Tailored Physicochemical Properties and Bioactive Value of Sweet Pepper Fruits from Controlled High Temperature
Abstract
1. Introduction
2. Materials and Methods
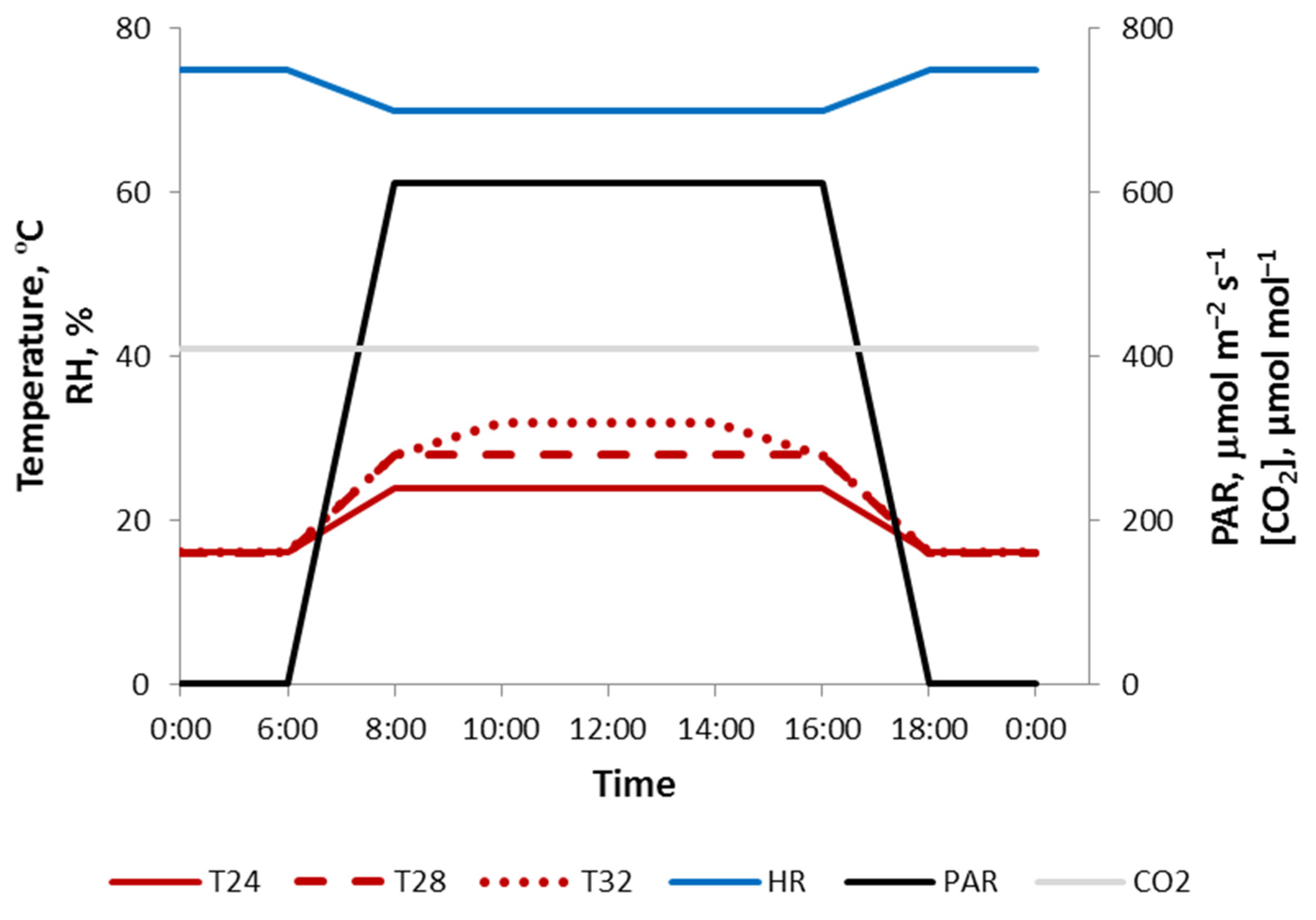
2.1. Plant Material and Growth Conditions
2.2. Fresh Weight
2.3. Skin Color
2.4. Total Soluble Solids
2.5. Phenolic Concentration
2.6. Mineral Content
2.7. Free Amino Acids
2.8. Statistical Analysis
3. Results and Discussion
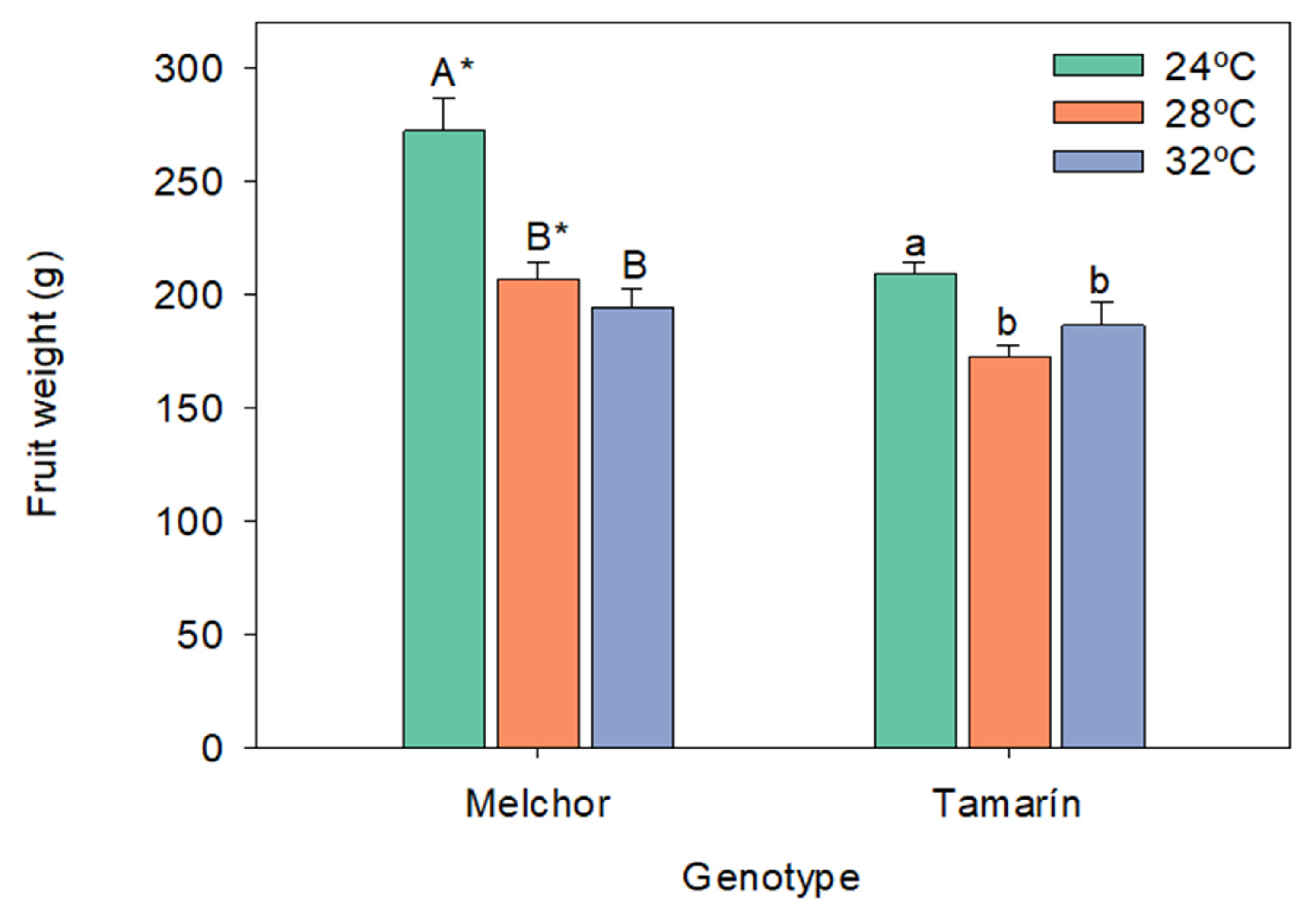
3.1. Fresh Weight
3.2. Skin Color
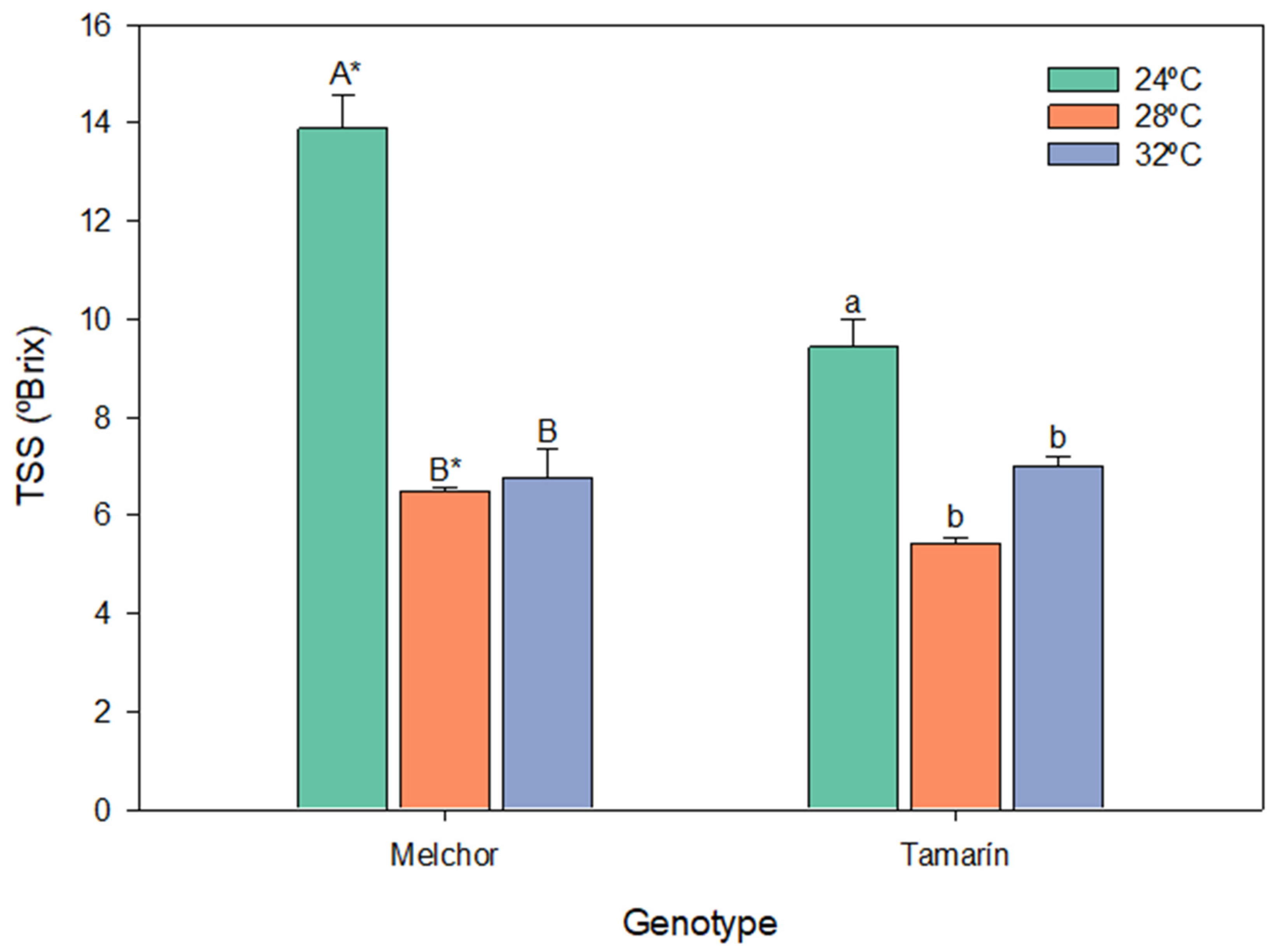
3.3. Total Soluble Solids
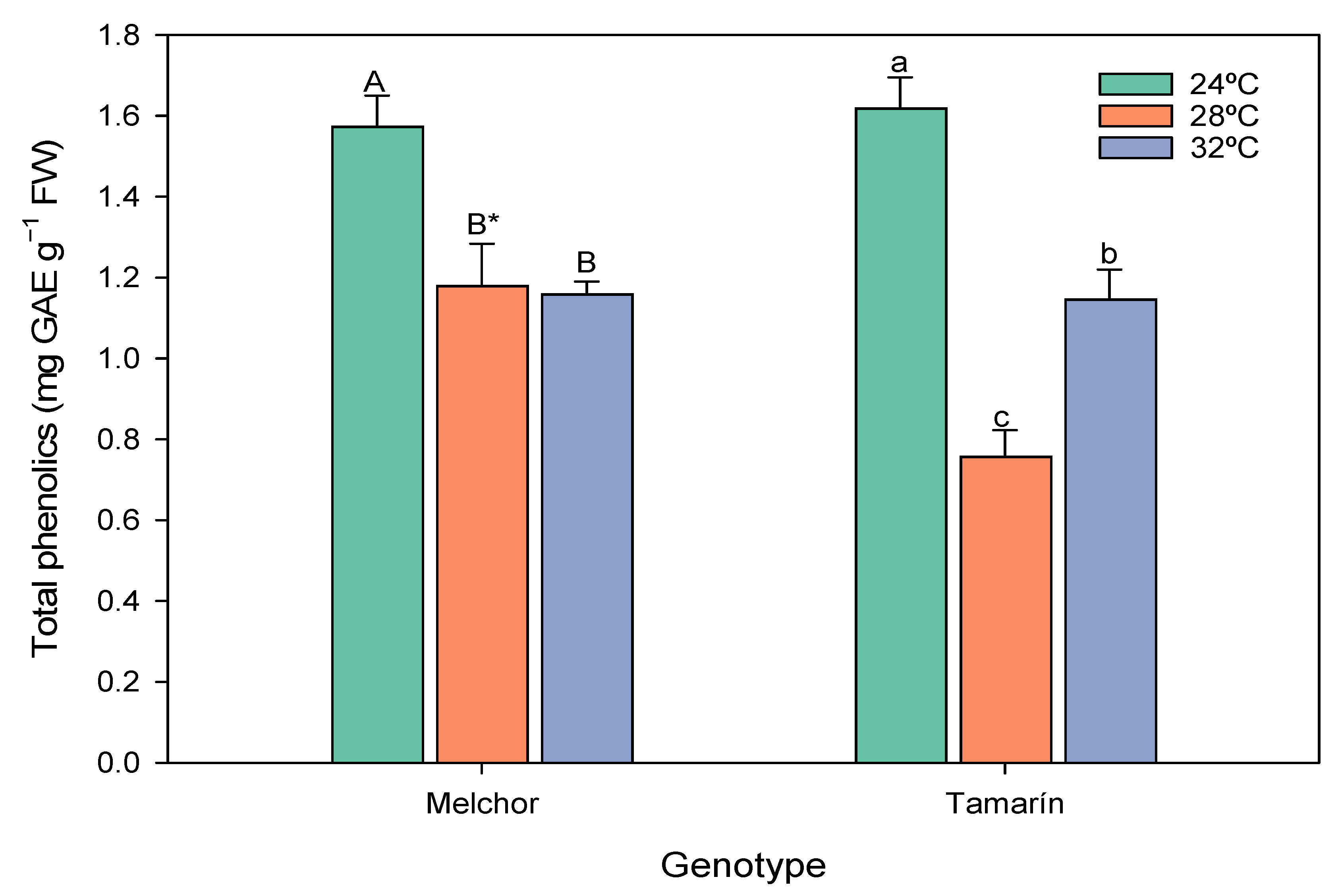
3.4. Phenolic Concentration
3.5. Mineral Content
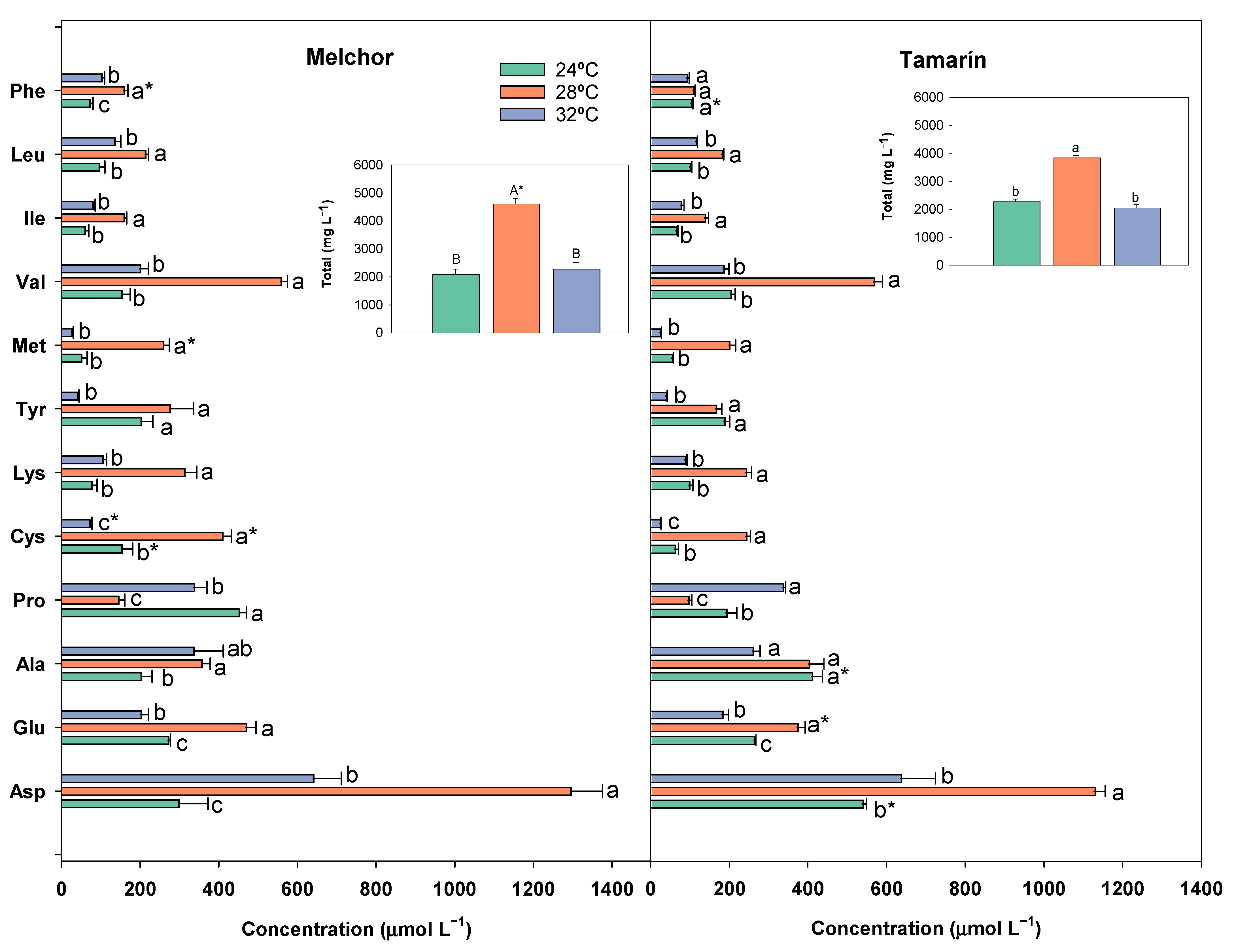
3.6. Free Amino Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Souza, R.; Peña-Fleitas, M.T.; Thompson, R.B.; Gallardo, M.; Grasso, R.; Padilla, F.M. The Use of Chlorophyll Meters to Assess Crop N Status and Derivation of Sufficiency Values for Sweet Pepper. Sensors 2019, 19, 2949. [Google Scholar] [CrossRef]
- Piñero, M.C.; Pérez-Jiménez, M.; López-Marín, J.; del Amor, F.M. Fruit Quality of Sweet Pepper as Affected by Foliar Ca Applications to Mitigate the Supply of Saline Water under a Climate Change Scenario. J. Sci. Food Agric. 2018, 98, 1071–1078. [Google Scholar] [CrossRef]
