Abstract
The use of synthetic fungicides to control fungal diseases has growing limitations due to eco-toxicological risks. Therefore, it is necessary to replace or integrate high risk chemicals with safer tools for human health and environment. Consequently, research on the selection, evaluation, characterization, and use of biocontrol agents (BCAs) has consistently increased in the last decades. BCA formulates, particularly in some countries, are still scarce in coping with the growing demand for their use in sustainable agricultural management. To foster development and utilization of new effective bioformulates, there is a need to optimize BCA activity, to share knowledge on their formulation processes and to simplify the registration procedures. Studies based on new molecular tools can significantly contribute to achieve such objectives. The present review provides the state of the art on biocontrol of fungal plant diseases with special emphasis on (i) features of the most studied BCAs; (ii) key strategies to optimize selection and use of BCAs (iii); mechanisms of action of the main BCAs; (iv) molecular tools and metagenomic studies in the selection and use of BCAs; (v) main issues and constraints in the registration and commercialization of BCAs, and (vi) perspectives in the biocontrol of fungal plant diseases.
1. Introduction
Fungi are responsible for a range of plant diseases that cause consistent damage and losses of vegetal crop products worldwide, both in the field and during storage. Pathogenic fungi can produce direct deterioration of plant edible products []. In addition, species of some fungi such as Alternaria, Aspergillus, Fusarium and Penicillium spp. produce secondary metabolites, i.e., mycotoxins, which can contaminate agricultural products and/or derived foods consumed by humans and/or animals [,].
For decades, synthetic fungicides have been the main control tool against fungal plant pathogens. However, in recent years, although the chemical approach is still prevalent over other control means, the use of synthetic fungicides in plant disease management has been progressively decreasing because of mounting global concerns on risks due to residues in the environment and foods. The use of synthetic fungicides is also discouraged by (i) increasing onset of fungicide-resistant pathogen strains, (ii) demand by consumers and vegetal product retailers for very low or even “zero” chemical residues, and (iii) restrictive international regulations on permitted levels of chemical residues and on registration and eco-toxicological impact of pesticides (e.g., EU Directive 2009/128 on sustainable use of pesticides and EU Green Deal 2019 Farm to Fork Strategy).
On the other hand, the growing demand for agri-food products due to the steady increase of the world population is causing an urgent need for new effective control tools/strategies. These should be capable of integrating, or even replacing, synthetic pesticides so that high production standards and higher sustainability in agricultural production are ensured [,].
Among natural products, beneficial microorganisms (biocontrol and/or plant biostimulant microorganisms) appear to be the most promising tools to ensure plant health, as well as quality and safety of vegetal products. In the last decades, the copious research carried out (thousands of published scientific articles) has focussed on evaluating the effectiveness of many selected BCAs against various harmful fungal pathogens, and derived BCA-based bioformulates are now available on the market [,]. The main mechanisms of action of BCAs have also been elucidated, although research on this topic is still ongoing with the aim to discover/elucidate better new mechanisms and optimize biocontrol activity and formulation of microbial antagonists []. Another way to enhance BCA effectiveness and to foster their use in agriculture is their integration/alternance with other control tools, including combination of BCAs with lower doses of fungicides [,].
Regarding the mechanisms of biocontrol activity of selected BCAs, a major recent contribution to a deeper understanding attained using new molecular and omic tools (see Section 5). Studies based on these tools can provide deeper insights into the complex interactions of BCAs with host plant, pathogens and other microorganisms, and allow drawing up of the criteria for the selection and use of new BCAs. Furthermore, molecular tools are being used for the detection and monitoring of BCAs on plant surfaces and in the environment, thus providing information on the persistence of these agents over time and contributing to collection of useful data for the registration procedure (see Section 6).
Major obstacles to the development and commercialization of new BCAs are the limited number of studies and amount of shared knowledge on the formulation of microbial agents, mainly due to patent and industrial issues, and last but not least, the complexity of the registration procedures in place in some countries, particularly in Europe [].
The aim of this review is to provide a state of the art information on biocontrol of fungal plant diseases with emphasis on (i) lists and features of the most studied BCAs, (ii) key strategies to optimize selection and use of BCAs, (iii) mechanisms of action of the main BCAs and their role in biocontrol activity, (iv) molecular tools and metagenomic studies in the selection and use of BCAs, (v) issues and constraints in the registration and commercialization of BCAs, and (vi) perspectives in the biocontrol of fungal plant diseases.
2. Biocontrol Agents and Their Activity against Fungal Plant Pathogens
World trends in the management of plant diseases are shifting towards biocontrol strategies through the gradual reduction of the use of synthetic pesticides. In this scenario, new and effective microbial formulations based on bacteria, fungi and/or yeasts [,] can play a key role in the sustainable management of plant diseases.
In the following sections, we report and describe biocontrol agents (BCAs) in relation to their ability to counteract soil-borne, air-borne pathogens and/or postharvest fungal pathogens.
2.1. BCAs against Soil-Borne Pathogens
Plant diseases caused by soil-borne phytopathogenic fungi and oomycetes are notoriously difficult to control with the traditional approaches such as crop rotation, use of resistant varieties, and even chemical control. The most important soil-borne fungal pathogens include Fusarium spp. (Fusarium wilt and root rot), Verticillium dahliae (Verticillium wilt) and damping-off diseases caused by Rhizoctonia solani, Pythium spp., Phytophthora spp., Sclerotinia spp. or Sclerotium rolfsii [,]. It is well known that the incidence of some fungal diseases in a susceptible host plant is considerably mitigated in specific soil types defined as suppressive soils []. This disease-suppressive ability mainly derives from the soil resident microbial community that antagonize telluric pathogens by different ways []. The suppressive activity is affected by microbial abundance, biodiversity, and the interactions within the microbial community [,]. For many years, suppressive soils from natural and agricultural environments have represented an important source in which to select microorganisms active in the control of soil-borne pathogens [,,]. Plant root diseases can be controlled by manipulation of indigenous microbes or by introducing selected antagonists. The efficacy of fungal and bacterial species in the control of soil borne fungal pathogens has been extensively studied over the years, both in in vitro and in vivo systems. Among the fungi, Trichoderma species have proven to be effective BCAs against numerous soil-borne phytopathogenic fungi []. Trichoderma-based bioformulations are widely used in the control of fungal pathogens affecting the root system and the collar of agricultural crops. The main Trichoderma species effective in the control of soil borne fungal pathogens are T. atroviride, T. hamatum, T. harzianum and T. viride [,], and new species are described for their beneficial actions in the soil [,]. The multitude of species and their mechanisms involved allow this fungal genus to display protective activity across a broad spectrum of pathosystems [,].
Among fungi, the ones forming mycorrhizas can also reduce damage caused by soil-borne pathogens through their symbiotic interactions with the plant roots [,]. Among these symbiotic fungi, arbuscular mycorrhizas are widely studied as biocontrol agents of roots and collar diseases. Their main mechanisms of actions are indirect since they are based on the induction of resistance in the host plant [] and on improving the nutritional status of the plant []. Several fungal and plant species in almost all ecosystems can form mycorrhizae [], so mycorrhizae generally can display broad biocontrol action. However, protective ability is correlated with a multitude of other factors (host plant, mycorrhizal species, pathogens, environment conditions and soil microflora) which often limit its effectiveness [,].
The rhizobacteria as BCAs active against soil-borne fungal pathogens have been extensively studied and are generally considered to be as a subgroup of PGPRs (Plant Growth-Promoting Rhizobacteria) [,]. Bacterial spp. have been studied for over 40 years, and many of them are currently known and characterised for their ability to reduce the incidence of root system and collar diseases [,,]. The most studied species belong to Bacillus, Pseudomonas, Streptomyces and Burkholderia spp., for which efficacy and mode of action in the soil ecosystem have been clearly defined [,,,]. Recently, new interactions have been described in the rhizosphere between roots and bacteria capable of mitigating the negative action of soil-borne fungal pathogens. Interesting results were shown by Mousa et al. [], and Palmieri et al. [] describing the interaction between Gram-negative endophytes (i.e., Enterobacter sp. strain M6 and Rahnella aquatilis strain 36, respectivly) roots-hair (on finger millet and tomato plants, respectively) and pathogenic fungi of the Fusarium genus. In both model studies, the relevance of a long coevolution in this tritrophic system is hypothesized.
Among soil microorganisms, yeasts have received less attention as BCAs of soil-borne fungal pathogens than bacteria and filamentous fungi. Probably, because yeasts, contrarily to fungi and bacteria, are unable to actively move in the soil and therefore quickly colonize the roots []. Nevertheless, yeasts have been recovered from various soil types [,], in the rhizosphere [,] and as endophytes [], playing a fundamental ecological role in this particular ecological niches. However, some yeast species have been described as effective in counteracting fungal pathogens causing root and collar diseases [,]. For example, El-Tarabily described the active role of Candida valida, Rhodotorula glutinis and Trichosporon asahii in the suppression of diseases caused by Rhizoctonia solani on sugar beet [].
2.1.1. Microbial Consortia
The intrinsic characteristics of the soil system, the heterogeneity of the soil types and the differences between fungal pathogens often make the inoculative and inundative approaches based on the application of a single microorganism (either a bacterium or a fungus) ineffective [,]. This is fundamentally due to both (i) inadequate colonization of hosts by useful microorganisms, and (ii) their inefficient effect on soil-borne pathogen growth and virulence. To circumvent these problems and increase the stability and efficiency of useful microorganisms introduced in the soil and rhizosphere, solutions based on combinations of BCAs, commonly called microbial consortia, consisting of two or more microbial strains, have been developed []. Recently, the design of microbial consortia is a major trend in biotechnology applied to the management of soil-borne diseases, and microbial communities consisting of separate application of prokaryotes [,] or fungi [] or use of a mix of fungi and bacteria [,,] are already available. Besides the natural microbiome, the application of selected mixtures of microorganisms has proven to be effective in biocontrol experiments [,]. It is likely that the enhanced antagonistic activity is due to the combination of different mechanisms of action that operates within these communities. Probably, as already highlighted for other sectors, in the artificial microbial community the deriving interactions alternate the metabolic activity of the involved microorganisms with a more effective effect on target pathogens [,,]. Much research has described microbial consortia active in the control of soil-borne fungal pathogens, and this topic has already been the subject of different reviews [,,]. The microorganisms (fungi and bacteria) most frequently used in effective microbial consortia belong to the following genus: Trichoderma, Bacillus, Pseudomonas, Rhizobium, Glomus, Serratia, Rahnella, Burkholderia [,,,,].
A further novelty in the management of soil-borne fungal pathogens could derive from the manipulation of indigenous microflora through practices that increase the abundance, the population complexity and the quantity of beneficial bacterial and fungal species []. In general, the dynamics of the telluric microbial populations are strongly influenced by soil porosity (affecting the distribution of moisture and O2) and organic matter quality and content []. Other anthropic activities (e.g., use of agrochemicals, fertilizers, tillage, irrigation, crop rotations, and other cultivation techniques) can also significantly affect soil microbial community abundance and composition [,]. It is important to highlight that a common caveat in many studies on microbial communities has been the difficulty in characterizing the composition and evolution of these communities under experimental conditions. With the advent of NGS techniques and the progress in the bioinformatic pipelines, it is now possible to characterize with high resolution the composition of the microbial communities not only at a taxonomical level through the analysis of selected genomics regions (usually the 16S rRNA for bacteria, and the 28S rRNA or the Internally Transcribed Spacer (ITS) for fungi) and metagenome shotgun sequencing, but also at a functional level through metatranscriptome sequencing analysis that aims at the identification of genes expressed in a microbial community. Details on the NGS techniques and their applications in the biocontrol field were recently reviewed by Massart, Martinez-Medina, and Jijakli [] (Section 5).
2.2. BCAs against Air-Borne Pathogens
A great number of bacterial, yeast and fungal species inhabit the aerial parts of plants known as the phyllosphere. This is a complex ecosystem in which microorganisms and host plants interact extensively to create dynamic communities of microorganism living as commensals and sometimes adapt to a specific plant species []. Microbial communities can inhabit both external surfaces (epiphytes) or internal plant tissues (endophytes), and these communities play an important role in protecting plants from diseases. Pathogens can also often have an epiphytic phase before entering plant tissues as endophytes []. The study of phyllosphere microbiology can be useful to better understand the behaviour and control of pathogens of aerial plant parts. Their mechanisms of diffusion, colonization, survival and pathogenicity have been the subject of many studies [,]. Much less is understood about the identity or property of the numerous phyllosphere-inhabiting non-pathogenic microbes that can play an important role in the biocontrol of many phytopathogens [].
The biological control of air-borne pathogens has advanced more slowly than biocontrol of soil-borne pathogens [], and this is probably due to the massive use of synthetic fungicides and copper-based products which, in the aerial plant part, have often shown to be more effective than biocontrol agents.
On the aerial plant surface, antagonists can (i) compete with the pathogen for nutrients, (ii) produce antibiotics that reduce germination of pathogen spores, (iii) kill the pathogen by contact or by direct penetration (mycoparasitism or microbial predation), and (iv) activate/stimulate plant defence responses against the pathogen.
For additional information on this topic we suggest the recent review of Legein et al. []. There are numerous examples of successful biocontrol of air-borne pathogens [,] that can be controlled by microorganisms naturally occurring on aerial surfaces of plants. In particular, Chaetomium sp. and Athelia bombacina suppress Venturia inaequalis; Rhodotorula kratochvilovae strain LS11 control Monilinia spp, Tuberculina maxima parasitizes the white pine blister rust fungus Cronartium ribicola; Darluca filum and Verticillium lecanii parasitize several rusts, Tilletiopsis sp. parasitizes the cucumber powdery mildew fungus Sphaerotheca fuliginea, and Nectria inventa and Gonatobotrys simplex parasitize two pathogenic species of Alternaria [].
Among the best known examples of biocontrol agents addressed to control air-borne diseases, the powdery mildew mycoparasite, Ampelomyces quisqualis, with its commercial product AQ10® WG, is one of the first commercialized BCAs []. Another example regarding is the biocontrol of the chestnut blight fungal pathogen Cryphonectria parasitica in Europe by hypovirulent strains of the pathogen, which are able to transfer a hypovirulence factor (a mycovirus) to virulent strains to induce cortical cankers healing [,]. On this topic, see also a recent article by Kunova et al. [] regarding new a formulation and delivery method of hypovirulent strains of C. parasitica for biological control of chestnut blight.
More recent research concerns the biocontrol activity of Pythium oligandrum and Trichoderma spp. against the “Esca” disease, a devastating grapevine trunk disease caused by a broad range of taxonomically unrelated wood fungal pathogens. The two BCAs induce plant resistance and outcompete the pathogen(s) by colonizing the same ecological niches (such as pruning wounds, xylem vessels and parenchymatic cells) [,].
Biocontrol bacteria belonging to the genera Pseudomonas and Bacillus have also proven to be effective against diseases caused by air-borne pathogens. Their activity appears to mainly rely on antibiosis and the induction of systemic resistance in several plant species [,]. In particular, Bacillus spp. are the most used BCAs and appear to be effective in a wide range of pathosystems []. Recently, Ramírez-Cariño et al. [] and Kazerooni et al. [] reported that tomato early blight and pepper leaf spot, caused by Alternaria alternata, can be controlled by Bacillus spp. protecting plants from pathogen attack through induction of systemic resistance in the host. Andreolli et al. [] report that Pseudomonas protegens strain MP12, a plant growth-promoting endophytic bacterium, shows a broad spectrum of activity in vitro against different grapevine fungal pathogens, such as Botrytis cinerea, A. alternata, Aspergillus niger, Penicillium expansum, Neofusicoccum parvum, Phaeomoniella chlamydospora and Phaeoacremonium aleophilum. Furthermore, the bacterium is able to reduce drastically B. cinerea necrosis on treated grapevine leaves []. Previous studies reported that P. protegens strains can synthesize antimicrobial molecules including pyrrolnitrin, pyoluteorin, 2,4-diacetylphloroglucinol, analogues of rhizoxin, hydrogen cyanide, monoacetylphloroglucinol, the lipopeptide orfamide A, and toxoflavin [,,,]. Similar results were obtained by Burkholderia phytofirmans strain PsJN [,], recently reclassified as Paraburkholderia phytofirmans DSM 17436T [], that demonstrates an induction of plant growth in parallel with an antagonistic effect on in vitro growth and development of B. cinerea. Study of the mechanisms of action of bacterial BCAs has also focussed on bacterial volatilome and its potential role in suppressing plant diseases. Vrieze et al. [] recently analysed bacterial-derived Volatile Organic Compounds (VOCs) against Phytophthora infestans from sixteen Pseudomonas strains evaluating the in vitro inhibition of P. infestans, and the protective effects against late blight on potato leaf disks [].
