In Vitro Micrografting of Horticultural Plants: Method Development and the Use for Micropropagation
Abstract
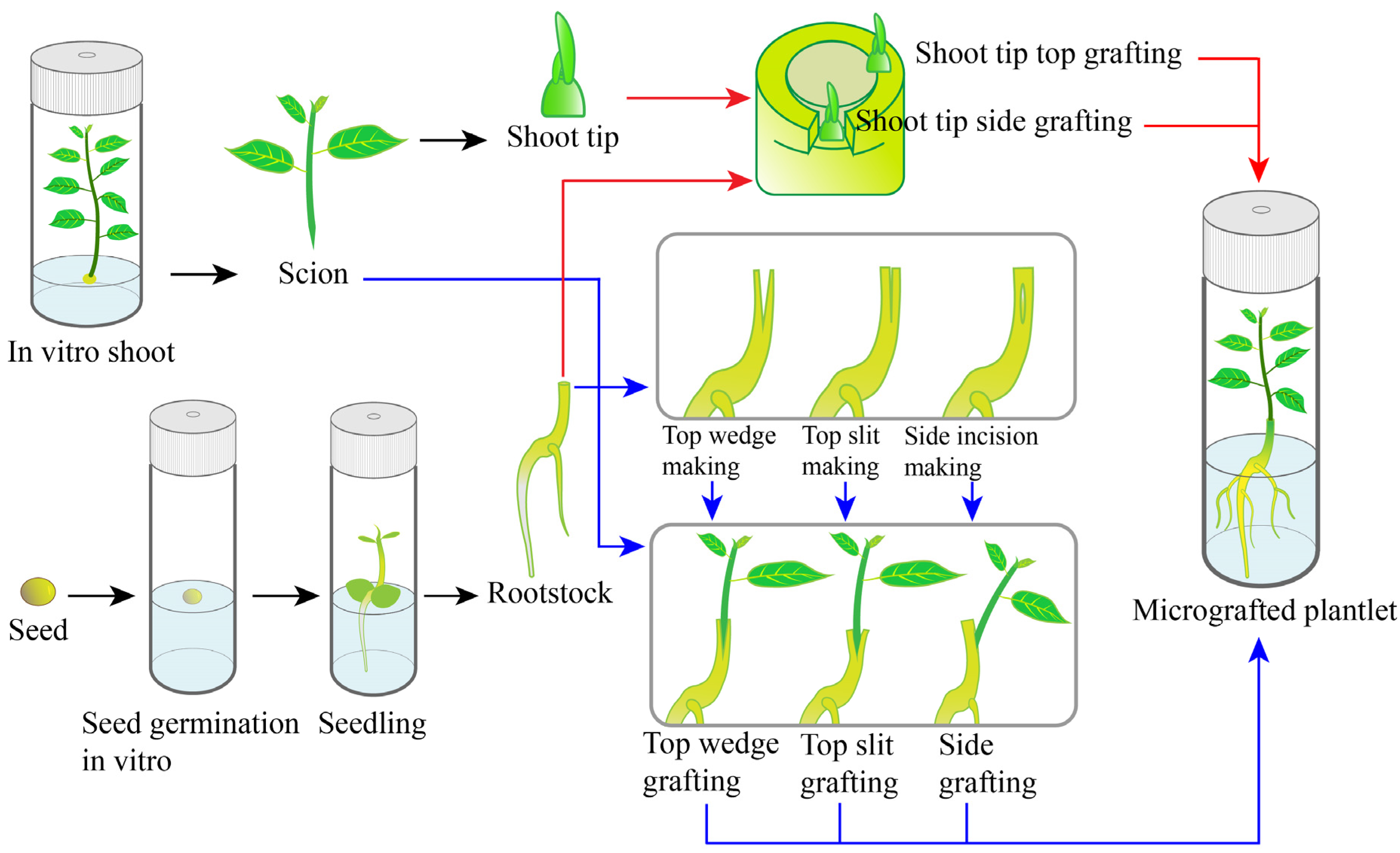
1. Overall Developments and Characters of Micrografting
2. Establishment of Micrografting
2.1. Preparation of Scions
2.2. Preparation of Rootstocks
| Plant Species (Scion) | Scion Source and Size | Rootstock Source and Age | Grafting Technique | Success Rate (%) and (No. Scions Tested) | Reference |
|---|---|---|---|---|---|
| Amygdalus communis (Almond) | Shoots of 1.5–2.0 cm in length | Almond seedlings of 2 weeks old | Top slit | 100 (1) | [18] |
| Anacardium occidentale (Cashew) | In vitro shoot apices | Cashew seedlings, 5–8 cm in height (age not specified) | Apical and side grafting | 45–73 (1) | [69] |
| Shoots of 3–15 mm in length | Cashew seedlings of 20–25 days old | Top slit and side grafting | 80 (top wedge) and 100 (side grafting) (1) | [31] | |
| Annona cherimola (Cherimoya) | Nodal section of 2 cm in length | Cherimoya seedling of 42 days old | Side insertion | 31–70 (3) | [19] |
| Citrus deliciosa (Kinnow mandarin) | Shoot tips less than 1.0 mm in size | C. jambhiri, C. carrizo and C. reshnii seedlings of 15–20 days old | Side reverse T insertion | Up to 66.5 (1) | [70] |
| Garcinia indica | Apical shoots of 0.5–1.0 cm in length for the initial grafting and 1.0–1.5 cm for the subsequent grafting | Garcinia indica seedlings of 2 months old | Top slit | 95 (1) | [71] |
| Malus domestica (Apple) | Field grown shoots (size not specified) | Apple shoots of 3 weeks old | Vertical slit | 42–93 (3) | [36] |
| Olea europea (Olive) | Greenhouse-grown shoots of 1.0–1.5 cm in length | Olive seedlings of 3 weeks old | Top slit | Up to 83 (1) | [45] |
| Opuntia ficus-indica (Cactus) | Shoots of 0.5 cm in length | Shoots of O. strepacantha, O. robusta, O. cochinera, O. leucotricha and O. ficus-indica, 1.0 cm in length (age not specified) | Top wedge and horizontal graft | 30 (top wedge) to 90 (horizontal) (1) | [54] |
| Passiflora edulis (Passion fruit) | Nodal segments of 1.5 cm in length | Passion fruit shoots of 2 months old | V-shaped joint with grafting devices | 73.3 (1) | [26] |
| Pelecyphoraaselliformis (Cactus) | Apical and subapical segments of 5 and 3 mm, respectively | O. ficus-indica shoots, 10 mm in length (age not specified) | Horizontal graft | 81 and 97 for the subapical and apical scions, respectively (1) | [72] |
| Pistacia vera var. Siirt (Pistachio) | Shoot tips of 0.5–10 mm in length | Pistachio seedlings of 10–14 days old | Top slit and top wedge | Up to 80 (1) | [17] |
| Protea cynaroides (King Protea) | Shoots of 5 mm in length | King Protea seedling of 30 days old | Top slit | 80 (1) | [55] |
| Prunus dulcis (Almond) | Apical shoots of 1.5–2.0 cm in length | Shoots of almond/peach hybrid rootstock of 3–7 weeks old | Top slit | 50–70 (2) | [65] |
| Shoots (size not specified) | Almond seedlings of 2 weeks old | Top slit | Up to 100 (1) | [63] | |
| Shoot tips of 4, 8 and 15 mm in length | Almond seedlings of 14 days old | Top slit and top wedge | 90–100 (2) | [33] | |
| Prunus avium (Cherry) | Shoot tips of 0.3–1.0 cm in length | P. avium × (P. canescens × P. tomentosa) shoots, 3–4 cm in length (age not specified) | Top slit | Up to 79 (1) | [21] |
| Pyrus communis (‘Old Home’ x ‘Farmingdale 333′) (Pear) | Shoots of 10 mm in length | P. elaeagrifolia seedlings of 10–14 days old | Cleft | 97.9 (1) | [24] |
| Rosa hybrida cvs./(Rose) | Shoots 10–15 mm in length | R. canina and R. multiflora shoots, 20 mm in length (age not specified) | With grafting devices | Up to 100 (2) | [59] |
| Theobroma cacao (Cacao) | Shoots of 4–6 mm in length | Cacao seedlings of 5–6 weeks old | Not specified | >50 (1) | [73] |
| Bud sticks with apical or axillary buds (sourced from potted plants) of 1 cm in length | Cacao seedlings of 3 weeks old | Top slit and side grafting | 55–95 (1) | [38] | |
| Vitis vinifera (Grape) | Shoot tips of 0.2–0.5 mm in length | White to slightly coloured hypocotyls from white somatic embryos | Side grafting | 18–30 (4) | [74] |
| In vitro/in vivo derived shoot tips of 0.3–0.8 mm in length | Shoots of V. vinifera × V. berlandieri, 1.0 cm in length (age not specified) | Not specified | 40–61 (in vitro shoot tips) and 12–17 (in vivo shoot tips) (4) | [34] | |
| Ziziphus mauritiana (Jujube) | Shoots of 5–10 mm in length | Jujube seedlings (7 spp.) of 4 weeks old | Top wedge | 28–100 (1) | [42] |
2.3. Grafting Techniques
2.4. Culture Conditions
2.5. Acclimatization of Micrografted Plants
3. Factors Affecting the Success of Micrografting
3.1. Scions
3.2. Rootstocks
3.3. Micrografting Methods
3.4. Culture Conditions