- Mateos, R.M.; Jiménez, A.; Román, P.; Romojaro, F.; Bacarizo, S.; Leterrier, M.; Gómez, M.; Sevilla, F.; Del Río, L.A.; Corpas, F.J.; et al. Antioxidant Systems from Pepper (Capsicum Annuum L.): Involvement in the Response to Temperature Changes in Ripe Fruits. Int. J. Mol. Sci. 2013, 14, 9556–9580. [Google Scholar] [CrossRef]
- Del Amor, F.M.; Serrano-Martínez, S.A.; Fortea, M.I.; Gómez-López, M.D.; Núñez-Delicado, E. Yield and Fruit Quality Response of Sweet Pepper Genotypes Grown under Soilless Cultivation. Artic. J. Plant Nutr. 2013, 36, 1247–1257. [Google Scholar] [CrossRef]
- Kim, E.H.; Lee, S.Y.; Baek, D.Y.; Park, S.Y.; Lee, S.G.; Ryu, T.H.; Lee, S.K.; Kang, H.J.; Kwon, O.H.; Kil, M.; et al. A Comparison of the Nutrient Composition and Statistical Profile in Red Pepper Fruits (Capsicums Annuum L.) Based on Genetic and Environmental Factors. Appl. Biol. Chem. 2019, 62, 48. [Google Scholar] [CrossRef]
- Otálora, G.; Piñero, M.C.; Collado-González, J.; López-Marín, J.; Del Amor, F.M. Exogenous Salicylic Acid Modulates the Response to Combined Salinity-Temperature Stress in Pepper Plants (Capsicum Annuum L. var. Tamarin). Plants 2020, 9, 1790. [Google Scholar] [CrossRef]
- IPCC. Summary for Poliymakers. In Global Warming of 1.5 °C: An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Preindustrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Contex of Strengthening the Global Response to the Threat of Climate Change; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; World Meteorological Organization: Geneva, Switzerland, 2018; p. 32. Available online: https://www.ipcc.ch/site/assets/uploads/sites/2/2018/07/SR15_SPM_High_Res.pdf (accessed on 16 July 2021).