As described below, although yeasts are well known as BCAs effective against postharvest diseases [], they are also potentially able to control phyllosphere diseases. Lima et al. and De Curtis et al. reported as different biocontrol yeasts (e.g., Rhodosporidium kratochvilovae, Cryptococcus laurentii, Aureobasidium pullulans and Rhodotorula glutinis) are also able to prevent powdery mildew of cucurbits [] and durum wheat []. De Curtis et al. [] showed that the integration of synthetic fungicdes with the biocontrol yeast Rhodotorula kratochvilovae strain LS11 reduced the incidence of brown rot of stone fruits caused by Monilinia spp. and minimize fungicide residues in derived juice [].
As shown in Section 2.1.1., recent studies focussing on plant soil microbiome and potential manipulation/use of microbial consortia have led to the establishment of new criteria in the selection of new combinations of BCAs to control soil-borne diseases. Similarly, some recent papers have highlighted the growing interest on the study of the phyllosphere microbiome as well as its potential manipulation in the protection of vegetal crops from air-borne pathogens []. For example, Ritpitakphong et al., explored the importance of the phyllosphere microbiome of the leaf surface of Arabidopsis, protecting plants from Botrytis cinerea infection [], whereas Schmidt et al. analyse the bacterial and fungal endophyte communities in healthy and diseased oilseed rape and their potential role for biocontrol of Sclerotinia and Phoma spp. [].
2.3. BCAs against Postharvest Pathogens
In the postharvest phase, several fungal pathogens, mostly wound pathogens, can compromise the shelf life of fruit and vegetables. Since the 1980s, the application of antagonistic organisms such as yeasts and bacteria have been tested against postharvest pathogens [,]. Among bacteria, the Gram-negative Pseudomonas syringae, commercially developed as Biosave®, was one of the first studied and formulated antagonists, and was used to prevent infections caused by Penicillium expansum and Botrytis cinerea on apple []. Furthermore, the Gram-positive bacteria Bacillus subtilis was developed on the marked as Serenade® for pre-harvest treatments aimed at reducing decay symptoms caused by postharvest fungal pathogens []. Although a lot of studies have reported the biocontrol properties of antagonistic bacteria (e.g., for their capacity to induce plant defense responses and for their host growth promotion) [], several authors have proposed yeasts as more suitable biocontrol agents against postharvest diseases because, contrary to bacterial BCAs, they usually do not produce antibiotics [,,]. Yeasts are able to colonize different habitats and ecological niches and they naturally occur on fruit and leaves surfaces. Moreover, the yeast community inhabiting the carposphere varies over time depending on the ripening stage of the fruit []. Several yeasts isolated from different matrices have been selected and studied for their ability to counteract different postharvest pathogens and were formulated and developed as biocontrol products [,].
An interesting BCA with broad range activity against postharvest pathogens, but also indicated for field applications, is the yeast-like fungus Aureobasidium pullulans. This microorganism was tested on strawberries, table grape berries and kiwifruit, and showed significant protection against major storage rot agents such as B. cinerea and Rhizopus stolonifera [,]. In particular, Lima et al. found field application of A. pullulans (isolate L47) on strawberries floral tissues increased the efficacy of the antagonist to counteract latent and quiescent infections []. Biofungicides based on A. pullulans strains indicated for biocontrol applications against fungal pathogens of fruit (e.g., Boni protect®) are now available on the marked []. Several scientific contributions have shown the efficacy of an integrated approach for disease management of postharvest diseases by the combination of biocontrol yeasts with different types of additives and fungicides [,,], e.g., the BCA, Candida sake, had antagonistic activity against B. cinerea tested in a controlled condition and in the field coupled with the film-forming adjuvant Fungicover® [,]. Furthermore, a review about the alternative to improve the biocontrol efficacy of BCAs with several non-conventional compounds (plant growth regulators and elicitors) was published by Zhang et al. []. Application of biocontrol yeasts also turns out to be effective in significantly decreasing mycotoxin contamination, as in the case of patulin and ochratoxin A (OTA) accumulation in apples and in wine grapes, respectively []. Interestingly, the presence of the biocontrol yeast Rhodosporidium kratochvilovae LS11 (now reclassified as Rhodotorula kratochvilovae) in apple wounds stimulates the specific rate of patulin biosynthesis (measured as ng patulin/g fungal DNA) by the mycotoxigenic pathogen P. expansum, yet the overall contamination of apples is decreased []. Likewise, other biocontrol yeasts that can degrade mycotoxins, LS11are able to degrade patulin in vitro [,] and the mycotoxin degradation by the yeast leads to the formation of less toxic desoxypatulinic acid []. This degradation pathway appears to be common within the subphylum Pucciniomycotina, since it has also been shown for Sporobolomyces sp. [].
A strain of the marine yeast Rhodosporidium paludigenum isolated in southeast China is a promising BCA, and has also the capacity to degrade patulin [,,]. Interestingly, R. paludigenum was also characterized for probiotic and antimicrobial properties of its polysaccharides []. The antagonistic yeast Cryptococcus podzolicus Y3 was recently sequenced and characterized for its ability related to OTA degradation []. Moreover, one of the main advantages related to BCA application with respect to chemical control of postharvest pathogen relies on their self-sustaining feature, although in the case of mycotoxins degradation it should be pointed out that degradation products are less toxic, but not proven be in the long term []. More recently, two papers reported that strains of the low temperature-adapted yeasts Leucosporidium scottii and Cryptococcus laurentii were highly effective in their biocontrol activity on apple and tomatoes inoculated with B. cinerea in cold storage conditions [,]. These studies reported labor-saving methods for the isolation of cold adapted BCAs for application on fruits (in this case on cold-stored fruits) reducing the scale of resources expense, and most importantly confirm that Basidiomycete yeasts belonging to the genera Cryptococcus are the most frequently isolated as cold-adapted yeasts []. During the isolation of a BCA, it is recommended to perform several samplings, because field management and abiotic factors may affect biocontrol properties of a potential BCA isolated in a single location, as shown for a strain of Metchnikowia pulcherrima isolated from apples by Janisiewicz et al. who highlighted how different strains of the same species isolated over time from the same orchard differed in their biocontrol potential []. Metschnikowia fructicola isolated by Kurtzman and Droby and described first as a “sister” species of M. pulcherrima that morphologically was not easy to distinguish, because the latter, being a biocontrol agent, was effective against B. cinerea []. Furthermore, its genome has not yet been sequenced and assembled. The strain was developed as Shemer® for commercial use as biofungicide against postharvest diseases [].
In theory, isolation of BCAs from the same region, plant or part of the plant on which they will be applied ensure their survival, reducing or avoiding the BCA adaptation phase and improving its fitness []. The BCA Papiliotrema terrestris strain LS28 was isolated from apple epiphytic microflora and selected for its ability to counteract fungal pathogens of plants and fruits, both in the field and in postharvest stages. Whole-genome sequencing was recently applied on LS28 for genomic studies and further investigation on its mechanism of action against phytopathogens [,].
In conclusion, yeast BCAs represent a concrete opportunity to accomplish the need of an eco-friendly strategy in order to reduce fruit losses and chemical residues in fruit during the postharvest stage, although preventive field applications of BCAs are also strongly recommended.
3. BCAs under Evaluation or Already Approved as Biofungicides in the EU
Due to the growing importance of the biocontrol means, currently, several biocontrol agents are available on the market in the European Community, and others are in the evaluation stage for their approval by the EC authorities [].
A list of bacterial, viral and fungal BCAs, with their main characteristics, are shown in Table 1 and Table 2. Data reported were taken in January 2022 and reorganized from the EU pesticide database (https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database accessed on 1 January 2022), which allows users to search for information on active substances used in plant protection products, maximum residue levels (MRLs) in food, and emergency authorizations for plant protection products in the EC Member States.

Table 1.
Bacterial and Viral BCAs approved or submitted for approval as Biofungicides in the EU.

Table 2.
Fungal BCAs approved or submitted for approval as BioFungicides in the EU.
4. Mechanisms of Action of Biocontrol Agents
A biocontrol agent (BCA) is a microorganism capable of counteracting one or more target plant pathogens, interfering with their life cycles [].
Several types of interactions can take place between BCAs and plant pathogens, and different mechanisms of action have been identified []. Among these mechanisms, a major categorization can be traced by (i) direct antagonism against the pathogen, such as parasitism, antibiosis and competition; and (ii) indirect biocontrol activity, such as induction of (systemic) mechanisms resistance []. These kinds of mechanisms are not mutually exclusive. Indeed, it is common that the activity of a single BCA relies on different mechanisms to counteract the pathogens. The ability of a BCA to deploy a given mechanism of action can vary depending on pathogen, and host-plant and environmental conditions (nutrient availability, pH, temperature, etc.) [,]. The elucidation of BCA mechanisms of action is crucial to set the criteria to be adopted in the search of new and more effective BCAs [] (see also Section 5 below). In this regard, Raymaekers et al. [] provided an overview of the screening methods adopted for selecting novel BCAs, which represents an important source of information about the different mechanisms of action and their characterization.
The major modes of actions of BCAs that have been identified and studied so far are summarized and described below in Figure 1.
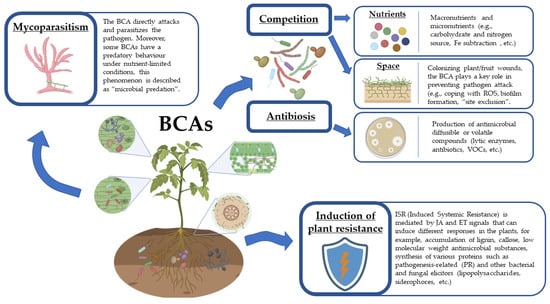
Figure 1.
The main modes of actions of biocontrol agents (BCAs) against plant pathogens. Created with Biorender (biorender.com accessed on 1 June 2022).
4.1. Mycoparasitism
Mycoparasitism is an interaction in which a fungal BCA directly attacks and parasitizes the pathogen, thus killing it or its propagules. Ampelomyces quisqualis is one the best known mycoparasitic fungi worldwide for its ability to control pathogens that cause powdery mildews on different plants. Other mycoparasites are Coniothyrium minitans that attacks sclerotia of Sclerotinia sclerotiorum [,], Trichoderma spp. and Pythium oligandrum that attack fungal hyphae of different phytopathogenic fungi [,,,]. In some cases, a single fungal pathogen can be attacked by multiple mycoparasites. For example, Acremonium alternatum, Acrodontium crateriforme, A. quisqualis, Cladosporium oxysporum and Gliocladium virens are just some representative cases of fungi able to parasitize powdery mildew pathogens [].
Some bacteria are also considered parasites of fungi. Strains belonging to Streptomyces spp. have been reported as hyperparasite of some phytophathogenic fungi (Alternaria brassicicola, Botrytis cinerea, Fusarium oxysporum, Mycocentrospora acerina, Rhizoctonia solani, S. sclerotiorum, Phomopsis sclerotioides and Pythium ultimum) [,]. A mycoparasite-like action was reported for Serratia marcescens that is able to inject antifungal effectors into the fungal hyphae causing fungal cell death, as described by Trunk et al. in witch these antifungal effectors can act against fungal cells, including human pathogenic Candida species [].
In addition to mycoparasitism, microbial predation, the capability to hunt and kill one organism from another for consumption and sustenance (usually through phagocytosis) has also been described []. Some BCAs have a predatory behaviour under nutrient-limited conditions. For example, some Trichoderma species produce a range of enzymes that are directed against cell walls of fungi. In the presence of mature bark compost Trichoderma spp. are able to produce chitinase(s) to parasitize R. solani by activating the expression of chitinase genes (due the reduction of easily accessible glucose and cellulose), but when fresh bark compost is used, Trichoderma spp. does not directly attack the plant pathogen R. solani [].
4.2. Antibiosis
Antibiosis (from the Greek words ἀντι, and βίος that collectively mean “against life”) is generally considered the property of a BCA to inhibit the growth or kill another microorganism by the production of diffusible or volatile antibiotic compounds with a variable target spectrum. The most common antibiotics have a natural origin, and new molecules have been discovered over the years []. Antibiotic application on plants is generally not allowed, although some BCAs able to produce these secondary metabolites were used in the past []. The opportunity of using antibiotic-producing BCAs is still debated, due to issues related to the possible onset of antibiotic resistance in microbial species that are potentially harmful to humans. Current strategies mainly pursue the selection of BCAs that do not produce antibiotics for their use on aerial parts of the plant, and particularly on edible ones (e.g., fruit and vegetables) [,]. On the other hand, the use of antibiotic-producing BCAs is considered to be more tolerable in the rhizosphere against soilborne pathogens [].
Bacterial species belonging to Pseudomonas and Bacillus genera are the most studied organisms for antibiotic production, and a lot of scientific literature is available on this topic [,]. Other bacterial genera, such as Streptomyces, Burkholderia, Serratia, Pantoea, Lysobacter and Enterobacter are known as producers of antibiotics with antibacterial and antifungal properties (e.g., iturin lipopeptides produced by Bacillus spp.) and have been tested against postharvest fungal pathogens [,,,,,,]. Among filamentous fungi, different Trichoderma species are known to produce antibiotic compounds active against a wide range of microorganisms [,]. Volatile antimicrobials compounds (VOCs) are low-molecular weight molecules, and like antibiotics, these substances work in a concentration-dependent manner having a cross kingdom spectrum of activity []. However, for yeasts and bacterial BCAs, VOC production includes several classes of chemicals with antimicrobial activities. VOCs that are involved in the biocontrol activity are alcohols, esters, aldehydes, ketones, terpenes and lactones. Most of the scientific contributions available that rely on VOCs treat VOC production as a good screening parameter for the selection of postharvest BCAs. Tests made on these BCAs were effective in vitro and in controlled storage conditions, but these encouraging results were not obtained in the field [,].
For some BCAs, the production of VOCs works synergistically with the secretion of killer toxins (KTs). Production of KTs by yeasts was described first in the 1960s [], and the ability of each strain to secrete more than one KT was subsequently discovered []. Structurally KTs are glycoproteins or proteins with a variable spectrum of activity; however, the list of yeasts producers of KTs is constantly being updated [,].