4. Applications of Micrografting in Micropropagation
4.1. Root Promotion
4.2. Promotion of Shoot Proliferation
4.3. Embryo Rescue or the Promotion of Organogenesis-Derived Shoot Regrowth
4.4. Shoot Regrowth after Cryopreservation
| Plant Species (Scion) | Scion Source and Size | Rootstock Source and Age | Grafting Technique | Success Rate (%) and (No. Scions Tested) | Reference |
|---|---|---|---|---|---|
| Citrus sinensis (Sweet orange) | Shoots of 1–2 mm in length (sourced from greenhouse plants) | Carrizo citrange seedlings of 2 weeks old | Side insertion | 90 (1) | [93] |
| Shoots recovered from germinated somatic embryos (size not specified) | Carrizo citrange seedlings (age not specified) | Not specified | 90 (1) | [117] | |
| Citrus spp. | Shoot tips (1–1.5 mm in length) cryopreserved by droplet-vitrification | Carrizo citrange seedlings up to 6 weeks old | Side grafting | 10–100 (32); average of 56% | [129] |
| Helianthus annuus (Sunflower) | Shoots (0.5–1 cm in length) from leaf explants | Sunflower seedlings of 7–10 days old | Side insertion | 47–85 (7) | [77] |
| Shoots (1 cm in length) from cotyledon explants | Sunflower seedlings of 1–2 weeks old | Side insertion | 69 (1) | [78] | |
| Lens culinaris (Lentil) | Shoots (1–1.5 cm in length) from cotyledonary nodes | Lentil seedlings of 5–6 days old | Top slit | 90–100 (3) | [16] |
| Persea americana (Avocado) | Shoots regenerated from somatic embryos (SEs) | Avocado seedlings of 7–12 days old | Top slit | 70.5 (1) | [37] |
| Shoots derived from previously micrografted SE shoots | 100 (1) | ||||
| Shoots of 5–10 mm in length | 59 (1) | ||||
| Shoots (size not specified) regenerated from genetically transformed SEs | Avocado seedlings of 3 weeks old | Top slit | 83.6 (1) | [112] | |
| Shoots of 3–5 mm in length from genetically transformed SEs | Avocado seedlings of 4 weeks old | Top slit | 60–80 (1) | [124] | |
| Solanum lycopersicum F1 hybrids (Tomato) | Shoots (size not specified) regenerated from cotyledon explants | Tomato seedlings of 3 weeks old | Top slit | 75–83 (3) | [131] |
| Ziziphus jujuba (Chinese jujube) | Shoot tips (0.2–1 mm in length) cryopreserved by droplet-vitrification | Z.spinosa seedling of 4 weeks old | Side grafting | 5–75 (1) | [132] |
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mudge, K.; Janick, J.; Scofield, S.; Goldschmidt, E.E. A History of Grafting. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; Volume 35, pp. 437–493. [Google Scholar]
- King, S.R.; Davis, A.R.; Liu, W.; Levi, A. Grafting for Disease Resistance. Hortscience 2008, 43, 1673–1676. [Google Scholar] [CrossRef]
- Goldschmidt, E.E. Plant grafting: New mechanisms, evolutionary implications. Front. Plant Sci. 2014, 5, 727. [Google Scholar] [CrossRef] [PubMed]
- Macan, G.P.F.; Cardoso, J.C. In vitro grafting of Psidium guajava in Psidium cattleianum for the Management of the Meloidogyne enterolobii. Int. J. Fruit Sci. 2020, 20, 106–116. [Google Scholar] [CrossRef]
- Fazio, G. Genetics, breeding, and genomics of apple rootstocks. In The Apple Genome, Compendium of Plant Genomes; Korban, S.S., Ed.; Springer: Cham, Switzerland, 2021; pp. 105–130. [Google Scholar]
- Martins, V.; Silva, V.; Pereira, S.; Afonso, S.; Oliveira, I.; Santos, M.; Ribeiro, C.; Vilela, A.; Bacelar, E.; Silva, A.P.; et al. Rootstock affects the fruit quality of ‘Early Bigi’ sweet cherries. Foods 2021, 10, 2317. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, T.A. History of Plant Tissue Culture. In Methods in Molecular Biology, 2nd ed.; Loyola-Vargas, V.M., Vázquez-Flota, F., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2006; Volume 318, pp. 9–32. [Google Scholar]
- Doorenbos, J. Rejuvenation of Hedera helix in graft combinations. Koninklijke Nederlandse Akademie van Wetenschappen: Series C. Biol. Med. Sci. 1953, 57, 99–102. [Google Scholar]
- Holmes, F.O. Elimination of spermivirus from the nightingale chrysanthemum. Phytopathology 1956, 46, 599–600. [Google Scholar]
- Murashige, T.; Bitters, W.P.; Rangan, E.M.; Nauer, E.M.; Roistacher, C.N.; Holliday, P.B. A technique of shoot apex grafting and its utilization towards recovering virus-free citrus clones. HortScience 1972, 7, 118–119. [Google Scholar] [CrossRef]
- Navarro, L.; Roistacher, C.N.; Murashige, T. Improvement of shoot-tip grafting in vitro for virus-free citrus. J. Am. Soc. Hortic. Sci. 1975, 100, 471–479. [Google Scholar]
- Pathirana, R.; McKenzie, M.J. Early detection of grapevine leafroll virus in Vitis vinifera using in vitro micrografting. Plant Cell Tiss. Organ Cult. 2005, 81, 11–18. [Google Scholar] [CrossRef]
