Nutrient Content with Different Fertilizer Management and Influence on Yield and Fruit Quality in Apple cv. Gala
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description, Plant Material and Experimental Design
2.2. Treatments Application
2.3. Sample Preparation of Buds, Flowers, Leaves and Fruits
2.4. Mineral Organ Analysis
2.5. Deviation from Optimum Percentage (DOP Index)
2.6. Fruit Production and Fruit Quality Attributes
2.7. Statistical Analysis
3. Results
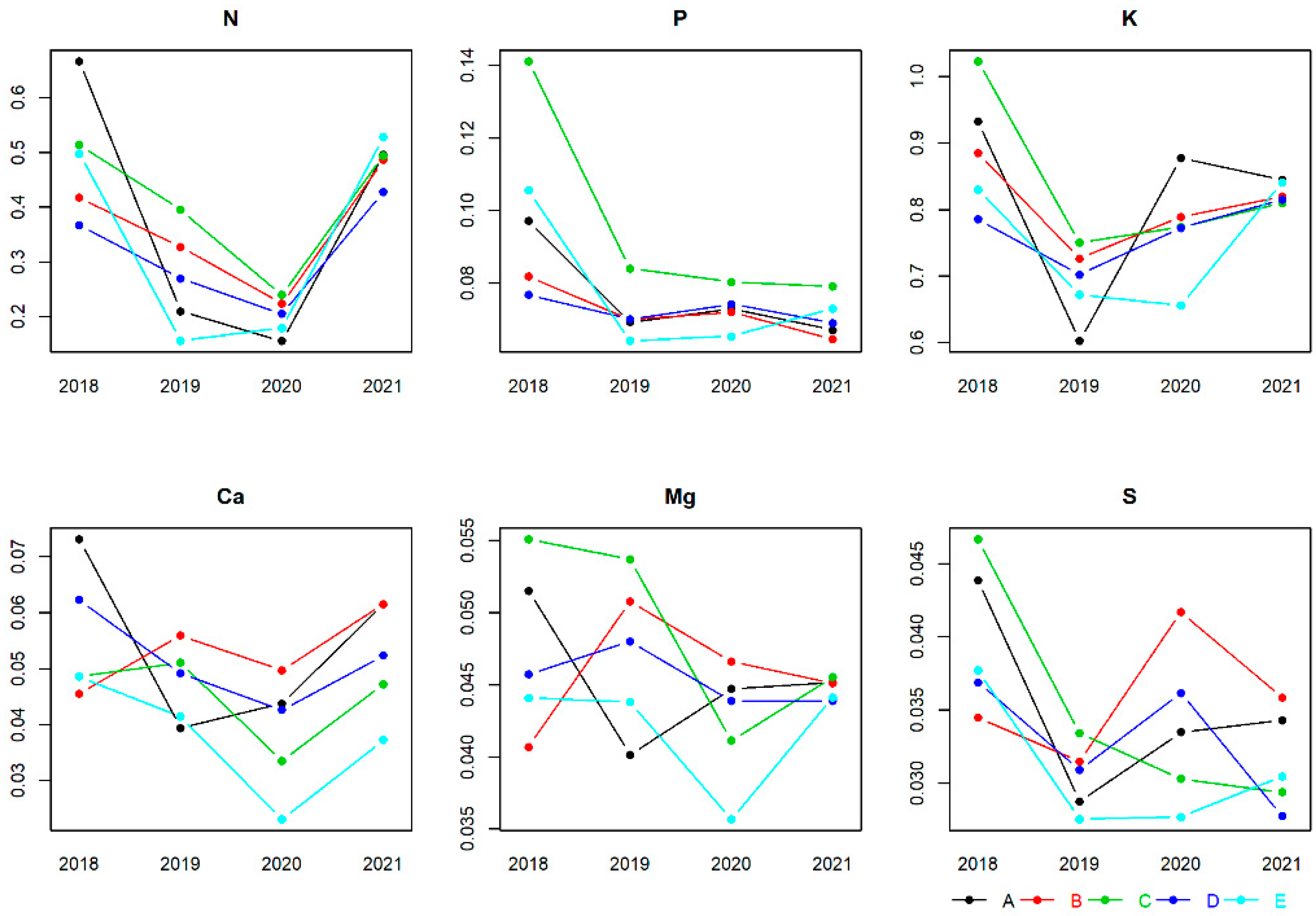
3.1. Nutrient Concentration Ranges in Buds, Flowers, Leaves and Fruits
3.2. Correlation of an Element in Different Organs
3.3. Macronutrients and Micronutrients in Leaf 90–110 DAFB and Deviation from Optimum Percentage (DOP Index)
3.4. Macronutrients in Fruits 15 DBH
3.5. Fruit Production
3.5.1. Yield and Fruit Size
3.5.2. Fruit Quality
4. Discussion
4.1. Nutrient Concentration Ranges in Buds, Flowers, Leaves and Fruits
4.2. Correlations between Nutrient Concentrations between Organs
4.3. Macronutrients and Micronutrients in Leaf 90–110 DAFB and Deviation from Optimum Percentage (DOP Index)
4.4. Macronutrients in Fruits 15 DBH
4.5. Yield
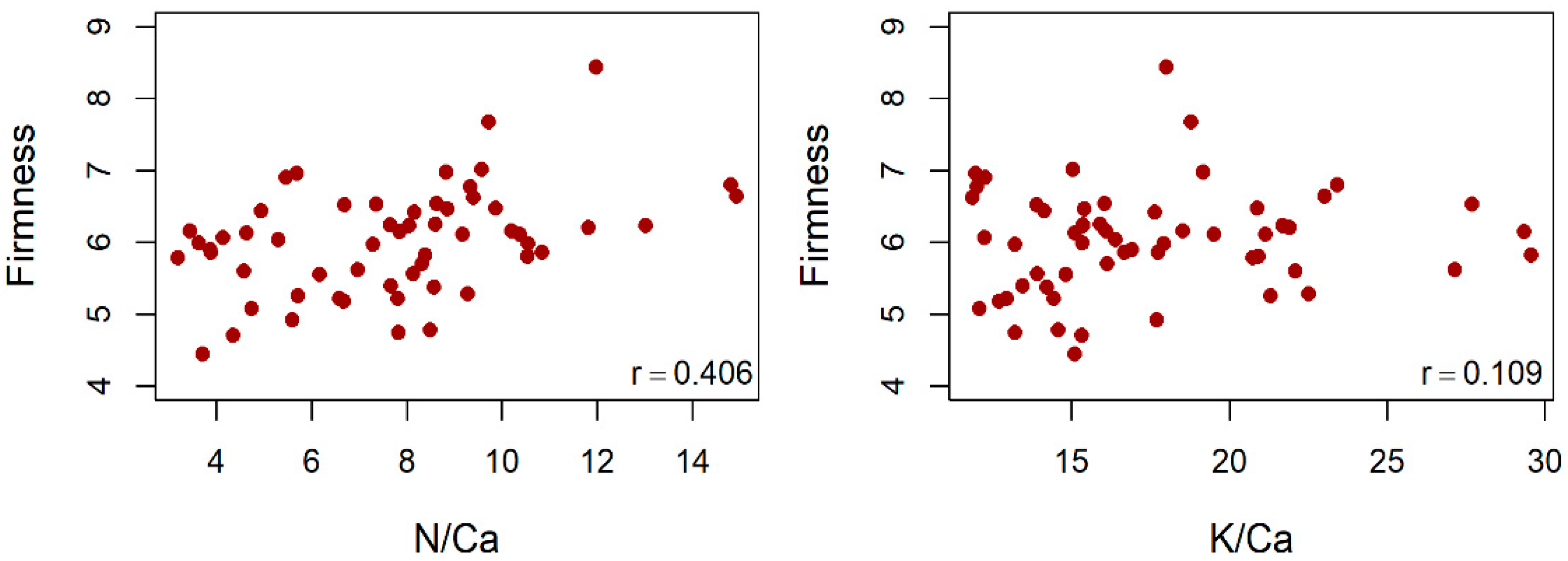
4.6. Fruit Size, TSS and Firmness
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). 2022. Available online: www.faostat.fao.org (accessed on 30 June 2022).
- Carranca, C.; Brunetto, G.; Tagliavini, M. Nitrogen nutrition of fruit trees to reconcile productivity and environmental concern. Plants 2018, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Ličina, V.; Krogstad, T.; Simić, A.; Akšić, M.F.; Meland, M. Precision fertilization to apple trees A review. NIBIO Rep. 2021, 7, 59–88. [Google Scholar]
- INIAP-Laboratório Químico Agrícola Rebelo da Silva. Manual de Fertilização das Culturas; Instituto Nacional de Investigação Agrária, Lisboa: Lisboa, Portugal, 2006; p. 281. ISBN 989-95131-0-5. [Google Scholar]
- Milošević, T.; Milošević, N. Apple fruit quality, yield and leaf macronutrients content as affected by fertilizer treatment. J. Soil Sci. Plant. Nut. 2015, 15, 76–83. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N. Influence of mineral fertilizer, farmyard manure, natural zeolite, and their mixture on fruit quality and leaf micronutrient levels of apple trees. Commun. Soil Sci. Plant Anal. 2017, 48, 539–548. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N.; Mladenović, J. The influence of organic, organo-mineral and mineral fertilizers on tree growth, yielding, fruit quality and leaf nutrient composition of apple cv. “Golden Delicious Reinders”. Sci. Hortic. 2022, 297, 110978. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, A.; Montaño-Carrasco, M.; Ojeda-Barrios, D.; Martínez-Rosales, A.; Cruz-Álvarez, O.; Martínez-Damián, M.T.; Rodríguez-Roque, M.J. Foliar nutritional content and apple fruit quality as affected by organic, conventional, or integrated management. J. Plant. Nutr. 2021, 44, 1886–1902. [Google Scholar] [CrossRef]
