Abstract
Top rot is a new fungal fruit disease in Rosa roxburghii production regions of southwest China. In this study, the pathogen of top rot disease in R. roxburghii fruits was firstly identified as Colletotrichum fructicola CXCDF-3 based on the pathogenicity, morphology, and multigene phylogenetic analysis. The biological property test results indicated that the optimal growth conditions of C. fructicola CXCDF-3 were 25 °C, pH 6.0~8.0, full light or darkness, D-(+) maltose, peptone, and PDA medium. Moreover, difenoconazole, tebuconazole, azoxystrobin, prothioconazole, thiophanate-methyl, prochloraz, carbendazim, and cyprodinil displayed superior toxicity activities to C. fructicola CXCDF-3 with EC50 values of 0.26, 0.64, 0.99, 2.15, 4.64, 4.89, 7.27, and 7.73 mg L−1, respectively. The field control efficacies of 80% tebuconazole water-dispersible granule (WG) 6000-fold liquid, 10% difenoconazole WG 5000-fold liquid, and 250 g/L azoxystrobin emulsifiable concentrate (SC) 1000-fold liquid against top rot disease of R. roxburghii fruits were 85.44%, 84.47%, and 83.50%, respectively. This study reports for the first time that the novel top rot disease in R. roxburghii is caused by C. fructicola and highlights that 80% tebuconazole WG, 10% difenoconazole WG, and 250 g/L azoxystrobin SC could be recommended for controlling top rot disease.
1. Introduction
Rosa roxburghii Tratt., a genus of rose in the Rosaceae family, is an emerging, healthy, and typical representative of the third-generation fruits rich in vitamin C, flavonoids, superoxide dismutase (SOD), and various minerals [1,2,3]. It is widely used to reduce blood pressure, improve immunity, protect the liver, and regulate digestion, as well as an antibacterial, antioxidation, antiradiation and anticancer agents, suggesting it has high medical, nutritional, and commercial values [4,5,6,7,8,9]. As a major agricultural industry for controlling rocky desertification, alleviating poverty, and vitalizing rural areas, the R. roxburghii industry has expanded rapidly in southwest China, especially in Guizhou Province, where the planting area reached 170,000 hm2 in 2021 [9,10,11]. With the development of the commercial cultivation of R. roxburghii, however, the diseases and insects that frequently occurred in R. roxburghii orchards became increasingly prominent and serious [11,12]. In Guizhou Province of southwest China, serious diseases of R. roxburghii include top rot, powdery mildew, and brown spot, which inevitably reduce the yield and quality of R. roxburghii and frequently cause severe economic losses [12,13].
Recently, top rot, a newly discovered fruit disease in R. roxburghii that mainly damages R. roxburghii fruits, was found to have an annual incidence of about 20% in the cultivation areas of southwest China since 2019. Its early, middle, late, and fruit internal symptoms are shown in Figure 1. The pathogen of top rot usually infects R. roxburghii fruits at the junction of the fruit and sepal, showing a small dark red diseased spot with a smooth surface. As the aggravation of the damage increases, the diseased spot spreads to the fruit’s middle and the sepal base turns red. The diseased spot that spreads on the fruit surface gradually turns black, and the pulp turns black and rotten and the sepal is dried and necrotic in the infected late stage. It occurs sporadically from early June to early July during the fruit enlargement period and spreads quickly in mid-/late July during the fruit’s basic forming period; in particular, the incidence increases rapidly after continuous rainy weather. Its damage characteristics are obvious and easy to identify, and the diseased fruits easily drop, which seriously affects the yield and quality of R. roxburghii fruits. At present, the pathogen of top rot is unclear and its control measures have not been established. Accordingly, it is of great significance to identify the pathogen of top rot in R. roxburghii fruits and develop its green, effective, and economical control practice for the sustainable development of the Rosa roxburghii industry.

Figure 1.
The early (a), middle (b), late (c), and internal fruit (d) symptoms of top rot in R. roxburghii fruits.
Generally, the combination of morphology and molecular biology is often used for the identification of microorganisms. Moreover, compared with single-gene phylogenetic analysis, multigene phylogenetic analysis is widely used due to its higher resolution and reliability [14,15]. For the control of R. roxburghii diseases, some chemical fungicides (triadimefon, myclobutanil, azoxystrobin, and tebuconazole) [16] and antibiotics (polyoxin and kasugamycin) [17] have been reported to control powdery mildew. Our previous study results indicated that the single or combined application of allicin and chitosan effectively controlled powdery mildew of R. roxburghii and reliably enhanced its disease resistance, photosynthesis, growth, yield, and quality [9,11]. To our knowledge, there have been no reports on effective and green control fungicides of top rot of R. roxburghii fruits. As a consequence, there is an urgent need to screen potentially cost-effective and environmentally friendly fungicides against top rot of R. roxburghii fruits.
Whether top rot disease is caused by a new pathogen and whether the commonly used fungicides are effective against it are worthy of further study. In this work, the pathogen of the novel top rot disease in R. roxburghii fruits was isolated and identified based on the pathogenicity, the morphology, and a multigene phylogenetic analysis for the first time. Moreover, its biological characteristics and potential control fungicides were studied and screened, respectively. Subsequently, the field control efficacy of nine potential fungicides against top rot disease of R. roxburghii fruits was evaluated. This study is the first to report on the pathogen of top rot disease in R. roxburghii and provides a theoretical basis for potentially effective green fungicides for controlling top rot disease.
