | 20.78 ± 0.73 de | 5.39 ± 1.4 h | 15.25 ± 1.12 fg | 17.07 ± 2.47 B |
| Callus induced in dark | 43.30 ± 0.13 a | 22.78 ± 0.53 cd | 11.96 ± 0.56 g | 22.92 ± 1.7 cd | 25.24 ± 3.44 A |
| Mean | 33.27 ± 2.64 A | 21.21 ± 0.61 B | 8.00 ± 1.27 C | 18.40 ± 1.35 D | |
3.2.3. Correlation Analysis between Variables
4. Discussion
4.1. In Vitro Callus Induction of B. glabra
4.2. Quantification of Secondary Metabolites and Antioxidant Activity of In Vitro-Induced Calli and Conventionally Propagated Nodal Segment of B. glabra
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saleem, H.; Thet, H.; Naidu, R.; Sirajudeen, A.; Ahmed, N. HPLC–PDA Polyphenolic Quantification, UHPLC–MS Secondary Metabolite Composition, and in Vitro Enzyme Inhibition Potential of Bougainvillea glabra. Plants 2020, 9, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asuk, A.A.; Agiang, M.A.; Dasofunjo, K.; Willie, A.J. The Biomedical Significance of the Phytochemical, Proximate and Mineral Compositions of the Leaf, Stem Bark and Root of Jatropha Curcas. Asian Pac. J. Trop. Biomed. 2015, 5, 650–657. [Google Scholar] [CrossRef] [Green Version]
- Arteaga Figueroa, L.; Abarca-Vargas, R.; García Alanis, C.; Petricevich, V.L. Comparison between Peritoneal Macrophage Activation by Bougainvillea X buttiana Extract and LPS and/or Interleukins. Biomed Res. Int. 2017, 2017, 4602952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, J.J.; Rodrigues, D.T.; Gonçalves, M.B.; Lemos, M.C.; Gallarreta, M.S.; Bianchini, M.C.; Gayer, M.C.; Puntel, R.L.; Roehrs, R.; Denardin, E.L.G. Paraquat Exposure-Induced Parkinson’s Disease-like Symptoms and Oxidative Stress in Drosophila Melanogaster: Neuroprotective Effect of Bougainvillea Glabra Choisy. Biomed. Pharmacother. 2017, 95, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Naito, F.Y.B.; de Nazaré Almeida dos Reis, L.; Batista, J.G.; Nery, F.M.B.; Rossato, M.; Melo, F.L.; de Cássia Pereira-Carvalho, R. Complete Genome Sequence of Bougainvillea Chlorotic Vein Banding Virus in Bougainvillea Spectabilis from Brazil. Trop. Plant Pathol. 2020, 45, 159–162. [Google Scholar] [CrossRef]
- Pavan Kumar, P.; Janakiram, T.; Bhat, K.V. Microsatellite Based DNA Fingerprinting and Assessment of Genetic Diversity in Bougainvillea Cultivars. Gene 2020, 753, 144794. [Google Scholar] [CrossRef]
- Marasini, P.; Khanal, A. Assessing Rooting Media and Hormone on Rooting Potential of Stem Cuttings of Bougainvillea. J. Inst. Agric. Anim. Sci. 2018, 35, 197–201. [Google Scholar] [CrossRef]
- Abarca-Vargas, R.; Petricevich, V.L. Extract from Bougainvillea Xbuttiana (Variety Orange) Inhibits Production of Lps-Induced Inflammatory Mediators in Macrophages and Exerts a Protective Effect in Vivo. Biomed Res. Int. 2019, 2019, 2034247. [Google Scholar] [CrossRef] [Green Version]
- Ogunwande, I.A.; Avoseh, O.N.; Olasunkanmi, K.N.; Lawal, O.A.; Ascrizzi, R.; Flamini, G. Chemical Composition, Anti-Nociceptive and Anti-Inflammatory Activities of Essential Oil of Bougainvillea gabra. J. Ethnopharmacol. 2019, 232, 188–192. [Google Scholar] [CrossRef]
- Kumara Swamy, M.; Sudipta, K.M.; Lokesh, P.; Neeki, A.M.; Rashmi, W.; Bhaumik, H.S.; Darshil, H.S.; Vijay, R.; Kashyap, S.S.N. Phytochemical Screening and in vitro Antimicrobial Activity of Bougainvillea Spectabilis Flower Extracts. Int. J. Phytomed. 2012, 4, 375–379. [Google Scholar]
