Characteristics of Interspecific Hybridization and Inbred Progeny of Pumpkin (Cucurbita moschata Duch.) and Winter Squash (Cucurbita maxima Duch.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pumpkin Lines and Breeding Methods
2.1.1. Laboratory Lines
2.1.2. Crossing Methods
2.2. Characteristics of Interspecific Hybridization and Inbred Progeny Plants
2.3. Methods for Assessing Nutritional Components of the Fruits
2.4. Statistical Analysis
3. Results
3.1. Analysis of Plant Characteristics of Interspecific F1 Hybrids
3.2. Analysis of Seed and Plant Characteristics of Back-Cross F1
3.3. Seed and Plant Characteristics of Triple-Cross or Four-Way-Cross F1 and F2
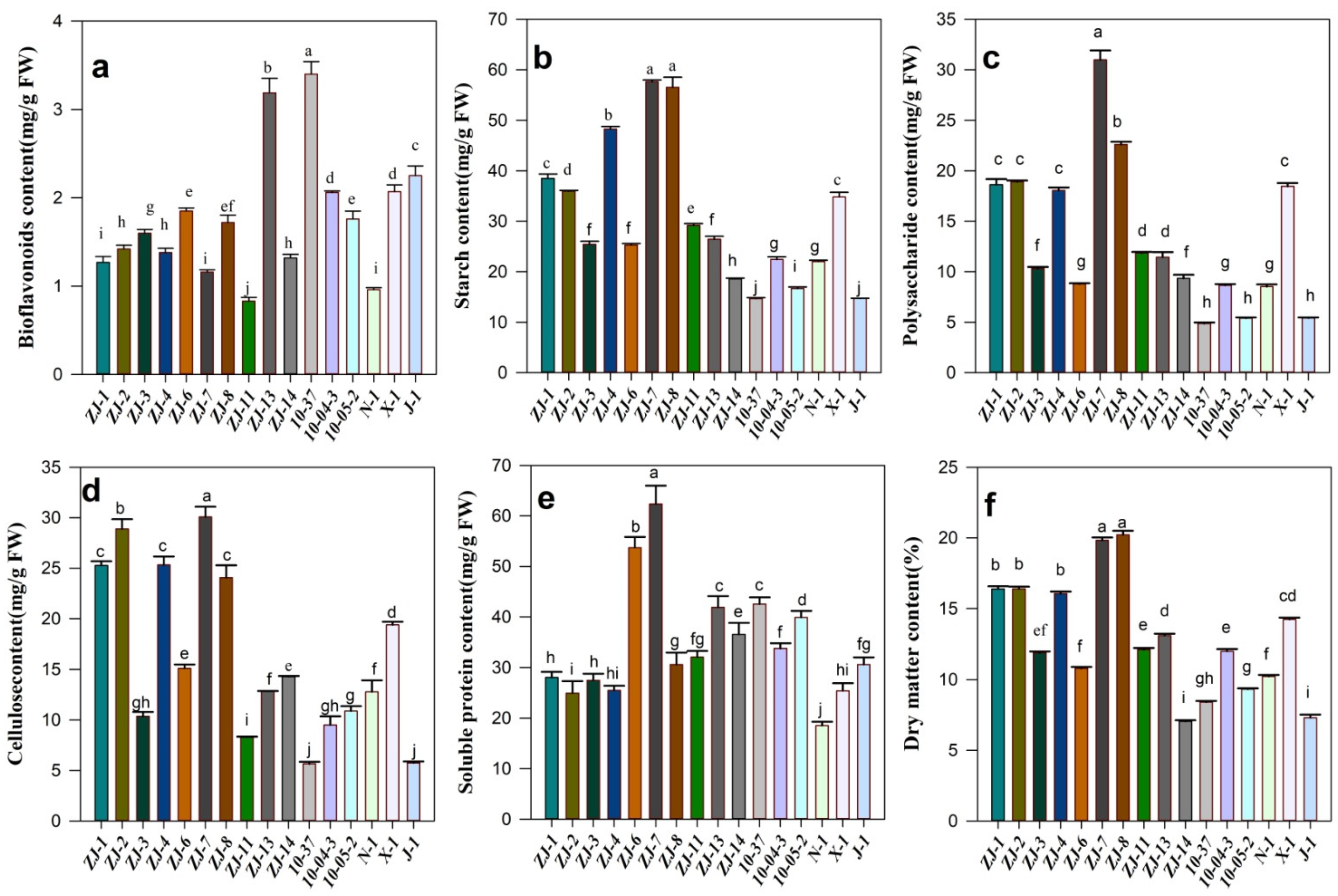
3.4. Comparative Analysis of Fruit Quality of Interspecific Hybridization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeffrey, C. A review of the Cucurbitaceae. Bot. J. Linn. Soc. 1980, 81, 233–247. [Google Scholar] [CrossRef]
- Naik, M.L.; Prasad, V.M.; Laxmi, R. A study on character association and path analysis in pumpkin (Cucurbita moschata Duch. ex Poir.). Int. J. Adv. Res. 2015, 3, 1030–1034. [Google Scholar]
- Kaźmińska, K.; Sobieszek, K.; Targońska-Karasek, M.; Korzeniewska, A.; Niemirowicz-Szczytt, K.; Bartoszewski, G. Genetic Diversity Assessment of a Winter Squash and Pumpkin (Cucurbita maxima Duchesne) Germplasm Collection Based on Genomic Cucurbita-conserved SSR Markers. Sci. Hortic. 2017, 219, 37–44. [Google Scholar] [CrossRef]
- Singh, A.; Abhilash, P.C. Wild relatives of cultivated plants in India. In A Reservoir of Alternative Genetic Resources and More; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2017; pp. 2049–2056. [Google Scholar]
- Jena, K.K.; Khush, G.S. Introgression of Genes from Oryza Officinalis Well ex Watt to Cultivated rice O. sativa L. Theor. Appl. Genet. 1990, 80, 737–745. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Sun, J.; Zhang Jia, Y.; Du, X. SSR and Agronomic Characters Analysis of the Introgressed Lines From Interspecific Hybridization in Cotton. J. Plant Genet. Resour. 2009, 10, 60–67. [Google Scholar]