- Oh, S.Y.; Koh, S.C. Fruit Development and Quality of Hot Pepper (Capsicum annuum L.) under Various Temperature Regimes. Hortic. Sci. Technol. 2019, 37, 313–321. [Google Scholar]
- Balasooriya, H.N.; Dassanayake, K.B.; Ajlouni, S. The Impact of Elevated CO2 and High Temperature on the Nutritional Quality of Fruits-A Short Review. Am. J. Agric. Res. 2019, 4, 26. [Google Scholar] [CrossRef][Green Version]
- Porras, M.E.; Lorenzo, P.; Medrano, E.; Sánchez-González, M.J.; Baeza, E.J.; Piñero, M.C.; Sánchez-Guerrero, M.C. Sweet Pepper Grown under Salinity Stress as Affected by CO2 Enrichment and Nitrogen Source. Acta Hortic. 2017, 1170, 805–812. [Google Scholar] [CrossRef]
- Piñero, M.C.; Lorenzo, P.; Sánchez-Guerrero, M.C.; Medrano, E.; López-Marín, J.; del Amor, F.M. Reducing Extreme Weather Impacts in Greenhouses: The Effect of a New Passive Climate Control System on Nutritional Quality of Pepper Fruits. J. Sci. Food Agric. 2021, 102, 2723–2730. [Google Scholar] [CrossRef]
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Piñero, M.C.; Otálora, G.; Collado-González, J.; López-Marín, J.; del Amor, F.M. Effects of Selenium on the Chlorophylls, Gas Exchange, Antioxidant Activity and Amino Acid Composition of Lettuce Grown under an Aquaponics System. Horticulturae 2021, 8, 30. [Google Scholar] [CrossRef]