- Saleem, H.; Htar, T.T.; Naidu, R.; Zengin, G.; Ahmad, I.; Ahemad, N. Phytochemical Profiling, Antioxidant, Enzyme Inhibition and Cytotoxic Potential of Bougainvillea glabra Flowers. Nat. Prod. Res. 2020, 34, 2602–2606. [Google Scholar] [CrossRef] [PubMed]
- Kalsum, N. Preliminary Studies of the Immunomodulator Effect of the Propolis Trigona Spp. Extract in a Mouse Model. IOSR J. Agric. Vet. Sci. 2017, 10, 75–80. [Google Scholar] [CrossRef]
- Ghogar, A.; Jiraungkoorskul, W. Antifertility Effect of Bougainvillea spectabilis or Paper Flower. Pharmacogn. Rev. 2017, 11, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, G.I.; Alabi, O.T.; Owoyele, B.V.; Soladoye, A.O. Anti-Diabetic Properties of the Aqueous Leaf Extract of Bougainvillea glabra (Glory of the Garden) on Alloxan-Induced Diabetic Rats. Rec. Nat. Prod. 2009, 3, 187–192. [Google Scholar]
- Abarca-Vargas, R.; Petricevich, V.L. Bougainvillea Genus: A Review on Phytochemistry, Pharmacology, and Toxicology. Evid. Based Complement. Altern. Med. 2018, 2018, 9070927. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, N.; Kapoor, H.C.; Lodha, M.L. Cloning and Expression of Antiviral/Ribosome-Inactivating Protein from Bougainvillea Xbuttiana. J. Biosci. 2008, 33, 91–101. [Google Scholar] [CrossRef]
- Abarca-Vargas, R.; Peña Malacara, C.F.; Petricevich, V.L. Characterization of Chemical Compounds with Antioxidant and Cytotoxic Activities in Bougainvillea x buttiana Holttum and Standl, (Var. Rose) Extracts. Antioxidants 2016, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Bortolotti, M.; Bolognesi, A.; Polito, L. Bouganin, an Attractive Weapon for Immunotoxins. Toxins 2018, 10, 323. [Google Scholar] [CrossRef] [Green Version]
- Jehani, M.; Pathak, D.M. In Vitro Antifungal Activity of Plant Extracts (Sterilized and Unsterilized) against Macrophomina Phaseolina (Tassi) Goid. Cause Stem Canker of Pigeonpea [Cajanus Cajan (L.) Millsp.]. Int. J. Plant Prot. 2019, 12, 105–109. [Google Scholar] [CrossRef]
- Vaquero, L.R.; Estrada, M.E.V.; Aparicio, A.R.J.; Arellano, S.L.E.; Lozano, S.E. Evaluation of Methanolic Extract of Bougainvillea glabra Choisy “Variegata” against Spodoptera Frugiperda1 under Laboratory Conditions. Southwest. Entomol. 2016, 41, 983–990. [Google Scholar] [CrossRef]
- Haida, Z.; Nakasha, J.J.; Hakiman, M. In Vitro Responses of Plant Growth Factors on Growth, Yield, Phenolics Content and Antioxidant Activities of Clinacanthus nutans (Sabah Snake Grass). Plants 2020, 9, 1030. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Vipul, A. Phytochemical Analysis and in Vitro Antioxidant Activities of Leaves, Stems, Flowers, and Roots Extracts of Bougainvillea spectabilis Willd. Int. J. Green Pharm. 2018, 12, 277–284. [Google Scholar] [CrossRef]
- Ahmad, A.; ul Qamar, M.T.; Shoukat, A.; Aslam, M.M.; Tariq, M.; Hakiman, M.; Joyia, F.A. The Effects of Genotypes and Media Composition on Callogenesis, Regeneration and Cell Suspension Culture of Chamomile (Matricaria chamomilla L.). PeerJ 2021, 9, e11464. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Yoga, L. Extraction, Isolation and Characterization of Bioactive Compounds from Plants Extracts. Afr. J. Tradit. Complementary Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Bilić, M.; Velić, D. Study of Solid-Liquid Extraction Kinetics of Total Polyphenols from Grape Seeds. J. Food Eng. 2007, 81, 236–242. [Google Scholar] [CrossRef]