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Marcelle, R. Mineral nutrition and fruit quality. Acta Hortic. 1995, 383, 219–226. [Google Scholar] [CrossRef]
- Casero, T.; Benavides, A.; Puy, J.; Recasens, I. Relationships between leaf and fruit nutrients and fruit quality attributes in Golden Smoothee apples using multivariate regression techniques. J. Plant. Nutr. 2005, 27, 313–324. [Google Scholar] [CrossRef]
- Nava, G.; Dechen, A.R.; Nachtigall, G.R. Nitrogen and potassium fertilization affect apple fruit quality in Southern Brazil. Commun. Soil Sci. Plant. Anal. 2008, 39, 96–107. [Google Scholar] [CrossRef]
- Fallahi, E.; Fallahi, B.; Neilsen, G.H.; Neilsen, D.; Peryea, F.J. Effects of mineral nutrition on fruit quality and nutritional disorders in apples. Acta Hortic. 2010, 868, 49–60. [Google Scholar] [CrossRef]
- Jivan, C.; Sala, F. Relationship between tree nutritional status and apple quality. Hortic. Sci. 2014, 41, 1–9. [Google Scholar] [CrossRef]
- Doryanizadeh, M.; Mahmood, G.; Sabouri, A. Estimation of postharvest quality of “Red Delicious” apple fruits based on fruit nutrient elements composition. J. Agric. Sci. 2017, 9, 164–173. [Google Scholar] [CrossRef]
- Fazio, G.; Lordan, J.; Grusak, M.A.; Francescatto, P.; Robinson, T.L.I. Mineral nutrient profiles and relationships of ‘Honeycrisp’ grown on a genetically diverse set of rootstocks under Western New York climatic conditions. Sci. Hortic. 2020, 266, 108477. [Google Scholar] [CrossRef]
- Mészáros, M.; Hnátková, H.; Čonka, P.; Náměstek, J. Linking mineral nutrition and fruit quality to growth intensity and crop load in apple. Agronomy 2021, 11, 506. [Google Scholar] [CrossRef]
- El-Jendoubi, H.; Igartua, E.; Abadía, J.; Abadía, A. Prognosis of iron chlorosis in pear (Pyrus communis L.) and peach (Prunus persica L. Batsch) trees using bud, flower and leaf mineral concentrations. Plant. Soil 2012, 354, 121–139. [Google Scholar] [CrossRef]
- Sanz, M.; Pérez, J.; Pascual, J.; Machín, J. Prognosis of iron chlorosis in apple trees by floral analysis. J. Plant. Nutr. 1998, 2, 1697–1703. [Google Scholar] [CrossRef]
- Wojcik, P. Boron analysis in tissues before apple tree bloom can be used to assess boron nutritional status. J. Plant. Nutr. 2002, 25, 1011–1020. [Google Scholar] [CrossRef]
- Uçgun, K.; Gezgin, S. Interpretation of leaf analysis performed in early vegetation in apple orchards. Commun. Soil Sci. Plant. Anal. 2017, 48, 1719–1725. [Google Scholar] [CrossRef]
- Egnér, H.; Riehm, H.; Domingo, W. Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktions methoden zur Phosphor und Kaliumbestimmung. K. Lantbr. Ann. 1960, 26, 199–215. [Google Scholar]
- Instituto Português do Mar e da Atmosfera (IPMA). Atlas climático Ibérico. 2022. Available online: https://www.ipma.pt/export/sites/ipma/bin/docs/institucionais/pr_atlas.clima.iberico.dmm11.pdf (accessed on 30 June 2022).