- Tu, Y.; Tang, J.; Tu, W.; Dai, X.; Zhang, T. Comprehensive Evaluation of Water Logging Tolerance of Progenies Between Brassica Napus L. and Rorippaindica Hiern. J. Plant Genet. Resour. 2015, 16, 895–902. [Google Scholar]
- Peterka, H.; Budahn, H.; Schrader, O.; Ahne, R.; Schütze, W. Transfer of Resistance Against the Beet Cyst Nematode from Radish (Raphanus sativus) to Rape (Brassica napus) by Monosomic Chromosome Addition. Theor. Appl. Genet. 2004, 109, 30–41. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, H.; Liu, W.; Han, H.-M.; Zhou, S.; Yang, X.; Li, X.; Zhang, J.; Li, L. Cytological Identification of Wheat-agropyron Cristatum Robertsonian Translocation Line. J. Plant Genet. Resour. 2018, 19, 1038–1044. [Google Scholar]
- Wu, J.; Yang, X.; Wang, H. The Introgression of Chromosome 6P Specifying for Increased Numbers of Florets and Kernels from Agropyron Cristatum Into Wheat. Theor. Appl. Genet. 2006, 114, 13–20. [Google Scholar] [CrossRef]
- Lin, D. Genetic Genes and Interspecific Hybridization of Pumpkin Plants. Chin. Watermelon Melon 2000, 3, 41–44. [Google Scholar]
- Lin, D. Origin and Classification of Pumpkin Plants. Chin. Watermelon Melon 2000, 1, 36–38. [Google Scholar]
- Cheng, Y.; Zhang, E.; Xu, Z.; Wang, Y.; Liang, B. A Preliminary Report on the Innovation of Pumpkin Germplasm Resources. Northwest Agric. J. 2001, 1, 100–102. [Google Scholar] [CrossRef]
- Singh, D.; Singh, A.; Singh, A. Wide Hybridization. Plant Breeding and Cultivar Development; Academic Press: London, UK, 2021; pp. 159–178. [Google Scholar]
- Li, B.; Liu, Y.; Wang, C. Basic Biological Research and Breeding Progress of Cucurbita Vegetables. China Veg. 1996, 6, 50–52. [Google Scholar]