Well documented is the production of KTs by a killer strain of Saccharomyces cerevisiae able to synthesizes several KTs, such as K1 and K2 (also called ionophoric KTs), that bind β-1-6-D-glucan and are thus able to create ion channels in plasma membrane affecting the electrochemical gradient across the membrane. KT K28 binds to α-1-3 linked mannose residues of the cell wall, later interacts with plasma membrane receptors, then travels the secretion pathway in the reverse direction, reaching the nucleus, and arresting at the G1/S phase the cell cycle of the host, blocking irreversibly DNA synthesis []. Several BCAs, such as Debaryomyces hansenii KI2a, D. hansenii MI1a and Wickerhamomyces anomalus BS91, were able to produce KTs which were tested against Monilinia fructigena and M. fructicola in vitro and in vivo []. Yeast KTs, as with bacteriocins produced by bacteria, confer on the producer strain (self-immune to their own) an advantage in terms of natural competition. The genetic information for KTs production in yeasts may be harboured on plasmids, but more often is based on cytoplasmic inheritance by satellite dsRNA of viral origin, or coded in the genome [,,]. Furthermore, a main feature is that chromosomally encoded KTs have a broad spectrum of activity against many fungal pathogens, such as those produced by Pichia spp. []. KTs produced by Pichia spp. (with a broad spectrum activity) includes panomycocin, which is a monomeric glycoprotein (49 KDa) with exo-β-1,3-glucanase activity, that binds glucans at the cell wall and, by glucandegradation, kills the susceptible host. Furthermore, given efficacy against dermatophytes causal agents such as Candida spp., panomycocin was proposed for topical application as an antifungal compound []. Among BCAs, another species that has the killer phenotype (K+) is the yeast-like fungus Aureobasidum pullulans, well characterized for its antagonistic activity against postharvest fungal pathogens [].
4.3. Competition
In microbial communities, the competition for nutrients and space is crucial since the availability of nutrients, space and other physical resources is generally limited []. In the soil, on the phyllosphere or fructoplane, accordingly to the biotrophic, hemi-biotrophic or necrotrophic lifestyles of the fungal pathogen, spore germination and growth require the presence of available sources of nutrients to start the infection process. The main sources of nutrient in the soil are represented by root exudates that are considered chemo-attractants for soil-borne pathogens []. At the same time, “positive interactions” are stimulated by root exudates, allowing the colonization of the roots by BCAs []. New strategies aiming to antagonize soil-borne pathogens, include the application of microbial consortia (see Section 2.1.1.), a combination of different microorganisms that may boost the ability of nutrients utilization, increasing competition, as in the case of syntrophy []. In the case of biocontrol of postharvest wound pathogens of fruit, wound competence, i.e., the ability of a BCA to rapidly colonize fruit wounds, plays a key role in preventing the pathogen attack []. Actually, fruit manipulation at harvest and during transportation may cause wounds, where the production of reactive oxygen species (ROS) occurs as a consequence of wounding []. Therefore, BCAs must be able to cope with the oxidative stress caused by ROS when they colonize fruit wounds, which is a prerequisite to exert biocontrol activity [,]. The growth of the BCA Papiliotrema terrestris LS28 in apple wounds is affected by ROS and makes necessary the expression of genes involved in resistance to ROS-generated oxidative stress. This was first suggested by Castoria et al. [], and recently corroborated with a functional genetic approach by knocking out the gene encoding the oxidative stress-responsive transcription factor YAP1. The deleted mutants displayed a significant reduction of biocontrol activity []. In the analogy with these results, the pre-treatment of the yeast Candida oleophila with sub-lethal concentration of H2O2 increased the biocontrol activity of this BCA against Penicillium expansum and Botrytis cinerea []. Summarizing, BCAs able to cope with ROS in the niche of interaction have more strength to compete for nutrients.
BCAs compete with pathogens mainly for carbohydrate and nitrogen since free forms of these macronutrients are available for microbial growth in the fructoplane and on the phyllosphere [,]. Concerning micronutrient competition, iron seems to be crucial according to several studies between BCAs (bacteria, yeasts and fungi) versus pathogens [,]. To this purpose the biocontrol yeasts Metschnikowia pulcherrima and M. fructicola can compete for iron through the production of the siderophore pulcherriminic acid, crucial for the control of P. expansum, B. cinerea and A. alternata []. The BCA Rhodotorula glutinis is able to sequester iron for its own growth in an apple wound by the production of Rhodotorulic acid []. Furthermore, the biocontrol of Monilinia laxa by A. pullulans is mediated by the production of siderophores that are independent by the presence of the pathogen [].
While it is true that ROS resistance allows for better competition for nutrients in the wound by BCAs, by contrast, iron allows BCAs to better cope with ROS, because the catalase enzyme is known to require iron for ROS detoxification [,].
Moreover, competition is based on rapid BCA growth and may involve biofilm formation, allowing the BCA to occupy the niche (i.e., the wound) covering it, causing “site exclusion” (180). For the BCA A. pullulans it was demonstrated that the production of extracellular polysaccharides (EPSs) may depend on the concentration of the nitrogen sources, and its dosage improve the competitive fitness in wound and biocontrol ability []. One of the first studies on competition for nutrient and space was carried out on Cryptococcus laurentii (now Papiliotrema terrestris strain LS28) []. Later by SEM observation carried out by Di Francesco and Ugolini [], it was demonstrated that the competition for nutrients and space by two strains of A. pullulans is involved in their biocontrol of M. laxa on peaches. This mode of action is based on the active metabolism of the BCA and may affect the less competitive pathogen in many ways []. The key advantage of this BCA mode of action is that the resistance of the pathogens to it is more difficult to develop.
4.4. Induced Resistance
Antagonistic microorganisms can induce resistance and biopriming in plants, thus providing systemic resistance against a broad spectrum of plant pathogens []. Biotic and abiotic diseases, and in some instances even damage caused by insects and nematodes, can be reduced in plants pre-stimulated with the application of non-pathogenic microorganisms (priming) [,,,,]. Plant defences can be induced by pathogenic and non-pathogenic microorganisms as pathogen-associated or microbe-associated molecular patterns (PAMPs or MAMPs), or with certain natural or synthetic chemical compounds []. Resistance can be induced locally and/or spread throughout the host plant via chemical signals. Non-pathogenic microorganisms can induce ISR (Induced Systemic Resistance) in plants, that is able to enhance their defensive capacity to multiple plant pathogens. ISR is phenotypically similar to the pathogen-induced SAR (Systemic Acquired Resistance) [,]. SAR and ISR generally act through different signalling pathways: SAR induction is mediated through salicylic acid (SA)-signalling pathways, while ISR requires jasmonic acid and ethylene signalling pathways and, in some cases, SA-dependent SAR pathway [].
Plant defence responses may include thickening of cell walls by lignification, deposition of callose, accumulation of low-molecular-weight antimicrobial substances (e.g., phytoalexins), synthesis of various proteins (e.g., pathogenesis-related (PR) such as chitinases, glucanases, and peroxidases) and other bacterial and fungal elicitors (lipopolysaccharides, siderophores, etc.) [,].
ISR can also be induced by treatment with microbial components and by a diverse group of structurally unrelated organic and inorganic compounds, such as microbial derived compounds, plant derived compounds (e.g., plants peptides), and synthetic lipopeptides. [,].
The activation of ISR by BCAs has been demonstrated against phytopathogenic fungi, bacteria, and viruses. Among the first reports concerning the ability to induce ISR, is the reduction of the susceptibility to Fusarium wilt mediated by Pseudomonas sp. on carnation [] and to the airborne disease of cucumber caused by Colletotrichum orbiculare mediated by certain strains of growth-promoting rhizobacteria []. Subsequently, much research reported that ISR can be triggered by a lot of potential BCAs, for example: (i) Burkholderia phytofirmans PsJN against Botrytis cinerea and Verticillium dahliae on grapevine and tomato, respectively [,]; (ii) Gliocladium roseum against Erysiphe orontii []; (iii) Pseudomonas spp. against Ceratocystis fagacearum on oak [], and (v) Bacillus spp. against Fusarium spp. [].
Raymaekers et al. [], provided an overview and discussion of the screening systems and reported on novel BCAs for biocontrol of microbial plant diseases, discriminating the indirect mechanism of action, the induction of resistance, between phenotype-based and marker-based approaches, which evaluate directly the intended phenotype (disease reduction) or the expression of a marker predictive for this phenotype, respectively. This second approach (that has gained relevance over the years due to the evolution of new techniques) is based on the use of molecular tools for the detection of ROS and phytoalexins using fluorescence measurements, enzymatic and proteomic analysis, and differentially expressed genes as markers. For example, Agostini et al. [] analysed the proteome and transcriptome on maize silks following priming induced by Trichoderma root colonization, showing that Trichoderma activates plant proteins to counteract Fusarium infection. Comparison between proteomic and transcriptomic data suggests differential response regulation. Proteins from the phenylpropanoid pathway are activated to quickly respond to pathogen attacks []. The RNA-seq analysis of the expression of genes involved in plant hormone signalling pathways related to ISR revealed active participation of JA and SA signalling pathways, which further indicated the involvement of ISR and SAR in the protection of tomato plants from Alternaria solani operated by Chaetomium globosum []. Roylawar et al. [] reported that the root-endophytic fungus Piriformospora indica (Pi) can reduce significantly the onion leaf blight caused by Stemphylium vesicarium. They attribute this phenomenon to the protective effect of Pi colonisation against peroxidative damage, and its role in oxidative stress signalling. A qPCR-based expression analysis of the defence-related genes, provided further indications of the ability to induce onion ISR.
5. Molecular Approaches to Potentiate the Effectiveness of BCAs against Fungal Pathogens
5.1. NGS Techniques to Elucidate the Mechanisms of Action of Fungal, Yeasts, and Bacterial BCAs
Understanding the mechanisms of biocontrol operated by BCAs against fungal pathogens at a molecular level is a key requirement to fully exploit their antagonistic activity. The rapid diffusion of next-generation sequencing (NGS) techniques has had a tremendous impact in the biocontrol field through the generation of whole genome sequencing, transcriptomics (RNAseq) and proteomics data, allowing comparative genome analysis and gene/protein expression analyses to identify molecular pathways and key genes potentially playing a critical role in biocontrol.
Several of these techniques have been applied alone or in combination to study biocontrol and plant-promoting mechanisms of filamentous fungi. For example, Shaw et al. determined the gene expression changes in the biocontrol and plant-growth promoting agent Trichoderma hamatum during antagonistic interactions with the pathogen Sclerotinia sclerotiorum in soil. They identified a biphasic response of T. hamatum during biocontrol characterized by the induction of genes involved in transport and oxidation-reduction, and genes encoding small secreted cysteine-rich proteins, secondary metabolite-producing gene clusters and genes unique to T. hamatum []. For other studies on application of omics approaches in Trichoderma we recommend the recent review of Sharma et al. []. In other filamentous fungi used as biocontrol agents, Zhao et al. applied comparative genomics and transcriptomics analysis to elucidate the mechanisms used by the mycoparasite Coniothyrium minitans to antagonize S. sclerotiorum, and found overexpression of fungal cell-wall-degrading enzymes (FCWDs) during parasitism []. Similar results were also obtained for Chaetomium globosum against Bipolaris sorokiniana [], and Clonostachys rosea against Fusarium graminearum []. In C. rosea, Demissie et al. [] applied RNAseq to identify the mechanisms of gene expression in response to F. graminearum secretome, and Broberg et al. used comparative genomics to demonstrate the role of drug efflux transporters in the biocontrol activity of C. rosea against F. graminearum []. While these studies applied the classical RNAseq protocol, other studies exploited this technology to perform dual RNAseq analysis [,,], i.e., to study changes in gene expression in the BCA while interacting with the pathogen and/or the host, as well as their response to the BCA. Moreover, an innovative application was reported by Lysøe et al. [] who performed a time course-based transcriptomic approach to identify at the same time genes expressed in a three-way interaction between the BCA C. rosea, the pathogen Helminthosporium solani, and the host Solanum tuberosum. This study provided an enormous amount of data enabling the identification of the differentially expressed transcripts in C. rosea that could be involved in biocontrol activity against the pathogen, pathogenicity factors from the pathogen H. solani that could be important for disease development, and potato response to the two microorganisms.
In biocontrol yeasts, Hershkovitz et al. applied RNAseq to study gene expression changes in the biocontrol agent Metschnikowia fructicola during its interaction with grapefruit peel tissues and with the mycelium of the postharvest pathogen Penicillium digitatum. During interaction with the host, genes involved in oxidative stress, iron and zinc homeostasis, and lipid metabolism were induced, while during interaction with the pathogen genes involved in multidrug transport and amino acid metabolism were induced []. In another study, Zhang et al. applied RNAseq to study the host response to the BCA Yarrowia lipolytica, and they found that this BCA induced host resistance through crosstalk between salicylic acid and ethylene/jasmonate pathways []. Rueda-Mejia et al. [] performed dual RNA-seq of A. pullulans NBB 7.2.1 during co-incubation with F. oxysporum NRRL 26381/CL57, and found that ~12% of all the A. pullulans genes were differentially expressed, with upregulated genes including secreted hydrolases such as glycosylases, esterases, and proteases, and genes encoding enzymes predicted to be involved in the synthesis of secondary metabolites. Conversely, only 80 genes were differentially expressed in F. oxysporum, with lipid and carbohydrate metabolism being the most represented Gene Ontology categories. Laur et al. [] performed three-way RNAseq during interaction of the BCA Pseudozyma flocculosa in the context of its biocontrol activity against Blumeria graminis f.sp. hordei as it parasitizes Hordeum vulgare. The authors found that P. flocculosa uses effectors to obtain nutrients extracted by B. graminis from barley leaves, indirectly parasitizing barley in a transient manner. The activity of these P. flocculosa effectors is synchronized with the activity of B. graminis haustorial effectors, and a rapid decline of the photosynthetic machinery of barley. The authors named this mechanism hyperbiotrophy because the ultimate host target of P. flocculosa is the plant, and parasitism that is achieved through the powdery mildew pathogen.
As regards bacterial biocontrol agents, comparative genomics was used to identify genes involved in phytohormone production, increased nutrient availability and biocontrol mechanisms in two strains of the plant growth-promoting rhizobacteria (PGPR) Paenibacillus polymyxa []. In addition to comparative genomics, Nelkner et al. [] applied RNAseq to verify the role of genes involved in secondary metabolite and siderophore biosynthesis, plant growth promotion, inorganic phosphate solubilization, biosynthesis of lipopolysaccharides and exopolysaccharides, exoproteases, volatiles and detoxification in the biocontrol of Pseudomonas brassicacearum against R. solani. Lastly, dual-RNAseq was used to study the mechanism underlying the antagonism of Pseudomonas fluorescens against Rhizoctonia solani and Pythium aphanidermatum, and upregulation of P. fluorescens genes involved in metabolite detoxification during co-cultivation with R. solani was found [].
5.2. Functional Genomics to Identify Fungal, Yeast, and Bacterial Genes Important for Biocontrol
Although the application of the omics approach provides a comprehensive knowledge of the molecular processes underlying the biocontrol activity of BCAs against plant pathogens, these studies serve also to prioritize further experiments through the application of functional genetics approaches (i.e., targeted mutagenesis, or overexpression analyses) to unequivocally confirm whether a certain gene/pathway is involved in the proposed biocontrol phenotype. Many studies on molecular mechanisms of biocontrol have been performed in the filamentous mycoparasitic fungi of the genus Trichoderma due to their early discovery and large impact on human welfare. For example, the first successful transformation of a Trichoderma species (T. reesei) was achieved in 1987 [], followed by a number of optimization strategies, genome sequencing, and molecular applications [reviewed in [,,,,]. To avoid redundancy with the listed reviews, in this work we aim to mention only the works that we consider key genetics discoveries that demonstrated a role of genes in the biocontrol activity of a Trichoderma mycoparasite species. Key studies regarded (i) the discovery of the function of the T. atroviridae G-protein encoding genes TGA1 and TGA3 in the development of contact area and coils around host hyphae [,], (ii) the inability of a gpr1-silenced transformant of T. atroviridae to detect, lyse and kill the host fungus [], (iii) the role of the ABC transporter Taabc2 from T. atroviridae in its biocontrol activity against several pathogens [], (iv) the identification of the Vel1 gene in T. virens as master regulator of morphogenesis and biocontrol activity [], and (v) the identification of a TBRG-1 Ras-like protein in T. virens, as being involved in conidiation, in negative regulation of antibiosis and mycoparasitism, and in biocontrol activity against R. solani []. Several studies of functional genetics have also been performed in C. rosea. Genes that were demonstrated to be important for mycoparasitism and biocontrol activity are (i) the MFS transporter gene mfs464 [], (ii) the gene encoding the cell wall biogenesis protein phosphatase CrSsd1 [], (iii) the nonribosomal peptide synthetase gene nps1 [], (iv) the polyketide synthase-encoding gene pks29 [], and (v) the mitogen-activated protein kinase gene Crmapk of C. chloroleuca []. Last, overexpression of the C. rosea endochitinase gene Chi67-1 increased its biocontrol activity against S. sclerotiorum []. In C. minitans, Zeng et al. [] found that the gene CmBCK1, encoding MAP kinase and homologous to BCK1 of Saccharomyces cerevisiae is required for conidiation and mycoparasitism against S. sclerotiorum. A complete review of fungal genes and metabolites associated with the biocontrol of soil-borne plant pathogenic fungi has been recently published []. All together these studies of functional genetics demonstrate that mycoparasitism operated by filamentous fungi is a complex biological process that involves genes with different cellular functions.