- Direção-Geral de Agricultura e Desenvolvimento Rural (DGADR) Normas Técnicas Para a Produção Integrada de Pomóideas; DGADR: Lisbon, Portugal, 2012; p. 252. Available online: https://www.dgadr.gov.pt/images/docs/prod_sust/normas_pi/i012008.pdf (accessed on 30 June 2022).
- EN 13650; Comite Europeen de Normalisation (CEN). Soil Improvers and Growing Media—Extraction of Aqua Regia Soluble Elements. European Standard: New York, NY, USA, 2001.
- Horneck, D.A.; Miller, R.O. Determination of Total Nitrogen in Plant Tissue. In Handbook of Reference Methods for Plant Analysis; Karla, Y.P., Ed.; Soil and Plant Science Council, CRC Press: Boca Raton, FL, USA, 1998; pp. 75–83. [Google Scholar]
- Houba, V.J.G.; Novozamsky, I.; van Dijk, D. Certification of an air-dry soil for pH and extractable nutrients using one hundredth molar calcium chloride. Commun. Soil Sci. Plant. Anal. 1998, 29, 1083–1090. [Google Scholar] [CrossRef]
- Montañés, L.; Heras, L.; Abadía, J.; Sanz, M. Plant analysis interpretation based on a new index: Deviation from optimum percentage (DOP). J. Plant. Nutr. 1993, 16, 1289–1308. [Google Scholar] [CrossRef]
- Nachtigall, G.R.; Dechen, A.R. Seasonality of nutrients in leaves and fruits of apple trees. Sci. Agric. 2006, 63, 493–501. [Google Scholar] [CrossRef]
- Cheng, L.; Raba, R. Nutrient requirements of Gala/M.26 apple trees for high yield and quality. N. Y. Fruit Quart. 2009, 17, 5–10. [Google Scholar]
- Casero, T.; Torres, E.; Alegre, S.; Recasens, I. Macronutrient accumulation dynamics in apple fruits. J. Plant. Nutr. 2017, 40, 2468–2476. [Google Scholar] [CrossRef]
- Faust, M. Nutrition of fruit trees. In Physiology of Temperature Fruit Trees; Wiley-Interscience publication: New York, NY, USA, 1989; pp. 53–132. [Google Scholar]
- Sanz, M.; Montañés, L.; Carrera, M. The possibility of using floral analysis to diagnose the nutritional status of pear trees. Acta Hortic. 1994, 367, 290–295. [Google Scholar] [CrossRef]
- Sanz, M.; Montañés, L. Flower analysis as a new approach to diagnosing the nutritional status of the peach tree. J. Plant. Nutr. 1995, 18, 1667–1675. [Google Scholar] [CrossRef]
- Belkhodja, R.; Morales, F.; Sanz, M.; Abadía, A.; Abadía, J. Iron deficiency in peach trees: Effects on leaf chlorophyll and nutrient concentrations in flowers and leaves. Plant. Soil 1998, 203, 257–268. [Google Scholar] [CrossRef]
- Jiménez, S.; Garín, A.; Gogorcenat, Y.; Betrán, J.A.; Moreno, M.A. Flower and foliar analysis for prognosis of sweet cherry nutrition: Influence of different rootstocks. J. Plant. Nutr. 2004, 27, 701–712. [Google Scholar] [CrossRef]
- Kucukyumuk, Z.; Erdal, I. Rootstock and cultivar effect on mineral nutrition, seasonal nutrient variation and correlations among leaf, flower and fruit nutrient concentrations in apple trees. Bulg. J. Agric. Sci. 2011, 17, 633–641. [Google Scholar]
- Uçgun, K.; Altındal, M.; Cansu, M. Usage of shoot analyses to assess early-season nutritional status of apple trees. Erwerbs-Obstbau 2018, 60, 113–117. [Google Scholar] [CrossRef]