- Cui, Z.-H.; Agüero, C.B.; Wang, Q.-C.; Walker, M.A. Validation of micrografting to identify incompatible interactions of rootstocks with virus-infected scions of Cabernet Franc. Aust. J. Grape Wine Res. 2019, 25, 268–275. [Google Scholar] [CrossRef]
- Hao, X.-Y.; Jiao, B.-L.; Wang, M.-R.; Wang, Y.-L.; Shang, B.-X.; Wang, J.-Y.; Wang, Q.-C.; Xu, Y. In vitro biological indexing of grapevine leafroll-associated virus 3 in red- and white-berried grapevines (Vitis vinifera). Aust. J. Grape Wine Res. 2021, 27, 483–490. [Google Scholar] [CrossRef]
- Chilukamarri, L.; Ashrafzadeh, S.; Leung, D.W.M. In Vitro grafting—Current applications and future prospects. Sci. Hortic. 2021, 280, 109899. [Google Scholar] [CrossRef]
- Gulati, A.; Schryer, P.; McHughen, A. Regeneration and micrografting of lentil shoots. In Vitro Cell. Dev. Biol.-Plant 2001, 37, 798–802. [Google Scholar] [CrossRef]
- Onay, A.; Pirinç, V.; Yıldırım, H.; Basaran, D. In vitro Micrografting of Mature Pistachio (Pistacia vera var. Siirt). Plant Cell Tiss. Organ Cult. 2004, 77, 215–219. [Google Scholar] [CrossRef]
- Işıkalan, Ç.; Namli, S.; Akbas, F.; Ak, B.E. Micrografting of almond (Amygdalus communis) cultivar ‘Nonpareil’. Aust. J. Crop. Sci. 2011, 5, 61–65. [Google Scholar]
- Padilla, I.M.G.; Encina, C.L. The use of consecutive micrografting improves micropropagation of cherimoya (Annona cherimola Mill.) cultivars. Sci. Hortic. 2011, 129, 167–169. [Google Scholar] [CrossRef]
- Luo, J.; Gould, J.H. In Vitro shoot-tip grafting improves recovery of cotton plants from culture. Plant Cell Tiss. Organ Cult. 1999, 57, 211–213. [Google Scholar] [CrossRef]
- Bourrain, L.; Charlot, G. In vitro micrografting of cherry (Prunus avium L.‘Regina’) onto ‘Piku® 1’rootstock [P. avium × (P. canescens × P. tomentosa)]. J. Hortic. Sci. Biotechnol. 2014, 89, 47–52. [Google Scholar] [CrossRef]
- Koufan, M.; Mazri, M.A.; Essatte, A.; Moussafir, S.; Belkoura, I.; El Rhaffari, L.; Toufik, I. A novel regeneration system through micrografting for Argania spinosa (L.) Skeels, and confirmation of successful rootstock-scion union by histological analysis. Plant Cell Tiss. Organ Cult. 2020, 142, 369–378. [Google Scholar] [CrossRef]
- Errea, P.; Garay, L.; Marín, J.A. Early detection of graft incompatibility in apricot (Prunus armeniaca) using in vitro techniques. Physiol. Plant 2001, 112, 135–141. [Google Scholar] [CrossRef]
- Dumanoğlu, H.; Çelik, A.; Büyükkartal, H.N.; Dousti, S. Morphological and anatomical investigations on in vitro micrografts of OHxF 333 / Pyrus elaeagrifolia interstock / rootstock combination in pears. J. Agric. Sci. 2014, 20, 269–279. [Google Scholar]
- Bao, W.W.; Zhang, X.C.; Zhang, A.L.; Zhao, L.; Wang, Q.C.; Liu, Z.D. Validation of micrografting to analyze compatibility, shoot growth, and root formation in micrografts of kiwifruit (Actinidia spp.). Plant Cell Tiss. Organ Cult. 2020, 140, 209–214. [Google Scholar] [CrossRef]
- Hieu, T.; Phong, T.H.; Khai, H.D.; Mai, N.T.N.; Cuong, D.M.; Luan, V.Q.; Tung, H.T.; Nam, N.B.; Nhut, D.T. Efficient production of vigorous passion fruit rootstock for in vitro grafting. Plant Cell Tiss. Organ Cult. 2022, 148, 635–648. [Google Scholar] [CrossRef]
- Fragoso, V.; Goddard, H.; Baldwin, I.T.; Kim, S.-G. A simple and efficient micrografting method for stably transformed Nicotiana attenuata plants to examine shoot-root signaling. Plant Meth. 2011, 7, 1–8. [Google Scholar] [CrossRef]
- Paultre, D.S.G.; Gustin, M.P.; Molnar, A.; Oparka, K.J. Lost in transit: Long-distance trafficking and phloem unloading of protein signals in Arabidopsis homografts. Plant Cell 2016, 28, 2016–2025. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, Y.; Wang, S.; Zhang, W.; Wang, S.; Xu, C.; Yu, Y.; Li, T.; Jiang, F.; Li, W. A constitutive and drought-responsive mRNA undergoes long-distance transport in pear (Pyrus betulaefolia) phloem. Plant Sci. 2020, 293, 110419. [Google Scholar] [CrossRef]
- Tsutsui, H.; Yanagisawa, N.; Kawakatsu, Y.; Ikematsu, S.; Sawai, Y.; Tabata, R.; Arata, H.; Higashiyama, T.; Notaguchi, M. Micrografting device for testing systemic signaling in Arabidopsis. Plant J. 2020, 103, 918–929. [Google Scholar] [CrossRef]
- Thimmappaiah; Shirly, R.A.; Sadhana, P.H. In vitro propagation of cashew from young trees. In Vitro Cell. Dev. Biol. -Plant 2002, 38, 152–156. [Google Scholar] [CrossRef]
- Perez-Tornero, O.; Burgos, L. Different media requirements for micropropagation of apricot cultivars. Plant Cell Tiss. Organ Cult. 2000, 63, 133–141. [Google Scholar] [CrossRef]
- Yıldırım, H.; Onay, A.; Süzerer, V.; Tilkat, E.; Ozden-Tokatli, Y.; Akdemir, H. Micrografting of almond (Prunus dulcis Mill.) cultivars “Ferragnes” and “Ferraduel”. Sci. Hortic. 2010, 125, 361–367. [Google Scholar] [CrossRef]