- Fatima, H.; Khan, K.; Zia, M.; Ur-Rehman, T.; Mirza, B.; Haq, I.U. Extraction Optimization of Medicinally Important Metabolites from Datura innoxia Mill.: An in Vitro Biological and Phytochemical Investigation. BMC Complement. Altern. Med. 2015, 15, 376. [Google Scholar] [CrossRef] [Green Version]
- Ishida, T.; Rossky, P.J. Solvent Effects on Solute Electronic Structure and Properties: Theoretical Study of a Betaine Dye Molecule in Polar Solvents. J. Phys. Chem. A 2001, 105, 558–565. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Gliszczyńska-͆wigło, A.; Barthet, V.; Skręty, J. Effect of Extraction Method on the Phenolic and Cyanogenic Glucoside Profile of Flaxseed Extracts and Their Antioxidant Capacity. J. Am. Oil Chem. Soc. 2015, 92, 1609–1619. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Vanden Berghe, D.; Maes, L. Anti-Infective Potential of Natural Products: How to Develop a Stronger in Vitro “Proof-of-Concept. ” J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Mahmoud, S.N.; Al-ani, N.K. Effect of Different Sterilization Methods on Contamination and Viability of Nodal Segments of Cestrum nocturnum L. Int. J. Res. Stud. Biosci. 2016, 4, 4–9. [Google Scholar] [CrossRef]
- Ahmad, I.; Zamir, R.; Shah, S.T.; Wali, S. In Vitro Surface Sterilization of the Shoot Tips of Bougainvillea Spectabilis Willd. Pure Appl. Biol. 2016, 5, 1171–1175. [Google Scholar] [CrossRef]
- Mostafiz, S.; Wagiran, A. Efficient Callus Induction and Regeneration in Selected Indica rice. Agronomy 2018, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Hakiman, M.; Maziah, M. Non Enzymatic and Enzymatic Antioxidant Activities in Aqueous Extract of Different Ficus deltoidea Accessions. J. Med. Plants Res. 2009, 3, 120–131. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry to Total Phenolics with Phosphomolybdic Acid Reagents. Am. J. Enol. Vinic. 1965, 16, 144–158. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total Phenolics and Total Flavonoids in Bulgarian Fruits and Vegtables. J. Univ. Chem. Technol. Metal 2005, 40, 255–260. [Google Scholar]
- Wong, S.P.; Leong, L.P.; William Koh, J.H. Antioxidant Activities of Aqueous Extracts of Selected Plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Re, R.; Brühwiler, P.; Mourad, S.; Verdejo, R.; Shaffer, M. Development and Characterisation of Carbon Nanotube-Reinforced Polyurethane Foams. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Sturm, A.; Tang, G.Q. The Sucrose-Cleaving Enzymes of Plants Are Crucial for Development, Growth and Carbon Partitioning. Trends Plant Sci. 1999, 4, 401–407. [Google Scholar] [CrossRef]
- Barneix, A.J.; Causin, H.F. The Central Role of Amino Acids on Nitrogen Utilization and Plant Growth. J. Plant Physiol. 1996, 149, 358–362. [Google Scholar] [CrossRef]
