Bio-Management of Root-Knot Nematodes on Cucumber Using Biocidal Effects of Some Brassicaceae Crops
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pre-Cultivation Treatments
2.2. Cucumber Cultivation
2.3. Horticultural Characteristics Assessment
2.4. Nematode Parameters Assessment
2.5. Chemical Compounds Assessment
2.6. Statistical Analysis
3. Results
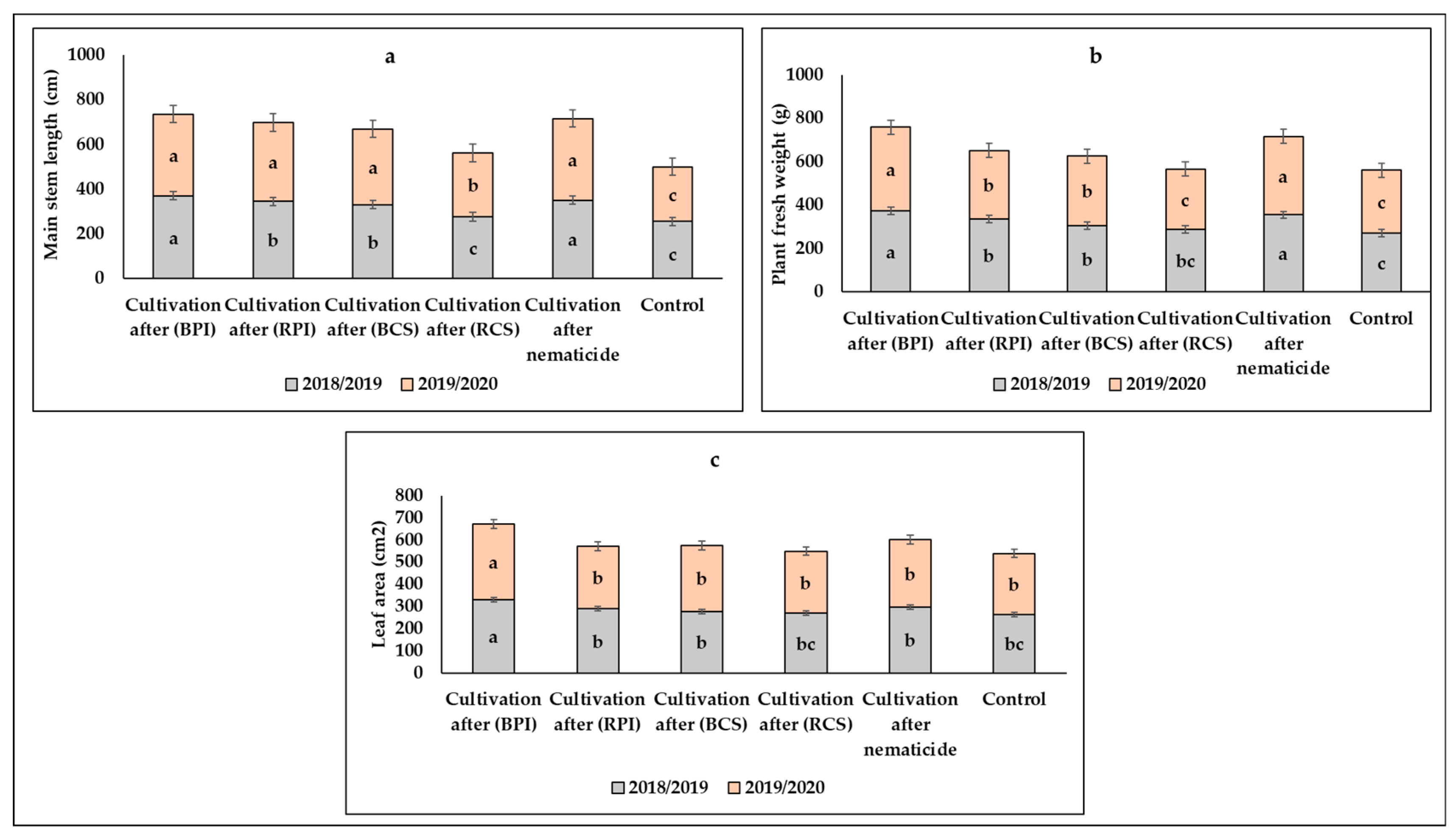
3.1. Effects of Biofumigants and Crop Sequence Approach on Horticultural Characteristics
3.1.1. Vegetative Characteristics
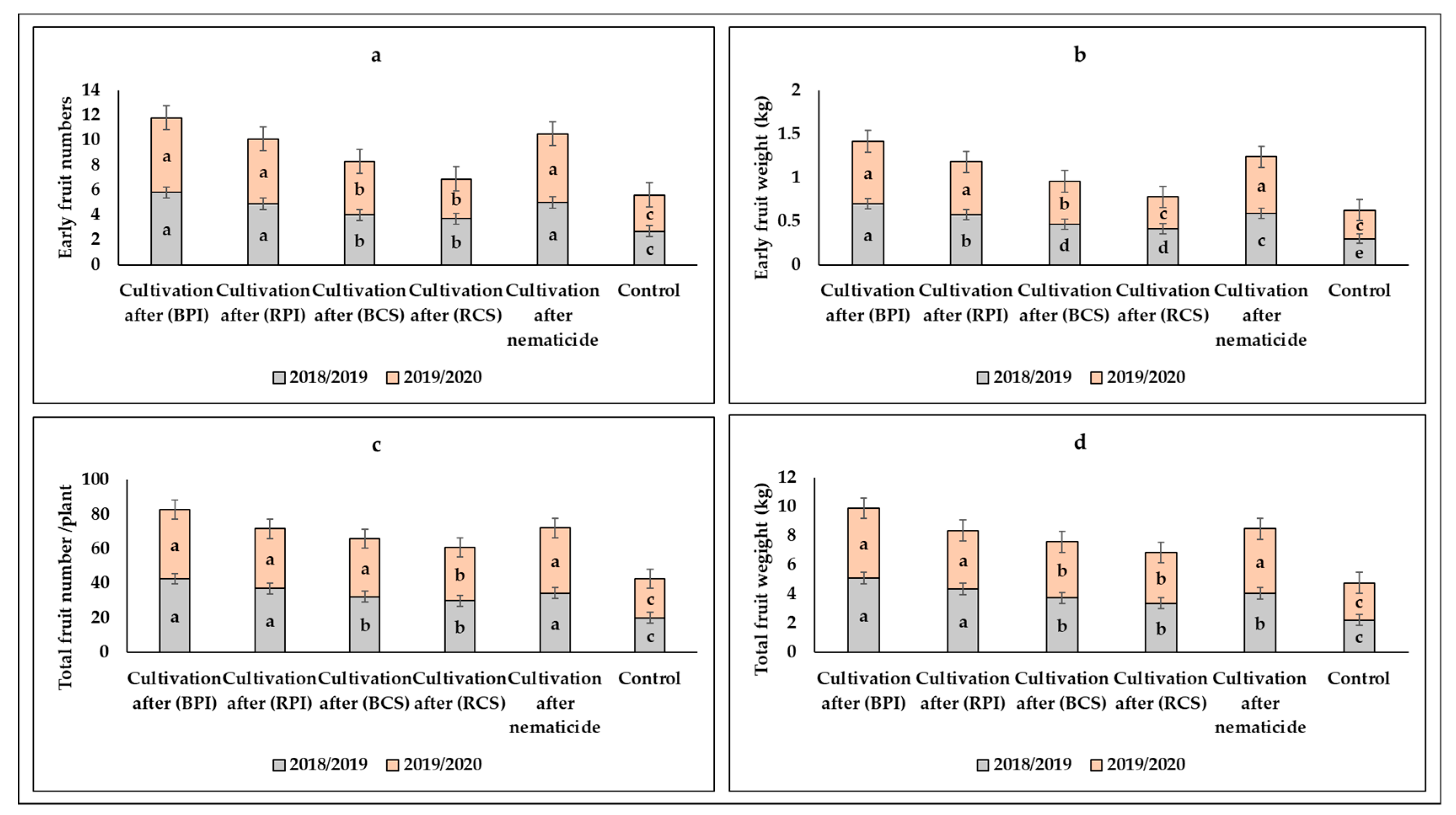
3.1.2. Flowering and Fruiting Characteristics
3.1.3. Yield and Its Components
3.2. The Effects of Bio-Fumigants and Crop Sequence Treatments on the Management of Root-Knot Nematode, Meloidogyne spp.
3.3. The Relationship between Biofumigant Crops as a Yield Enhancer and Nematode Suppressor and Their Total Phenols, Glucosinolates, Isothiocyanate Contents, and Myrosinase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Remaly, E.B. Inheritance of Nematode Resistance in Some Cucumber Cultivars. Master’s Thesis, Cairo University, Cairo, Egypt, 2009. [Google Scholar]
- El-Remaly, E.B. Production of Cucumber Inbred lines Resistant to Root-Knot Nematodes, Meloidogyne spp. and Studying Nature of Resistance. Ph.D. Thesis, Cairo University, Cairo, Egypt, 2016. [Google Scholar]
- Mukherjee, A. Eco-friendly management of plant parasitic nematodes. Indian J. Sci. Technol. 2020, 13, 2883–2891. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Matthiessen, J.N. Developing and refining the biofumigation concept. Agroindustria 2004, 3, 233–239. [Google Scholar]
- Youssef, M.M. Biofumigation as a promising tool for managing plant-parasitic nematodes. A review. Sci. Agric. 2015, 10, 115–118. [Google Scholar]
- Sasanelli, N.; Konrat, A.; Migunova, V.; Toderas, I.; Iurcu-Straistaru, E.; Rusu, S.; Bivol, A.; Andoni, C.; Veronico, P. Review on Control Methods against Plant Parasitic Nematodes Applied in Southern Member States (C Zone) of the European Union. Agriculture 2021, 11, 602. [Google Scholar] [CrossRef]
- Zasada, I.; Ferris, H. Nematode suppression with brassicaceous amendments: Application based upon glucosinolate profiles. Soil Biol. Biochem. 2004, 36, 1017–1024. [Google Scholar] [CrossRef]
- Brown, P.D.; Morra, M.J. Control of soil- borne plant pests using glucosinolate-containing plants. Adv. Agron. 1997, 61, 167–231. [Google Scholar] [CrossRef]