- Aazami, M.A.; Hassanpouraghdam, M.B. In Vitro Micro-Grafting of Some Iranian Grapevine Cultivars. Rom. Biotech. Lett. 2010, 15, 5576–5580. [Google Scholar]
- Ashrafzadeh, S. In Vitro grafting–twenty-first century’s technique for fruit tree propagation. Acta Agric. Scand. B Soil Plant Sci. 2020, 74, 584–587. [Google Scholar] [CrossRef]
- Dobránszki, J.; Jámbor-Benczúr, E.; Hudák, I.; Magyar-Tábori, K. Model experiments for establishment of in vitro culture by micrografting in apple. Int. J. Hortic. Sci. 2005, 11, 47–49. [Google Scholar] [CrossRef]
- Raharjo, S.H.T.; Litz, R.E. Micrografting and ex vitro grafting for somatic embryo rescue and plant recovery in avocado (Persea americana). Plant Cell Tiss. Organ Cult. 2005, 82, 1–9. [Google Scholar] [CrossRef]
- Miguelez-Sierra, Y.; Hernández-Rodríguez, A.; Acebo-Guerrero, Y.; Baucher, M.; El Jaziri, M. In vitro micrografting of apical and axillary buds of cacao. J. Hort. Sci. Biotech. 2017, 92, 25–30. [Google Scholar] [CrossRef]
- Thimmappaiah; Puthra, G.T.; Anil, S.R. In vitro grafting of cashew (Anacardium occidentale). Sci. Hortic. 2002, 92, 177–182. [Google Scholar] [CrossRef]
- Ali, H.M.A.; Elamin, O.M.; Ali, M.A. Propagation of grapefruit (Citrus paradisi macf) by shoot tip micrografting. Gezira J. Agric. 2004, 2, 37–49. [Google Scholar]
- Singh, P.; Patel, R.M. Factors affecting in vitro degree of browning and culture establishment of pomegranate. Afr. J. Plant Sci. 2016, 10, 43–49. [Google Scholar] [CrossRef]
- Danthu, P.; Touré, M.; Soloviev, P.; Sagna, P. Vegetative propagation of Ziziphus mauritiana var. Gola by micrografting and its potential for dissemination in the Sahelian Zone. Agrofor. Syst. 2004, 60, 247–253. [Google Scholar] [CrossRef]
- Ma, F.; Guo, C.; Liu, Y.; Li, M.; Ma, T.; Mei, L.; Hsiao, A.I. In vitro shoot-apex grafting of mulberry (Morus alba L.). Hortscience 1996, 31, 460–462. [Google Scholar] [CrossRef]
- Nas, M.N.; Read, P.E. Simultaneous micrografting, rooting and acclimatization of micropropagated American chestnut, grapevine and hybrid hazelnut. Eur. J. Hortic. Sci. 2003, 68, 234–237. [Google Scholar]
- Farahani, F.; Razeghi, S.; Peyvandi, M.; Attaii, S.; Mazinani, M.H. Micrografting and micropropagation of olive (Olea europea L.) Iranian cultivar: Zard. Afri. J. Plant Sci. 2011, 5, 671–675. [Google Scholar]
- Edriss, M.H.; Baghdady, G.A.; Abdrabboh, G.A.; Abd El-Razik, A.M.; Abdel Aziz, H.F. Micro-grafting of Florida prince peach cultivar. Nat. Sci. 2015, 13, 54–60. [Google Scholar]
- Hassanen, S.A. In vitro grafting of pear (Pyrus spp.). World Appl. Sci. J. 2013, 24, 705–709. [Google Scholar] [CrossRef]
- Vozárová, Z.; Nagyová, A.; Nováková, S. In vitro micrografting of different Prunus species by cherry-adapted Plum pox virus isolate. Acta Virol. 2018, 62, 109–111. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Chen, Q.; Tian, J. Studies on factors affecting the microshoot grafting survival of walnut. Acta Hortic. 2010, 861, 327–332. [Google Scholar] [CrossRef]
- Ribeiro, H.; Ribeiro, A.; Pires, R.; Cruz, J.; Cardoso, H.; Barrroso, J.M.; Peixe, A. Ex vitro rooting and simultaneous micrografting of the walnut hybrid rootstock ‘Paradox’ (Juglans hindsi × Juglans regia) cl. ‘Vlach’. Agronomy 2022, 12, 595. [Google Scholar] [CrossRef]
- Zhang, N.; Zeng, H.X.; Shi, X.F.; Yang, Y.X.; Cheng, W.S.; Ren, J.; Li, Y.H.; Li, A.C.; Tang, M.; Sun, Y.H.; et al. An efficient in vitro micrografting technology of watermelon. Acta Hortic. 2015, 1086, 65–70. [Google Scholar] [CrossRef]
- Edriss, M.H.; Burger, D.W. Micro-grafting shoot-tip culture of citrus on three trifoliolate rootstocks. Sci. Hort. 1984, 23, 255–259. [Google Scholar] [CrossRef]
- Deogratias, J.M.; Castellani, V.; Dosba, F.; Juarez, J.; Arregui, J.M.; Ortega, C.; Ortega, V.; Llacer, G.; Navarro, L. Study of growth parameters on apricot shoot-tip grafting in vitro (STG). Acta Hortic. 1991, 293, 363–372. [Google Scholar] [CrossRef]
- Estrada-Luna, A.A.; López-Peralta, C.; Cárdenas-Soriano, E. In vitro micrografting and the histology of graft union formation of selected species of prickly pear cactus (Opuntia spp.). Sci. Hortic. 2002, 92, 317–327. [Google Scholar] [CrossRef]
- Wu, H.C.; du Toit, E.S.; Reinhardt, C.F. Micrografting of Protea cynaroides. Plant Cell Tiss. Organ Cult. 2007, 89, 23–28. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Gulyás, A.; Magyar-Tábori, K.; Wang, M.-R.; Wang, Q.-C.; Dobránszki, J. In vitro tissue culture of apple and other Malus species: Recent advances and applications. Planta 2019, 249, 975–1006. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Saxena, P.K. Inhibition of phenylpropanoid biosynthesis in Artemisia annua L.: A novel approach to reduce oxidative browning in plant tissue culture. PLoS ONE 2013, 8, e76802. [Google Scholar] [CrossRef]