2. Materials and Methods
2.1. Culture Media and Fungicides
The potato dextrose agar (PDA) and nutrient agar (NA) were produced by Bo-Microbe Technology Co. Ltd. (Shanghai, China). The Czapek-Dox medium (CDM, g L−1) contained: K2HPO4 (1 g), MgSO4·7H2O (0.5 g,) KCl (0.5 g), FeSO4 (0.01 g), agar (20 g), sucrose (30 g), NaNO3 (3 g), and distilled water (1 L). The potato sugar agar (PSA, g L−1) contained: agar (20 g), sucrose (20 g), potato (200 g), and distilled water (1 L). The oatmeal agar (OA, g L−1) contained: oat powder (20 g), agar (20 g), and distilled water (1 L). The Sabouraud medium (SADY, g L−1) contained: yeast extract (10 g), glucose (40 g), peptone (10 g), agar (10 g), and distilled water (1 L). The PDA was used for the isolation, purification, and biological characteristic determination of the pathogenic strain; the NA, ACM, OA, SDAY, and PSA were used for its biological characteristic determination. All media were sterilized at 121 °C for 30 min. All other used chemicals and reagents were of analytical reagent grade.
The 25% myclobutanil EC (emulsifiable concentrate) was produced by Haoshouchengweien Agrochemical Co. Ltd. (Jiangsu, China). The 10% difenoconazole water-dispersible granule (WG) and 250 g/L azoxystrobin SC (suspension concentrate) were obtained from Syngenta Nantong Crop Protection Co. Ltd. (Jiangsu, China). The 40% cyprodinil SC was produced by Hansheng Biotechnology Co. Ltd. (Qingdao, China). The 40% chlorothalonil SC and 240 g/L thifluzamide SC were provided by Limin Chemical Co. Ltd. (Jiangsu, China). The 10% prothioconazole SC was purchased from Daoyuan Biotechnology Co. Ltd. (Guizhou, China). The 50% carbendazim wettable powder (WP) was produced by Runer Technology Co. Ltd. (Sichuan, China). The 50% tebuconazole WG and 30% picoxystrobin SC were obtained from Meibang Pesticide Co. Ltd. (Shanxi, China). The 25% prochloraz EC was provided by Huifeng Biological Agriculture Co. Ltd. (Jiangsu, China). The 50% iprodione WP was purchased from Meiluofu Agricultural Science and Technology Co. Ltd. (Shandong, China). The 80% mancozeb WP was produced by Taoshi Yinong Agricultural Technology Co. Ltd. (Jiangsu, China). The 50% thiram WP was obtained from Jinnonghua Pharmaceutical Co. Ltd. (Shandong, China). The 70% thiophanate-methyl WP was provided by Yunfa Chemical Co. Ltd. (Shanghai, China). The 50% imazalil EC was purchased from Maoming lvying Agrochemical Co. Ltd. (Guangdong, China).
2.2. Pathogen
The pathogen of top rot with a high pathogenicity was isolated from diseased R. roxburghii fruits obtained from an orchard of R. roxburghii with a ‘Guinong 5′ cultivar in Chaxiang Village, Gujiao Town, Longli Country, Guizhou Province, China (26°54′3648″ N, 106°95′1381″ E). The pathogen was kept at −20 °C in the Institute of Crop Protection, Guizhou University, Guizhou Province, China.
2.3. Field Experiment Site
The field experiments were carried out in 2021 in an orchard of R. roxburghii with a tree age of 8 years (‘Guinong 5′ cultivar in Chaxiang Village, Gujiao Town, Longli Country, Guizhou Province, China (26°54′36″ N, 106°95′13″ E)). The planting density of the R. roxburghii trees was 106 plants per 666.7 m2. The annual rainfall, annual sunshine duration, mean temperature, frostless season, and mean altitude of the field site were about 1100 mm, 1160 h, 17~29.3 °C, 280 d, and 1390 m, respectively. The fertility of the planting soils is shown in Table 1.

Table 1.
The fertility of planting soils of R. roxburghii.
2.4. Isolation and Identification of Top Rot Pathogen in R. roxburghii
2.4.1. Isolation and Purification of Top Rot Pathogen
Pathogens of diseased R. roxburghii fruits were isolated by the tissue separation method. On an ultraclean worktable (SW-CJ-3FD, Fuxia Medical Technology Co. Ltd., Shaoxing, China), 3 mm × 3 mm lesion tissues were cut from the junction of disease and health in diseased fruits using a sterile scalpel, soaked in 75% alcohol for disinfection for 30~35 s, and then washed with sterile water 3 times and transferred to sterilized filter paper to drain the water. Subsequently, the lesion tissue was transferred to the fresh PDA plate, and the plate was cultured at 28 °C for 4 d. A small number of new mycelia at the pathogen edge were selected and transferred to a fresh PDA plate for the purification culture. A single strain was obtained using single-spore culturing that was named strain CXCDF-3. The PDA plate and single spore of strain CXCDF-3 were stored at 4 °C for further research.
2.4.2. Pathogenicity Determination of Strain CXCDF-3
The pathogenicity of strain CXCDF-3 was determined by using the strain to infect the injured and noninjured R. roxburghii fruits in vivo and in planta. The healthy R. roxburghii fruits were sterilized with 75% ethanol and then washed with sterile water. We cut 3 wounds near the top of injured R. roxburghii fruits using a sterile blade. A 5 mm disc was cut from a 7-day-old strain CXCDF-3 PDA plate and then placed in the above solidified PDA center with the mycelium side down. Subsequently, a 5 mm pathogen disc cut from a 7-day-old strain CXCDF-3 PDA plate was inoculated on the injured or noninjured surface of each R. roxburghii fruit in vivo or in planta. The 5 mm sterilized PDA disc was set in four replicates as a control. For the fruit, 5 fruits were inoculated in each treatment; this also was repeated 4 times. In the in vivo experiment, the fruit and pathogen discs (or PDA disc) were wrapped with film for 2 cycles to prevent the interference of rain scouring and wind blowing. In the planta experiment, the treated R. roxburghii fruits were cultured alternately in an incubator at 28 °C, 12 h light /12 h darkness, and 80% relative humidity. According to Koch’s rule, the incidence of top rot in the R. roxburghii fruits was observed every day. After discovering the incidence, the pathogen was separated using the same method as mentioned above, and we then observed whether it was the same as strain CXCDF-3.