In biocontrol yeasts, only few functional genetics studies have been performed so far. Mutation and overexpression of the C. oleophila β-exoglucanase-encoding gene EXG1 did not result in different biocontrol activity in vitro and in vivo against Penicillium digitatum compared to the wild type (WT) strain [,]. A following study in Pichia anomala revealed that single or double mutants for the exo-β-1,3-glucanase-encoding genes EXG1 and EXG2 displayed some reduction in the antagonistic activity of B. cinerea on apples compared to the WT when applied at low cellular concentrations and on young apples [,]. Overall, these studies revealed that the production of exo-β-1,3-glucanases has a minor role in the biocontrol operated by Ascomycetes BCAs, and in certain conditions their contribution might be masked by more relevant modes of action, such as competition for nutrients and space. As a note, analogous studies should be performed on endo-glucanases that are expected to cause more dramatic damage to pathogen cell walls.
Two other studies aimed at underlining the molecular bases of competition for nutrients. Fiori et al. [] reported that a leucine-auxotrophic mutant of the biocontrol yeast P. angusta was unable to control brown rot lesion caused by Metschnikowia fructicola compared to its parental WT strain. The addition of exogenous L-leucine to the infected wounds restored antagonistic activity in the leucine-auxotrophic mutant, suggesting that amino acids utilization by the BCA might be important for nutrients competition. In another study, a spontaneous colorless mutant of M. pulcherrima with a premature stop codon in the transcriptional regulator gene SNF2 was found to lack pulcherrimin and exhibited reduced biocontrol activity against B. caroliana in vitro and in vivo. The reduced antifungal activity of the pigmentless M. pulcherrima cells supports a role for pulcherrimin in the antagonistic phenotype through an uncharacterized interaction with iron []. Of note, pigmentless mutants only showed reduced antifungal activity and still strongly inhibited the growth of filamentous fungi, indicating that biocontrol is the result of a complex interaction that involves the coexistence of several different mechanisms.
Lastly, there are two other studies that have characterized the role of transcription factors in biocontrol activity through their involvement in resistance to abiotic stresses associated with antagonistic traits. Sui et al. [] mutated the transcription factor RML1 in C. oleophila and found that rml1Δ mutants displayed reduced resistance to heat stress (40 °C), salt stress, and oxidative stress induced by hydrogen peroxide in vitro, and reduced ability of wound colonization and antagonistic activity against B. cinerea in vivo in kiwi fruit. In another study, Castoria et al. [] mutated the Papiliotrema terrestris transcription factors RIM101 and YAP1 and found that, in vitro, the yap1Δ mutant displayed increased sensitivity to oxidative, genotoxic and nitrosative stresses, while the rim101Δ mutant was unable to grow at alkaline pH and was sensitive to cell wall-stressors. In vivo, both yap1Δ and rim101Δ mutants displayed reduced ability of apple wound colonization, but only the yap1Δ displayed reduced antagonistic activity against P. expansum and Monilinia fructigena. Both of these studies demonstrated that resistance to abiotic stresses by the BCAs, in particular to oxidative stress, is an important factor to outcompete the pathogen through the rapid and timely colonization of wounded fruit tissues (wound competence) that are characterized by the production of a high level of reactive oxygen species as a consequence of wounding. These molecular studies confirmed previous biochemical and phenotypical studies [,].
Lastly, bacteria allow easier genetic manipulation compared to fungi, so several molecular studies are available, and only the most important ones are reported in this review. Palmieri et al. elucidated the genetic mechanisms of the antagonistic activity of Rahnella aquatilis against the root-infecting fungal pathogen Fusarium oxysporum f. sp. lycopersici []. R. aquatilis induces a rapid acidification of the rhizosphere through the production of gluconic acid, which counteracts F. oxysporum-induced alkalinization. The authors found that an R. aquatilis mutant for the gene gcd encoding a glucose dehydrogenase responsible for gluconic acid production was unable to acidify the medium and prevent F. oxysporum infection. Furthermore, mutation of the flagellin gene fliC, essential for flagellum function and bacterial motility, failed to show a chemotactic response toward external stimuli, including exudates from tomato roots or fungal mycelium. Glucose dehydrogenase (gcd) and gluconate dehydrogenase (gad) encoding genes were characterized also in Pseudomonas fluorescens CHA0 []. A transposon library in P. fluorescens NBC275 identified genes involved in pyoverdine biosynthesis (pvdI and pvdD), chitin-binding protein (gbpA), and in polyketide biosynthesis (phlD) as essential for antifungal activity and biocontrol capacity of this beneficial bacterium []. In a recent study, deletion of Pseudomonas protegens proA, a protegenin biosynthetic gene, resulted in the reduction of the anti-oomycete activity [].
6. Issues and Constraints in the Registration and Commercial Development of Biocontrol Agents against Phytopathogenic Fungi
Microbiol-based biopesticides are the best candidates to replace or integrate synthetic pesticides and to promote a sustainable agri-food production. Nevertheless, only few microbial biofungicides are currently available compared to conventional fungicides based on chemical active ingredients (Table 1 and Table 2). Therefore, to implement the disease management of many crops, there is an urgent need to develop other biocontrol products. From the laboratory stage, the development of a commercial microbe-based biopesticide consists of three complex phases: (i) development of a viable and stable formulation; (ii) patent application; (iii) registration of the active ingredient and its formulation (Figure 2).
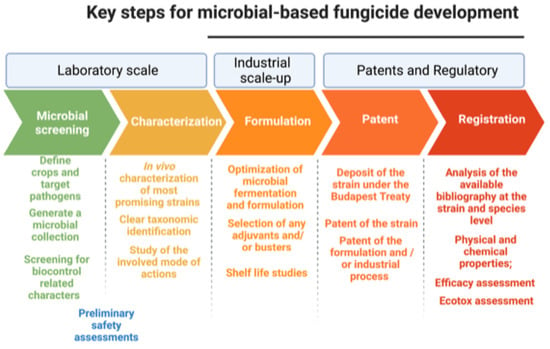
Figure 2.
Timeline and main steps needed to develop a new microbial-based fungicide from laboratory investigation to the registration of an active ingredient and its formulation. For each step the most important required activities are reported.
6.1. Development of a Stable Formulate
The formulation process is a key step for pesticide development in terms of production costs and effectiveness. To date, not much information is available on formulation processes and technologies, as they are often protected by trade secrets. The plant protection exerted by most BCAs against fungal pathogens is usually due to the presence of viable cells and the consequent biological processes underlying the involved mode(s) of actions. Consequently, the main purpose of the formulation process of a microbe-based biopesticide must be the stabilization of the microbial cells by maintaining their viability at acceptable levels over time, i.e., during storage until utilization. The formulation process significantly affects microbial viability, since during this stage microbial cells are subjected to multiple stresses. Today, many alternative formulation processes are available, and it is possible to evaluate and choose for each microorganism the process that has the lowest impact on viability []. Furthermore, to mitigate the negative effect of stressful conditions on microbial cells, the growth medium is usually mixed with protectant compounds []. The improvement of stability during storage can be achieved by treatments preceding the formulation, such as the adoption of appropriate growth conditions. In addition, chemical additives or suitable packaging are very useful to preserve formulate stability (i.e., cell viability) []. Biopesticide stabilization achieved by the formulation process also has the purpose of limiting microbial contamination, which must be kept below the limits during the entire storage period. Moreover, the physical state of the formulate must remain unaltered during shelf life; for example, particle aggregation and formation of clumps are undesirable in both solid and liquid formulates []. The other two relevant functions of the formulation process are to aid in the handling and application of the biopesticide as well as to increase persistence in the environment after application. For these purposes, many adjuvant compounds are already commercially available, which allow a fast dissolution in water, a uniform distribution of BCA cells on vegetable surfaces and have a protective action against abiotic stresses affecting microbial viability after application []. For some biocontrol agents, the ability of some compounds to increase their antagonistic action against the target fungal pathogens has also been characterized []. Numerous types of formulates are currently available, and these can be divided into solid or liquid, depending on the inert carrier mixed with the active ingredient (i.e., BCA cells). Regardless of the specific formulate, the nature of the final product can be of four types: liquid, slurry, granular, or powder. For the solid ones, the inert carriers can be classified into organic (e.g., starch, lignin, humic acids, cellulose, polysaccharides, and skim milk) and inorganic (e.g., silica, vermiculite, zeolite, and clay). The formulation process of a solid product inevitably involves the dehydration of the microbial biomass, which can be achieved by vacuum-drying or freeze-drying process. The vacuum drying process can be more cost-efficient as compared to freeze-drying [,,]. However, it generally yields a lower cell concentration in terms of CFU (colony forming units) per gram of formulate. The liquid formulations essentially consist of suspensions of microbial cells amended with substances that may improve stickiness, stabilization, surfactant and dispersal abilities [,]. The main advantage of liquid formulates over solid ones is that they are easier to handle. Unlike solid carrier-based inoculants, liquid formulates allow the manufacturer to include enough nutrients, cell protectants, and other adjuvants to improve final product stability and performance. Furthermore, the formulation process is a very important step for the development of alternative microbe-based products to be used as seed coatings, for bumble and bees vectoring, or slow-release formulas.
6.2. Patent Application
A patent confers, by law, a temporary privilege (generally 20 years), for the exclusive industrial or commercial exploitation to the inventors for the inventions that meet the standards of novelty, non-obviousness, and industrial applicability. In return, applicants are obliged to disclose their inventions to the public. Although the regulation of the patent is dictated by the individual state, there is a certain homogeneity regarding the cardinal principles. The European regulation provides four basic requirements for patentability: (i) there must be an “invention”, belonging to any field of technology; (ii) the invention must be susceptible of industrial application; (iii) the invention must be new, and (iv) the invention must involve an inventive step. A microorganism that is the active ingredient of a biopesticide can be patented as a biotechnological invention; specifically, a product consisting of or containing biological material or a process by means of which biological material is produced, processed, or used. As regards the novelty requirement, in the case of microorganisms this is applicable when the microorganism is artificially generated by genetic modifications or other techniques, or if the microorganism already described, is isolated from its natural environment []. When an invention involves microorganisms, national laws in most countries, as a disclosure action, require that the applicant deposit the strain at a designated and recognized international depositary authority. To avoid the requirement to deposit the microorganisms in each country in which patent protection is requested, the Budapest Treaty provides that the deposit of a microorganism in any international depositary authority suffices for the purposes of patent procedures at national patent offices of all the states that signed the treaty. Adopted in 1977, the Budapest Treaty concerns a specific topic regarding the international patent process for microorganisms. Currently, the Budapest Treaty assembly has 85 member states (https://wipolex.wipo.int/en/treaties/ShowResults?search_what=B&bo_id=15 accessed on 1 January 2022), while the international depositary authorities for the microorganisms has 39 member states (https://www.wipo.int/budapest/en/idadb/ accessed on 1 January 2022). According to the International Patent Classification (IPC) of the World Intellectual Property Organization (WIPO), microbial biopesticides are included in the group “Human Necessities” (IPC A), subgroup “AGRICULTURE” (IPC A01) and are identified with the IPC code A01N63/00 (including biocides, pest repellants or attractants, plant growth regulators containing microorganisms, viruses, microbial fungi, animals (e.g., nematodes), or substances produced by or obtained from microorganisms, viruses, microbial fungi, enzymes or fermenters). To date, there are 48736 patents involving biopesticide (IPC A01N63/00) in the world, and the countries with the highest number of patent filings are China (25%) and USA (17%), and the main applicants are large multinational companies of the agrochemical sector such as Bayer, Monsanto, Mycogen, and Syngenta, among others. Only considering the biofungicides subdivision (IPC A01N63/00 AND fungicides) the patents amount to about 3500, of which 35% have been deposited in the United States, and even in this case, the major applicants are multinational companies. Classifying biopesticides based on the active ingredient, there are about 2920 bacterial-based, 1658 fungal-based and 234 viral-based biopesticides, of which 227 bacterial-based and 169 fungal-based are fungicides. For all categories, there has been a considerable increasing trend in the last few years.
6.3. Registration of Active Ingredients and Formulation Process
Despite the relatively high number of patent applications for biopesticides, only a few of them have materialized in registration for agricultural use. The registration depends on specific rules within each country. Before commercialization, a pesticide must be carefully evaluated to ensure that it meets safety standards to protect human health and the environment. For this purpose, the registration process is the key step for safety verification allowing the pesticide’s distribution, sale, and use only after the company meets the scientific and regulatory requirements. In most countries, biopesticides are evaluated and registered following the same system as conventional synthetic pesticides. However, this approach can pose an unnecessarily high and inappropriate regulatory burden for microbe-based biopesticides, considerably limiting their commercial diffusion. At present, a separate registration process for biological control agents is being considered. For registration of biopesticides, each jurisdiction has its own requirements for the data package to be submitted. However, the European Union (EU) and the USA Environmental Protection Agency (EPA), although with slight differences in the registration procedures, have enough similarities so it is possible to generalize. In both EU and USA, it is necessary to meet specific a regulation for any pesticide, including the registration of microbe-based products. The purposes of this regulation are:
- -
- Protection of human and environmental safety;
- -
- Ensuring and maintaining quality standards;
- -
- Protection of technological invention and rights [].
- -
- In the registration dossier, the data required are differentiated for the active ingredient and the formulate [].
- -
- Data requirements for an active substance (Technical Grade Active Ingredient, TGAI) usually include:
- -
- Identity and purity;
- -
- Physical and chemical or biological properties;
- -
- Further information on use, production processes, and related areas;
- -
- Analytical methods used to identify the active ingredient;
- -
- Effects on Human health;
- -
- Residues (often confused with persistence);
- -
- Fate and behavior in the environment;
- -
- Effects on non-target organisms;
- -
- Summary of all.
- -
- Data requirements for the formulated product (FP):
- -
- Identity and composition of the formulation;
- -
- Physical and chemical properties;
- -
- Application, labelling, and packaging;
- -
- Further information;
- -
- Analytical methods;
- -
- Efficacy data;
- -
- Toxicology and exposure;
- -
- Residues;
- -
- Fate and behavior in the environment;
- -
- Effects on non-target organisms;
- -
- Summary.
For many microbial-based pesticides, the TGAI cannot be identified because the pure cell biomass is not stable, and its stabilization involves a formulation process and therefore a FP. In these cases, the coincidence between the TGAI and the FP allows that the registration studies are carried out only for the FP. The required information for each section must be provided through studies conducted under Good Laboratory Practice (GLP) from the scientific literature.
Although formulation and registration are the last steps in the development process of a microbe-based fungicide, much can be done in the previous experimental phase. Many issues that arise during the of formulate development and the registration can be anticipated in the experimental phase by facilitating these issues which constitute an important obstacle to the commercial development of a bio fungicide.