- Jonard, R.; Hugard, J.; Macheix, J.J.; Martinez, J.; Mosella-Chancel, L.; Poessel, J.L.; Villemur, P. In Vitro micrografting and its applications to fruit science. Sci. Hortic-Amst. 1983, 20, 147–159. [Google Scholar] [CrossRef]
- Davoudi Pahnekolayi, M.; Tehranifar, A.; Samiei, L.; Shoor, M. Optimizing culture medium ingredients and micrografting devices can promote in vitro micrografting of cut roses on different rootstocks. Plant Cell Tiss. Organ Cult. 2019, 137, 265–274. [Google Scholar] [CrossRef]
- Naddaf, M.E.; Rabiei, G.; Moghadam, E.G.; Mohammadkhani, A. In vitro production of PPV-free Sweet cherry (Prunus avium cv. Siahe-Mashhad) by meristem culture and micro-grafting. J. Plant Bioinform. Biotechnol. 2021, 1, 51–59. [Google Scholar] [CrossRef]
- Nunes, J.C.O.; Abreu, M.F.; Dantas, A.C.M.; Pereira, A.J.; Pedrotti, E.L. Caracterização morfológica de microenxertia em macieira. Rev. Bras. Frutic. 2005, 27, 80–83. [Google Scholar] [CrossRef]
- Navarro, L. Application of shoot-tip grafting in vitro to woody species. Acta Hortic. 1988, 227, 43–55. [Google Scholar] [CrossRef]
- Asadi Zargh Abad, M.; Shekafandeh, A. In vitro grafting of ‘Sahand’ cultivar on two wild almond rootstocks and evaluation of its some physiological and biochemical traits vis-a-vis different rootstocks. Plant Cell Tiss. Organ Cult. 2021, 145, 507–516. [Google Scholar] [CrossRef]
- Juárez, J.; Aleza, P.; Navarro, L. Applications of citrus shoot-tip grafting in vitro. Acta Hort. 2015, 1065, 635–642. [Google Scholar] [CrossRef]
- Channuntapipat, C.; Sedgley, M.; Collins, G. Micropropagation of almond cultivars Nonpareil and Ne Plus Ultra and the hybrid rootstock Titan x Nemaguard. Sci. Hortic. 2003, 98, 473–484. [Google Scholar] [CrossRef]
- Obeidy, A.A.; Smith, M.A.L. A versatile new tactic for fruit tree micrografting. Hortic. Sci. 1991, 26, 776. [Google Scholar] [CrossRef]
- Nkanaunena, G.A.; Kwapata, M.B.; Bokosi, J.M.; Maliro, M.F.A. The effect of age and type of rootstock, method of scion placement and light intensity levels on the performance of Uapaca kirkiana (Muell. Arg) micrografts cultured in vitro. UNISWA J. Agric. 2001, 10, 22–29. [Google Scholar] [CrossRef]
- Sammona, O.S.; Abde Elhamid, N.A.; Samaan, M.S.F. Effects of some factors on the micropropagation and micrografting of some grape rootstocks in vitro. J. Agric. Sci. 2018, 26, 133–146. [Google Scholar] [CrossRef]
- Mneney, E.E.; Mantell, S.H. In Vitro micrografting of cashew. Plant Cell Tiss. Organ Cult. 2001, 66, 49–58. [Google Scholar] [CrossRef]
- Kumar, R.A.J.; Kaul, M.K.; Saxena, S.N.; Singh, A.K.; Khadda, B.S. Standardization of protocol for in Vitro shoot tip grafting in Kinnow mandarin (Citrus deliciosa). Indian J. Agric. Sci. 2014, 84, 1376–1381. [Google Scholar]
- Chabukswar, M.M.; Deodhar, M.A. Restoration of rooting competence in a mature plant of Garcinia indica through serial shoot tip grafting in vitro. Sci. Hortic. 2006, 108, 194–199. [Google Scholar] [CrossRef]
- Badalamenti, O.; Carra, A.; Oddo, E.; Carimi, F.; Sajeva, M. Is in vitro micrografting a possible valid alternative to traditional micropropagation in Cactaceae? Pelecyphora aselliformis as a case study. SpringerPlus 2016, 5, 1–4. [Google Scholar] [CrossRef]
- Jenderek, M.M.; Holman, G.E.; Irish, B.M.; Souza Vidigal, F. In Vitro Seed Germination and Rootstock Establishing for Micrografting of Theobroma cacao L. In Proceedings of the ASHS 2015 Annual Conference, New Orleans, LA, USA, 4–7 August 2015. [Google Scholar]
- Torres-Viñals, M.; Sabaté-Casaseca, S.; Aktouche, N.; Grenan, S.; Lopez, G.; Porta-Falguera, M.; Torregrosa, L. Large-scale production of somatic embryos as a source of hypocotyl explants for Vitis vinifera micrografting. Vitis 2004, 43, 163–168. [Google Scholar] [CrossRef]
- Mhatre, M.; Bapat, V.A. Micrografting in grapevine (Vitis spp.). In Protocols for Micropropagation of Woody Trees and Fruits; Jain, S.M., Häggman, H., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 249–258. [Google Scholar]
- Liu, X.; Liu, M.; Ning, Q.; Liu, G. Reverse-cleft in vitro micrografting of Ziziphus jujuba Mill. Infected with jujube witches’ broom (JWB). Plant Cell Tiss. Organ Cult. 2012, 108, 339–344. [Google Scholar] [CrossRef]
- Zhang, Z.; Finer, J.J. Sunflower (Helianthus annuus L.) organogenesis from primary leaves of young seedlings preconditioned by cytokinin. Plant Cell Tiss. Organ Cult. 2015, 123, 645–655. [Google Scholar] [CrossRef]