2.4.3. Identification of Strain CXCDF-3
Strain CXCDF-3 was inoculated into the PDA medium for 5~7 d, and its colony morphology and color characteristics were observed. Subsequently, it was transferred to OA medium to induce sporulation, and the morphological and structural characteristics of mycelium, sporulation, and conidia were also observed using an optical microscope. Strain CXCDF-3 DNA was extracted with a fungal gDNA extraction kit. The DNA gene fragments of strain CXCDF-3 were amplified with the fungal universal and specific primers including ITS1/ITS4, ACT512/ACT783, GDF1/GDR1, Bt2a/Bt2b, CHS-79/CHS-345, and CL1/CL2A. The primer sequences are shown in Table 2. The sequencing results of the positive and negative primers were spliced together for homology comparison in NCBI, and the model and published strains were downloaded as reference sequences (Table S1). BioEdit was used to process the sequence combination data of 6 conserved genes. The sequence information was spliced end to end according to the order of ACT–CAL–CHS–GADPH–ITS–TU by SequenceMatrix. Then, the polygenic phylogenetic tree was constructed using the maximum likelihood method in CIPRES; FigTree V1.4.3 was used to view and adjust the phylogenetic tree type. Finally, we combined the results of the morphological identification to define the classification status of strain CXCDF-3.

Table 2.
Primers used for PCR amplification.
2.5. Biological Characteristics of Colletotrichum fructicola CXCDF-3
The mycelium-growth-rate method was used to determine the biological characteristics of C. fructicola CXCDF-3. The mycelial growth status of strain CXCDF-3 inoculated on PDA medium for 5 d at different culture temperatures (10, 15, 20, 25, 30, and 35 °C), light (24 h light, 24 h darkness, 12 h light /12 h darkness), and pH values (3, 4, 5, 6, 7, 8, 9, and 10) were measured. Meanwhile, the mycelial growth status of strain CXCDF-3 inoculated on CDM medium for 5 d with different carbon sources (sucrose, glucose, D-(+)-maltose, D-fructose, starch, D-mannitol, lactose, and glycerol) and nitrogen sources (NaNO3, peptone, (NH4)2SO4, yeast extract, beef extract, urea, KNO3, and NH4NO3) was also determined. Moreover, its mycelial growth status inoculated on PDA, NA, ACM, OA, SDAY, and PSA media for 5 d at 25 °C was also checked.
2.6. Toxicity Tests of Fungicides to C. fructicola CXCDF-3
The toxicities of 16 fungicides to C. fructicola CXCDF-3 were determined by the mycelium-growth-rate method [18]. The tested solutions of different fungicides at five gradient levels were prepared with sterile water. First, 1 mL of tested solution of each fungicide was homogeneously blended into 9 mL of fresh PDA liquid (45~55 °C); 1 mL of sterile water was used in four replicates as a control. Then, 10 mL of blended fungicide–PDA or sterile water–PDA liquid was introduced into the Petri dishes with a 90 mm diameter, and we awaited their solidification. Subsequently, a 5 mm disc was cut from a 7-day-old C. fructicola CXCDF-3 PDA plate and then placed in the above-mentioned solidified PDA center with the mycelium side down. After the treated plates were inoculated at 28 °C for 7 d, the diameters of C. fructicola CXCDF-3 growth in the PDA plates were monitored using the crossing method. The growth inhibition of C. fructicola CXCDF-3 by 16 fungicides was calculated using Equation (1):
Inhibition rate (%) = 100 × [(Mycelium diameter of control − Mycelium diameter of fungicide)/(Mycelium diameter of control − 5)]
The EC50 (effective fungicide concentration of 50% inhibition rate) values of 16 fungicides against C. fructicola CXCDF-3 were calculated using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA).
2.7. Field Control Experiment of Top Rot in R. roxburghii Fruits
Nine fungicides with excellent toxic activity to C. fructicola CXCDF-3 were used for controlling top rot of R. roxburghii fruits under open-field conditions. The control experiment of top rot of R. roxburghii fruits was carried out using the foliar spray method. The experiment used 28 different treatments; these are shown in Table 3. A total of 112 plots were arranged randomly with four replicates; each plot had six trees. Considering the incidence period of top rot in R. roxburghii fruits, about 0.47 L of fungicide dilution liquid was sprayed on each R. roxburghii plant (including leaves, stems, and fruits) on 8 July and 15 July in 2021, respectively.

Table 3.
The experimental treatments of the tested fungicides.
The diseased fruit rates and control efficacies of the tested fungicides for top rot of R. roxburghii fruits were investigated 1 d before the first spraying and after 3, 7, 14, 21, 28, and 45 d (preharvest interval, PHI) of the second spraying. A total of 25 fixed fruits on the east, west, south, and north sides of each R. roxburghii tree were used for investigation. The incidence rate and control effect were calculated using Equations (2) and (3), respectively:
Diseased fruit rate (%) = 100 × Number of disease fruits/Total number of fruits
Control effect (%) = 100 × (Diseased fruit rate of control − Diseased fruit rate of fungicide)/Diseased fruit rate of control
2.8. Statistical Analyses
All data are expressed as the mean ± standard deviation (SD) of four replicates. Significant differences were determined using a one-way analysis of variance (ANOVA) in SPSS 18.0 software. Origin 10.0 was used to draw the chart.