- Lazzeri, L.; Curto, G.; Dallavalle, E.; D’Avino, L.; Malaguti, L.; Santi, R.; Patalano, G. Nematicidal efficacy of biofumigation by defatted Brassicaceae meal for control of Meloidogyne incognita (Kofoid et White) Chitw. on a full field Zucchini crop. J. Sustain. Agric. 2009, 33, 349–358. [Google Scholar] [CrossRef]
- Liebman, M.; Dyck, E. Crop Rotation and Intercropping Strategies for Weed Management. Ecol. Appl. 1993, 3, 92–122. [Google Scholar] [CrossRef] [PubMed]
- Matthiessen, J.N.; Kirkegaard, J. Biofumigation and Enhanced Biodegradation: Opportunity and Challenge in Soilborne Pest and Disease Management. Crit. Rev. Plant. Sci. 2006, 25, 235–265. [Google Scholar] [CrossRef]
- Gimsing, A.L.; Kirkegaard, J. Glucosinolates and biofumigation: Fate of glucosinolates and their hydrolysis products in soil. Phytochem. Rev. 2008, 8, 299–310. [Google Scholar] [CrossRef]
- Viglierchio, D.R.; Schmitt, R.V. On the methodology of nematode extraction from field samples: Baermann funnel modifications. J. Nematol. 1983, 15, 438–444. [Google Scholar] [PubMed]
- Franklin, M.T.; Goodey, J.B. A Cotton Blue-Lactophenol Technique for Mounting Plant-Parasitic Nematodes. J. Helminthol. 1949, 23, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Piedrabuena, A.; García-Álvarez, A.; Díez-Rojo, M.; Bello, A. Use of crop residues for the control of Meloidogyne incognita under laboratory conditions. Pest. Manag. Sci. 2006, 62, 919–926. [Google Scholar] [CrossRef]
- Du, G.; Li, M.; Ma, F.; Liang, D. Antioxidant capacity and the relationship with polyphenol and Vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Jia, C.-G.; Xu, C.-J.; Wei, J.; Yuan, J.; Yuan, G.-F.; Wang, B.-L.; Wang, Q.-M. Effect of modified atmosphere packaging on visual quality and glucosinolates of broccoli florets. Food Chem. 2009, 114, 28–37. [Google Scholar] [CrossRef]
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D. Biotic elicitors effectively increase the glucosinolates content in Brassicaceae sprouts. J. Agric. Food Chem. 2014, 62, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H. Participles and Procedures of Statistics, 2nd ed.; McGraw-Hill Co.: Singapore, 1984; Printing; 633p. [Google Scholar]
- Sarrantonio, M. Building soil fertility and tilth with cover crops. In Managing Cover Crops Profitably; Clark, A., Ed.; Sustainable Agriculture Network: College Park, MD, USA, 2007; pp. 16–24. [Google Scholar]
- Magdoff, F.; Van, E. Building Soils for Better Crops: Sustainable Soil Management, 3rd ed.; Sustainable Agriculture Research and Education: Waldorf, MD, USA, 2009. [Google Scholar]