- Zhang, Z.; Finer, J.J. Use of cytokinin pulse treatments and micrografting to improve sunflower (Helianthus annuus L.) plant recovery from cotyledonary tissues of mature seeds. In Vitro Cell. Dev. Biol. Plant 2016, 52, 391–399. [Google Scholar] [CrossRef]
- Gebhardt, K.; Goldbach, H. Establishment, graft union characteristics and growth of Prunus micrografts. Physiol. Plant. 1988, 72, 153–159. [Google Scholar] [CrossRef]
- Hao, X.-Y.; BI, W.-L.; Cui, Z.-H.; Pan, C.; Xu, Y.; Wang, Q.-C. Development, histological observations and Grapevine leafroll-associated virus-3 localisation in in vitro grapevine micrografts. Ann. Appl. Biol. 2017, 170, 379–390. [Google Scholar] [CrossRef]
- Saleh, S.S.; AbdAllah, M.N.; El Botaty, E.S.M. Using new micro grafting techniques in grape. J. Plant Prod. 2019, 10, 1015–1022. [Google Scholar] [CrossRef]
- Dobránszky, J.; Magyar-Tábori, K.; Jámbor-Benczúr, E.; Lazányi, J. New in vitro micrografting method for apple by sticking. Int. J. Hort. Sci. 2000, 6, 79–83. [Google Scholar] [CrossRef]
- Ponsonby, D.J.; Mantell, S.H. In vitro establishment of Picea pungens f. glauca and P. sitchensis seedling rootstocks with an assessment of their suitabilities for micrografting with scions of various Picea species. J. Hortic. Sci. 1993, 68, 463–475. [Google Scholar] [CrossRef]
- Cortizo, M.; Afonso, P.; Fernández, B.; Rodríguez, A.; Centeno, M.; Ordás, R. Micrografting of mature stone pine (Pinus pinea L.) trees. Ann. For. Sci. 2004, 61, 843–845. [Google Scholar] [CrossRef]
- Sertkaya, G. Effects of different rootstocks in micrografting on growing of Washington Navel orange plants obtained by shoot tip grafting. Biotechnol. Biotechnol. Equip. 2004, 18, 82–88. [Google Scholar] [CrossRef]
- Volk, G.M.; Jenderek, M.M.; Walters, C.; Bonnart, R.; Shepherd, A.; Skogerboe, D.; Hall, B.D.; Moreland, B.; Krueger, R.; Polek, M. Implementation of Citrus shoot tip cryopreservation in the USDA-ARS National Plant Germplasm System. Acta Hortic. 2019, 1234, 329–334. [Google Scholar] [CrossRef]
- Suárez, I.E.; Álvarez, C.; Díaz, C.L. Micrografting of Valencia orange and Tahiti lime. Temas Agrar. 2021, 26, 26–35. [Google Scholar] [CrossRef]
- Lahoty, P.; Singh, J.; Bhatnagar, P.; Raipurohit, D.; Singh, B. In vitro multiplication of Nagpur mandarin (Citrus reticulata Blanco) through STG. Vegetos 2013, 26, 318–324. [Google Scholar] [CrossRef]
- Bhatt, K.M.; Banday, F.A.; Mir, M.A.; Rather, Z.A.; Hussain, G. In vitro grafting in apple (Malus domestica Borkh) cv. Lal Ambri. Karnataka J. Agric. Sci. 2013, 26, 399–402. [Google Scholar]
- Prasaei, Z.; Hedayat, M.; Rastgoo, S.; Bayat, F. Evaluation rootstock, sucrose concentration and culture medium supporting systems in the micrografting and acclimatization of lime (Citrus aurantifulia). Agric. Biotechnol. J. 2018, 10, 1–17. [Google Scholar]
- Zhu, B.; Cao, H.-N.; Zong, C.-W.; Piao, R.-Z.; Chen, L.; Zhou, L. Micrografting technology in grapevine (Vitis vinifera L.). Fruit Veg. Cereal Sci. Biotechnol. 2007, 1, 60–63. [Google Scholar]
- Yıldırım, H.; Akdemir, H.; Süzerer, V.; Ozden, Y.; Onay, A. In vitro micrografting of the almond cultivars “Texas”, “Ferrastar” and “Nonpareil”. Biotechnol. Biotechnol. Equip. 2013, 27, 3493–3501. [Google Scholar] [CrossRef]
- Kobayashi, A.K.; Bespalhok, J.C.; Pereira, L.F.P.; Vieira, L.G.E. Plant regeneration of sweet orange (Citrus sinensis) from thin section of mature stem segments. Plant Cell Tiss. Organ Cult. 2003, 74, 99–102. [Google Scholar] [CrossRef]
- Chand, L.; Sharma, S.; Kajla, S. Effect of rootstock and age of seedling on success of in vitro shoot tip grafting in Suárez. Indian J. Hort. 2016, 73, 8–12. [Google Scholar] [CrossRef]
- Benson, E.E. In vitro plant recalcitrance in vitro plant recalcitrance: An introduction. In Vitro Cell. Dev. Biol.-Plant 2000, 36, 141–148. [Google Scholar] [CrossRef]
- Naz, A.A.; Jaskani, M.J.; Abbas, H.; Qasim, M. In vitro studies on micrografting technique in two cultivars of citrus to produce virus free plants. Pak. J. Bot. 2007, 39, 1773–1778. [Google Scholar]
- Singh, A.D.; Meetei, N.T.; Kundu, S.; Salma, U.; Mandal, N. In vitro micrografting using three diverse indigenous rootstocks for the production of Citrus tristeza virus-free plants of Khasi mandarin. In Vitro Cell. Dev. Biol.-Plant 2019, 55, 180–189. [Google Scholar] [CrossRef]
- Conejero, A.; Romero, C.; Cunill, M.; Mestre, M.A.; Martínez-Calvo, J.; Badenes, M.L.; Llácer, G. In vitro shoot-tip grafting for safe Prunus budwood exchange. Sci. Hortic. 2013, 150, 265–370. [Google Scholar] [CrossRef]
- Revilla, M.A.; Pacheco, J.; Casares, A.; Rodríguez, R. In vitro reinvigoration of mature olive trees (Olea europaea L.) through micrografting. In Vitro Cell. Dev. Biol. Plant 1996, 32, 257–261. [Google Scholar] [CrossRef]