3. Results
3.1. Pathogenicity of Top Rot Pathogen in R. roxburghii Fruits
The pathogenicity symptoms of the top rot pathogen in R. roxburghii fruits are shown in Figure 2. The statistical results showed that strain CXCDF-3 had a strong pathogenicity; the infection incidences of the wounded and nonwounded fruits inoculated with strain CXCDF-3 were 100% and 93.33%, respectively. The symptoms of top rot disease, which gradually appeared around the inoculation site after inoculation for 3 d, were obvious 6 d after inoculation and consistent with natural symptoms in the field (Figure 2a–c). Moreover, the control group fruits inoculated with sterilized PDA showed no symptoms of top rot disease (Figure 2c–e). These results showed that strain CXCDF-3 is the pathogenic fungus of top rot in R. roxburghii and has a strong pathogenicity to R. roxburghii fruits.

Figure 2.
Pathogenicity symptoms of top rot pathogen in R. roxburghii fruits. (a) Symptoms of in vivo fruits wound-inoculated with strain CXCDF-3; (b) symptoms of in vivo fruits non-wound-inoculated with strain CXCDF-3; (c) symptoms of in vivo fruits wound-inoculated with sterilized PDA; (d) symptoms of in vivo fruits non-wound-inoculated with sterilized PDA; (e) symptoms of in planta fruits wound-inoculated with sterilized PDA.
3.2. Morphological and Molecular Identification of Strain CXCDF-3
The morphological characteristics of strain CXCDF-3 are shown in Figure 3. As shown in Figure 3a–c, the colony of strain CXCDF-3 was nearly round on the PDA medium and showed a white radioactive colony and a serrated outer ring at the initial stage, which then gradually turned light gray. Moreover, its colony diameter reached 7.6~8.4 cm (n = 10) after culturing for 6 d at 25 °C in darkness, and its colony surface began to produce orange-yellow spore piles and the back side produced a small amount of black metabolites after culturing for 3 d. As shown in Figure 3d, the conidium of strain CXCDF-3 was nearly cylindrical, colorless or grayish black, single-spore, blunt at both ends, and slightly coarse at one end. In addition, its size was 16.2~23.9 μm × 4.8~5.3 μm (average 19.9 μm × 5.0 μm, n = 50); some conidia included 1~2 oil spots and their light projection or refraction spots could be seen in the microscopic field.
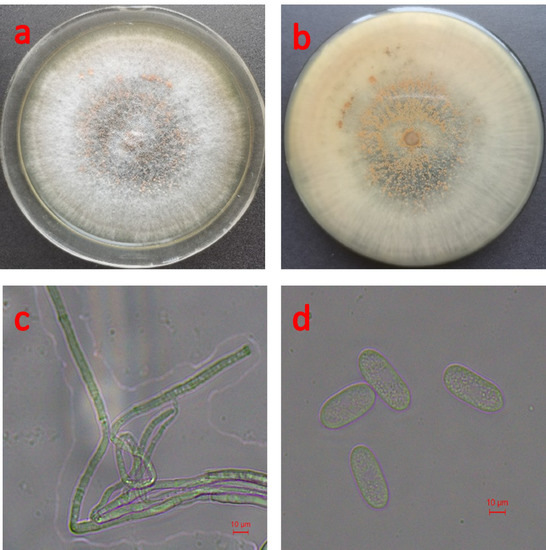
Figure 3.
Morphological characteristics of strain CXCDF-3: (a) front side of strain CXCDF-3 grown on PDA plate; (b): back side of strain CXCDF-3 grown on PDA plate; (c) mycelium of strain CXCDF-3; (d) conidium of strain CXCDF-3.
The sequenced results indicated that the target sequences of ITS, ACT, GAPDH, TUB, CHS, and CAL of strain CXCDF-3 were amplified to 552, 283, 306, 489, 304, and 773 basepairs, respectively. The blast searches in GenBank indicated that the sequences of strain CXCDF-3 had a high homology with those of Colletotrichum fructicola. The phylogenetic tree of strain CXCDF-3 based on ACT–CAL–CHS–GADPH–ITS–TUB multigene association are shown in Figure 4. Strain CXCDF-3, C. fructicola Y3, and C. fructicola ZC102-5 were clustered on one branch with a support rate of 98 (Figure 4) and also were on the same branch as Colletotrichum gloeosporioides 30206. Combined with the results for the pathogenicity and morphological characteristics, strain CXCDF-3 was identified as C. fructicola of the Colletotrichum gloeosporioides species complex. The gene sequences of C. fructicola CXCDF-3 were uploaded into the GenBank database under the accession numbers OK189592 (ITS), OK432513 (ACT), OK338006 (GAPDH), OK318654 (TUB), OK357987 (CHS), and OK381604 (CAL).
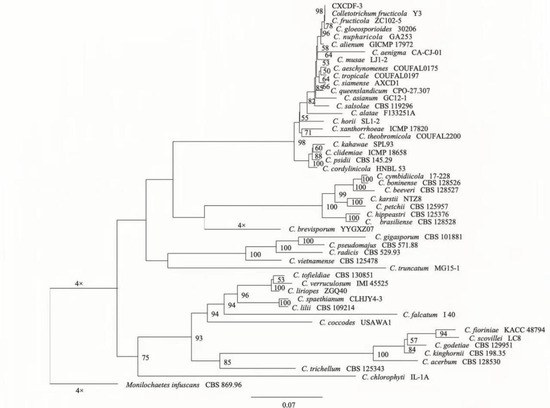
Figure 4.
