Impact of Artificial Polyploidization in Ajuga reptans on Content of Selected Biologically Active Glycosides and Phytoecdysone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction of the Plant Materials
2.3. HPLC and LC/MS Analyses
2.4. NMR Analysis-Identification of trans-Verbascoside and trans-Teupolioside
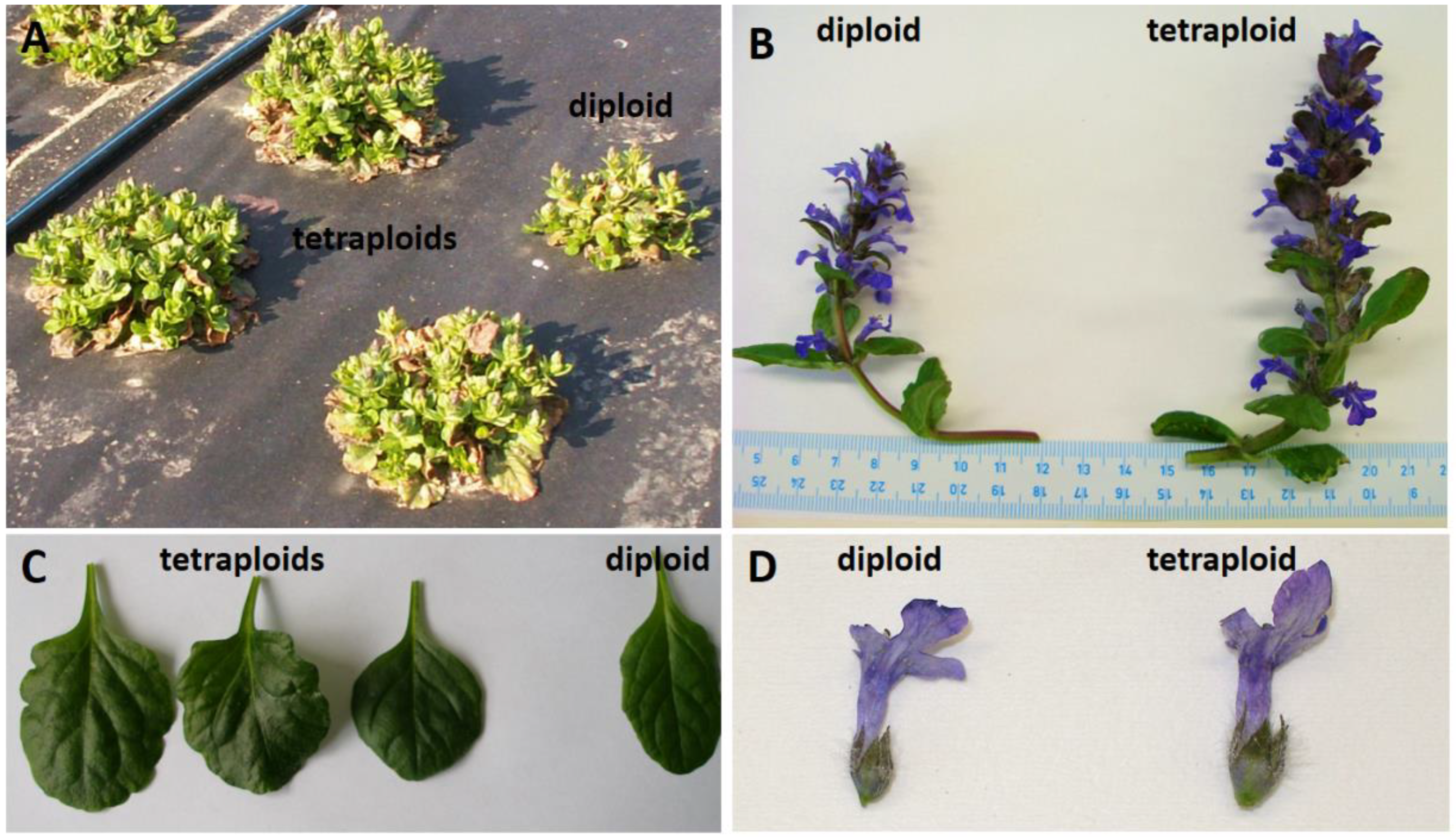
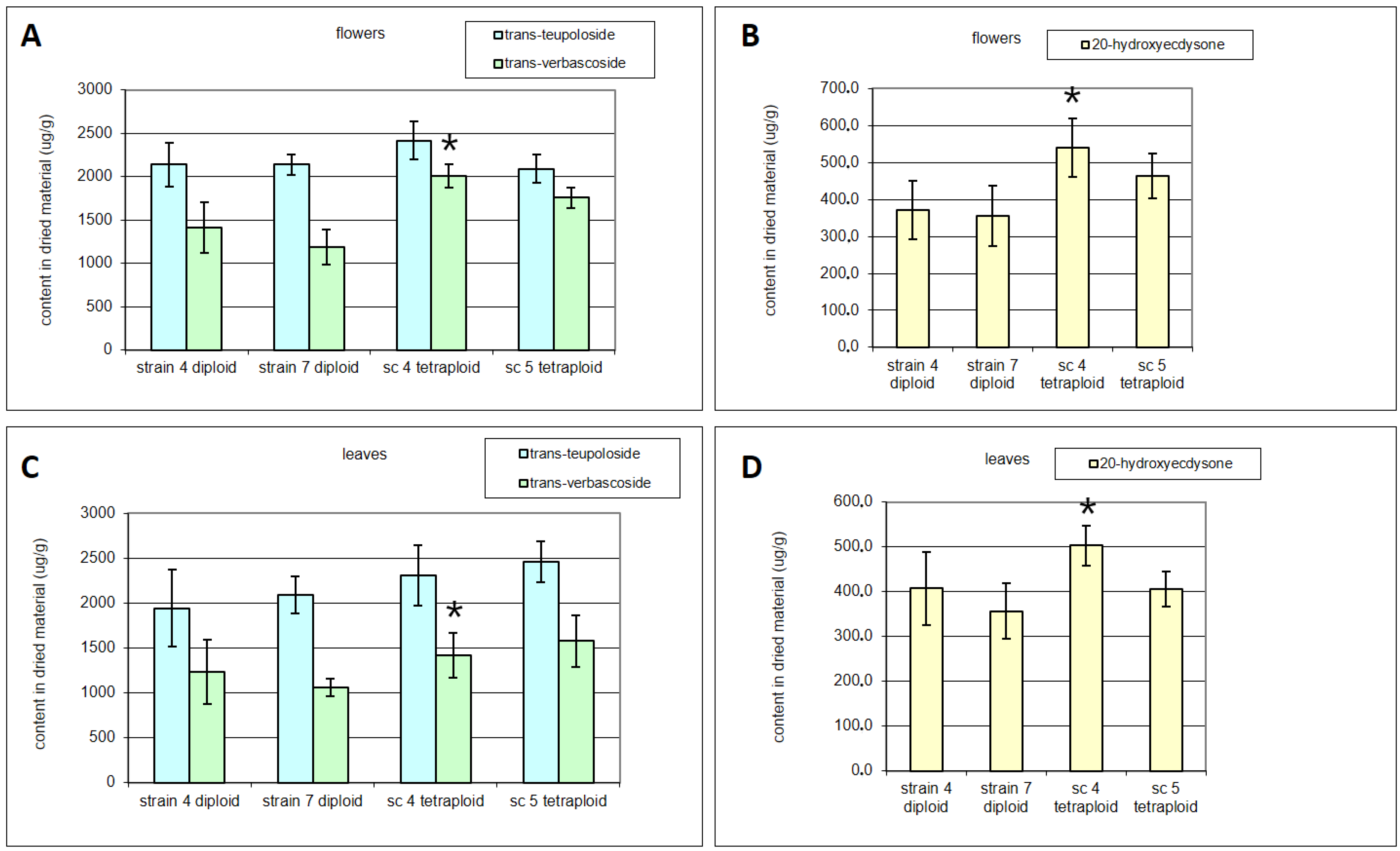
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Duren, M.; Morpurgo, R.; Dolezel, J.; Afza, R. Induction and verification of autotetraploids in diploid banana (Musa acuminata) by in vitro techniques. Euphytica 1996, 88, 25–34. [Google Scholar] [CrossRef]
- Xing, S.H.; Guo, X.B.; Wang, Q.; Pan, Q.F.; Tian, Y.S.; Liu, P.; Zhao, J.Y.; Wang, G.F.; Sun, X.F.; Tang, K.X. Induction and flow cytometry identification of tetraploids from seed-derived explants through colchicine treatments in Catharanthus roseus (L.) G. don. BioMed. Res. Internat. 2011, 2011, 10. [Google Scholar] [CrossRef] [Green Version]
- Tavan, M.; Mirjalili, M.H.; Karimzadeh, G. In vitro polyploidy induction: Changes in morphological, anatomical and phytochemical characteristics of Thymus persicus (Lamiaceae). Plant Cell Tissue Org. Cult. 2015, 122, 573–583. [Google Scholar] [CrossRef]
- Švécarová, M.; Navrátilová, B.; Hašler, P.; Ondřej, V. Artificial induction of tetraploidy in Humulus lupulus L. using oryzalin. Acta Agrobot. 2019, 72, 1764. [Google Scholar] [CrossRef]
- Pavela, R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 2015, 114, 3835–3853. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Esposito, E.; Mazzon, E.; Riccardi, L.; Caminiti, R.; Dal Toso, R.; Pressi, G.; Cuzzocrea, S. Teupolioside, a phenylpropanoid glycosides of Ajuga reptans, biotechnologically produced by IRBN22 plant cell line, exerts beneficial effects on a rodent model of colitis. Biochem. Pharmacol. 2009, 77, 845–857. [Google Scholar] [CrossRef] [Green Version]