- Gao, X.; Yang, D.; Cao, D.; Ao, M.; Sui, X.; Wang, Q.; Kimatu, J.N.; Wang, L. In vitro micropropagation of Freesia hybrid and the assessment of genetic and epigenetic stability in regenerated plantlets. J. Plant Growth Regul. 2010, 29, 257–267. [Google Scholar] [CrossRef]
- Sales, E.K.; Butardo, N.G. Molecular analysis of somaclonal variation in tissue culture derived bananas using MSAP and SSR markers. Int. J. Biol. Vet. Agric. Food Eng. 2014, 8, 572–579. [Google Scholar] [CrossRef]
- Krishna, H.; Alizadeh, M.; Singh, D.; Singh, U.; Chauhan, N.; Eftekhari, M.; Sadah, R.K. Somaclonal variation and their applications in horticultural crops improvement. 3 Biotech 2016, 6, 54. [Google Scholar] [CrossRef]
- Souza, J.A.; Bettoni, J.C.; Dalla Costa, M.; Baldissera, T.C.; dos Passos, J.M.; Primieri, S. In vitro rooting and acclimatization of ‘Marubakaido’ apple rootstock using indole-3-acetic acid from rhizobacteria. Commun. Plant Sci. 2022, 12, 16–23. [Google Scholar] [CrossRef]
- Amghar, I.; Ibriz, M.; Ibrahimi, M.; Boudra, A.; Gaboun, F.; Meziani, R.; Iraqi, D.; Mazri, M.A.; Diria, G.; Abdelwahd, R. In vitro root induction from Argan (Argania spinosa (L.) Skeels) adventitious shoots: Influence of ammonium nitrate, auxins, silver nitrate and putrescine, and evaluation of plantlet acclimatization. Plants 2021, 10, 1062. [Google Scholar] [CrossRef]
- Hussain, G.; Wani, M.S.; Mir, M.A.; Rather, Z.A.; Bhat, K.M. Micrografting for fruit crop improvement. Afr. J. Biotechnol. 2014, 13, 2474–2483. [Google Scholar] [CrossRef][Green Version]
- Vidoy-Mercado, I.; Narváez, I.; Palomo-Ríos, E.; Litz, R.E.; Barceló-Muñoz, A.; Pliego-Alfaro, F. Reinvigoration/rejuvenation induced through micrografting of tree species: Signaling through graft union. Plants 2021, 10, 1197. [Google Scholar] [CrossRef] [PubMed]
- Dolcet-Sanjuan, R.; Claveria, E.; Gruselle, R.; Meier-Dinkel, A.; Jay-Allemand, C.; Gaspar, T. Practical factors controlling in vitro adventitious root formation from walnut shoot microcuttings. J. Am. Soc. Hort. Sci. 2004, 129, 198–203. [Google Scholar] [CrossRef]
- Hackett, W.P. Juvenility, maturation, and rejuvenation in woody plants. Hortic. Rev. 1985, 7, 109–155. [Google Scholar] [CrossRef]
- Chakraborty, T.; Chaitanya, K.V.; Akhtar, N. Analysis of regeneration protocols for micropropagation of Pterocarpus santalinus. Plant Biotechnol. Rep. 2022, 16, 1–15. [Google Scholar] [CrossRef]
- Abousalim, A.; Mantell, S.H. Micrografting of pistachio (Pistacia vera L. cv. Mateur). Plant Cell Tiss. Organ Cult. 1992, 29, 231–234. [Google Scholar] [CrossRef]
- Huang, L.-C.; Hsiao, C.-K.; Lee, S.-H.; Huang, B.-L.; Murashige, T. Restoration of vigor and rooting competence in stem tissues of mature citrus by repeated grafting of their shoot apices onto freshly germinated seedlings in vitro. In Vitro Cell. Dev. Biol. Plant 1992, 28, 30–32. [Google Scholar] [CrossRef]
- Raharjo, S.H.T.; Witjaksono, N.F.N.; Gomez-Lim, M.A.; Padilla, G.; Litz, R.E. Recovery of avocado (Persea americana Mill.) plants transformed with the antifungal plant defensin gene PDF1.2. In Vitro Cell. Dev. Biol.-Plant 2008, 44, 254–262. [Google Scholar] [CrossRef]
- Duclercq, J.; Sangwan-Norreel, B.; Catterou, M.; Sangwan, R.S. De novo shoot organogenesis: From art to science. Trends Plant Sci. 2011, 16, 597–606. [Google Scholar] [CrossRef]
- Tiwari, V.; Chaturvedi, A.K.; Mishra, A.; Jha, B. An efficient method of Agrobacterium-mediated genetic transformation and regeneration in local Indian cultivar of groundnut (Arachis hypogaea) using grafting. Appl. Biochem. Biotechnol. 2015, 175, 436–453. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Zhao, Y.; Yang, Y.; Liu, Z. An effective method for Agrobacterium tumefaciens-mediated transformation of Jatropha curcas L. using cotyledon explants. Bioengineered 2020, 11, 1146–1158. [Google Scholar] [CrossRef]
- De Pasquale, F.; Giuffrida, S.; Carimi, F. Minigrafting of shoots, roots, inverted roots, and somatic embryos for rescue of in vitro Citrus regenerants. J. Am. Soc. Hort. Sci. 1999, 124, 152–157. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Curtolo, M.; Latado, R.R.; Martinelli, A.P. Somatic embryogenesis of a seedless sweet orange (Citrus sinensis (L.) Osbeck). In Vitro Cell. Dev. Biol.-Plant 2017, 53, 619–623. [Google Scholar] [CrossRef]
- Almeida, W.A.B.; Mourão Filho, F.A.A.; Pino, L.E.; Boscariol, R.L.; Rodriguez, A.P.M.; Mendes, B.M.J. Genetic transformation and plant recovery from mature tissues of Citrus sinensis L. Osbeck. Plant Sci. 2003, 164, 203–211. [Google Scholar] [CrossRef]