Phylogenetic tree of strain CXCDF-3 based on ACT–CAL–CHS–GADPH–ITS–TUB multigene phylogenetic analysis. Distance scale indicates the unit length of difference values between organisms or sequences.
3.3. Biological Characteristics of C. fructicola CXCDF-3
Figure 5 depicts the effects of temperature, light, pH values, carbon sources, nitrogen sources, and culture media on the growth of C. fructicola CXCDF-3. In Figure 5a, it can be observed that the colony diameter of C. fructicola CXCDF-3 initially increased and then decreased with the increase in temperature; its optimum growth temperature was about 25 °C with an average colony diameter of 6.46 cm after culturing for 5 d. C. fructicola CXCDF-3 grew slowly in the temperature ranges of 10~20 °C and 30~35 °C, indicating that high or low temperatures could inhibit its mycelium growth. The colony diameters of C. fructicola CXCDF-3 treated with 24 h of light and darkness were 5.74 and 5.61 cm, which were significantly (p < 0.05) higher than the 3.68 cm that resulted for 12 h light/12 h darkness, respectively (Figure 5b). As displayed in Figure 5c, the colony growth of C. fructicola CXCDF-3 was greater in a neutral, slightly alkaline, or slightly acidic environment; a colony diameter of 5.70~6.32 cm was observed when the pH value was 6.0~8.0. The inhibiting effect of an acidic environment on its growth was stronger than that of an alkaline environment. C. fructicola CXCDF-3 exhibited the best utilization on D-(+) maltose with a colony diameter of 5.48 cm after culturing for 5 d, followed by glucose, sucrose, and starch; while its utilization on D-fructose, D-mannitol, lactose, and glycerol was relatively low (Figure 5d). As shown in Figure 5e, C. fructicola CXCDF-3 also had a superior utilization on peptone, with a colony diameter of 5.94 cm after culturing for 5 d, followed by yeast extract, (NH4)2SO4, NaNO3, beef extract, KNO3, NH4NO3, and urea. As shown in Figure 5f, the colony growth of C. fructicola CXCDF-3 on PDA medium was more effective, with a colony diameter of 6.73 cm after culturing for 5 d, followed by OA, SDAY, NA, CDM, ACM, and PSA media. These results indicated that the optimal growth conditions for C. fructicola CXCDF-3 were 25 °C, pH 6.0~8.0, full light or darkness, D-(+) maltose, peptone, and PDA medium.
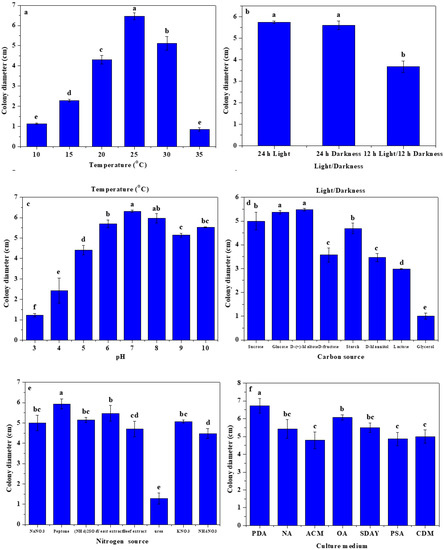
Figure 5.
The effects of temperature (a), light (b), pH values (c), carbon sources (d), nitrogen sources (e), and culture media (f) on the growth of strain CXCDF-3. Values and error bars indicate the mean and SD of four replicates, respectively. Different small letters indicate significant differences at the 5% level (p < 0.05).
3.4. Toxicity of Different Fungicides to C. fructicola CXCDF-3
The toxicities of 16 fungicides to C. fructicola CXCDF-3 are shown in Table 4. The 10% difenoconazole WG, 80% tebuconazole WG, 250 g/L azoxystrobin SC, 10% prothioconazole SC, 70% thiophanate-methyl WP, 25% prochloraz EC, 50% carbendazim WP, and 40% cyprodinil SC exhibited excellent toxicity activities toward C. fructicola CXCDF-3 of R. roxburghii, with EC50 values of 0.26, 0.64, 0.99, 2.15, 4.64, 4.89, 7.27, and 7.73 mg L−1, which were higher by 354.69-, 143.90-, 93.93-, 43.22-, 19.98-, 18.97-, 12.76- and 12.00-fold than the lowest 240 g/L thifluzamide SC with that of 92.71 mg L −1, respectively. Although 80% mancozeb WP had a relatively inferior toxicity to C. fructicola, its EC50 value was 6.24-fold higher than that of 240 g/L thifluzamide SC. The results presented here indicated that difenoconazole, tebuconazole, azoxystrobin, prothioconazole, thiophanate-methyl, prochloraz, carbendazim, cyprodinil, and mancozeb had notable potentials for controlling top rot disease of R. roxburghii fruits in the field.

Table 4.
The toxicities of sixteen fungicides to C. fructicola CXCDF-3.