- Marchev, A.S.; Georgiev, M.I. Plant In vitro Systems as a sustainable source of sctive ingredients for cosmeceutical application. Molecules 2020, 25, 2006. [Google Scholar] [CrossRef]
- Dinan, L. Phytoecdysteroids: Biological aspects. Phytochemistry 2001, 57, 325–339. [Google Scholar] [CrossRef]
- Aly, R.; Ravid, U.; Abu-Nassar, J.; Botnick, I.; Lebedev, G.; Gal, S.; Ziadna, H.; Achdari, G.; Smirov, E.; Meir, A.; et al. Biological activity of natural phytoecdysteroids from Ajuga iva against the sweetpotato whitefly Bemisia tabaci and the persea mite Oligonychus perseae. Pest Manag. Sci. 2011, 67, 1493–1498. [Google Scholar] [CrossRef]
- Gubser, G.; Vollenweider, S.; Eibl, D.; Eibl, R. Food ingredients and food made with plant cell and tissue cultures: State-of-the art and future trends. Eng. Life Sci. 2021, 21, 87–98. [Google Scholar] [CrossRef]
- Švécarová, M.; Navrátilová, B.; Ondřej, V. In vitro polyploidization of Ajuga reptans L. using oryzalin. Acta Biol. Crac. Bot. 2018, 60, 69–73. [Google Scholar]
- Rungsimakan, S.; Rowan, M.G. Terpenoids, flavonoids and caffeic acid derivatives from Salvia viridis L. cvar. Blue Jeans. Phytochemistry 2014, 108, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.L.; Chen, B.J.; Zhu, D.N. In vitro production and identification of autotetraploids of Scutellaria baicalensis. Plant Cell Tissue Org. Cult. 2002, 70, 289–293. [Google Scholar] [CrossRef]
- Navrátilová, B.; Švécarová, M.; Bednář, J.; Ondřej, V. In vitro polyploidization of Thymus vulgaris L. and its effect on composition of essential oils. Agronomy 2021, 11, 596. [Google Scholar] [CrossRef]