Some microbial species can potentially be considered as low-risk active substances. Therefore, the new EPPO directive PP1/296 contemplates for these microbes a slightly different and simplified registration process compared to conventional active ingredients: longer data protection periods and reduced amount of efficacy data to support the registration process. A caveat is that a microorganism may be considered as being of low-risk active ingredient unless at a strain level it has demonstrated multiple resistance to antimicrobials used in human or veterinary medicine (Commission Regulation (EU) 2017/1432 of 7 August 2017).
7. Conclusions and Perspectives
Synthetic pesticides, because of their eco-toxicological risks, are facing increasing limitations in their use worldwide.
Biocontrol products based on microbial antagonists are safer alternative tools to replace or integrate chemical products. Therefore, studies on the selection, characterization and commercial development of BCAs have been steadily increasing over the last decades. Among the most studied microbial antagonists are bacteria, yeasts and filamentous fungi. In this review, we list the most studied genera and species of biocontrol microorganisms and describe their main characteristics in their use as biocontrol tools against fungal diseases of vegetal crops both in the field and in postharvest. Some possible key strategies to optimize selection and use of new BCAs are also discussed.
Despite the large number of studies conducted on microbial antagonists, BCA formulates are still too scarce to cope with the growing demand for their use in sustainable agricultural systems. The main constraints limiting/delaying the development of new microbe-based formulates are (i) the activity of microbial antagonists, which is sometimes lower than that of synthetic pesticides, (ii) the scarcity of information available on the microbial formulation protocols, due to industrial secrecy, and (iii) the complex registration and patent procedures in place in some countries (e.g., in Europe). The use of molecular and omic tools can increasingly contribute to a more efficient and faster selection of microbial antagonists by providing a detailed comprehension of their mechanisms of action, a crucial aspect to optimize BCAs activity and facilitating the registration procedures.
Despite the technical and bureaucratic difficulties associated with development of microbial BCAs, there is a strong tendency to switch to the control of plant diseases with a lower environmental impact and with fewer risks for human health, as well as increasing political support from various governments to find solutions and funding of research on new technologies to solve the more general problems related to climate change and the conservation of biodiversity and environment. Therefore, research into the design and development of more efficient bioproducts, including microbial formulations to be used against fungal diseases, will attract more and more attention in the near future.
Author Contributions
Conceptualization, G.L. and D.P.; writing—original draft preparation, D.P., G.I., C.D.G. and G.B.; review and editing, G.L., R.C. and F.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rajaram, S.; Dubin, H.J. Plant Diseases, Global Food Security and the Role of R. Glenn Anderson. In Plant Diseases and Food Security in the 21st Century; Scott, P., Strange, R., Korsten, L., Gullino, M.L., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 35–45. ISBN 978-3-030-57899-2. [Google Scholar]
- Castoria, R.; Logrieco, A. Mycotoxins in fruits and major fruit-derived products-an overview. Microb. Biotechnol. Hortic. 2007, 2, 305–344. [Google Scholar]
- Sanzani, S.M.; Reverberi, M.; Geisen, R. Mycotoxins in harvested fruits and vegetables: Insights in producing fungi, biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Biol. Technol. 2016, 122, 95–105. [Google Scholar] [CrossRef]
- Pirttilä, A.M.; Mohammad Parast Tabas, H.; Baruah, N.; Koskimäki, J.J. Biofertilizers and Biocontrol Agents for Agriculture: How to Identify and Develop New Potent Microbial Strains and Traits. Microorganisms 2021, 9, 817. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Khan, A.; Asif, M.; Khan, F.; Ansari, T.; Shariq, M.; Siddiqui, M.A. Biological control: A sustainable and practical approach for plant disease management. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2020, 70, 507–524. [Google Scholar] [CrossRef]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of Some Biopesticides and Their Importance in Plant Protection for Commercial Acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [Green Version]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining Biocontrol Agents with Chemical Fungicides for Integrated Plant Fungal Disease Control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- RBH, W.; Golding, J. Advances in Postharvest Fruit and Vegetable Technology; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-1-4822-1697-7. [Google Scholar]
- Frederiks, C.; Wesseler, J.H.H. A comparison of the EU and US regulatory frameworks for the active substance registration of microbial biological control agents. Pest Manag. Sci. 2019, 75, 87–103. [Google Scholar] [CrossRef] [Green Version]
- Shoda, M. Bacterial control of plant diseases. J. Biosci. Bioeng. 2000, 89, 515–521. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Ampt, E.A.; van Ruijven, J.; Raaijmakers, J.M.; Termorshuizen, A.J.; Mommer, L. Linking ecology and plant pathology to unravel the importance of soil-borne fungal pathogens in species-rich grasslands. Eur. J. Plant Pathol. 2019, 154, 141–156. [Google Scholar] [CrossRef] [Green Version]
- Cacciola, S.O.; Gullino, M.L. Emerging and re-emerging fungus and oomycete soil-borne plant diseases in Italy. Phytopathol. Mediterr. 2019, 58, 451–472. [Google Scholar] [CrossRef]
- Schlatter, D.; Kinkel, L.; Thomashow, L.; Weller, D.; Paulitz, T. Disease Suppressive Soils: New Insights from the Soil Microbiome. Phytopathology 2017, 107, 1284–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastida, F.; Eldridge, D.J.; García, C.; Kenny Png, G.; Bardgett, R.D.; Delgado-Baquerizo, M. Soil microbial diversity–biomass relationships are driven by soil carbon content across global biomes. ISME J. 2021, 15, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.; Naorem, A.K.; Lal, R.; Dalal, R.C.; Sinha, N.K.; Patra, A.K.; Chaudhari, S.K. Disease-Suppressive Soils—Beyond Food Production: A Critical Review. J. Soil Sci. Plant Nutr. 2021, 21, 1437–1465. [Google Scholar] [CrossRef]
- Montesinos, E.; Bonaterra, A. Pesticides, Microbial. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: Oxford, UK, 2009; pp. 110–120. ISBN 978-0-12-373944-5. [Google Scholar]
- Rodríguez, M.A.; Rothen, C.; Lo, T.E.; Cabrera, G.M.; Godeas, A.M. Suppressive soil against Sclerotinia sclerotiorum as a source of potential biocontrol agents: Selection and evaluation of Clonostachys rosea BAFC1646. Biocontrol Sci. Technol. 2015, 25, 1388–1409. [Google Scholar] [CrossRef] [Green Version]
- Brescia, F.; Pertot, I.; Puopolo, G. Lysobacter. In Beneficial Microbes in Agro-Ecology: Bacteria and Fungi; Amaresan, N., Senthil Kumar, M., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; Chapter 16; pp. 313–338. ISBN 978-0-12-823414-3. [Google Scholar]
- Alfiky, A.; Weisskopf, L. Deciphering Trichoderma–Plant–Pathogen Interactions for Better Development of Biocontrol Applications. J. Fungi 2021, 7, 61. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- del Carmen, H.; Rodríguez, M.; Evans, H.C.; de Abreu, L.M.; de Macedo, D.M.; Ndacnou, M.K.; Bekele, K.B.; Barreto, R.W. New species and records of Trichoderma isolated as mycoparasites and endophytes from cultivated and wild coffee in Africa. Sci. Rep. 2021, 11, 5671. [Google Scholar] [CrossRef]
- Kandula, D.R.W.; Jones, E.E.; Stewart, A.; McLean, K.L.; Hampton, J.G. Trichoderma species for biocontrol of soil-borne plant pathogens of pasture species. Biocontrol Sci. Technol. 2015, 25, 1052–1069. [Google Scholar] [CrossRef]
- González-Pérez, E.; Ortega-Amaro, M.A.; Salazar-Badillo, F.B.; Bautista, E.; Douterlungne, D.; Jiménez-Bremont, J.F. The Arabidopsis-Trichoderma interaction reveals that the fungal growth medium is an important factor in plant growth induction. Sci. Rep. 2018, 8, 16427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrin, R. Interactions between mycorrhizae and diseases caused by soil-borne fungi. Soil Use Manag. 1990, 6, 189–194. [Google Scholar] [CrossRef]
- Singh, R.; Adholeya, A.; Mukerji, K.G. Mycorrhiza in Control of Soil Borne Pathogens. In Mycorrhizal Biology; Mukerji, K.G., Chamola, B.P., Singh, J., Eds.; Springer: Boston, MA, USA, 2000; pp. 173–196. ISBN 978-1-4615-4265-0. [Google Scholar]
- Harrier, L.A.; Watson, C.A. The potential role of arbuscular mycorrhizal (AM) fungi in the bioprotection of plants against soil-borne pathogens in organic and/or other sustainable farming systems. Pest Manag. Sci. 2004, 60, 149–157. [Google Scholar] [CrossRef]
- Bücking, H. The Role of the Mycorrhizal Symbiosis in Nutrient Uptake of Plants and the Regulatory Mechanisms Underlying These Transport Processes. In Plant Science; Liepold, E., Ed.; IntechOpen: Rijeka, Croatia, 2012; Chapter 4. [Google Scholar]
- Soudzilovskaia, N.A.; Vaessen, S.; Barcelo, M.; He, J.; Rahimlou, S.; Abarenkov, K.; Brundrett, M.C.; Gomes, S.; Merckx, V.; Tedersoo, L. FungalRoot: Global online database of plant mycorrhizal associations. New Phytol. 2020, 227, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, N. Mycorrhizal Fungi as Bioprotectors of Crops Against Verticillium Wilt—A Hypothetical Scenario Under Changing Environmental Conditions. Plants 2020, 9, 1468. [Google Scholar] [CrossRef] [PubMed]
- Azcón-Aguilar, C.; Jaizme-Vega, M.C.; Calvet, C. The contribution of arbuscular mycorrhizal fungi to the control of soil-borne plant pathogens. In Mycorrhizal Technology in Agriculture; Gianinazzi, S., Schüepp, H., Barea, J.M., Haselwandter, K., Eds.; Birkhäuser Basel: Basel, Switzerland, 2002; pp. 187–197. ISBN 978-3-0348-8117-3. [Google Scholar]
- Shaikh, S.S.; Sayyed, R.Z. Role of Plant Growth-Promoting Rhizobacteria and Their Formulation in Biocontrol of Plant Diseases. In Plant Microbes Symbiosis: Applied Facets; Arora, N.K., Ed.; Springer: New Delhi, India, 2015; pp. 337–351. ISBN 978-81-322-2068-8. [Google Scholar]
- Labuschagne, N.; Pretorius, T.; Idris, A.H. Plant Growth Promoting Rhizobacteria as Biocontrol Agents against Soil-Borne Plant Diseases. In Plant Growth and Health Promoting Bacteria; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 211–230. ISBN 978-3-642-13612-2. [Google Scholar]
- Weller, D.M. Pseudomonas Biocontrol Agents of Soilborne Pathogens: Looking Back Over 30 Years. Phytopathology 2007, 97, 250–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M. Bacillus spp.: A Promising Biocontrol Agent of Root, Foliar, and Postharvest Diseases of Plants. In Bacilli and Agrobiotechnology; Islam, M.T., Rahman, M., Pandey, P., Jha, C.K., Aeron, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 113–141. ISBN 978-3-319-44409-3. [Google Scholar]
- Bukhat, S.; Imran, A.; Javaid, S.; Shahid, M.; Majeed, A.; Naqqash, T. Communication of plants with microbial world: Exploring the regulatory networks for PGPR mediated defense signaling. Microbiol. Res. 2020, 238, 126486. [Google Scholar] [CrossRef]
- LeBlanc, N. Bacteria in the genus Streptomyces are effective biological control agents for management of fungal plant pathogens: A meta-analysis. BioControl 2021, 67, 111–121. [Google Scholar] [CrossRef]
- Barea, J.-M.; Pozo, M.J.; Azcón, R.; Azcón-Aguilar, C. Microbial co-operation in the rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef] [Green Version]
- Handelsman, J.; Stabb, E.V. Biocontrol of Soilborne Plant Pathogens. Plant Cell 1996, 8, 1855–1869. [Google Scholar] [CrossRef]
- De Curtis, F.; Lima, G.; Vitullo, D.; De Cicco, V. Biocontrol of Rhizoctonia solani and Sclerotium rolfsii on tomato by delivering antagonistic bacteria through a drip irrigation system. Crop Prot. 2010, 29, 663–670. [Google Scholar] [CrossRef]
- Mousa, W.K.; Shearer, C.; Limay-Rios, V.; Ettinger, C.L.; Eisen, J.A.; Raizada, M.N. Root-hair endophyte stacking in finger millet creates a physicochemical barrier to trap the fungal pathogen Fusarium graminearum. Nat. Microbiol. 2016, 1, 16167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, D.; Vitale, S.; Lima, G.; Di Pietro, A.; Turrà, D. A bacterial endophyte exploits chemotropism of a fungal pathogen for plant colonization. Nat. Commun. 2020, 11, 5264. [Google Scholar] [CrossRef] [PubMed]
- El-Tarabily, K.A.; Sivasithamparam, K. Potential of yeasts as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Mycoscience 2006, 47, 25–35. [Google Scholar] [CrossRef]
- Botha, A. Yeasts in Soil. In Biodiversity and Ecophysiology of Yeasts; Péter, G., Rosa, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 221–240. ISBN 978-3-540-30985-7. [Google Scholar]
- Yurkov, A.M. Yeasts of the soil–obscure but precious. Yeast 2018, 35, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Robbins, C.; Thiergart, T.; Hacquard, S.; Garrido-Oter, R.; Gans, W.; Peiter, E.; Schulze-Lefert, P.; Spaepen, S. Root-Associated Bacterial and Fungal Community Profiles of Arabidopsis thaliana Are Robust Across Contrasting Soil P Levels. Phytobiomes J. 2017, 2, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Cecilia Mestre, M.; Rosa, C.A.; Safar, S.V.B.; Libkind, D.; Fontenla, S.B. Yeast communities associated with the bulk-soil, rhizosphere and ectomycorrhizosphere of a Nothofagus pumilio forest in northwestern Patagonia, Argentina. FEMS Microbiol. Ecol. 2011, 78, 531–541. [Google Scholar] [CrossRef]
- Joubert, P.M.; Doty, S.L. Endophytic Yeasts: Biology, Ecology and Applications. In Endophytes of Forest Trees: Biology and Applications; Pirttilä, A.M., Frank, A.C., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 3–14. ISBN 978-3-319-89833-9. [Google Scholar]