3.5. Field Control Efficacy of Different Fungicides against Top Rot in R. roxburghii Fruits
The field control efficacies of nine fungicides against top rot disease of R. roxburghii fruits are shown in Table 5. Each dilution concentration of the nine fungicides could effectively decrease the diseased fruit rate of R. roxburghii caused by top rot; of these, 80% tebuconazole WG, 10% difenoconazole WG, and 250 g/L azoxystrobin SC had superior control efficacies. At the harvest period, the control effects of 80% tebuconazole WG 6000-fold liquid, 10% difenoconazole WG 5000-fold liquid, 250 g/L azoxystrobin SC 1000-fold liquid, and 250 g/L azoxystrobin SC 800-fold liquid against top rot disease of R. roxburghii fruits were 85.44%, 84.47%, 83.50%, and 82.53% respectively; their differences were not significant. Moreover, except for the fact that the control efficacies of the 10% difenoconazole WG 6000-fold liquid were more than 80%, those of the other fungicide treatments were less than 80% at the harvest period. These results indicated that 80% tebuconazole WG, 10% difenoconazole WG, and 250 g/L azoxystrobin SC effectively controlled top rot disease of R. roxburghii fruits and can be recommended for application in R. roxburghii production.

Table 5.
The control effect of nine fungicides on top rot in R. roxburghii.
4. Discussion
Top rot, which has become increasingly serious with the expansion of the R. roxburghii planting area in the past two years, seriously affects the plant’s yield and quality. The traditional classification of fungi is mainly based on morphological characteristics and ITS sequence analysis [19]. Whereas for the similar species of C. Gloeosporioides, their ITS sequence differences are small and their morphological classification standards are also more vague in the co-evolution between the host and pathogen [20,21]. Moreover, the support rate of the phylogenetic tree constructed for the most related and complex species by the single ITS sequence analysis was low in some key evolutionary nodes, so it could not be identified [22]. Compared with a single-gene phylogenetic analysis, a multigene phylogenetic analysis is more effective and accurate in determining the systematic relationships of a species, especially related and complex species [14,15,23]. In this study, the pathogen of top rot disease in R. roxburghii fruits isolated from Guizhou Province of southwest China was identified on the basis of the ACT–CAL–CHS–GADPH–ITS–TUB multigene phylogenetic tree method. The results showed that strain CXCDF-3, C. fructicola Y3, and C. fructicola ZC102-5 were clustered on one branch with a support rate of 98, and also were on the same branch as C. gloeosporioides 30206. Combined with the results of the pathogenicity and morphological characteristics, the pathogen of top rot disease in R. roxburghii fruit was identified as C. fructicola CXCDF-3 of the C. Gloeosporioides species complex.
Many previous studies have shown that as a widespread pathogenic fungus, C. fructicola can damage various crops such as fruits and vegetables, coffee [22], celery [23], apple [24], pear [25], tea-oil [26], and grape [27]. To our knowledge, this is the first report that C. fructicola can damage R. roxburghii. Rui et al. [28] found that the temperature for the mycelial growth of C. gloeosporioides ranged from 10 °C to 35 °C with an optimum of 25 °C; the suitable pH ranged from 3 to 7 with an optimum of 7. Song et al. [29] reported that the optimum growth temperature and pH of C. fructicola in strawberry were 25~30 °C and 6~7 respectively, and that it could utilize various carbon and nitrogen sources. The results in this work indicated that the optimal growth conditions of C. fructicola CXCDF-3 were 25 °C, pH 6.0~8.0, and full light or darkness, and that it also could utilize various carbon sources, nitrogen sources, and media, with the optimum for these being D-(+) maltose, peptone, and PDA medium, respectively. These results were consistent with the above-mentioned results.
The application of chemical fungicides to control plant diseases is an integral component of crop management [30,31]. Top rot is a novel fungal fruit disease in Rosa roxburghii production areas of southwest China for which no effective chemical control fungicides have been reported. An outstanding antifungal activity is the application basis of chemical fungicides for controlling plant fungal diseases in the field. The present results showed that 10% difenoconazole WG, 80% tebuconazole WG, 250 g/L azoxystrobin SC, 10% prothioconazole SC, 70% thiophanate-methyl WP, 25% prochloraz EC, 50% carbendazim WP, and 40% cyprodinil SC exhibited excellent toxicity activities toward C. fructicola CXCDF-3 of R. roxburghii with EC50 values of 0.26, 0.64, 0.99, 2.15, 4.64, 4.89, 7.27, and 7.73 mg L−1, respectively, indicating they had a notable potential to control top rot disease of R. roxburghii fruits in the field. Tebuconazole and difenoconazole are two triazole fungicides used widely to control plant pathogenic fungi; their action mechanism is to inhibit the sterol biosynthesis of fungi [31,32]. Azoxystrobin belongs to the group of mitochondrial respiration inhibitor fungicides and possesses broad-spectrum systemic activity against the four major classes of pathogenic fungi [33,34]. The present results demonstrated that the control efficacies of 80% tebuconazole WG 6000-fold liquid, 10% difenoconazole WG 5000-fold liquid, 250 g/L azoxystrobin SC 1000-fold liquid, and 250 g/L azoxystrobin SC 800-fold liquid against top rot disease of R. roxburghii fruits were 85.44%, 84.47%, 83.50%, and 82.53% at the harvest period, respectively. Meanwhile, some of tested fungicides in this study such as carbendazim, chlorothalonil, thiram, tiophanate methyl, and mancozeb are now forbidden in Europe. Considering the potential risks of above forbidden fungicides, 80% tebuconazole WG, 10% difenoconazole WG, and 250 g/L azoxystrobin SC can accordingly be recommended to control top rot disease in R. roxburghii production.
Currently, chemical fungicides with a high efficiency and a low risk are still the most effective and frequent measures for controlling plant diseases. However, increasing attention has been focused on chemical fungicide residuals as potential risks to environment, wildlife, and human beings. In this study, the field concentration of 80% tebuconazole WG (6000-fold liquid), 10% difenoconazole WG 5000-fold liquid), and 250 g/L azoxystrobin SC (1000-fold liquid) was relatively low, and the PHI of 45 d was also very long. Therefore, the potential food safety risk caused by tebuconazole, difenoconazole, or azoxystrobin should be minute or almost nonexistent. This study was the first to report the pathogen of top rot disease in R. roxburghii and to highlight that 80% tebuconazole WG, 10% difenoconazole WG, and 250 g/L azoxystrobin SC could be used for controlling top rot disease.