- Johnson, R.S.; Andris, H.; Day, K.; Bede, R. Using dormant shoots to determine the nutritional status of the nutritional status of peach trees. Acta Hortic. 2006, 721, 285–290. [Google Scholar] [CrossRef]
- Pestana, M.; Varennes, A.; De Goss, M.J.; Abadía, J.; Faria, E.A. Floral analysis as a tool to diagnose iron chlorosis in orange trees. Plant. Soil 2004, 259, 287–295. [Google Scholar] [CrossRef]
- Karagiannidis, N.; Thomidis, T.; Zakinthinos, G.; Tsipouridis, C. Prognosis and correction of iron chlorosis in peach trees and relationship between iron concentration and Brown Rot. Sci. Hortic. 2008, 118, 212–217. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Chorianopoulou, S.N.; Zakynthinos, G.; Sarlis, G.; Drossopoulos, J.B. Flower analysis for prognosis of nutritional dynamics of almond tree. J. Plant. Nutr. 2001, 24, 705–716. [Google Scholar] [CrossRef]
- Kalcsits, L.; Lotze, E.; Tagliavini, M.; Hannam, K.D.; Mimmo, T.; Neilsen, D.; Neilsen, G.; Atkinson, D.; Casagrande Biasuz, E.; Borruso, L.; et al. Recent achievements and new research opportunities for optimizing macronutrient availability, acquisition, and distribution for perennial fruit crops. Agronomy 2020, 10, 1738. [Google Scholar] [CrossRef]
- Kowalczyk, W.; Wrona, D.; Przybyłko, S. Content of minerals in soil, apple tree leaves and fruits depending on nitrogen fertilization. J. Elem. 2017, 22, 67–77. [Google Scholar] [CrossRef]
- Sete, P.B.; Comin, J.J.; Ciotta, M.N.; Salume, J.A.; Thewes, F.; Brackmann, A.; Toselli, M.; Nava, G.; Rozane, D.E.; Loss, A.; et al. Nitrogen fertilization affects yield and fruit quality in pear. Sci. Hortic. 2019, 258, 108782. [Google Scholar] [CrossRef]
- Nava, G.; Dechen, A.R. Long-term annual fertilization with nitrogen and potassium affect yield and mineral composition of ‘Fuji’ apple. Sci. Agric. 2009, 66, 377–385. [Google Scholar] [CrossRef]
- Brunetto, G.; de Melo, W.G.B.; Toselli, M.; Quartieri, M.; Tagliavini, M. The role of mineral nutrition on yields and fruit quality in grapevine, pear and apple. Rev. Bras. Frutic. 2015, 37, 1089–1104. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Neilsen, D.; Herbert, L. Nitrogen fertigation concentration and timing of application affect nitrogen nutrition, yield, firmness, and color of apples grown at high density. HortScience 2009, 44, 1425–1431. [Google Scholar] [CrossRef]
- Bennewitz, E.; Cazanga-Solar, R.; Carrasco-Benavides, M.; Fredes-Monsalve, C.; Alba-Mejía, J.E.; Losak, T. Vegetative and productive responses of organic apple (Malus domestica L.) to fossilized red guano and a controlled-release fertilizer. Chil. J. Agric. Anim. Sci. 2017, 33, 213–220. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Wang, Y.; Zhang, N.; Guo, Y.; Ren, X.; Zhao, Z. Potassium fertilization arrests malate accumulation and alters soluble sugar metabolism in apple fruit. Biol. Open 2018, 7, bio024745. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Shen, Y.; Huang, Y. Advances in mineral nutrition transport and signal transduction in Rosaceae fruit quality and postharvest storage. Front. Plant. Sci. 2021, 12, 620018. [Google Scholar] [CrossRef] [PubMed]