- Trojak-Goluch, A.; Kawka-Lipińska, M.; Wielgusz, K.; Praczyk, M. Polyploidy in industrial crops: Applications and perspectives in plant breeding. Agronomy 2021, 11, 2574. [Google Scholar] [CrossRef]
- Dhawan, O.P.; Lavania, U.C. Enhancing the productivity of secondary metabolites via induced polyploidy: A review. Euphytica 1996, 87, 81–89. [Google Scholar] [CrossRef]
- Banyai, W.; Sangthong, R.; Karaket, N.; Inthima, P.; Mii, M.; Supaibulwatana, K. Overproduction of artemisinin in tetraploid Artemisia annua L. Plant Biotechnol. J. 2010, 27, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.G.; Tang, T.X.; Chen, R.; Liang, C.H.; Liu, X.Y.; Wu, C.L.; Yang, Y.S.; Yang, D.P.; Wu, H. A comparative study of bioactive secondary metabolite production in diploid and tetraploid Echinacea purpurea (L.) Moench. Plant Cell Tissue Org. Cult. 2014, 116, 323–332. [Google Scholar] [CrossRef]
- Taha-Salaime, L.; Lebedev, G.; Abo-Nassar, J.; Marzouk, S.; Inbar, M.; Ghanim, M.; Aly, R. Activity of Ajuga iva extracts against the African cotton leafworm Spodoptera littoralis. Insects 2020, 11, 726. [Google Scholar] [CrossRef]
- Mishra, B.K.; Pathak, S.; Sharma, A.; Trivedi, P.K.; Shukla, S. Modulated gene expression in newly synthesized autotetraploid of Papaver somniferum L. South Afr. J. Bot. 2010, 76, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Parson, J.L.; Martin, S.L.; Golenia, G.; James, T. Polyploidization for the genetic improvement of Cannabis sativa. Front. Plant Sci. 2019, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Caruso, I.; Lepore, L.; De Tommasi, N.; Dal Piaz, F.; Frusciante, L.; Aversano, R.; Garramone, R.; Carputo, D. Secondary metabolite profile in induced tetraploids of wild Solanum commersonii Dun. Chem. Biodivers. 2011, 8, 2226–2237. [Google Scholar] [CrossRef] [PubMed]