- Ferraz, P.; Cássio, F.; Lucas, C. Potential of Yeasts as Biocontrol Agents of the Phytopathogen Causing Cacao Witches’ Broom Disease: Is Microbial Warfare a Solution? Front. Microbiol. 2019, 10, 1766. [Google Scholar] [CrossRef] [Green Version]
- El-Tarabily, K.A. Suppression of Rhizoctonia solani diseases of sugar beet by antagonistic and plant growth-promoting yeasts. J. Appl. Microbiol. 2004, 96, 69–75. [Google Scholar] [CrossRef]
- Niu, B.; Wang, W.; Yuan, Z.; Sederoff, R.R.; Sederoff, H.; Chiang, V.L.; Borriss, R. Microbial Interactions within Multiple-Strain Biological Control Agents Impact Soil-Borne Plant Disease. Front. Microbiol. 2020, 11, 2452. [Google Scholar] [CrossRef]
- Xu, X.-M.; Jeffries, P.; Pautasso, M.; Jeger, M.J. Combined Use of Biocontrol Agents to Manage Plant Diseases in Theory and Practice. Phytopathology 2011, 101, 1024–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, D.; Vitullo, D.; De Curtis, F.; Lima, G. A microbial consortium in the rhizosphere as a new biocontrol approach against fusarium decline of chickpea. Plant Soil 2017, 412, 425–439. [Google Scholar] [CrossRef]
- Liu, K.; McInroy, J.A.; Hu, C.-H.; Kloepper, J.W. Mixtures of Plant-Growth-Promoting Rhizobacteria Enhance Biological Control of Multiple Plant Diseases and Plant-Growth Promotion in the Presence of Pathogens. Plant Dis. 2017, 102, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas-Inciarte, L.; Fuenmayor-Arrieta, Y.; Luzardo-Méndez, M.; Costa-Jardin, M.D.; Vera, A.; Carmona, D.; Homen-Pereira, M.; Costa-Jardin, P.D.; San-Blas, E. Use of different Trichoderma species in cherry type tomatoes (Solanum lycopersicum L.) against Fusarium oxysporum wilt in tropical greenhouses. Agron. Costarric. 2019, 43, 85–100. [Google Scholar]
- Minchev, Z.; Kostenko, O.; Soler, R.; Pozo, M.J. Microbial Consortia for Effective Biocontrol of Root and Foliar Diseases in Tomato. Front. Plant Sci. 2021, 12, 2428. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Grunau, A.; Minerdi, D.; Gehrig, P.; Roschitzki, B.; Eberl, L.; Garibaldi, A.; Gullino, M.L.; Riedel, K. A proteomics approach to study synergistic and antagonistic interactions of the fungal–bacterial consortium Fusarium oxysporum wild-type MSA 35. Proteomics 2010, 10, 3292–3320. [Google Scholar] [CrossRef]
- Win, T.T.; Bo, B.; Malec, P.; Fu, P. The effect of a consortium of Penicillium sp. and Bacillus spp. in suppressing banana fungal diseases caused by Fusarium sp. and Alternaria sp. J. Appl. Microbiol. 2021, 131, 1890–1908. [Google Scholar] [CrossRef]
- Gonda, M.; Garmendia, G.; Rufo, C.; León Peláez, Á.; Wisniewski, M.; Droby, S.; Vero, S. Biocontrol of Aspergillus flavus in Ensiled Sorghum by Water Kefir Microorganisms. Microorganisms 2019, 7, 253. [Google Scholar] [CrossRef] [Green Version]
- Zhimo, V.Y.; Biasi, A.; Kumar, A.; Feygenberg, O.; Salim, S.; Vero, S.; Wisniewski, M.; Droby, S. Yeasts and Bacterial Consortia from Kefir Grains Are Effective Biocontrol Agents of Postharvest Diseases of Fruits. Microorganisms 2020, 8, 428. [Google Scholar] [CrossRef] [Green Version]
- Jawed, K.; Yazdani, S.S.; Koffas, M.A.G. Advances in the development and application of microbial consortia for metabolic engineering. Metab. Eng. Commun. 2019, 9, e00095. [Google Scholar] [CrossRef]
- García-Jiménez, B.; Torres-Bacete, J.; Nogales, J. Metabolic modelling approaches for describing and engineering microbial communities. Comput. Struct. Biotechnol. J. 2021, 19, 226–246. [Google Scholar] [CrossRef] [PubMed]
- Ponomarova, O.; Patil, K.R. Metabolic interactions in microbial communities: Untangling the Gordian knot. Curr. Opin. Microbiol. 2015, 27, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzola, M.; Freilich, S. Prospects for Biological Soilborne Disease Control: Application of Indigenous versus Synthetic Microbiomes. Phytopathology 2016, 107, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Sarma, B.K.; Yadav, S.K.; Singh, S.; Singh, H.B. Microbial consortium-mediated plant defense against phytopathogens: Readdressing for enhancing efficacy. Soil Biol. Biochem. 2015, 87, 25–33. [Google Scholar] [CrossRef]
- Izquierdo-García, L.F.; González-Almario, A.; Cotes, A.M.; Moreno-Velandia, C.A. Trichoderma virens Gl006 and Bacillus velezensis Bs006: A compatible interaction controlling Fusarium wilt of cape gooseberry. Sci. Rep. 2020, 10, 6857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pliego, C.; Ramos, C.; de Vicente, A.; Cazorla, F.M. Screening for candidate bacterial biocontrol agents against soilborne fungal plant pathogens. Plant Soil 2011, 340, 505–520. [Google Scholar] [CrossRef] [Green Version]
- Sayeed Akhtar, M.; Siddiqui, Z.A. Biocontrol of a root-rot disease complex of chickpea by Glomus intraradices, Rhizobium sp. and Pseudomonas straita. Crop Prot. 2008, 27, 410–417. [Google Scholar] [CrossRef]
- Szczech, M.; Shoda, M. Biocontrol of Rhizoctonia Damping-off of Tomato by Bacillus subtilis Combined with Burkholderia cepacia. J. Phytopathol. 2004, 152, 549–556. [Google Scholar] [CrossRef]
- Frąc, M.; Hannula, S.E.; Bełka, M.; Jędryczka, M. Fungal Biodiversity and Their Role in Soil Health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef] [Green Version]
- Sleutel, S.; Bouckaert, L.; Buchan, D.; Van Loo, D.; Cornelis, W.M.; Sanga, H.G. Manipulation of the soil pore and microbial community structure in soil mesocosm incubation studies. Soil Biol. Biochem. 2012, 45, 40–48. [Google Scholar] [CrossRef]
- Rachwał, K.; Gustaw, K.; Kazimierczak, W.; Waśko, A. Is soil management system really important? comparison of microbial community diversity and structure in soils managed under organic and conventional regimes with some view on soil properties. PLoS ONE 2021, 16, e0256969. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Milner, H.; Díaz-Pérez, J.C.; Ji, P. Effects of soil management practices on soil microbial communities and development of southern blight in vegetable production. Appl. Soil Ecol. 2015, 91, 58–67. [Google Scholar] [CrossRef]
- Massart, S.; Martinez-Medina, M.; Jijakli, M.H. Biological control in the microbiome era: Challenges and opportunities. Biol. Control 2015, 89, 98–108. [Google Scholar] [CrossRef]
- Janakiev, T.; Dimkić, I.; Unković, N.; Ljaljević Grbić, M.; Opsenica, D.; Gašić, U.; Stanković, S.; Berić, T. Phyllosphere Fungal Communities of Plum and Antifungal Activity of Indigenous Phenazine-Producing Pseudomonas synxantha against Monilinia laxa. Front. Microbiol. 2019, 10, 2287. [Google Scholar] [CrossRef] [Green Version]
- Legein, M.; Smets, W.; Vandenheuvel, D.; Eilers, T.; Muyshondt, B.; Prinsen, E.; Samson, R.; Lebeer, S. Modes of Action of Microbial Biocontrol in the Phyllosphere. Front. Microbiol. 2020, 11, 1619. [Google Scholar] [CrossRef]
- Parasuraman, P.; Pattnaik, S.; Busi, S. Phyllosphere Microbiome: Functional Importance in Sustainable Agriculture. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J.S., Singh, D.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 10; pp. 135–148. ISBN 978-0-444-64191-5. [Google Scholar]
- Lindow, S. Phyllosphere microbiology: A perspective. In Microbial Ecology of Aerial Plant Surfaces; Athenaeum Press: Cambridge, MA, USA, 2006; pp. 1–20. [Google Scholar] [CrossRef]
- Lindow, S.E.; Leveau, J.H.J. Phyllosphere microbiology. Curr. Opin. Biotechnol. 2002, 13, 238–243. [Google Scholar] [CrossRef]
- Rishbeth, J.; Lumsden, R.D.; Gibbs, J.N.; Hamilton, W.D.; Cook, R.J. Biological Control of Air-Borne Pathogens [and Discussion]. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1988, 318, 265–281. [Google Scholar]
- Bacterial Weapons of Fungal Destruction: Phyllosphere-Targeted Biological Control of Plant Diseases, with Emphasis on Sclerotinia Stem Rot and Blackleg Diseases in Canola (Brassica napus L.). In Environmental and Microbial Relationships; Kubicek, C.P.; Druzhinina, I.S. (Eds.) Springer: Berlin/Heidelberg, Germany, 2007; pp. 189–199. ISBN 978-3-540-71840-6. [Google Scholar]
- Griffin, E.A.; Carson, W.P. The Ecology and Natural History of Foliar Bacteria with a Focus on Tropical Forests and Agroecosystems. Bot. Rev. 2015, 81, 105–149. [Google Scholar] [CrossRef]
- Agrios, G.N. Control of Plant Diseases. In Plant Pathology, 5th ed.; Agrios, G.N., Ed.; Academic Press: San Diego, CA, USA, 2005; Chapter 9; pp. 293–353. ISBN 978-0-12-044565-3. [Google Scholar]
- Wilson, M. Biocontrol of aerial plant diseases in agriculture and horticulture: Current approaches and future prospects. J. Ind. Microbiol. Biotechnol. 1997, 19, 188–191. [Google Scholar] [CrossRef]
- Heiniger, U.; Rigling, D. Biological Control of Chestnut blight in Europe. Annu. Rev. Phytopathol. 1994, 32, 581–599. [Google Scholar] [CrossRef]
- Choi, G.H.; Nuss, D.L. Hypovirulence of Chestnut Blight Fungus Conferred by an Infectious Viral cDNA. Science 1992, 257, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Kunova, A.; Pizzatti, C.; Cerea, M.; Gazzaniga, A.; Cortesi, P. New formulation and delivery method of Cryphonectria parasitica for biological control of chestnut blight. J. Appl. Microbiol. 2017, 122, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Carro-Huerga, G.; Compant, S.; Gorfer, M.; Cardoza, R.E.; Schmoll, M.; Gutiérrez, S.; Casquero, P.A. Colonization of Vitis vinifera L. by the Endophyte Trichoderma sp. Strain T154: Biocontrol Activity against Phaeoacremonium minimum. Front. Plant Sci. 2020, 11, 1170. [Google Scholar] [CrossRef]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Ceballos, I.; Mosquera, S.; Angulo, M.; Mira, J.J.; Argel, L.E.; Uribe-Velez, D.; Romero-Tabarez, M.; Orduz-Peralta, S.; Villegas, V. Cultivable Bacteria Populations Associated with Leaves of Banana and Plantain Plants and Their Antagonistic Activity against Mycosphaerella fijiensis. Microb. Ecol. 2012, 64, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Lawhon, S.D.; Drake, K.L.; Nunes, J.E.S.; Figueiredo, J.F.; Rossetti, C.A.; Gull, T.; Everts, R.E.; Lewin, H.A.; Galindo, C.L.; et al. Systems Biology Analysis of Gene Expression during In Vivo Mycobacterium avium paratuberculosis Enteric Colonization Reveals Role for Immune Tolerance. PLoS ONE 2012, 7, e42127. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Cariño, H.F.; Guadarrama-Mendoza, P.C.; Sánchez-López, V.; Cuervo-Parra, J.A.; Ramírez-Reyes, T.; Dunlap, C.A.; Valadez-Blanco, R. Biocontrol of Alternaria alternata and Fusarium oxysporum by Trichoderma asperelloides and Bacillus paralicheniformis in tomato plants. Antonie Van Leeuwenhoek 2020, 113, 1247–1261. [Google Scholar] [CrossRef] [PubMed]
- Kazerooni, E.A.; Maharachchikumbura, S.S.N.; Al-Sadi, A.M.; Kang, S.-M.; Yun, B.-W.; Lee, I.-J. Biocontrol Potential of Bacillus amyloliquefaciens against Botrytis pelargonii and Alternaria alternata on Capsicum annuum. J. Fungi 2021, 7, 472. [Google Scholar] [CrossRef] [PubMed]
- Andreolli, M.; Zapparoli, G.; Angelini, E.; Lucchetta, G.; Lampis, S.; Vallini, G. Pseudomonas protegens MP12: A plant growth-promoting endophytic bacterium with broad-spectrum antifungal activity against grapevine phytopathogens. Microbiol. Res. 2019, 219, 123–131. [Google Scholar] [CrossRef]
- Demissie, Z.A.; Witte, T.; Robinson, K.A.; Sproule, A.; Foote, S.J.; Johnston, A.; Harris, L.J.; Overy, D.P.; Loewen, M.C. Transcriptomic and Exometabolomic Profiling Reveals Antagonistic and Defensive Modes of Clonostachys rosea Action against Fusarium graminearum. Mol. Plant-Microbe Interact. 2020, 33, 842–858. [Google Scholar] [CrossRef]
- Nowak-Thompson, B.; Gould, S.J.; Kraus, J.; Loper, J.E. Production of 2,4-diacetylphloroglucinol by the biocontrol agent Pseudomonas fluorescens Pf-5. Can. J. Microbiol. 1994, 40, 1064–1066. [Google Scholar] [CrossRef]
- Kidarsa, T.A.; Goebel, N.C.; Zabriskie, T.M.; Loper, J.E. Phloroglucinol mediates cross-talk between the pyoluteorin and 2,4-diacetylphloroglucinol biosynthetic pathways in Pseudomonas fluorescens Pf-5. Mol. Microbiol. 2011, 81, 395–414. [Google Scholar] [CrossRef]
- Gross, H.; Stockwell, V.O.; Henkels, M.D.; Nowak-Thompson, B.; Loper, J.E.; Gerwick, W.H. The Genomisotopic Approach: A Systematic Method to Isolate Products of Orphan Biosynthetic Gene Clusters. Chem. Biol. 2007, 14, 53–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miotto-Vilanova, L.; Jacquard, C.; Courteaux, B.; Wortham, L.; Michel, J.; Clément, C.; Barka, E.A.; Sanchez, L. Burkholderia phytofirmans PsJN Confers Grapevine Resistance against Botrytis cinerea via a Direct Antimicrobial Effect Combined with a Better Resource Mobilization. Front. Plant Sci. 2016, 7, 1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ait Barka, E.; Gognies, S.; Nowak, J.; Audran, J.-C.; Belarbi, A. Inhibitory effect of endophyte bacteria on Botrytis cinerea and its influence to promote the grapevine growth. Biol. Control 2002, 24, 135–142. [Google Scholar] [CrossRef]
- Sawana, A.; Adeolu, M.; Gupta, R.S. Molecular signatures and phylogenomic analysis of the genus Burkholderia: Proposal for division of this genus into the emended genus Burkholderia containing pathogenic organisms and a new genus Paraburkholderia gen. nov. harboring environmental species. Front. Genet. 2014, 5, 429. [Google Scholar] [CrossRef] [Green Version]
- De Vrieze, M.; Pandey, P.; Bucheli, T.D.; Varadarajan, A.R.; Ahrens, C.H.; Weisskopf, L.; Bailly, A. Volatile Organic Compounds from Native Potato-associated Pseudomonas as Potential Anti-oomycete Agents. Front. Microbiol. 2015, 6, 1295. [Google Scholar] [CrossRef] [Green Version]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Lima, G.; De Curtis, F.; Piedimonte, D.; Spina, A.M.; De Cicco, V. Activity of antagonists and natural compounds against powdery mildew of cucurbits: Laboratory and field trials. J. Plant Pathol. 2002, 84, 185. [Google Scholar]
- De Curtis, F.; De Cicco, V.; Lima, G. Efficacy of biocontrol yeasts combined with calcium silicate or sulphur for controlling durum wheat powdery mildew and increasing grain yield components. Field Crops Res. 2012, 134, 36–46. [Google Scholar] [CrossRef]