- Fan, L. Characteristics and Application of Interspecific Hybrids Between Indian Pumpkin and Chinese Pumpkin. China Veg. 2016, 2, 86–88. [Google Scholar]
- Shen, W.; Xu, X.; Huang, W. Primary Study of Interspecific Hybrids Between Cucurbita Maxima and Cucurbita moschata. Zhejiang Agric. Sci. 2007, 4, 392–394. [Google Scholar]
- Jiang, Y.; Li, H.; Liu, A. Study on the Distant Hybridization Affinity and Change of Physiological and Biochemical of Chinese and Indian Pumpkins. J. Northeast. Agric. Univ. 2009, 40, 23–27. [Google Scholar]
- Pujar, D. Distant Hybridization in Fruit Crops: A Review. Int. J. Pure Appl. Biosci. 2017, 5, 1312–1315. [Google Scholar]
- Zhang, Q.; Yu, E.; Medina, A. Development of Advanced Interspecific-bridge Lines among Cucurbita pepo, C. maxima, and C. moschata. Hortscience 2012, 47, 452–458. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Zhang, L.; Yuan, Z.; Hao, M.; Zheng, Y. Distant Hybridization: A Tool for Interspecific Manipulation of Chromosomes. In Alien Gene Transfer in Crop Plants; Springer: New York, NY, USA, 2014; Volume 1, pp. 25–42. [Google Scholar]
- Dinesh, M.; Rekha, A.; Ravishankar, K.; Praveen, K.; Santosh, L. Breaking the Intergeneric Crossing Barrier in Papaya Using Sucrose Treatment. Sci. Hortic. 2007, 114, 33–36. [Google Scholar]
- Tian, L.; Wang, Y.; Niu, L.; Tang, D. Breeding of DiseaseResistant Seedless Grapes Using Chinese Wild Vitis spp.: I. In Vitro Embryo Rescue and Plant Development. Sci. Hortic. 2008, 117, 136–141. [Google Scholar] [CrossRef]
- Khush, G.; Brar, D. Overcoming the barriers in hybridization. In Distant Hybridization of Crop Plants; Springer: Berlin/Heidelberg, Germany, 1992; pp. 47–61. [Google Scholar]
- McCandless, L. Geneva Releases ‘Whitaker’ Summer Squash at the NYS Vegetable Conference. 10 February 1998. Available online: https://ecommons.cornell.edu/bitstream/handle/1813/35439/980210_New_Squash_Variety.pdf;jsessionid=06E459F51A10CD98485A0C0FF078B3EF?sequence=1 (accessed on 5 June 2022).