- Deadman, M.; Al Hasani, H.; Al Sa’di, A. Solarization and biofumigation reduce Pythium aphanidermatum induced damping-off and enhance vegetative growth of greenhouse cucumber in Oman. J. Plant Pathol. 2006, 88, 335–337. [Google Scholar] [CrossRef]
- Monfort, W.; Csinos, A.; Desaeger, J.; Seebold, K.; Webster, T.; Diaz-Perez, J. Evaluating Brassica species as an alternative control measure for root-knot nematode (M. incognita) in Georgia vegetable plasticulture. Crop Prot. 2007, 26, 1359–1368. [Google Scholar] [CrossRef]
- Anita, B. Crucifer vegetable leaf wastes as biofumigants for the management of root-knot nematode (Meloidogyne hapla Chitwood) in celery (Apium graveolens L.). J. Biopestic. 2012, 5, 111–114. [Google Scholar]
- Youssef, M.M.A.; Lashein, A. Effect of Cabbage (Brassica Oleracea) Leaf Residue as a Biofumigant, on Root Knot Nematode, Meloidogyne Incognita Infecting Tomato. J. Plant Prot. Res. 2013, 53, 271–274. [Google Scholar] [CrossRef]
- Waisen, P. Management of Plant-Parasitic Nematodes and Soil Health Using Oil Radish (Raphanus sativus) and Brown Mustard (Brassica Juncea) Cover Crops. Ph.D. Thesis, University of Hawai‘i at Mānoa, Honolulu, HI, USA, 2019. [Google Scholar]
- El-Sherbiny, A.A.; Awd Allah, S.F. Management of the root-knot nematode, Meloidogyne incognita on tomato plants by pre-planting soil bio fumigation with harvesting residues of some winter crops and waste residues of oyster mushroom cultivation under field conditions Egypt. J. Agron. 2014, 13, 189–202. [Google Scholar]
- Zhang, D.; Yan, D.; Cheng, H.; Fang, W.; Huang, B.; Wang, X.; Wang, X.; Yan, Y.; Ouyang, C.; Li, Y.; et al. Effects of multi-year biofumigation on soil bacterial and fungal communities and strawberry yield. Environ. Pollut. 2019, 256, 113415. [Google Scholar] [CrossRef] [PubMed]
- Ploeg, A.; Stapleton, J. Glasshouse studies on the effects of time, temperature and amendment of soil with broccoli plant residues on the infestation of melon plants by Meloidogyne incognita and M. javanica. Nematology 2001, 3, 855–861. [Google Scholar] [CrossRef]
- Halbrendt, J.M. Allelopathy in the management of plant-parasitic nematodes. J. Nematol. 1996, 28, 8–14. [Google Scholar]
- Kirkegaard, J.A.; Sarwar, M. Biofumigation potential of Brassicas. I. Variation in glucosinolate profiles of diverse field-grown Brassicas. Plant Soil 1998, 201, 71–89. [Google Scholar] [CrossRef]