- Amarante, C.V.T.; Steffens, C.A.; Ernani, P.R. Identificação pré-colheita do risco de ocorrência de “bitter pit” em maçãs ‘Gala’ por meio de infiltração com magnésio e análise dos teores de cálcio e nitrogênio nos frutos. Rev. Bras. Frutic. 2010, 32, 27–34. [Google Scholar] [CrossRef]
- Marcelle, R.D. Is there any relationship between the flesh mineral content and the peel hexane extracts of apple fruit? Acta Hortic. 1993, 326, 251–258. [Google Scholar] [CrossRef]
- Kuzin, A.; Solovchenko, A. Essential role of potassium in apple and its implications for management of orchard fertilization. Plants 2021, 10, 2624. [Google Scholar] [CrossRef]




| Orchard | pH | EC | Texture | OM | N-NH4 | N-NO3 | P2O5 | K2O |
|---|---|---|---|---|---|---|---|---|
| (mS cm−1) | (%) | ---------- mg kg−1 ---------- | ||||||
| A | 8.0 | 0.15 | Clay | 1.44 | 10.9 | 3.3 | 552 | 437 |
| B | 7.3 | 0.32 | Clay | 1.60 | 9.5 | 12.7 | 958 | 393 |
| C | 7.2 | 0.14 | clay loam | 1.50 | 8.4 | 7.4 | 920 | 203 |
| D | 8.3 | 0.24 | Clay | 1.67 | 11.1 | 13.2 | 323 | 305 |
| E | 7.8 | 0.21 | Clay | 1.80 | 9.7 | 15.7 | 391 | 213 |
| Orchard | Planting Date | Latitude Longitude | Clone | Rootstock | Spacing (m) (m × m) |
|---|---|---|---|---|---|
| A | 2016 | 39°26′59.95″ N | Schniga SchniCo | M9 T337 | 3.80 × 0.70 |
| 9°01′05.14″ W | |||||
| 9°01′05.14″ W | |||||
| B | 2016 | 39°30′55.01″ N | Gala Schnico | M9 T337 | 3.30 × 0.85 |
| 9°00′54.71″ W | |||||
| C | 2015 | 39°35′20.06″ N 8°59′11.40″ W | Venus Fengal | M9 T337 | 3.80 × 0.80 |
| D | 2015 | 39°28′30.48″ N 9°07′12.72″ W | Gala Brookfield | M9 T337 | 4.00 × 1.00 |
| E | 2004 | 39°32′55.36″ N 8°57′22.52″ W | Galaxy Selecta | M9 EMLA | 4.50 × 1.20 |
| Orchard | ||||||
|---|---|---|---|---|---|---|
| Treatment | (kg ha−1) | A | B | C | D | E |
| Standard | N | 64.4 ± 3.1 | 47.8 ± 5.3 | 55.6 ± 5.1 | 39 ± 5.1 | 49.8 ± 4.2 |
| P2O5 | 51.0 ± 2.7 | 18.3 ± 1.7 | 35.2 ± 4.4 | 28.3 ± 5.4 | 26.0 ± 11.7 | |
| K2O | 75.5 ± 5.7 | 76.5 ± 5.2 | 81.5 ± 19.7 | 62.1 ± 11.4 | 74.4 ± 2.8 | |
| 2× Standard | N | 101.7 ± 2.5 | 92.5 ± 13.1 | 103.2 ± 1.1 | 73.0 ± 23.6 | 98.6 ± 5.1 |
| P2O5 | 64.9 ± 5.3 | 37.3 ± 5.7 | 65.7 ± 17.9 | 52.6 ± 12.7 | 41.8 ± 4.0 | |
| K2O | 160.0 ± 0.7 | 158.5 ± 21.2 | 154.3 ± 10.0 | 112.6 ± 18.2 | 144.6 ± 4.4 | |
| 2× Standard OM | N | 107.7 ± 9.5 | 94.0 ± 12.4 | 103.4 ± 1.0 | 80.4 ± 17.7 | 103.0 ± 4.7 |
| P2O5 | 69.1 ± 3.3 | 40.0 ± 5.0 | 65.6 ± 17.9 | 57.2 ± 7.3 | 57.5 ± 19.4 | |
| K2O | 166.0 ± 12.7 | 149.5 ± 9.9 | 154.3 ± 10.0 | 120.8 ± 15.6 | 141.3 ± 8.8 | |
| Buds | Flowers | Leaves 45 DAFB | Leaves 90–110 DAFB | Fruits 60 DABF | Fruits 15 DBH | |||
|---|---|---|---|---|---|---|---|---|
| Buds | N | 0.521 | 0.523 | 0.498 | 0.316 | 0.734 | N | |
| P | 0.325 | 0.196 | −0.008 | −0.182 | 0.169 | P | ||