- Wessel-Beaver, L.; Cuevas, H.; Andres, T.; Piperno, D. Genetic Compatibility between Cucurbita moschata and C. argyrosperma. Progress in cucurbit genetics and breeding research. In Proceedings of the 8th EUCAPRIA Meeting on Cucurbit Genetics and Breeding, Palacky University, Olomouc, Czech Republic, 12–17 July 2004; pp. 393–400. [Google Scholar]
- Chetelat, R.; De Verna, J. Expression of Unilateral Incompatibility in Pollen of Lycopericon pennellii is Determined by Major Loci on Chromosomes 1, 6 and 10. Theor. Appl. Genet. 1991, 82, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Finkers, R.; Heusden, A.; Fien, M.; Van, J.; Maris, P.; Lindhout, P. The Construction of a Solanum Habrochaites LYC4 introgression line population and the identification of QTLs for resistance to Botrytis cinerea. Theor. Appl. Genet. 2007, 114, 1071–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.P.; Ge, X.H.; Yao, X.C.; Feng, Y.H.; Li, X.Y. Synthesis and characterization of interspecific trigenomic hybrids and allohexaploids between three cultivated Brassica allotetroploids and wild species Brassica fruticulosa. Afr. J. Biotechnol. 2011, 10, 12171–12176. [Google Scholar]
- Parisi, M.; Iovene, M.; Frusciante, L. Production and morpho-cytological characterization of BC2 progenies from 5X × 4X and 4X × 5X crosses in Solanum. In Proceedings of the XLV Italian Society of Agricultural Genetics-SIGA Annual Congress, Salsomaggiore Term, Italy, 26–29 September 2001. [Google Scholar]
- Stebbins, G.L. Artificial polyploidy as a tool in plant breeding. Brookhaven Symp. Biol. 1956, 9, 35–52. [Google Scholar]
- Wang, Y.; Scarth, R.; Campbell, C. Interspecific hybridization between Fagopyrum tataricum (L.) Gaertn. and F. esculentum Moench. Fagopyrum 2002, 19, 31–35. [Google Scholar]
- Whitaker, T.W.; Robinson, R.W. Breeding Vegetable Crops; Bassett, M.J., Ed.; AVI Publishing Company, Inc.: Westport, CT, USA, 1986; pp. 209–238. [Google Scholar]
- Singh, S.P.; Terán, H.; Schwartz, H.F.; Otto, K.; Lema, M. White mold-resistant interspecific common bean germplasm lines VCW 54 and VCW 55. J. Plant Regist. 2009, 3, 191–197. [Google Scholar] [CrossRef]
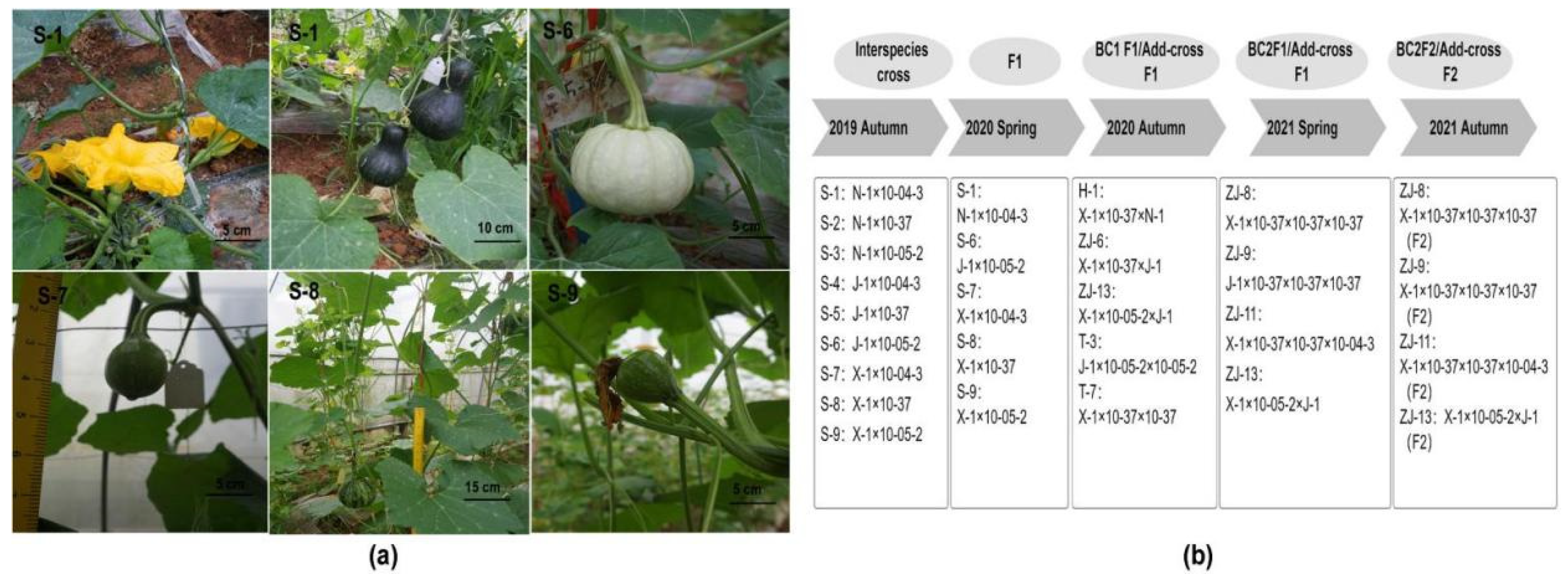
| Inbred Line Name | Pumpkin Taxon | Growth Potential | Powdery Mildew Resistance Level | Maturity | Fruit Quality Level | Fruit Shape and Color |
|---|---|---|---|---|---|---|
| J-1 | C. moschata | Strong | High | Late maturity | Moderate | Round with ribbed |