- Heo, Y.; Park, E.G.; Son, B.G.; Choi, Y.W.; Lee, Y.J.; Park, Y.H.; Suh, J.M.; Cho, J.H.; Hong, C.O.; Lee, S.G.; et al. The influence of abnormally high temperatures on growth and yield of hot pepper (Capsicum annuum L.). J. Agric. Life Sci. 2013, 47, 9–15. [Google Scholar] [CrossRef]
- Sato, S.; Kamiyama, M.; Iwata, T.; Makita, N.; Furukawa, H.; Ikeda, H. Moderate increase of mean daily temperature adversely affects fruit set of Lycopersicon esculentum by disrupting specific physiological processes in male reproductive development. Ann. Bot. 2006, 97, 731–738. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, A.; Halim, R.; Aziz, T. Alleviation of temperature stress by nutrient management in crop plants: A review. J. Soil Sci. Plant Nutri. 2012, 12, 221–244. [Google Scholar] [CrossRef]
- Maso Hornero-Méndez, D.; Gómez-Ladrón De Guevara, R.; Isabel Mínguez-Mosquera, M. Carotenoid Biosynthesis Changes in Five Red Pepper (Capsicum Annuum L.) Cultivars during Ripening. Cultivar Selection for Breeding. J. Agric. Food Chem. 2000, 48, 3857–3864. [Google Scholar] [CrossRef]