| K | 0.266 | 0.734 | 0.120 | −0.155 | 0.225 | K | ||
| Mg | 0.141 | 0.423 | 0.280 | 0.280 | 0.217 | Mg | ||
| Ca | 0.328 | 0.462 | 0.152 | 0.215 | 0.069 | Ca | ||
| S | −0.047 | −0.366 | −0.065 | −0.267 | 0.058 | S | ||
| Flowers | Fe | −0.191 | Fe N | 0.376 | 0.392 | 0.448 | 0.581 | N |
| Cu | 0.27 | Cu P | 0.275 | 0.015 | −0.290 | 0.151 | P | |
| Zn | 0.491 | Zn K | 0.195 | −0.468 | −0.675 | −0.375 | K | |
| Mn | 0.125 | Mn Mg | 0.134 | −0.097 | −0.085 | 0.456 | Mg | |
| B | 0.317 | B Ca | 0.235 | −0.094 | −0.138 | 0.069 | Ca | |
| S | −0.062 | 0.180 | −0.221 | −0.164 | S | |||
| Leaves 45 DAFB | Fe | 0.285 | 0.094 | Fe N | 0.281 | 0.178 | 0.549 | N |
| Cu | 0.131 | 0.114 | Cu P | 0.503 | 0.536 | 0.407 | P | |
| Zn | 0.16 | 0.525 | Zn K | 0.156 | −0.073 | 0.067 | K | |
| Mn | 0.18 | −0.045 | Mn Mg | 0.496 | 0.190 | 0.29 | Mg | |
| B | 0.397 | 0.449 | B Ca | 0.486 | 0.237 | 0.066 | Ca | |
| S | 0.081 | 0.461 | −0.213 | S | ||||
| Leaves 90–110 DAFB | Fe | 0.462 | 0.024 | 0.512 | Fe N | 0.307 | 0.447 | N |
| Cu | 0 | 0.12 | 0.159 | Cu P | 0.380 | 0.057 | P | |
| Zn | 0.273 | −0.07 | 0.077 | Zn K | 0.363 | 0.378 | K | |
| Mn | 0.281 | −0.151 | 0.312 | Mn Mg | 0.208 | −0.017 | Mg | |
| B | 0.522 | 0.382 | 0.747 | B Ca | 0.421 | 0.058 | Ca | |
| S | −0.006 | 0.005 | S | |||||
| Fruits 60 DABF | Fe | −0.083 | 0.181 | 0 | −0.076 | Fe N | 0.343 | N |
| Cu | −0.016 | 0.151 | 0.078 | 0.134 | Cu P | 0.19 | P | |
| Zn | 0.214 | 0.501 | 0.706 | 0.244 | Zn K | 0.154 | K | |
| Mn | 0.165 | −0.223 | 0.005 | 0.302 | Mn Mg | 0.225 | Mg | |
| B | 0.515 | 0.528 | 0.871 | 0.794 | B Ca | 0.034 | Ca | |
| S | −0.139 | S | ||||||
| Fruits 15 DBH | Fe | 0.157 | 0.105 | 0.145 | 0.179 | 0.126 | Fe | |
| Cu | −0.405 | −0.007 | 0.366 | 0.259 | 0.048 | Cu | ||
| Zn | 0.3 | 0.171 | 0.118 | 0.345 | 0.148 | Zn | ||
| Mn | −0.019 | −0.057 | 0.271 | 0.624 | 0.101 | Mn | ||
| B | 0.559 | 0.312 | 0.664 | 0.853 | 0.781 | B | ||
| Significance | Leaves 90–110 DAFB | Fruits 15 DBH | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | N | P | K | Ca | Mg | S | |
| Y: Year | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| O: Orchard | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| F: Fertilization | n.s. | 0.000 | n.s. | 0.019 | 0.009 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| YO | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| YF | n.s. | 0.043 | 0.001 | 0.001 | n.s. | n.s. | 0.001 | n.s. | n.s. | n.s. | n.s. | n.s. |
| OF | 0.047 | 0.000 | n.s. | 0.023 | 0.000 | 0.000 | 0.048 | 0.026 | n.s. | n.s. | n.s. | n.s. |
| YOF | n.s. | n.s. | n.s. | 0.002 | n.s. | 0.001 | 0.002 | n.s. | n.s. | n.s. | n.s. | n.s. |
| Year | Orchard | N | P | K | Ca | Mg | S | Sum | Group |
|---|---|---|---|---|---|---|---|---|---|
| 2018 | A | −17.0 ± 11.6 | −7.6 ± 8.4 | −9.0 ± 4.7 | +9.3 ± 8.3 | −9.2 ± 9.9 | −36.3 ± 7.3 | 88.5 | defg |
| B | −21.8 ± 6.9 | −6.8 ± 5.7 | −9.7 ± 5.1 | −14.8± 14.4 | −7.0 ± 16.2 | −29.0 ± 9.8 | 89.1 | defg | |