- Caruso, I.; Dal Piaz, F.; Malafronte, N.; De Tommasi, N.; Aversano, R.; Wulff Zottele, C.; Scarano, M.-T.; Carputo, D. Impact of ploidy change on secondary metabolites and photochemical efficiency in Solanum bulbocastanum Dun. Nat. Prod. Commun. 2013, 8, 1387–1392. [Google Scholar] [PubMed] [Green Version]
- Alipieva, K.; Korkina, L.; Orhan, I.; Georgiev, M. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotech. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navrátilová, B.; Ondřej, V.; Vrchotová, N.; Tříska, J.; Horník, Š.; Pavela, R. Impact of Artificial Polyploidization in Ajuga reptans on Content of Selected Biologically Active Glycosides and Phytoecdysone. Horticulturae 2022, 8, 581. https://doi.org/10.3390/horticulturae8070581
Navrátilová B, Ondřej V, Vrchotová N, Tříska J, Horník Š, Pavela R. Impact of Artificial Polyploidization in Ajuga reptans on Content of Selected Biologically Active Glycosides and Phytoecdysone. Horticulturae. 2022; 8(7):581. https://doi.org/10.3390/horticulturae8070581
Chicago/Turabian StyleNavrátilová, Božena, Vladan Ondřej, Naděžda Vrchotová, Jan Tříska, Štěpán Horník, and Roman Pavela. 2022. "Impact of Artificial Polyploidization in Ajuga reptans on Content of Selected Biologically Active Glycosides and Phytoecdysone" Horticulturae 8, no. 7: 581. https://doi.org/10.3390/horticulturae8070581
APA StyleNavrátilová, B., Ondřej, V., Vrchotová, N., Tříska, J., Horník, Š., & Pavela, R. (2022). Impact of Artificial Polyploidization in Ajuga reptans on Content of Selected Biologically Active Glycosides and Phytoecdysone. Horticulturae, 8(7), 581. https://doi.org/10.3390/horticulturae8070581