- Takahashi, M.; Yoshida, C.; Komoda, T. Establishing an Efficient Fruit Ripening Method for Sweet Pepper (Capsicum Anuum L.) through Light Irradiation and Dark Processing. Hortic. J. 2018, 87, 73–79. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant Tolerance to High Temperature in a Changing Environment: Scientific Fundamentals and Production of Heat Stress-Tolerant Crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Rahman, M.; Rahaman, S.; Islam, R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Kim, M.; Kang, S.; Yun, S.; Kim, S.; Joa, J.; Park, Y. Influence of Excessively High Temperatures on the Fruit Growth and Physicochemical Properties of Shiranuhi Mandarin in Plastic-Film Greenhouse Cultivation. Plants 2021, 10, 1525. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Vitaglione, P.; Giordano, M.; Pannico, A.; Colantuono, A.; De Pascale, S. Genotypic variation in nutritional and antioxidant profile among iceberg lettuce cultivars. Acta Sci. Pol. Hortorum Cultus 2017, 16, 37–45. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional Value, Bioactive Compounds and Health Benefits of Lettuce (Lactuca Sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Rosales, M.A.; Cervilla, L.M.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.d.M.; Blasco, B.; Ríos, J.J.; Soriano, T.; Castilla, N.; Romero, L.; Ruiz, J.M. The Effect of Environmental Conditions on Nutritional Quality of Cherry Tomato Fruits: Evaluation of Two Experimental Mediterranean Greenhouses. J. Sci. Food Agric. 2011, 91, 152–162. [Google Scholar] [CrossRef]