- Tariq, U.; Ali, M.; Abbasi, B.H. Morphogenic and Biochemical Variations under Different Spectral Lights in Callus Cultures of Artemisia absinthium L. J. Photochem. Photobiol. B Biol. 2014, 130, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Daneshvar, M.H. In Vitro Adventitious Shoot Regeneration through Direct and Indirect Organogenesis from Seedling-Derived Hypocotyl Segments of Ficus religiosa L.: An Important Medicinal Plant. HortScience 2018, 53, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.; Zhu, S.; Guan, R.; Deng, X. Identification of 2,4-D-Responsive Proteins in Embryogenic Callus of Valencia Sweet Orange (Citrus sinensis Osbeck) Following Osmotic Stress. Plant Cell. Tissue Organ Cult. 2010, 103, 145–153. [Google Scholar] [CrossRef]
- Mok, D.W.S.; Mok, M.C. Cytokinin Metabolism and Action. Annu. Rev. Plant Physiol. Plant Mol. Biol 2001, 52, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Renu, S.; Kharb, P.; Rani, K. Rapid Micropropagation and Callus Induction of Catharanthus Roseus in Vitro Using Different Explants. World J. Agric. Sci. 2011, 7, 699–704. [Google Scholar]
- Behbahani, M.; Shanehsazzadeh, M.; Hessami, M.J. Optimization of Callus and Cell Suspension Cultures of Barringtonia racemosa (Lecythidaceae Family) for Lycopene Production. Sci. Agric. 2011, 68, 69–76. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Yokota, S.; Ohkubo, T.; Andoh, Y.; Yahara, S.; Yoshizawa, N. In Vitro Regeneration of the Medicinal Woody Plant Phellodendron Amurense Rupr. through Excised Leaves. Plant Cell. Tissue Organ Cult. 2005, 80, 43–50. [Google Scholar] [CrossRef]
- Hoque, A.; Razvy, M.; Biswas, M.; Kbir, A. Micropropagation of Water Chestnut (Trapa Sp.) through Local Varieties of Rajshahi Division. Asian J. Plant Sci. 2006, 5, 409–413. [Google Scholar]
- Thammina, C.; He, M.; Lu, L.; Cao, K.; Yu, H.; Chen, Y.; Tian, L.; Chen, J.; Mcavoy, R.; Ellis, D.; et al. In Vitro Regeneration of Triploid Plants of Euonymus alatus “compactus” (Burning Bush) from Endosperm Tissues. HortScience 2011, 46, 1141–1147. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.; Verma, O.; Chand, S. In Vitro Propagation of Boerhaavia diffusa L.: An Important Medicinal Plant of Family Nyctagimaceae. Indian J. Genet. Plant Breed. 2019, 79, 89–95. [Google Scholar] [CrossRef]
- Rameshkumar, R.; Satish, L.; Pandian, S.; Rathinapriya, P.; Rency, A.S.; Shanmugaraj, G.; Pandian, S.K.; Leung, D.W.M.; Ramesh, M. Production of Squalene with Promising Antioxidant Properties in Callus Cultures of Nilgirianthus ciliatus. Ind. Crops Prod. 2018, 126, 357–367. [Google Scholar] [CrossRef]
- Bhojwani, S.S.; Dantu, P.K. Plant Tissue Culture: An Introductory Text; Springer India: New Delihi, India, 2013; ISBN 9788132210252. [Google Scholar]
- Younas, M.; Drouet, S.; Nadeem, M.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Differential Accumulation of Silymarin Induced by Exposure of Silybum Marianum L. Callus Cultures to Several Spectres of Monochromatic Lights. J. Photochem. Photobiol. B Biol. 2018, 184, 61–70. [Google Scholar] [CrossRef]
- Gao, J.; Li, J.; Luo, C.; Yin, L.; Li, S.; Yang, G.; He, G. Callus Induction and Plant Regeneration in Alternanthera philoxeroides. Mol. Biol. Rep. 2011, 38, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, J.; Summers, W.L. Dark–Light Treatments Influence Induction of Tomato Anther Callus. HortScience 2019, 26, 915–916. [Google Scholar] [CrossRef] [Green Version]