- De Curtis, F.; Ianiri, G.; Raiola, A.; Ritieni, A.; Succi, M.; Tremonte, P.; Castoria, R. Integration of biological and chemical control of brown rot of stone fruits to reduce disease incidence on fruits and minimize fungicide residues in juice. Crop Prot. 2019, 119, 158–165. [Google Scholar] [CrossRef]
- Stone, B.W.G.; Weingarten, E.A.; Jackson, C.R. The Role of the Phyllosphere Microbiome in Plant Health and Function. Annu. Plant Rev. Online 2018, 1, 533–556. [Google Scholar]
- Ritpitakphong, U.; Falquet, L.; Vimoltust, A.; Berger, A.; Métraux, J.-P.; L’Haridon, F. The microbiome of the leaf surface of Arabidopsis protects against a fungal pathogen. New Phytol. 2016, 210, 1033–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, C.S.; Mrnka, L.; Lovecká, P.; Frantík, T.; Fenclová, M.; Demnerová, K.; Vosátka, M. Bacterial and fungal endophyte communities in healthy and diseased oilseed rape and their potential for biocontrol of Sclerotinia and Phoma disease. Sci. Rep. 2021, 11, 3810. [Google Scholar] [CrossRef]
- Wisniewski, M.; Wilson, C.L. Biological control of postharvest diseases of fruits and vegetables: Recent advances. Hortscience 1992, 27, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Roberts, R.G. Integrating biological control into postharvest disease management strategies. Hortscience 1994, 29, 758–762. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Jeffers, S.N. Efficacy of commercial formulation of two biofungicides for control of blue mold and gray mold of apples in cold storage. Crop Prot. 1997, 16, 629–633. [Google Scholar] [CrossRef]
- Obagwu, J.; Korsten, L. Integrated control of citrus green and blue molds using Bacillus subtilis in combination with sodium bicarbonate or hot water. Postharvest Biol. Technol. 2003, 28, 187–194. [Google Scholar] [CrossRef]
- Stéphane, C.; Brion, D.; Jerzy, N.; Christophe, C.; Ait, B.E. Use of Plant Growth-Promoting Bacteria for Biocontrol of Plant Diseases: Principles, Mechanisms of Action, and Future Prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [Green Version]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef]
- Pimenta, R.S.; Morais, P.B.; Rosa, C.A.; Corrêa, A. Utilization of Yeasts in Biological Control Programs. In Yeast Biotechnology: Diversity and Applications; Satyanarayana, T., Kunze, G., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 199–214. ISBN 978-1-4020-8292-4. [Google Scholar]
- Raaijmakers, J.M.; Vlami, M.; de Souza, J.T. Antibiotic production by bacterial biocontrol agents. Antonie Van Leeuwenhoek 2002, 81, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Lachance, M.-A. Yeast Biodiversity: How Many and How Much? In Biodiversity and Ecophysiology of Yeasts; Péter, G., Rosa, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–9. ISBN 978-3-540-30985-7. [Google Scholar]
- Shanmuganathan, N. Yeasts as a Biocontrol for Microbial Diseases of Fruit. U.S. Patent No. 5,525,132, 11 June 1996. [Google Scholar]
- Wilson, C.; Ghaouth, A.E. Composition containing 2-deoxy-D-glucose and Candida saitoana and a method of use for the biological control of postharvest diseases. U.S. Patent No. 5,591,429, 7 January 1997. [Google Scholar]
- Lima, G.; Ippolito, A.; Nigro, F.; Romanazzi, G.; Schena, L.; Gatto, M.; Salerno, M. Lotta biologica contro marciumi postraccolta di uva da tavola, fragola e actinidia con Aureobasidium pullulans e Candida oleophila. Inf. Agrar. 1996, 45, 79–84. [Google Scholar]
- Lima, G.; Ippolito, A.; Nigro, F.; Salerno, M. Effectiveness of Aureobasidium pullulans and Candida oleophila against postharvest strawberry rots. Postharvest Biol. Technol. 1997, 10, 169–178. [Google Scholar] [CrossRef]
- Weiss, A.; Mögel, G.; Kunz, S. Development of “Boni-Pro-tect”-a Yeast Preparation For Use In the Control of Post-harvest Diseases of Apples. In Proceedings of the 12th International Conference on Cultivation Technique and Phytopathological Problems in Organic Fruit-Growing, Weinsberg, Germany, 31 January–2 February 2006. [Google Scholar]
- Spadaro, D.; Gullino, M.L. State of the art and future prospects of the biological control of postharvest fruit diseases. Int. J. Food Microbiol. 2004, 91, 185–194. [Google Scholar] [CrossRef]
- Lima, G.; Spina, A.M.; Castoria, R.; De Curtis, F.; De Cicco, V. Integration of Biocontrol Agents and Food-Grade Additives for Enhancing Protection of Stored Apples from Penicillium expansum. J. Food Prot. 2005, 68, 2100–2106. [Google Scholar] [CrossRef]
- Lima, G.; De Curtis, F.; De Cicco, V. Interaction of microbial biocontrol agents and fungicides in the control of postharvest disease. Stewart Postharvest Rev. 2008, 4, 1–7. [Google Scholar] [CrossRef]
- Cañamás, T.P.; Viñas, I.; Torres, R.; Usall, J.; Solsona, C.; Teixidó, N. Field applications of improved formulations of Candida sake CPA-1 for control of Botrytis cinerea in grapes. Biol. Control 2011, 56, 150–158. [Google Scholar] [CrossRef]
- Calvo-Garrido, C.; Viñas, I.; Elmer, P.; Usall, J.; Teixidó, N. Candida sake CPA-1 and other biologically based products as potential control strategies to reduce sour rot of grapes. Lett. Appl. Microbiol. 2013, 57, 356–361. [Google Scholar] [CrossRef]
- Zhang, H.; Mahunu, G.K.; Castoria, R.; Yang, Q.; Apaliya, M.T. Recent developments in the enhancement of some postharvest biocontrol agents with unconventional chemicals compounds. Trends Food Sci. Technol. 2018, 78, 180–187. [Google Scholar] [CrossRef]
- Castoria, R.; Wright, S.A.I.; Droby, S. Biological Control of Mycotoxigenic Fungi in Fruits. In Mycotoxins in Fruits and Vegetables; Barkai-Golan, R., Paster, N., Eds.; Academic Press: San Diego, CA, USA, 2008; Chapter 16; pp. 311–333. ISBN 978-0-12-374126-4. [Google Scholar]
- Zheng, X.; Yang, Q.; Zhang, X.; Apaliya, M.T.; Ianiri, G.; Zhang, H.; Castoria, R. Biocontrol Agents Increase the Specific Rate of Patulin Production by Penicillium expansum but Decrease the Disease and Total Patulin Contamination of Apples. Front. Microbiol. 2017, 8, 1240. [Google Scholar] [CrossRef]
- Lima, G.; Castoria, R.; De Curtis, F.; Raiola, A.; Ritieni, A.; De Cicco, V. Integrated control of blue mould using new fungicides and biocontrol yeasts lowers levels of fungicide residues and patulin contamination in apples. Postharvest Biol. Technol. 2011, 60, 164–172. [Google Scholar] [CrossRef]
- Castoria, R.; Morena, V.; Caputo, L.; Panfili, G.; De Curtis, F.; De Cicco, V. Effect of the Biocontrol Yeast Rhodotorula glutinis Strain LS11 on Patulin Accumulation in Stored Apples. Phytopathology 2005, 95, 1271–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castoria, R.; Mannina, L.; Durán-Patrón, R.; Maffei, F.; Sobolev, A.P.; De Felice, D.V.; Pinedo-Rivilla, C.; Ritieni, A.; Ferracane, R.; Wright, S.A.I. Conversion of the Mycotoxin Patulin to the Less Toxic Desoxypatulinic Acid by the Biocontrol Yeast Rhodosporidium kratochvilovae Strain LS11. J. Agric. Food Chem. 2011, 59, 11571–11578. [Google Scholar] [CrossRef] [Green Version]
- Ianiri, G.; Idnurm, A.; Castoria, R. Transcriptomic responses of the basidiomycete yeast Sporobolomyces sp. to the mycotoxin patulin. BMC Genom. 2016, 17, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Bao, Y.; Shen, D.; Feng, W.; Yu, T.; Zhang, J.; Zheng, X.D. Biocontrol of Alternaria alternata on cherry tomato fruit by use of marine yeast Rhodosporidium paludigenum Fell & Tallman. Int. J. Food Microbiol. 2008, 123, 234–239. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Xia, J.; Yu, T.; Lou, B.; Wang, J.; Zheng, X.D. Effect of water activity on stress tolerance and biocontrol activity in antagonistic yeast Rhodosporidium paludigenum. Int. J. Food Microbiol. 2010, 143, 103–108. [Google Scholar] [CrossRef]
- Zhu, R.; Yu, T.; Guo, S.; Hu, H.A.O.; Zheng, X.; Karlovsky, P. Effect of the Yeast Rhodosporidium paludigenum on Postharvest Decay and Patulin Accumulation in Apples and Pears. J. Food Prot. 2015, 78, 157–163. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, W.; Yao, J.; Niu, J. Production, purification, characterization, and biological properties of Rhodosporidium paludigenum polysaccharide. PLoS ONE 2021, 16, e0246148. [Google Scholar] [CrossRef]
- Wei, M.; Dhanasekaran, S.; Godana, E.A.; Yang, Q.; Sui, Y.; Zhang, X.; Ngolong Ngea, G.L.; Zhang, H. Whole-genome sequencing of Cryptococcus podzolicus Y3 and data-independent acquisition-based proteomic analysis during OTA degradation. Food Control 2022, 136, 108862. [Google Scholar] [CrossRef]
- Ngolong Ngea, G.L.; Yang, Q.; Castoria, R.; Zhang, X.; Routledge, M.N.; Zhang, H. Recent trends in detecting, controlling, and detoxifying of patulin mycotoxin using biotechnology methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2447–2472. [Google Scholar] [CrossRef]
- Vero, S.; Garmendia, G.; González, M.B.; Bentancur, O.; Wisniewski, M. Evaluation of yeasts obtained from Antarctic soil samples as biocontrol agents for the management of postharvest diseases of apple (Malus × domestica). FEMS Yeast Res. 2013, 13, 189–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Wisniewski, M.E.; Abdelfattah, A.; Zheng, X. Biocontrol activity of a cold-adapted yeast from Tibet against gray mold in cherry tomato and its action mechanism. Extremophiles 2017, 21, 789–803. [Google Scholar] [CrossRef] [PubMed]
- De García, V.; Brizzio, S.; Libkind, D.; Buzzini, P.; Van Broock, M. Biodiversity of cold-adapted yeasts from glacial meltwater rivers in Patagonia, Argentina. FEMS Microbiol. Ecol. 2007, 59, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Janisiewicz, W.J.; Tworkoski, T.J.; Kurtzman, C.P. Biocontrol Potential of Metchnikowia pulcherrima Strains against Blue Mold of Apple. Phytopathology 2001, 91, 1098–1108. [Google Scholar] [CrossRef] [Green Version]
- Kurtzman, C.P.; Droby, S. Metschnikowia fructicola, a New Ascosporic Yeast with Potential for Biocontrol of Postharvest Fruit Rots. Syst. Appl. Microbiol. 2001, 24, 395–399. [Google Scholar] [CrossRef] [Green Version]
- Piombo, E.; Sela, N.; Wisniewski, M.; Hoffmann, M.; Gullino, M.L.; Allard, M.W.; Levin, E.; Spadaro, D.; Droby, S. Genome Sequence, Assembly and Characterization of Two Metschnikowia fructicola Strains Used as Biocontrol Agents of Postharvest Diseases. Front. Microbiol. 2018, 9, 593. [Google Scholar] [CrossRef] [Green Version]
- Pereyra, M.M.; Díaz, M.A.; Soliz-Santander, F.F.; Poehlein, A.; Meinhardt, F.; Daniel, R.; Dib, J.R. Screening Methods for Isolation of Biocontrol Epiphytic Yeasts against Penicillium digitatum in Lemons. J. Fungi 2021, 7, 166. [Google Scholar] [CrossRef]
- Lima, G.; De Curtis, F.; Castoria, R.; De Cicco, V. Activity of the Yeasts Cryptococcus laurentii and Rhodotorula glutinis against Post-harvest Rots on Different Fruits. Biocontrol Sci. Technol. 1998, 8, 257–267. [Google Scholar] [CrossRef]
- Palmieri, D.; Barone, G.; Cigliano, R.A.; De Curtis, F.; Lima, G.; Castoria, R.; Ianiri, G. Complete genome sequence of the biocontrol yeast Papiliotrema terrestris strain LS28. G3 Genes|Genomes|Genetics 2021, 11, jkab332. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Corke, A.T.K. Biological Control of Plant Pathogens. By Kenneth F. Baker and R. James Cook. San Francisco: Freeman (1974), pp. 433, £5.90. Exp. Agric. 1975, 11, 159. [Google Scholar] [CrossRef]
- Raymaekers, K.; Ponet, L.; Holtappels, D.; Berckmans, B.; Cammue, B.P.A. Screening for novel biocontrol agents applicable in plant disease management—A review. Biol. Control 2020, 144, 104240. [Google Scholar] [CrossRef]
- Benl tez, T.A.; Rincón, A.M.; Limón, M.C.; Codón, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar]
- Huang, H.C.; Erickson, R.S. Overwintering of Coniothyrium minkans, a mycoparasite of Sclerotinia sclerotiorum, on the Canadian Prairies. Australas. Plant Pathol. 2011, 31, 291–293. [Google Scholar] [CrossRef]
- Tu, J.C. Hyperparasitism of Streptomyces albus on a Destructive Mycoparasite Nectria inventa. J. Phytopathol. 1986, 117, 71–76. [Google Scholar] [CrossRef]
- Nofal, A.M.; El-Rahman, M.A.; Abdelghany, T.M.; Abd El-Mongy, M. Mycoparasitic nature of Egyptian Trichoderma isolates and their impact on suppression Fusarium wilt of tomato. Egypt. J. Biol. Pest Control 2021, 31, 103. [Google Scholar] [CrossRef]
- Moreno-Ruiz, D.; Lichius, A.; Turrà, D.; Di Pietro, A.; Zeilinger, S. Chemotropism Assays for Plant Symbiosis and Mycoparasitism Related Compound Screening in Trichoderma atroviride. Front. Microbiol. 2020, 11, 3006. [Google Scholar] [CrossRef] [PubMed]
- Horner, N.R.; Grenville-Briggs, L.J.; van West, P. The oomycete Pythium oligandrum expresses putative effectors during mycoparasitism of Phytophthora infestans and is amenable to transformation. Fungal Biol. 2012, 116, 24–41. [Google Scholar] [CrossRef] [Green Version]
- Deacon, J.W.; Henry, C.M. Methods used to study Pythium oligandrum, an aggressive parasite of other fungi. Ann. Appl. Biol. 1978, 89, 141–142. [Google Scholar] [CrossRef]
- Heydari, A.; Pessarakli, M. A review on biological control of fungal plant pathogens using microbial antagonists. J. Biol. Sci. 2010, 10, 273–290. [Google Scholar] [CrossRef] [Green Version]
- Tapio, E.; Pohto-Lahdenperä, A. Scanning electron microscopy of hyphal interaction between Streptomyces griseoviridis and some plant pathogenic fungi. Agric. Food Sci. 1991, 63, 435–441. [Google Scholar] [CrossRef]
- Ziedan, E.-S.H.; Farrag, E.S.; El-Mohamedy, R.S.; Abd Alla, M.A. Streptomyces alni as a biocontrol agent to root-rot of grapevine and increasing their efficiency by biofertilisers inocula. Arch. Phytopathol. Plant Prot. 2010, 43, 634–646. [Google Scholar] [CrossRef]
- Trunk, K.; Peltier, J.; Liu, Y.-C.; Dill, B.D.; Walker, L.; Gow, N.A.R.; Stark, M.J.R.; Quinn, J.; Strahl, H.; Trost, M.; et al. The type VI secretion system deploys antifungal effectors against microbial competitors. Nat. Microbiol. 2018, 3, 920–931. [Google Scholar] [CrossRef]
- Pal, K.K.; McSpadden Gardener, B. Biological Control of Plant Pathogens. Plant Health Instr. 2006, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Benhamou, N.; Chet, I. Cellular and Molecular Mechanisms Involved in the Interaction between Trichoderma harzianum and Pythium ultimum. Appl. Environ. Microbiol. 1997, 63, 2095–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Di Francesco, A.; Martini, C.; Mari, M. Biological control of postharvest diseases by microbial antagonists: How many mechanisms of action? Eur. J. Plant Pathol. 2016, 145, 711–717. [Google Scholar] [CrossRef]