- Underhill, E.W. Glucosinolates. In Encyclopedia of Plant Physiology; New Series; Bell, E.A., Charlwood, B.V., Eds.; Springer: Berlin/Heidelberg, Germany, 1980; Volume 8, pp. 493–511. [Google Scholar]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2019, 169, 112100. [Google Scholar] [CrossRef]
- Mojtahedi, H.; Santo, G.S.; Hang, A.N.; Wilson, J.H. Suppression of Root-knot Nematode Populations with Selected Rapeseed Cultivars as Green Manure. J. Nematol. 1991, 23, 170–174. [Google Scholar] [PubMed]
- Jeschke, V.; Burow, M. Glucosinolates. In ELS; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 1–8. [Google Scholar] [CrossRef]
- Fenwick, G.R.; Heaney, R.K.; Mullin, W.J.; VanEtten, C.H. Glucosinolates and their breakdown products in food and food plants. CRC Crit. Rev. Food Sci. Nutr. 1983, 18, 123–201. [Google Scholar] [CrossRef]
- Angus, F.; Gardner, A.; Kirkegaard, J.A.; Desmarchelier, J.M. Bio fumigation: Isothiocyanates released from brassica roots inhibit the growth of the take-all fungus. Plant Soil 1994, 162, 107–112. [Google Scholar] [CrossRef]
- Ploeg, A. Bio-fumigation to manage plant-parasitic nematodes. In Integrated Management and Biocontrol of Vegetable and Grain Crops Nematodes; Cianco, A., Mukerji, K.G., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 239–248. [Google Scholar] [CrossRef]
- Zasada, I.A.; Ferris, H. Sensitivity of Meloidogyne javanica and Tylenchulus semipenetrans to Isothiocyanates in Laboratory Assays. Phytopathology 2003, 93, 747–750. [Google Scholar] [CrossRef] [Green Version]
- Wato, T. The Role of Allelopathy in Pest Management and Crop Production—A Review. Food Sci. Qual. Manag. 2020, 93, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Dallavalle, E.; Lazzeri, L.; Curto, G. Life cycle duration of Meloidogyne incognita and host status of Brassicaceae and Capparaceae selected for glucosinate content. Nematology 2005, 7, 203–212. [Google Scholar] [CrossRef]
- Oka, Y. Mechanisms of nematode suppression by organic soil amendments—A review. Appl. Soil Ecol. 2010, 44, 101–115. [Google Scholar] [CrossRef]
- Chindo, P.S.; Bello, L.Y.; Kumar, N. Utilization of organic wastes for the management of phyto-parasitic nematodes in devel-oping economies. In Management of Organic Waste; Kumar, S., Bharti, A., Eds.; InTech: London, UK, 2012; p. 198. [Google Scholar] [CrossRef] [Green Version]