- Habibah, N.A.; Moeljopawiro, S.; Dewi, K.; Indrianto, A. Callus Induction and Flavonoid Production on the Immature Seed of Stelechocarpus burahol. J. Phys. Conf. Ser. 2018, 983, 012186. [Google Scholar] [CrossRef] [Green Version]
- Mahendra, C.; Murali, M.; Manasa, G.; Sudarshana, M.S. Biopotentiality of Leaf and Leaf Derived Callus Extracts of Salacia macrosperma Wight—An Endangered Medicinal Plant of Western Ghats. Ind. Crops Prod. 2020, 143, 111921. [Google Scholar] [CrossRef]
- Esmaeili, A.K.; Taha, R.M.; Mohajer, S.; Banisalam, B. Antioxidant Activity and Total Phenolic and Flavonoid Content of Various Solvent Extracts from in Vivo and in Vitro Grown Trifolium pratense L. (Red Clover). Biomed Res. Int. 2015, 2015, 643285. [Google Scholar] [CrossRef] [Green Version]
- Arezki, O.; Boxus, P.; Kevers, C.; Gaspar, T. Changes in Peroxidase Activity, and Level of Phenolic Compounds during Light-Induced Plantlet Regeneration from Eucalyptus camaldulensis Dehn. Nodes in Vitro. Plant Growth Regul. 2001, 33, 215–219. [Google Scholar] [CrossRef]
- Zahid, N.A.; Jaafar, H.Z.E.; Hakiman, M. Micropropagation of Ginger (Zingiber Officinale Roscoe) ‘Bentong’ and Evaluation of Its Secondary Metabolites and Antioxidant Activities Compared with the Conventionally Propagated Plant. Plants 2021, 10, 630. [Google Scholar] [CrossRef]
- Islam, M.Z.; Hossain, M.T.; Hossen, F.; Akter, M.S.; Mokammel, M.A. In-Vitro Antioxidant and Antimicrobial Activity of Bougainvillea glabra Flower. Res. J. Med. Plant 2016, 10, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Murali, M.; Prabakaran, G. Effect of Different Solvents System on Antioxidant Activity and Phytochemical Screening in Various Habitats of Ocimum basilicum L. (Sweet Basil) Leaves. Int. J. Zool. Appl. Biosci. 2018, 3, 375–381. [Google Scholar] [CrossRef]
- López-Laredo, A.R.; Ramírez-Flores, F.D.; Sepúlveda-Jiménez, G.; Trejo-Tapia, G. Comparison of Metabolite Levels in Callus of Tecoma stans (L.) Juss. Ex Kunth. Cultured in Photoperiod and Darkness. Vitr. Cell. Dev. Biol. Plant 2009, 45, 550–558. [Google Scholar] [CrossRef]
- Shah, M.; Ullah, M.A.; Drouet, S.; Younas, M.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Interactive Effects of Light and Melatonin on Biosynthesis of Silymarin and Anti-Inflammatory Potential in Callus Cultures of Silybum marianum (L.) Gaertn. Molecules 2019, 24, 1207. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, S.; Khan, M.A.; Ali, A.; Khan, L.; Khan, M.S.; Mashwani, Z.u.R. Feasible Production of Biomass and Natural Antioxidants through Callus Cultures in Response to Varying Light Intensities in Olive (Olea europaea L.) Cult. Arbosana. J. Photochem. Photobiol. B Biol. 2019, 193, 140–147. [Google Scholar] [CrossRef]
- Hakkim, F.; Shankar, C.; Girija, S. Chemical Composition and Antioxidant Property of Holy Basil (Ocimum sanctum L.) Leaves, Stems, and Inflorescence and Their in Vitro Callus Cultures. J. Agric. Food Chem. 2007, 55, 9109–9117. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Coelho, N.; Romano, A. Thymus lotocephalus Wild Plants and in Vitro Cultures Produce Different Profiles of Phenolic Compounds with Antioxidant Activity. Food Chem. 2012, 135, 1253–1260. [Google Scholar] [CrossRef]