5. Conclusions
In conclusion, the pathogen of the novel top rot fruit disease in R. roxburghii was identified for the first time as C. fructicola CXCDF-3 based on the pathogenicity, the morphology, and a multigene phylogenetic analysis. The optimal growth conditions of C. fructicola CXCDF-3 were 25 °C, pH 6.0~8.0, full light or darkness, D-(+) maltose, peptone, and PDA medium. Additionally, difenoconazole, tebuconazole, azoxystrobin, prothioconazole, thiophanate-methyl, prochloraz, carbendazim, cyprodinil, and mancozeb had notable toxicity activities against C. fructicola CXCDF-3. Moreover, 80% tebuconazole WG, 10% difenoconazole WG, and 250 g/L azoxystrobin SC could effectively control top rot disease of R. roxburghii fruits in the field. This work, which was the first to reports the pathogen of top rot disease in R. roxburghii fruits, provides a feasible and effective practice for controlling top rot disease. Considering the serious harmfulness of top rot disease in R. roxburghii, research on its diversified alternative and environmentally friendly control strategies is a major concern for the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8111036/s1, Table S1: The serial and login numbers of the participating comparison strains of strain CXCDF-3.
Author Contributions
X.W. and H.A. constructed the project; X.W., H.A. and Y.L. designed the experiments; Y.L. and J.L. performed the experiments; X.W., Y.L. and M.L. analyzed the data; X.W. and H.A. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 32160656), the “Hundred” Level Innovative Talent Foundation of Guizhou Province (no. GCC [2022]023-1), the Science-Technology Support Program of Guizhou Province (no. (2020)1Y134, (2021) YB243), and the Cultivation Program of Guizhou University (no. (2019)09).
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
We declare that we do not have any commercial or associative interest that represent a conflict of interest in connection with the work submitted.
References
- Wang, L.; Lv, M.; An, J.; Fan, X.; Dong, M.; Zhang, S.; Wang, J.; Wang, Y.; Cai, Z.; Fu, Y. Botanical characteristics, phytochemistry and related biological activities of Rosa roxburghii Tratt fruit, and its potential use in functional foods: A review. Food Funct. 2021, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Zhou, R. The healthcare function and development trend of toxburgh rose. Food Res. Dev. 2016, 37, 212–214. [Google Scholar]
- Wang, D.; Lu, M.; Ludlow, R.A.; Zeng, J.; Ma, W.; An, H. Comparative ultrastructure of trichomes on various organs of Rosa roxburghii. Microsc. Res. Tech. 2021, 84, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, H.; Li, Y.; Yu, Z.; Liu, X.; Huang, M. Identification and oenological properties analysis of a strain of Hanseniaspora uvarum from Rosa roxburghii. Food Ferment. Ind. 2020, 46, 97–104. [Google Scholar]
- Huang, X.; Yan, H.; Zhai, L.; Yang, Z.; Yi, Y. Characterization of the Rosa roxburghii Tratt transcriptome and analysis of MYB genes. PLoS ONE 2019, 14, e0203014. [Google Scholar] [CrossRef]
- Hao, M.; Zhang, F.; Liu, X.; Zhang, F.; Wang, L.; Xu, S.; Zhang, J.; Ji, H.; Xu, P. Qualitative and quantitative analysis of catechin and quercetin in flavonoids extracted from Rosa roxburghii Tratt. Trop. J. Pharm. Res. 2018, 17, 71–76. [Google Scholar] [CrossRef]
- Xu, P.; Liu, X.; Xiong, X.; Zhang, W.; Cai, X.; Qiu, P.; Hao, M.; Wang, L.; Lu, D.; Zhang, X.; et al. Flavonoids of Rosa roxburghii Tratt exhibit anti-apoptosis properties by regulating PARP-1/AIF. J. Cell. Biochem. 2017, 118, 3943–3952. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Liu, J.; Liu, L.; Zhang, E.; Li, W. Inhibition of metastasis and invasion of ovarian cancer cells by crude polysaccharides from rosa roxburghii tratt in vitro. Asian Pac. J. Cancer Prev. 2014, 15, 10351–10354. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z.; Luo, Y.; Wu, X.; An, H. Chitosan Can Induce Rosa roxburghii Tratt. against Sphaerotheca sp. and Enhance Its Resistance, Photosynthesis, Yield, and Quality. Horticulturae 2021, 7, 289. [Google Scholar] [CrossRef]
- Fan, W.; Pan, X.; Chen, H.; Yang, H.; Gong, F.; Guan, J.; Wang, M.; Mu, R. Effects of oxalic acid on the nutrient of calcareous cultivated soil and leaf, fruit yield and quality of Rosa roxburghii Tratt. J. Fruit Sci. 2021, 38, 1113–1122. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Zhang, C.; Guo, Z.; Wu, X.; An, H. Co-application of allicin and chitosan increases resistance of Rosa roxburghii against powdery mildew and enhances its yield and quality. Antibiotics 2021, 10, 1449. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, X.D.; Huang, W.Y.; Wu, X.M. Occurrence and Control Technology of Powdery Mildew in Rose roxburgh Tratt. China Fruits 2021, 1, 6–10. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Q.; Li, J.; Su, Y.; Wu, X. Chitosan as an Adjuvant to Enhance the Control Efficacy of Low-Dosage Pyraclostrobin against Powdery Mildew of Rosa roxburghii and Improve Its Photosynthesis, Yield, and Quality. Biomolecules 2022, 12, 1304. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Miao, Y.; Liu, Y.; Zhang, L.; Mahendra, S. Identification of novel 1,4-dioxane degraders and related genes from activated sludge by taxonomic and functional gene sequence analysis. J. Hazard. Mater. 2021, 412, 125157. [Google Scholar] [CrossRef] [PubMed]