- Amor, F.M.; Marcelis, L.F.M. Regulation of Nutrient Uptake, Water Uptake and Growth under Calcium Starvation and Recovery. J. Hortic. Sci. Biotechnol. 2003, 78, 343–349. [Google Scholar] [CrossRef]
- Mach, J. Calcium Channels and Acquired Thermotolerance: Here Comes the Sun and It’s All Right. Plant Cell 2012, 24, 3167. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Zhao, L.; Shen, T.; Sun, J.; Chen, H.; Kong, Q.; Nawaz, M.A.; Bie, Z. Melon Fruit Sugar and Amino Acid Contents Are Affected by Fruit Setting Method under Protected Cultivation. Sci. Hortic. (Amst.) 2017, 214, 288–294. [Google Scholar] [CrossRef]

| Week | Cl− | SO42− | NO3− | P | NH4+ | Mg2+ | Ca2+ | Na+ | K+ |
|---|---|---|---|---|---|---|---|---|---|
| W1–W6 | 3.6 | 4.2 | 7.7 | 1.2 | 2.5 | 1.4 | 4.2 | 4.1 | 4.5 |
| W7–W15 | 3.6 | 4.2 | 8.4 | 0.9 | 0.5 | 1.4 | 4.2 | 4.1 | 5.5 |
| Temp | Experiment | T, °C | RH, % | [CO2], μmol mol−1 |
|---|---|---|---|---|
| 24 °C | Daytime average | 19.3 | 73 | 451 |
| Average maximum | 24.2 | 79 | 500 | |
| Average minimum | 15.8 | 65 | 406 | |
| 28 °C | Daytime average | 21.0 | 75 | 437 |
| Average maximum | 28.0 | 83 | 482 | |
| Average minimum | 16.0 | 69 | 391 | |
| 32 °C | Daytime average | 22.1 | 80 | 407 |
| Average maximum | 32.0 | 89 | 442 | |
| Average minimum | 16.0 | 71 | 361 |
| Temp | Genotype | L* | a* | b* | C* | Hab |
|---|---|---|---|---|---|---|