| N-1 | C. moschata | Weak | Susceptible | Early maturity | High | Short, rod-shaped, and smooth |
| X-1 | C. moschata | Medium | Moderate | Medium maturity | Moderate | Highly spherical and ribbed |
| 10-37 | C. maxima | Strong | Susceptible | Late maturity | High | Oblate with grey white skin |
| 10-04-3 | C. maxima | Weak | Susceptible | Medium maturity | High | Oblate with green skin and dark green spots |
| 10-05-2 | C. maxima | Strong | Moderate | Late maturity | High | Oblate with light green skin and green spots |
| Hybridization No. | Hybridization | Average Seed Acquisition Rate of F1 (%) ± SD | F1 Plant Acquisition Rate (%) ± SD | F2 Seed Acquisition Rate (%) | F1 Male Flower Fertility | F1 Plant Characteristics | F1 Seed Characteristics | F1 Fruit Characteristics |
|---|---|---|---|---|---|---|---|---|
| S-1 | N-1 × 10-04-3 | 22.1 ± 0.5 d | 8.4 ± 0.7 d | 0 | Sterile | Similar to female parent | Shape is similar to the male parent and the color is similar to the female parent | Fruit shape is a combined type, the color is similar to male parent and the flesh quality is similar to male parent |
| S-2 | N-1 × 10-37 | 0 | - | - | - | - | - | - |
| S-3 | N-1 × 10-05-2 | 0 | - | - | - | - | - | - |
| S-4 | J-1 × 10-04-3 | 0 | - | - | - | - | - | - |
| S-5 | J-1 × 10-37 | 0 | - | - | - | - | - | - |
| S-6 | J-1 × 10-05-2 | 59.7 ± 1.9 c | 67.7 ± 2.1 b | 0 | Fertile | Similar to female parent | Shape and color are similar to the female parent | Fruit shape, color, and flesh quality are of combined type |
| S-7 | X-1 × 10-04-3 | 56.4 ± 0.7 c | 29.9 ± 1.0 c | 0 | Partly fertile | Similar to female parent | Shape and color are similar to the female parent | Fruit shape, color, and flesh quality are similar to female parent |
| S-8 | X-1 × 10-37 | 79.8 ± 0.8 a | 72.2 ± 1.0 a | 0 | Sterile | Similar to female parent | Shape and color are similar to the male parent | Fruit shape and flesh are similar to male parent, color is of combined type |
| S-9 | X-1 × 10-05-2 | 65.2 ± 2.7 b | 62.6 ± 2.4 b | 0 | Fertile | Similar to female parent | Shape and color are similar to the female parent | Fruit shape is similar to female parent, color and flesh are of combined type |
| Hybridization No. | Back-Crossing | BC1F1 Seed Acquisition Rate (%) ± SD | BC1F1 Plant Acquisition Rate (%) ± SD | BC1F2 Seed Acquisition Rate (%) ± SD | BC1F1 Male Fertility | BC1F1 Plant Characteristics | BC1F1 Seed Characteristics | BC1F1 Fruit Characteristics |
|---|---|---|---|---|---|---|---|---|