- Song, K.; Sivanesan, I.; Ak, G.; Zengin, G.; Cziáky, Z.; Jekő, J.; Rengasamy, K.R.R.; Lee, O.N.; Kim, D.H. Screening of Bioactive Metabolites and Biological Activities of Calli, Shoots, and Seedlings of Mertensia maritima (L.) Gray. Plants 2020, 9, 1551. [Google Scholar] [CrossRef]
- Zengin, G.; Aktumsek, A. Investigation of Antioxidant Potentials of Solvent Extracts from Different Anatomical Parts of Asphodeline anatolica E. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 481–488. [Google Scholar] [CrossRef] [Green Version]

| Treatment | Days to Callus Initiation | Callus Frequency (%) | FW of Callus (g) | DW of Callus (mg) |
|---|---|---|---|---|
| Control | --- | --- | --- | --- |
| 2,4-D (µM) | ||||
| 2.5 | 12.09 ± 0.72 b | 88.54 ± 1.89 a | 2.98 ± 0.17 c | 122.12 ± 3.76 c |
| 5 | 11.72 ± 0.76 b | 90.08 ± 1.57 a | 3.85 ± 0.15 ab | 164.58 ± 8.90 a |
| 7.5 | 14.26 ± 0.82 a | 76.04 ± 0.77 b | 4.06 ± 0.26 a | 150.09 ± 6.77 b |
| BAP (µM) | ||||
| 0.5 | 13.02 ± 0.85 a | 86.39 ± 2.56 a | 3.15 ± 0.19 b | 132.89 ± 8.13 b |
| 1 | 12.79 ± 0.79 a | 85.67 ± 1.69 a | 3.51 ± 0.12 b | 140.39 ± 19.10 b |
| 1.5 | 12.73 ± 0.86 a | 81.77 ± 2.56 b | 4.23 ± 0.24 a | 167.50 ± 12.48 a |
| Culture Condition | ||||
| Light | 14.73 ± 0.73 a | 75.69 ± 3.81 b | 3.52 ± 0.23 a | 141.56 ± 17.44 a |
| Dark | 8.45 ± 0.73 b | 81.03 ± 4.05 a | 3.43 ± 0.20 b | 132.77 ± 8.63 b |
| F-value | ||||
| 2,4-D | 744.57 *** | 3020.89 *** | 121.66 *** | 143.7 *** |
| BAP | 6.72 *** | 17.97 *** | 16.42 *** | 15.05 *** |
| Culture Condition | 1836.00 *** | 117.57 *** | 5.91 ** | 4.35 * |
| 2,4-D*BAP | 22.19 *** | 83.51 *** | 31.82 *** | 42.68 *** |
| 2,4-D*Condition | 53.02 *** | 4.19 ** | 0.40 ns | 0.81 ns |
| BAP*Condition | 27.05 *** | 1.38 ** | 2.48 ns | 1.56 ns |
| 2,4-D*BAP*Condition | 7.5 *** | 6.35 *** | 0.96 ns | 0.53 ns |
| CV (%) | 5.58 | 2.77 | 12.56 | 12.15 |
| Condition | 2,4-D (µM) | BAP (µM) | Days to Callus Initiation | Callus Frequency % | FW of Callus (g) | DW of Callus (mg) | Callus Morphology |
|---|---|---|---|---|---|---|---|
| Light | Control | 0 | --- | --- | --- | --- | --- |
| 2.5 | 0.5 | 15.17 ± 0.44 fg | 78.33 ± 1.67 de | 2.53 ± 0.15 hi | 120.00 ± 11.54 fg | Y, B, & C | |
| 1 | 15.33 ± 0.44 ef | 76.67 ± 1.67 def | 3.21 ± 0.24 fgh | 141.67 ± 20.48 de | Y, B, & C | ||
| 1.5 | 14.12 ± 0.56 g | 93.33 ± 1.67 b | 3.03 ± 0.03 gh | 93.33 ± 3.33 i | Y, B, & C | ||
| 5 | 0.5 | 16.01 ± 0.18 def | 100.00 ± 0 a | 3.67 ± 0.17 defgh | 165.00 ± 7.63 bc | Y, B, & C | |
| 1 | 13.00 ± 0.58 h | 86.67 ± 1.67 c | 3.7 ± 0.53 defg | 113.33 ± 8.82 ghi | Y, B, & C | ||
| 1.5 | 16.00 ± 0.58 def | 80.67 ± 1.2d | 3.77 ± 0.34 defg | 156.00 ± 7.81 bcd | Y, B, & C | ||
| 7.5 | 0.5 | 17.67 ± 0.88 ab | 73.33 ± 1.67 f | 3.00 ± 0.06 gh | 116.67 ± 8.81 fgh | R, Y, & C | |
| 1 | 16.73 ± 0.50 bcd | 73.33 ± 1.67 f | 3.55 ± 0.24 efgh | 146.67 ± 9.28 de | R, Y, & C | ||
| 1.5 | 17.27 ± 0.43 bcd | 73.33 ± 1.67 f | 5.23 ± 0.16 a | 221.67 ± 13.01 a | R, Y, & C | ||
| Dark | Control | 0 | --- | --- | --- | --- | --- |
| 2.5 | 0.5 | 10.17 ± 0.30 j | 86.67 ± 1.67 c | 2.11 ± 0.39 i | 100.00 ± 5.7 hi | Y, B, & F | |