| C | −0.6 ± 6.1 | +12.2 ± 6.8 | −10.9 ± 4.9 | −4.0 ± 5.5 | +24.3 ± 12.4 | −31.5 ± 4.9 | 83.5 | efgh | |
| D | −8.7 ± 8.8 | −1.3 ± 6.0 | −25.8 ± 5.4 | +19.3 ± 9.0 | +5.3 ± 8.8 | −11.3 ± 10.4 | 71.6 | fgh | |
| E | −15.0 ± 12.3 | +14.3 ± 9.7 | −22.0 ± 4.8 | +17.6 ± 9.1 | +11.5 ± 9.6 | −18.8 ± 8.1 | 99.0 | defg | |
| 2019 | A | −26.7 ± 6.1 | +8.7 ± 4.7 | −13.2 ± 4.3 | +17.0 ± 8.8 | +31.6 ± 10.6 | −22.8 ± 6.1 | 120.1 | cdef |
| B | −24.7 ± 6.3 | +7.4 ± 7.7 | −19.1 ± 3.2 | −13.0 ± 7.4 | +20.2 ± 9.6 | −24.2 ± 5.5 | 108.7 | cdefg | |
| C | +6.7 ± 15.8 | +39.4 ± 22.3 | −23.0 ± 9.7 | +12.7 ± 13.0 | +66.0 ± 24.3 | −9.5 ± 7.8 | 157.4 | ab | |
| D | −18.5 ± 24.7 | +11.4 ± 4.4 | −23.3 ± 4.1 | +39.2 ± 9.7 | +61.0 ± 16.5 | −22.4 ± 6.9 | 175.7 | a | |
| E | −22.0 ± 10.2 | +24.8 ± 6.6 | −34.4 ± 3.2 | +22.9 ± 6.7 | +52.3 ± 7.9 | −9.7 ± 20.9 | 166.2 | a | |
| 2020 | A | −30.6 ± 27.8 | −6.1 ± 8.3 | −7.7 ± 9.8 | +20.6 ± 11.0 | +26.9 ± 4.8 | −19.1 ± 6.8 | 111.0 | cde |
| B | −23.7 ± 13.1 | +10.8 ± 5.8 | +12.3 ± 9.1 | −24.2 ± 16.9 | +0.7 ± 12.6 | +1.2 ± 15.4 | 73.0 | defg | |
| C | −34.2 ± 20.5 | +20.6 ± 11.6 | −12.0 ± 6.1 | −1.8 ± 9.3 | +56.1 ± 13.3 | −17.9 ± 4.8 | 142.5 | abc | |
| D | −36.6 ± 35.5 | +23.4 ± 5.4 | −18.2 ± 4.5 | +21.6 ± 5.6 | +37.1 ± 7.6 | +1.9 ± 3.8 | 138.8 | abc | |
| E | −41.5 ± 21.6 | +0.5 ± 5.0 | −26.0 ± 3.1 | +7.3 ± 4.2 | +19.2 ± 8.9 | −12.4 ± 7.4 | 107.1 | cdefg | |
| 2021 | A | −7.5 ± 4.3 | +1.0 ± 2.3 | +1.5 ± 5.4 | +22.6 ± 9.5 | +21.6 ± 6.6 | −19.3 ± 7.4 | 73.5 | gh |
| B | −13.0 ± 4.5 | −0.3 ± 4.2 | −0.3 ± 5.2 | +6.0 ± 10.1 | +13.8 ± 7.2 | −4.4 ± 5.7 | 37.8 | h | |
| C | +2.1 ± 4.5 | +30.4 ± 9.2 | −10.0 ± 8.9 | +31.6 ± 10.2 | +73.8 ± 14.5 | −24.8 ± 3.8 | 172.7 | a | |
| D | −15.7 ± 10.1 | +8.6 ± 3.4 | −7.4 ± 10.1 | +3.9 ± 6.8 | +18.4 ± 5.9 | −30.6 ± 6.3 | 84.6 | defgh | |
| E | +2.9 ± 5.2 | +20.5 ± 8.3 | −5.0 ± 11.5 | +29.7 ± 7.9 | +43.1 ± 13.2 | −23.0 ± 9.4 | 124.1 | bcd |
| Year | Orchard | Fe | Cu | Zn | Mn | B |
|---|---|---|---|---|---|---|
| 2018 | A | 84.7 ± 29.9 | 4.0 ± 2.1 | 13.4 ± 1.0 | 75.9 ± 10.2 | 20.6 ± 2.2 |
| B | 105.4 ± 15.0 | 5.6 ± 2.1 | 14.6 ± 2.1 | 69.1 ± 5.5 | 27.4 ± 1.9 | |
| C | 94.5 ± 15.8 | 7.4 ± 1.0 | 36.5 ± 2.9 | 191.7 ± 13.6 | 22.8 ± 2.1 | |
| D | 129.3 ± 74.8 | 7.3 ± 2.2 | 62.3 ± 6.2 | 306.1 ± 45.4 | 15.3 ± 2.0 | |
| E | 67.7 ± 7.7 | 7.5 ± 3.1 | 35.8 ± 4.0 | 134.3 ± 79.9 | 18.9 ± 1.3 | |
| 2019 | A | 90.1 ± 8.6 | 8.7 ± 1.0 | 18.0 ± 2.4 | 105.5 ± 21.1 | 15.6 ± 1.1 |
| B | 106.7 ± 45.2 | 9.0 ± 1.6 | 27.3 ± 9.5 | 108.9 ± 14.9 | 18.9 ± 2.3 | |
| C | 90.6 ± 14.6 | 8.1 ± 1.4 | 39.7 ± 4.2 | 263.4 ± 30.2 | 19.3 ± 4.2 | |
| D | 263.7 ± 51.4 | 9.9 ± 1.1 | 40.0 ± 3.5 | 183.3 ± 27.4 | 13.2 ± 1.1 | |
| E | 82.4 ± 17.1 | 8.8 ± 0.9 | 29.2 ± 6.3 | 115.9 ± 68.1 | 12.9 ± 0.8 | |
| 2020 | A | 94.6 ± 11.7 | 7.8 ± 0.4 | 22.7 ± 4.0 | 158.3 ± 22.3 | 18.6 ± 1.4 |
| B | 89.4 ± 13.8 | 8.3 ± 0.9 | 56.3 ± 14.4 | 387.7 ± 99.8 | 26.0 ± 3.1 | |
| C | 92.5 ± 32.2 | 5.7 ± 0.7 | 32.4 ± 4.4 | 238.5 ± 12.2 | 23.8 ± 1.4 | |
| D | 225.5 ± 53.6 | 8.4 ± 0.3 | 53.0 ± 4.5 | 269.2 ± 33.4 | 17.5 ± 0.7 | |