| T-1 | N-1 × 10-04-3 × 10-04-3 | 2.2 ± 0.2 d | 0.0 | - | - | - | - | - |
| T-2 | N-1 × 10-04-3 × N-1 | 0.0 | - | - | - | - | - | - |
| T-3 | J-1 × 10-05-2 × 10-05-2 | 69.7 ± 0.2 b | 61.8 ± 2.9 c | 0.0 | Sterile | Similar to C. moschata, strong growth potential | Light brown color, not smooth with ribbed edge | Black skinskin, long pendant shape |
| T-4 | J-1 × 10-05-2 × J-1 | 0.0 ± 0.0 | - | - | - | - | - | - |
| T-5 | X-1 × 10-04-3 × 10-04-3 | 43.1 ± 1.6 c | 0.0 | - | - | - | - | - |
| T-6 | X-1 × 10-04-3 × X-1 | 0.0 ± 0.0 | - | - | - | - | - | - |
| T-7 | X-1 × 10-37 × 10-37 | 81.8 ± 0.6 a | 75.1 ± 3.0 a | 0.0 | Fertile | Similar to C. moschata, medium growth potential | Light brown color, smooth | Oblate shape, green or grey skin |
| T-8 | X-1 × 10-37 × X-1 | 0.0 | - | - | - | - | - | - |
| T-9 | X-1 × 10-05-2 × 10-05-2 | 78.2 ± 3.5 a | 67.6 ± 5.7 b | 0.0 | Fertile | Similar to C. moschata, medium growth potential | White color, smooth | Oblate shape, dark yellow with brown spots |
| T-10 | X-1 × 10-05-2 × X-1 | 0.0 ± 0.0 | - | - | - | - | - | - |
| Hybridization No. | Method of Hybridization | Triple- and Four-Way-Cross F1 Seed Acquisition Rate (%) ± SD | Triple- and Four-Way-Cross F1 Plant Acquisition Rate (%) ± SD | Triple- and Four-Way-Cross F2 Seed Acquisition Rate (%) ± SD | Triple- and Four-Way-Cross F1 Male Fertility | Triple- and Four-Way-Cross F1 Plant Characteristics | Triple- and Four-Way-Cross F1 Seed Characteristics | Triple- and Four-Way-Cross F1 Fruit Characteristics |
|---|---|---|---|---|---|---|---|---|
| ZJ-1 | X-1 × 10-37 × N-1 | 41.8 ± 4.7 f | 94.9 ± 5.3 b | 0.0 | Fertile | Similar to C. moschata | Light yellow color and smooth skin | Round shape with green skin with spots |
| ZJ-2 | X-1 × 10-37 × N-1 × 10-37 | 67.7 ± 0.9 d | 97.3 ± 4.7 a | 0.0 | Fertile | Similar to C. moschata | Light yellow color and smooth skin | Pendant shape, yellow smooth skin |
| ZJ-3 | (J-1 × 10-05-2 × 10-05-2) × 10-05-2 | 90.9 ± 1.7 b | 97.0 ± 1.4 a | 0.0 | Sterile | Similar to C. moschata, strong growth potential | Light brown and not smooth with ribbed edge | Long pendant with black skin |
| ZJ-4 | X-1 × 10-37 × J-1 × 10-37 | 67.8 ± 2.2 d | 95.7 ± 2.0 b | 0.0 | Fertile | Similar to C. moschata | Brown color and smooth skin | Round shape with green spotted skin |
| ZJ-5 | X-1 × 10-37 × J-1 × 10-05-2 | 0.0 | - | - | - | - | - | - |
| ZJ-6 | X-1 × 10-37 × J-1 | 47.1 ± 1.2 e | 98.4 ± 2.7 a | - | Fertile | Similar to C. moschata | Light brown color and smooth skin | Round shape with green spotted skin |