- Balázs, E.; Bukovinszki, Á.; Csányi, M.; Csilléry, G.; Divéki, Z.; Nagy, I.; Mitykó, J.; Salánki, K.; Mihálka, V. Evaluation of a wide range of pepper genotypes for regeneration and transformation with an Agrobacterium tumefaciens shooter strain. S. Afr. J. Bot. 2008, 74, 720–725. [Google Scholar] [CrossRef]
- Das, A.; Kumar, S.; Nandeesha, P.; Yadav, I.S.; Saini, J.; Chaturvedi, S.K.; Datta, S. An efficient in vitro regeneration system of fieldpea (Pisum sativum L.) via shoot organogenesis. J. Plant Biochem. Biotechnol. 2014, 23, 184–189. [Google Scholar] [CrossRef]
- Roy, P.K.; Lodha, M.L.; Mehta, S.L. In vitro regeneration from internodal explants and somaclonal variation in chickpea (Cicer arietinum L). J. Plant Biochem. Biotechnol. 2001, 10, 107–112. [Google Scholar] [CrossRef]
- Nakajima, I.; Ito, A.; Moriya, S.; Saito, T.; Moriguchi, T.; Yamamoto, T. Adventitious shoot regeneration in cotyledons from Japanese pear and related species. In Vitro Cell. Dev. Biol.-Plant 2012, 48, 396–402. [Google Scholar] [CrossRef]
- Aguilar, M.E.; Villalobos, V.M.; Vasquez, N. Production of cocoa plants (Theobroma cacao L.) via micrografting of somatic embryos. In Vitro Cell. Dev. Biol.-Plant 1992, 28, 15–19. [Google Scholar] [CrossRef]
- Palomo-Ríos, E.; Cerezo, S.; Mercado, J.A.; Pliego-Alfaro, F. Agrobacterium-mediated transformation of avocado (Persea americana Mill.) somatic embryos with fluorescent marker genes and optimization of transgenic plant recovery. Plant Cell Tissue Organ Cult. 2017, 128, 447–455. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell Tissue Organ Cult. 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Wang, M.-R.; Lambardi, M.; Engelmann, F.; Pathirana, R.; Panis, B.; Volk, G.M.; Wang, Q.-C. Advances in cryopreservation of in vitro-derived propagules: Technologies and explant sources. Plant Cell Tissue Organ Cult. 2021, 144, 7–20. [Google Scholar] [CrossRef]
- Panis, B. Sixty years of plant cryopreservation: From freezing hardy mulberry twigs to establishing reference crop collections for future generations. Acta Hortic. 2019, 1234, 1–7. [Google Scholar] [CrossRef]
- Volk, G.M.; Bonnart, R.; Krueger, R.; Lee, R. Cryopreservation of citrus shoot tips using micrografting for recovery. Cryoletters 2012, 33, 418–426. [Google Scholar] [PubMed]
- Volk, G.M.; Bonnart, R.; Shepherd, A.; Yin, Z.; Lee, R.; Polek, M.; Krueger, R. Citrus cryopreservation: Viability of diverse taxa and histological observations. Plant Cell Tissue Organ Cult. 2017, 128, 327–334. [Google Scholar] [CrossRef]
- Yi, J.-Y.; Balaraju, K.; Baek, H.-J.; Yoon, M.-S.; Kim, H.-H.; Lee, Y.-Y. Cryopreservation of Citrus limon (L.) Burm. F Shoot Tips. Using a Droplet-vitrification Method. Korean J. Plant Res. 2018, 31, 684–694. [Google Scholar]
- Grigoriadis, I.; Nianiou-Obeidat, I.; Tsaftaris, A.S. Shoot regeneration and micrografting of micropropagated hybrid tomatoes. J. Hortic. Sci. Biotechnol. 2005, 80, 183–186. [Google Scholar] [CrossRef]
- Wang, R.R.; Mou, H.Q.; Gao, X.X.; Chen, L.; Li, C.; Li, M.F.; Wang, Q.C. Cryopreservation for eradication of Jujube witches’ broom phytoplasma from Chinese jujube (Ziziphus jujuba). Ann. Appl. Biol. 2015, 166, 218–228. [Google Scholar] [CrossRef]
- Akdemir, H.; Onay, A. Biotechnological Approaches for Conservation of the Genus Pistacia. In Sustainable Development and Biodiversity-Biodiversity and Conservation of Woody Plants; Ahuja, M.R., Mohan Jain, S., Eds.; Springer Nature: Cham, Switzerland, 2017; Volume 17, pp. 221–244. [Google Scholar]
- O’Brien, C.; Hiti-Bandaralage, J.; Folgado, R.; Hayward, A.; Lahmeyer, S.; Folsom, J.; Mitter, N. Cryopreservation of woody crops: The avocado case. Plants 2021, 10, 934. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.-R.; Bettoni, J.C.; Zhang, A.-L.; Lu, X.; Zhang, D.; Wang, Q.-C. In Vitro Micrografting of Horticultural Plants: Method Development and the Use for Micropropagation. Horticulturae 2022, 8, 576. https://doi.org/10.3390/horticulturae8070576
Wang M-R, Bettoni JC, Zhang A-L, Lu X, Zhang D, Wang Q-C. In Vitro Micrografting of Horticultural Plants: Method Development and the Use for Micropropagation. Horticulturae. 2022; 8(7):576. https://doi.org/10.3390/horticulturae8070576
Chicago/Turabian StyleWang, Min-Rui, Jean Carlos Bettoni, A-Ling Zhang, Xian Lu, Dong Zhang, and Qiao-Chun Wang. 2022. "In Vitro Micrografting of Horticultural Plants: Method Development and the Use for Micropropagation" Horticulturae 8, no. 7: 576. https://doi.org/10.3390/horticulturae8070576
APA StyleWang, M.-R., Bettoni, J. C., Zhang, A.-L., Lu, X., Zhang, D., & Wang, Q.-C. (2022). In Vitro Micrografting of Horticultural Plants: Method Development and the Use for Micropropagation. Horticulturae, 8(7), 576. https://doi.org/10.3390/horticulturae8070576