| E | 139.3 ± 17.0 | 8.9 ± 1.4 | 30.3 ± 2.3 | 156.2 ± 77.1 | 17.4 ± 0.6 | |
| 2021 | A | 83.5 ± 12.9 | 10.0 ± 0.6 | 38.5 ± 8.0 | 264.5 ± 65.7 | 26.3 ± 3.2 |
| B | 149.5 ± 20.8 | N.D. | 36.7 ± 4.5 | 166.9 ± 16.9 | 35.7 ± 2,4 | |
| C | 122.2 ± 62.6 | 14.1 ± 1.5 | 20.2 ± 8.3 | 76.3 ± 7.3 | 33.6 ± 0.9 | |
| D | 122.2 ± 16.0 | 11.3 ± 0.8 | 38.4 ± 2.7 | 200.1 ± 31.4 | 23.5 ± 0.7 | |
| E | 83.9 ± 10.1 | 13.1 ± 1.3 | 32.7 ± 8.4 | 168.8 ± 89.6 | 26.7 ± 0.8 |
| Year | Orchard | TSS (°Brix) | Firmness (kg cm−2) | ||
|---|---|---|---|---|---|
| Mean | sd | Mean | sd | ||
| 2018 | A | 13.2 ghij | 1.02 | 6.6 bc | 0.61 |
| B | 13.3 fgh | 0.86 | 6.4 bcde | 0.97 | |
| C | 13.1 hij | 0.93 | 6.2 cdef | 0.57 | |
| D | 12.2 kl | 0.90 | 6.8 b | 0.55 | |
| E | 13.3 fghi | 0.84 | 7.7 a | 0.96 | |
| 2019 | A | 12.7 ijk | 0.92 | 5.9 efg | 0.66 |
| B | 12.1 kl | 0.78 | 5.1 ijk | 0.43 | |
| C | 13.1 hij | 0.69 | 6.2 cdef | 0.66 | |
| D | 13.9 def | 0.64 | 6.3 bcdef | 0.79 | |
| E | 14.0 cde | 0.42 | 5.9 efg | 0.35 | |
| 2020 | A | 12.7 jk | 0.85 | 5.8 fgh | 0.59 |
| B | 12.0 l | 0.69 | 4.7 k | 0.36 | |
| C | 14.2 bcd | 0.67 | 5.4 hij | 0.61 | |
| D | 13.8 defg | 0.50 | N.D. | N.D. | |
| E | 15.4 a | 0.63 | 6.2 cdef | 0.84 | |
| 2021 | A | 13.4 efgh | 1.01 | 5.4 ghi | 0.55 |
| B | 12.1 kl | 0.92 | 4.9 jk | 0.61 | |
| C | 13.5 efgh | 0.92 | 6.0 ef | 0.55 | |
| D | 14.7 b | 1.09 | 6.1 def | 0.92 | |
| E | 14.6 bc | 0.81 | 6.6 bcd | 0.75 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, M.; Martins, M.J.; Policarpo, G.; Sprey, L.; Pastaneira, M.; Almeida, P.; Maurício, A.; Rosa, C.; Faria, J.; Martins, M.B.; et al. Nutrient Content with Different Fertilizer Management and Influence on Yield and Fruit Quality in Apple cv. Gala. Horticulturae 2022, 8, 713. https://doi.org/10.3390/horticulturae8080713
Mota M, Martins MJ, Policarpo G, Sprey L, Pastaneira M, Almeida P, Maurício A, Rosa C, Faria J, Martins MB, et al. Nutrient Content with Different Fertilizer Management and Influence on Yield and Fruit Quality in Apple cv. Gala. Horticulturae. 2022; 8(8):713. https://doi.org/10.3390/horticulturae8080713
Chicago/Turabian StyleMota, Mariana, Maria João Martins, Gonçalo Policarpo, Layanne Sprey, Mafalda Pastaneira, Patrícia Almeida, Anabela Maurício, Cristina Rosa, João Faria, Miguel B. Martins, and et al. 2022. "Nutrient Content with Different Fertilizer Management and Influence on Yield and Fruit Quality in Apple cv. Gala" Horticulturae 8, no. 8: 713. https://doi.org/10.3390/horticulturae8080713
APA StyleMota, M., Martins, M. J., Policarpo, G., Sprey, L., Pastaneira, M., Almeida, P., Maurício, A., Rosa, C., Faria, J., Martins, M. B., de Sousa, M. L., Santos, R., de Sousa, R. M., da Silva, A. B., Ribeiro, H., & Oliveira, C. M. (2022). Nutrient Content with Different Fertilizer Management and Influence on Yield and Fruit Quality in Apple cv. Gala. Horticulturae, 8(8), 713. https://doi.org/10.3390/horticulturae8080713