| 24 °C | Melchor | 29.91 ± 0.32 B,* | 29.10 ± 0.36 C,* | 14.97 ± 0.32 B,* | 32.73 ± 0.45 C,* | 27.20 ± 0.30 B |
| Tamarín | 28.17 ± 0.25 b | 24.31 ± 0.58 b | 13.42 ± 0.45 a | 27.78 ± 0.70 b | 28.82 ± 0.45 a,* | |
| 28 °C | Melchor | 32.33 ± 0.24 A,* | 30.73 ± 0.33 B,* | 14.81 ± 0.31 B,* | 34.12 ± 0.42 B,* | 25.71 ± 0.28 C,* |
| Tamarín | 30.64 ± 0.18 a | 26.97 ± 0.32 a | 12.64 ± 0.17 a | 29.78 ± 0.36 ab | 25.10 ± 0.11 b | |
| 32 °C | Melchor | 31.27 ± 0.35 A,* | 36.10 ± 0.32 A,* | 20.33 ± 0.29 A,* | 41.43 ± 0.40 A,* | 29.04 ± 0.25 A,* |
| Tamarín | 27.55 ± 0.74 b | 27.40 ± 0.99 a | 12.89 ± 0.84 a | 30.30 ± 1.22 a | 25.08 ± 0.84 b |
| Temp | Genotype | K | Mg | Ca | Fe | Cu | Mn | Zn | B |
|---|---|---|---|---|---|---|---|---|---|
| g Kg−1 DW | mg Kg−1 DW | ||||||||
| 24 °C | Melchor | 25.5 ± 0.4 A | 1.3 ± 0.0 A | 499 ± 23 A | 69.5 ± 3.4 A | 9.2 ± 0.6 B | 14.0 ± 0.6 A | 31.0 ± 0.5 A | 8.0 ± 0.4 A |
| Tamarín | 27.5 ± 0.5 a,* | 1.3 ± 0.0 a | 691 ± 41 a,* | 106.0 ± 3.4 a,* | 12.6 ± 1.4 a | 20.1 ± 1.4 a,* | 34.6 ± 0.9 a,* | 7.3 ± 0.2 b | |
| 28 °C | Melchor | 23.8 ± 0.7 B | 1.2 ± 0.1 AB | 290 ± 23 B | 57.0 ± 5.0 B | 12.9 ± 0.6 A | 9.9 ± 0.8 B | 24.4 ± 0.5 B | 9.0 ± 0.3 A |
| Tamarín | 24.7 ± 0.6 b | 1.2 ± 0.0 ab | 395 ± 48 b | 57.5 ± 7.9 b | 12.7 ± 0.2 a | 11.6 ± 0.3 b | 25.07 ± 0.6 b | 8.6 ± 0.3 a | |
| 32 °C | Melchor | 21.2 ± 0.6 C | 1.1 ± 0.0 B | 219 ± 30 B | 39.7 ± 2.5 C | 5.6 ± 0.5 C | 10.1 ± 0.2 B | 29.4 ± 1.0 A | 8.6 ± 0.2 A |