| 1 | 10.58 ± 0.33 ij | 80.00 ± 1.53 d | 3.68 ± 0.16 defgh | 159.00 ± 2.3 bc | Y, B, & F | ||
| 1.5 | 7.00 ± 0 k | 100.00 ± 0 a | 3.30 ± 0.3 fgh | 118.00 ± 18.9 fgh | Y, B, & F | ||
| 5 | 0.5 | 8.00 ± 0.12 k | 100.00 ± 0 a | 4.36 ± 0.46 bcd | 182.00 ± 9.6 bc | W, B, & F | |
| 1 | 7.00 ± 0 k | 93.33 ± 1.2 b | 2.93 ± 0.03 h | 126.67 ± 3.38 ef | R, Y, & F | ||
| 1.5 | 10.28 ± 0.17 j | 93.33 ± 0.88 b | 4.71 ± 0.54 abc | 185.33 ± 12.81 b | R, Y, & F | ||
| 7.5 | 0.5 | 11.11 ± 0.11 ij | 80.00 ± 1.53 d | 3.25 ± 0.05 fgh | 113.67 ± 3.18 fgh | Y, B, & F | |
| 1 | 11.08 ± 0.22 ij | 80.00 ± 1.53 d | 3.98 ± 0.02 cdef | 154.67 ± 3.18 cd | R, Y, & F | ||
| 1.5 | 11.72 ± 0.03 i | 73.33 ± 0.67 f | 5.33 ± 0.16 a | 230.67 ± 5.20 a | W, B, & F |
| Source of Sample | Type of Solvent | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic Acids (mg GAE/g DW) | Flavonoids (mg RE/g DW) | |||||||||
| Aqueous | Ethanol | Acetone | Hexane | Mean | Aqueous | Ethanol | Acetone | Hexane | Mean | |
| Node | 21.88 ± 0.57 a | 3.33 ± 0.38 cd | 1.84 ± 0.22 ef | 0.25 ± 0.05 g | 6.82 ± 1.87 A | 42.05 ± 0.18 a | 21.46 ± 0.31 b | 8.92 ± 0.04 d | 1.00 ± 0.04 i | 18.36 ± 4.67 A |
| Callus induced in light | 6.43 ± 0.26 b | 2.73 ± 0.26 d | 1.52 ± 0.03 ef | 0.20 ± 0.02 g | 2.72 ± 0.7 B | 10.30 ± 0.11 c | 8.46 ± 0.22 e | 7.34 ± 0.14 f | 0.92 ± 0.11 i | 6.75 ± 1.07 B |
| Callus induced in dark | 3.90 ± 0.17 c | 2.63 ± 0.08 d | 1.00 ± 0.27 f | 0.25 ± 0.07 g | 1.95 ± 0.43 C | 6.67 ± 0.11 g | 6.67 ± 0.15 g | 4.46 ± 0.07 h | 0.88 ± 0.17 i | 4.67 ± 0.72 C |
| Mean | 10.74 ± 1.83 A | 2.90 ± 0.17 B | 1.45 ± 0.16 C | 0.24 ± 0.03 D | 19.67 ± 5.62 A | 12.20 ± 2.33 B | 6.91 ± 0.65 C | 0.93 ± 0.06 D | ||
| Variable | TPC | TFC | ABTS | DPPH | Fe2+ |
|---|---|---|---|---|---|
| TPC | 1 | ||||
| TFC | 0.92 ** | 1 | |||
| ABTS | 0.90 ** | 0.80 ** | 1 | ||
| DPPH | 0.79 ** | 0.87 ** | 0.84 ** | 1 | |
| Fe2+ | 0.46 ** | 0.30 ns | 0.54 ** | 0.24 ns | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasrat, M.N.; Sakimin, S.Z.; Hakiman, M. Phytochemicals and Antioxidant Activities of Conventionally Propagated Nodal Segment and In Vitro-Induced Callus of Bougainvillea glabra Choisy Using Different Solvents. Horticulturae 2022, 8, 712. https://doi.org/10.3390/horticulturae8080712
Nasrat MN, Sakimin SZ, Hakiman M. Phytochemicals and Antioxidant Activities of Conventionally Propagated Nodal Segment and In Vitro-Induced Callus of Bougainvillea glabra Choisy Using Different Solvents. Horticulturae. 2022; 8(8):712. https://doi.org/10.3390/horticulturae8080712
Chicago/Turabian StyleNasrat, Mohammad Nasim, Siti Zaharah Sakimin, and Mansor Hakiman. 2022. "Phytochemicals and Antioxidant Activities of Conventionally Propagated Nodal Segment and In Vitro-Induced Callus of Bougainvillea glabra Choisy Using Different Solvents" Horticulturae 8, no. 8: 712. https://doi.org/10.3390/horticulturae8080712
APA StyleNasrat, M. N., Sakimin, S. Z., & Hakiman, M. (2022). Phytochemicals and Antioxidant Activities of Conventionally Propagated Nodal Segment and In Vitro-Induced Callus of Bougainvillea glabra Choisy Using Different Solvents. Horticulturae, 8(8), 712. https://doi.org/10.3390/horticulturae8080712