- Bazhenova, A.E.; Rudenko, O.S.; Pesterev, M.A.; Shcherbakova, N.A.; Misteneva, S.Y. Identification of the yeast strain cystobasidium slooffiae isolated from the cake test sample. Food Syst. 2021, 4, 111–116. [Google Scholar] [CrossRef]
- Yan, K.; Wang, J.L.; Zhou, Y.; Fu, D.P.; Huang, R.M. Efficacy of Five Fungicides in Rosa roxburghii Tratt against Sphaerotheca sp. Agrochemicals 2018, 57, 609–610. [Google Scholar]
- Xiang, J.; He, B. Toxicity Determination of Several Bio-fungicides to Powdery Mildew in Laboratory. Sci. Technol. Modern Agric. 2013, 19, 147. [Google Scholar]
- Wang, Q.; Zhang, C.; Long, Y.; Wu, X.; Su, Y.; Lei, Y.; Ai, Q. Bioactivity and control efficacy of the novel antibiotic tetramycin against various kiwifruit diseases. Antibiotics 2021, 10, 289. [Google Scholar] [CrossRef]
- Bellemain, E.; Carlsen, T.; Brochmann, C. ITS as an environmental DNA barcode for fungi: An insilico approach reveals potential PCR biases. BMC Microbiol. 2010, 10, 189. [Google Scholar] [CrossRef]
- Cai, L.; Hyde, K.D.; Taylor, P.; Weir, B.S.; Liu, Z.Y. A polyphasic approach for studying Colletotrichum. Fungal Divers. 2009, 39, 183–204. [Google Scholar] [CrossRef]
- Cao, X.; Xu, X.; Che, H.; West, J.; Luo, D. Characteristics and distribution of Colletotrichum species in coffee plantations in hainan, china. Plant Pathol. 2019, 6, 68. [Google Scholar] [CrossRef]
- Jouji, M.; Takao, T.; Toyozo, S. Grouping of Colletotrichum species in japan based on rDNA sequences. J. Gen. Plant Pathol. 2002, 68, 307–320. [Google Scholar] [CrossRef]
- Liu, B.; Pavel, J.; Hausbeck, M.K.; Feng, C.; Correll, J.C. Phylogenetic analysis, vegetative compatibility, virulence, and fungal filtrates of leaf curl pathogen Colletotrichum fioriniae from celery. Phytopathology 2020, 4, 111. [Google Scholar] [CrossRef]
- Alaniz, S.; Rodríguez, L.I.H.; Mondino, P. Colletotrichum fructicola, is the dominant and one of the most aggressive species causing bitter rot of apple in Uruguay. Trop. Plant Pathol. 2015, 40, 265–274. [Google Scholar] [CrossRef]
- Zhao, F.; Shen, L.; Min, F.; Hong, N.; Wang, G. Studies on the biological characteristics and pathogenicity for the monospora strains from ascospores of Colletotrichum fructicola infecting pear. Acta Phytopathol. Sin. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Li, H.; Zhou, G.; Liu, J.; Xu, J. Population genetic analyses of the fungal pathogen Colletotrichum fructicola on tea-oil trees in China. PLoS ONE 2016, 11, e015684. [Google Scholar] [CrossRef] [PubMed]
- Echeverrigaray, S.; Delamare, A.P.L.; Fontanella, G.; Favaron, F.; Stella, L.; Scariot, F.J. Colletotrichum species associated to ripe rot disease of grapes in the “Serra Gaucha” region of Southern Brazil. BIO Web Conf. 2019, 12, 01008. [Google Scholar] [CrossRef]
- Yang, R.; Shi, M.; Zhao, R.; Zhai, F. Identification and biological characteristics of Colletotrichum gloeosporioides on garlic bolt. Chin. Agric. Sci. Bull. 2011, 27, 160–164. [Google Scholar]
- Song, L.; Zhang, L.; Cao, Q.; Duan, K. Biological characteristics and pathogenicity of strawberry Colletotrichum fructicola. Acta Agric. Shanghai 2019, 35, 88–96. [Google Scholar] [CrossRef]
- Leadbeater, A. Recent developments and challenges in chemical disease control. Plant Protect. Sci. 2015, 51, 163–169. [Google Scholar] [CrossRef]
- Hirooka, T.; Ishii, H. Chemical control of plant diseases. J. Gen. Plant Pathol. 2013, 79, 390–401. [Google Scholar] [CrossRef]
- Fustinoni, S.; Mercadante, R.; Polledri, E.; Rubino, F.M.; Mandic-Rajcevic, S.; Vianello, G.; Colosio, C.; Moretto, A. Biological monitoring of exposure to tebuconazole in winegrowers. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Fustinoni, S.; Mercadante, R.; Polledri, E.; Rubino, F.M.; Mandic-Rajcevic, S.; Vianello, G.; Rodrigues, E.T.; Lopes, I.; Pardal, M.N. Occurrence, fate and effects of azoxystrobin in aquatic ecosystems: A review. Environ. Int. 2013, 53, 18–28. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y.; Wang, J.; Chen, X.; Wei, K.; Wang, M.; Shang, S. Activities of azoxystrobin and difenoconazole against Alternaria alternata and their control efficacy. Crop Prot. 2016, 90, 54–58. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).