| ZJ-7 | X-1 × 10-37 × J-1 × JG-1 | 20.8 ± 1.6 g | 0.0 | - | - | - | - | - |
| ZJ-8 | X-1 × 10-37 × 10-37 × 10-37 | 92.1 ± 2.5 b | 98.3 ± 2.9 a | 92.3 ± 8.4 b | Fertile | Similar to C. moschata | Light brown color and smooth skin | Round shape with green spotted skin |
| ZJ-10 | (X-1 × 10-37 × 10-37) × (X-1 × 10-05-2 × J-1) | 0.0 | - | - | - | - | - | - |
| ZJ-11 | (X-1 × 10-37 × 10-37) × 10-04-3 | 86.3 ± 2.3 c | 97.7 ± 2.0 a | 97.2 ± 2.6 a | Fertile | Similar to C. moschata | Black brown color and smooth skin | Oblate shape with green spotted skin |
| ZJ-12 | X-1 × 10-05-2 × J-1 × JG-1 | 0.0 | - | - | - | - | - | - |
| ZJ-13 | X-1 × 10-05-2 × J-1 | 98.0 ± 0.5 a | 99.0 ± 1.7 a | 97.2 ± 1.5 a | Fertile | Similar to C. moschata, resistance to powdery mildew and strong growth potential | Grayish white color and smooth skin | Pendant shape with brown smooth spotted skin |
| ZJ-14 | X-1 × 10-05-2 × J-1 × 10-05-2 | 86.0 ± 2.3 c | 97.3 ± 1.0 a | 0.0 | Sterile | Similar to C. moschata, strong growth potential | Light brown color and smooth skin | Round shape with dark green spotted skin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Jiang, Y.; Yang, X.; Deng, X.; Dang, J.; Wang, Z.; Yusop, M.R.; Abdullah, S. Characteristics of Interspecific Hybridization and Inbred Progeny of Pumpkin (Cucurbita moschata Duch.) and Winter Squash (Cucurbita maxima Duch.). Horticulturae 2022, 8, 596. https://doi.org/10.3390/horticulturae8070596
Liu Z, Jiang Y, Yang X, Deng X, Dang J, Wang Z, Yusop MR, Abdullah S. Characteristics of Interspecific Hybridization and Inbred Progeny of Pumpkin (Cucurbita moschata Duch.) and Winter Squash (Cucurbita maxima Duch.). Horticulturae. 2022; 8(7):596. https://doi.org/10.3390/horticulturae8070596
Chicago/Turabian StyleLiu, Zefa, Yanfang Jiang, Xipeng Yang, Xin Deng, Jiancheng Dang, Zhijun Wang, Mohd Rafii Yusop, and Shamsiah Abdullah. 2022. "Characteristics of Interspecific Hybridization and Inbred Progeny of Pumpkin (Cucurbita moschata Duch.) and Winter Squash (Cucurbita maxima Duch.)" Horticulturae 8, no. 7: 596. https://doi.org/10.3390/horticulturae8070596
APA StyleLiu, Z., Jiang, Y., Yang, X., Deng, X., Dang, J., Wang, Z., Yusop, M. R., & Abdullah, S. (2022). Characteristics of Interspecific Hybridization and Inbred Progeny of Pumpkin (Cucurbita moschata Duch.) and Winter Squash (Cucurbita maxima Duch.). Horticulturae, 8(7), 596. https://doi.org/10.3390/horticulturae8070596