- Antonious, G.F.; Bomford, M.; Vincelli, P. Screening Brassica species for glucosinolate content. J. Environ. Sci. Health B 2009, 44, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Avato, P.; D’Addabbo, T.; Leonetti, P.; Argentieri, M.P. Nematicidal potential of Brassicaceae. Phytochem. Rev. 2013, 12, 791–802. [Google Scholar] [CrossRef]
- Bors, M.D.; Semeniuc, C.A.; Socaci, S.; Varva, L.; Moldovan, O.; Vlaic, R.; Tofana, M. Total Phenolic Content and Antioxidant Capacity of Radish as Influenced by the Variety and Vegetative Stage. Bull. UASVM Food Sci. Technol. 2015, 72, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einhellig, F.A.; Leather, G.R. Potentials for exploiting allelopathy to enhance crop production. J. Chem. Ecol. 1988, 14, 1829–1844. [Google Scholar] [CrossRef]
- Einhellig, F.A.; Souza, I.F. Phytotoxicity of sorgoleone found in grain Sorghum root exudates. J. Chem. Ecol. 1992, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.-X.; Guo, Q.-H.; Gu, Y.-J. Factors Influencing Glucoraphanin and Sulforaphane Formation in Brassica Plants: A Review. J. Integr. Agric. 2012, 11, 1804–1816. [Google Scholar] [CrossRef]
- Sang, J.P.; Minchinton, I.R.; Johnstone, P.K.; Truscott, R.J.W. Glucosinolate profiles in the seed, root and leaf tissue of cabbage, mustard, rapeseed radish and swede. Can. J. Plant Sci. 1984, 64, 77–93. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Remaly, E.; Osman, A.A.; El-Gawad, H.G.A.; Althobaiti, F.; Albogami, S.; Dessoky, E.S.; El-Mogy, M.M. Bio-Management of Root-Knot Nematodes on Cucumber Using Biocidal Effects of Some Brassicaceae Crops. Horticulturae 2022, 8, 699. https://doi.org/10.3390/horticulturae8080699
El-Remaly E, Osman AA, El-Gawad HGA, Althobaiti F, Albogami S, Dessoky ES, El-Mogy MM. Bio-Management of Root-Knot Nematodes on Cucumber Using Biocidal Effects of Some Brassicaceae Crops. Horticulturae. 2022; 8(8):699. https://doi.org/10.3390/horticulturae8080699
Chicago/Turabian StyleEl-Remaly, Eman, Ahmed A. Osman, Hany G. Abd El-Gawad, Fayez Althobaiti, Sarah Albogami, Eldessoky S. Dessoky, and Mohamed M. El-Mogy. 2022. "Bio-Management of Root-Knot Nematodes on Cucumber Using Biocidal Effects of Some Brassicaceae Crops" Horticulturae 8, no. 8: 699. https://doi.org/10.3390/horticulturae8080699
APA StyleEl-Remaly, E., Osman, A. A., El-Gawad, H. G. A., Althobaiti, F., Albogami, S., Dessoky, E. S., & El-Mogy, M. M. (2022). Bio-Management of Root-Knot Nematodes on Cucumber Using Biocidal Effects of Some Brassicaceae Crops. Horticulturae, 8(8), 699. https://doi.org/10.3390/horticulturae8080699