| Tamarín | 22.5 ± 0.3 c | 1.1 ± 0.0 b | 170 ± 22 c | 35.3 ± 2.8 c | 5.8 ± 0.5 b | 11.3 ± 0.5 b | 25.3 ± 1.8 b | 8.2 ± 0.2 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piñero, M.C.; Lorenzo, P.; Sánchez-Guerrero, M.C.; Medrano, E.; López-Marín, J.; del Amor, F.M. Tailored Physicochemical Properties and Bioactive Value of Sweet Pepper Fruits from Controlled High Temperature. Horticulturae 2022, 8, 582. https://doi.org/10.3390/horticulturae8070582
Piñero MC, Lorenzo P, Sánchez-Guerrero MC, Medrano E, López-Marín J, del Amor FM. Tailored Physicochemical Properties and Bioactive Value of Sweet Pepper Fruits from Controlled High Temperature. Horticulturae. 2022; 8(7):582. https://doi.org/10.3390/horticulturae8070582
Chicago/Turabian StylePiñero, María Carmen, Pilar Lorenzo, María Cruz Sánchez-Guerrero, Evangelina Medrano, Josefa López-Marín, and Francisco M. del Amor. 2022. "Tailored Physicochemical Properties and Bioactive Value of Sweet Pepper Fruits from Controlled High Temperature" Horticulturae 8, no. 7: 582. https://doi.org/10.3390/horticulturae8070582
APA StylePiñero, M. C., Lorenzo, P., Sánchez-Guerrero, M. C., Medrano, E., López-Marín, J., & del Amor, F. M. (2022). Tailored Physicochemical Properties and Bioactive Value of Sweet Pepper Fruits from Controlled High Temperature. Horticulturae, 8(7), 582. https://doi.org/10.3390/horticulturae8070582