- Nunes, C.A. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.T.; Khan, A.; Chung, E.J.; Rashid, M.H.-O.; Chung, Y.R. Biological Control of Rice Bakanae by an Endophytic Bacillus oryzicola YC7007. Plant Pathol. J. 2016, 32, 228–241. [Google Scholar] [CrossRef] [Green Version]
- Meziane, H.; Gavriel, S.; Ismailov, Z.; Chet, I.; Chernin, L.; Höfte, M. Control of green and blue mould on orange fruit by Serratia plymuthica strains IC14 and IC1270 and putative modes of action. Postharvest Biol. Technol. 2006, 39, 125–133. [Google Scholar] [CrossRef]
- Pretorius, D.; van Rooyen, J.; Clarke, K.G. Enhanced production of antifungal lipopeptides by Bacillus amyloliquefaciens for biocontrol of postharvest disease. New Biotechnol. 2015, 32, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.; Keel, C. Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease. Annu. Rev. Phytopathol. 2003, 41, 117–153. [Google Scholar] [CrossRef] [PubMed]
- Brescia, F.; Vlassi, A.; Bejarano, A.; Seidl, B.; Marchetti-Deschmann, M.; Schuhmacher, R.; Puopolo, G. Characterisation of the Antibiotic Profile of Lysobacter capsici AZ78, an Effective Biological Control Agent of Plant Pathogenic Microorganisms. Microorganisms 2021, 9, 1320. [Google Scholar] [CrossRef] [PubMed]
- Poppe, L.; Vanhoutte, S.; Höfte, M. Modes of Action of Pantoea agglomerans CPA-2, an Antagonist of Postharvest Pathogens on Fruits. Eur. J. Plant Pathol. 2003, 109, 963–973. [Google Scholar] [CrossRef]
- Pacios-Michelena, S.; Aguilar González, C.N.; Alvarez-Perez, O.B.; Rodriguez-Herrera, R.; Chávez-González, M.; Arredondo Valdés, R.; Ascacio Valdés, J.A.; Govea Salas, M.; Ilyina, A. Application of Streptomyces Antimicrobial Compounds for the Control of Phytopathogens. Front. Sustain. Food Syst. 2021, 5, 1–13. [Google Scholar] [CrossRef]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Antagonistic fungi, Trichoderma spp.: Panoply of biological control. Biochem. Eng. J. 2007, 37, 1–20. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Choińska, R.; Piasecka-Jóźwiak, K.; Chabłowska, B.; Dumka, J.; Łukaszewicz, A. Biocontrol ability and volatile organic compounds production as a putative mode of action of yeast strains isolated from organic grapes and rye grains. Antonie Van Leeuwenhoek 2020, 113, 1135–1146. [Google Scholar] [CrossRef]
- Song, G.C.; Ryu, C.-M. Two Volatile Organic Compounds Trigger Plant Self-Defense against a Bacterial Pathogen and a Sucking Insect in Cucumber under Open Field Conditions. Int. J. Mol. Sci. 2013, 14, 9803–9819. [Google Scholar] [CrossRef] [Green Version]
- Bevan, E.A.; Makower, M. The physiological basis of the killer character in yeast. In Proceedings of the International Congress of Genetics, The Hague, The Netherland, 2–10 September 1963; Pergamon Press: Oxford, UK, 1963; Volume 1, pp. 202–203. [Google Scholar]
- Farkas, Z.; Márki-Zay, J.; Kucsera, J.; Vágvölgyi, C.; Golubev, W.; Pfeiffer, I. Characterization of two different toxins of Wickerhamomyces anomalus (pichia anomala) VKM Y-159. Acta Biol. Hung. 2012, 63, 277–287. [Google Scholar] [CrossRef] [PubMed]
- de Lima, J.R.; Gonçalves, L.R.B.; Brandão, L.R.; Rosa, C.A.; Viana, F.M.P. Isolation, identification, and activity in vitro of killer yeasts against Colletotrichum gloeosporioides isolated from tropical fruits. J. Basic Microbiol. 2013, 53, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Giovati, L.; Ciociola, T.; De Simone, T.; Conti, S.; Magliani, W. Wickerhamomyces Yeast Killer Toxins’ Medical Applications. Toxins 2021, 13, 655. [Google Scholar] [CrossRef]
- Schmitt, M.J.; Breinig, F. The viral killer system in yeast: From molecular biology to application. FEMS Microbiol. Rev. 2002, 26, 257–276. [Google Scholar] [CrossRef]
- Grzegorczyk, M.; Żarowska, B.; Restuccia, C.; Cirvilleri, G. Postharvest biocontrol ability of killer yeasts against Monilinia fructigena and Monilinia fructicola on stone fruit. Food Microbiol. 2017, 61, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Fredericks, L.R.; Lee, M.D.; Crabtree, A.M.; Boyer, J.M.; Kizer, E.A.; Taggart, N.T.; Roslund, C.R.; Hunter, S.S.; Kennedy, C.B.; Willmore, C.G.; et al. The Species-Specific Acquisition and Diversification of a K1-like Family of Killer Toxins in Budding Yeasts of the Saccharomycotina. PLOS Genet. 2021, 17, e1009341. [Google Scholar] [CrossRef]
- Brown, D.W. The KP4 killer protein gene family. Curr. Genet. 2011, 57, 51–62. [Google Scholar] [CrossRef]
- Schmitt, M.J.; Breinig, F. Yeast viral killer toxins: Lethality and self-protection. Nat. Rev. Microbiol. 2006, 4, 212–221. [Google Scholar] [CrossRef]
- Walker, G.M. Pichia anomala: Cell physiology and biotechnology relative to other yeasts. Antonie Van Leeuwenhoek 2011, 99, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Moura, V.S.; Pollettini, F.L.; Ferraz, L.P.; Mazzi, M.V.; Kupper, K.C. Purification of a killer toxin from Aureobasidium pullulans for the biocontrol of phytopathogens. J. Basic Microbiol. 2021, 61, 77–87. [Google Scholar] [CrossRef]
- Ghoul, M.; Mitri, S. The Ecology and Evolution of Microbial Competition. Trends Microbiol. 2016, 24, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Turrà, D.; Di Pietro, A. Chemotropic sensing in fungus–plant interactions. Curr. Opin. Plant Biol. 2015, 26, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The Role of Root Exudates in Rhizosphere Interactions with Plants and Other Organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Liu, Y. Review: Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 2013, 167, 153–160. [Google Scholar] [CrossRef]
- Castoria, R.; Caputo, L.; De Curtis, F.; De Cicco, V. Resistance of Postharvest Biocontrol Yeasts to Oxidative Stress: A Possible New Mechanism of Action. Phytopathology 2003, 93, 564–572. [Google Scholar] [CrossRef] [Green Version]
- Castoria, R.; Miccoli, C.; Barone, G.; Palmieri, D.; De Curtis, F.; Lima, G.; Heitman, J.; Ianiri, G. Molecular Tools for the Yeast Papiliotrema terrestris LS28 and Identification of Yap1 as a Transcription Factor Involved in Biocontrol Activity. Appl. Environ. Microbiol. 2021, 87, e02910-20. [Google Scholar] [CrossRef]
- Liu, J.; Wisniewski, M.; Droby, S.; Norelli, J.; Hershkovitz, V.; Tian, S.; Farrell, R. Increase in antioxidant gene transcripts, stress tolerance and biocontrol efficacy of Candida oleophila following sublethal oxidative stress exposure. FEMS Microbiol. Ecol. 2012, 80, 578–590. [Google Scholar] [CrossRef]
- Filonow, A.B. Role of Competition for Sugars by Yeasts in the Biocontrol of Gray Mold of Apple. Biocontrol Sci. Technol. 1998, 8, 243–256. [Google Scholar] [CrossRef]
- van Loon, L.C. Helping Plants To Defend Themselves: Biocontrol By Disease-Suppressing Rhizobacteria. In Highlights in European Plant Biotechnology Research and Technology Transfer; de Vries, G.E., Metzlaff, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 6, pp. 203–213. ISBN 0168-7972. [Google Scholar]
- Segarra, G.; Casanova, E.; Avilés, M.; Trillas, I. Trichoderma asperellum Strain T34 Controls Fusarium Wilt Disease in Tomato Plants in Soilless Culture through Competition for Iron. Microb. Ecol. 2010, 59, 141–149. [Google Scholar] [CrossRef]
- Saravanakumar, D.; Ciavorella, A.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Metschnikowia pulcherrima strain MACH1 outcompetes Botrytis cinerea, Alternaria alternata and Penicillium expansum in apples through iron depletion. Postharvest Biol. Technol. 2008, 49, 121–128. [Google Scholar] [CrossRef]
- Calvente, V.; Benuzzi, D.; de Tosetti, M.I.S. Antagonistic action of siderophores from Rhodotorula glutinis upon the postharvest pathogen Penicillium expansum. Int. Biodeterior. Biodegrad. 1999, 43, 167–172. [Google Scholar] [CrossRef]
- Di Francesco, A.; Baraldi, E. How siderophore production can influence the biocontrol activity of Aureobasidium pullulans against Monilinia laxa on peaches. Biol. Control 2021, 152, 104456. [Google Scholar] [CrossRef]
- Oberegger, H.; Schoeser, M.; Zadra, I.; Abt, B.; Haas, H. SREA is involved in regulation of siderophore biosynthesis, utilization and uptake in Aspergillus nidulans. Mol. Microbiol. 2001, 41, 1077–1089. [Google Scholar] [CrossRef]
- Klein, M.N.; Kupper, K.C. Biofilm production by Aureobasidium pullulans improves biocontrol against sour rot in citrus. Food Microbiol. 2018, 69, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castoria, R.; De Curtis, F.; Lima, G.; De Cicco, V. β-1,3-glucanase activity of two saprophytic yeasts and possible mode of action as biocontrol agents against postharvest diseases. Postharvest Biol. Technol. 1997, 12, 293–300. [Google Scholar] [CrossRef]
- Di Francesco, A.; Ugolini, L.; D’Aquino, S.; Pagnotta, E.; Mari, M. Biocontrol of Monilinia laxa by Aureobasidium pullulans strains: Insights on competition for nutrients and space. Int. J. Food Microbiol. 2017, 248, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F. Facing Climate Change: Application of Microbial Biostimulants to Mitigate Stress in Horticultural Crops. Agronomy 2020, 10, 794. [Google Scholar] [CrossRef]
- Kerry, B.R. Rhizosphere Interactions and the Exploitation of Microbial Agents for the Biological Control of Plant-Parasitic Nematodes. Annu. Rev. Phytopathol. 2000, 38, 423–441. [Google Scholar] [CrossRef] [Green Version]
- Ramamoorthy, V.; Viswanathan, R.; Raguchander, T.; Prakasam, V.; Samiyappan, R. Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pests and diseases. Crop Prot. 2001, 20, 1–11. [Google Scholar] [CrossRef]
- Khanna, K.; Kohli, S.K.; Ohri, P.; Bhardwaj, R. Plants-nematodes-microbes crosstalk within soil: A trade-off among friends or foes. Microbiol. Res. 2021, 248, 126755. [Google Scholar] [CrossRef]
- Bhavanam, S.; Stout, M.J. Assessment of Silicon-and Mycorrhizae-Mediated Constitutive and Induced Systemic Resistance in Rice, Oryza sativa L., against the Fall Armyworm, Spodoptera frugiperda Smith. Plants 2021, 10, 2126. [Google Scholar] [CrossRef] [PubMed]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for Enhanced Defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef] [PubMed]
- van Loon, L.C.; Bakker, P.A.H.M.; Pieterse, C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef] [Green Version]
- Yariv, B.; Arik, M.; Yechiel, S.; Ilan, C.; Ada, V. Synthetic Ultrashort Cationic Lipopeptides Induce Systemic Plant Defense Responses against Bacterial and Fungal Pathogens. Appl. Environ. Microbiol. 2009, 75, 5373–5379. [Google Scholar] [CrossRef] [Green Version]
- Kuć, J. Concepts and Direction of Induced Systemic Resistance in Plants and its Application. Eur. J. Plant Pathol. 2001, 107, 7–12. [Google Scholar] [CrossRef]
- van Peer, R.; Niemann, G.J.; Schippers, B. Induced resistance and phytoalexin accumulation in biological control of Fusarium wilt of carnation by Pseudomonas sp. strain WCS417r. Phytopathology 1991, 81, 728–734. [Google Scholar] [CrossRef]
- Wei, G. Induction of systemic resistance of cucumber to Colletotrichum orbiculare by select strains of plant growth-promoting rhizobacteria. Phytopathology 1991, 81, 1508–1512. [Google Scholar] [CrossRef]
- Barka, E.A.; Belarbi, A.; Hachet, C.; Nowak, J.; Audran, J.-C. Enhancement of in vitro growth and resistance to gray mould of Vitis vinifera co-cultured with plant growth-promoting rhizobacteria. FEMS Microbiol. Lett. 2000, 186, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Lahoz, E.; Contillo, R.; Porrone, F. Induction of Systemic Resistance to Erysiphe orontii Cast in Tobacco by Application on Roots of an Isolate of Gliocladium roseum Bainier. J. Phytopathol. 2004, 152, 465–470. [Google Scholar] [CrossRef]
- Brooks, D.S.; Gonzalez, C.F.; Appel, D.N.; Filer, T.H. Evaluation of Endophytic Bacteria as Potential Biological-Control Agents for Oak Wilt. Biol. Control 1994, 4, 373–381. [Google Scholar] [CrossRef]
- Khan, N.; Martínez-Hidalgo, P.; Ice, T.A.; Maymon, M.; Humm, E.A.; Nejat, N.; Sanders, E.R.; Kaplan, D.; Hirsch, A.M. Antifungal Activity of Bacillus Species against Fusarium and Analysis of the Potential Mechanisms Used in Biocontrol. Front. Microbiol. 2018, 9, 2363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostini, R.B.; Rius, S.P.; Vargas, W.A.; Campos-Bermudez, V.A. Proteome impact on maize silks under the priming state induced by Trichoderma root colonization. Planta 2021, 253, 115. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Aggarwal, R.; Bashyal, B.M.; Darshan, K.; Parmar, P.; Saharan, M.S.; Hussain, Z.; Solanke, A.U. Transcriptome Reprogramming of Tomato Orchestrate the Hormone Signaling Network of Systemic Resistance Induced by Chaetomium globosum. Front. Plant Sci. 2021, 12, 721193. [Google Scholar] [CrossRef]
- Roylawar, P.; Khandagale, K.; Randive, P.; Shinde, B.; Murumkar, C.; Ade, A.; Singh, M.; Gawande, S.; Morelli, M. Piriformospora indica Primes Onion Response against Stemphylium Leaf Blight Disease. Pathogen 2021, 10, 1085. [Google Scholar] [CrossRef]
- Shaw, S.; Le Cocq, K.; Paszkiewicz, K.; Moore, K.; Winsbury, R.; de Torres Zabala, M.; Studholme, D.J.; Salmon, D.; Thornton, C.R.; Grant, M.R. Transcriptional reprogramming underpins enhanced plant growth promotion by the biocontrol fungus Trichoderma hamatum GD12 during antagonistic interactions with Sclerotinia sclerotiorum in soil. Mol. Plant Pathol. 2016, 17, 1425–1441. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Salwan, R.; Sharma, P.N.; Gulati, A. Integrated Translatome and Proteome: Approach for Accurate Portraying of Widespread Multifunctional Aspects of Trichoderma. Front. Microbiol. 2017, 8, 1602. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, T.; Xie, J.; Cheng, J.; Chen, T.; Jiang, D.; Fu, Y. Mycoparasitism illuminated by genome and transcriptome sequencing of Coniothyrium minitans, an important biocontrol fungus of the plant pathogen Sclerotinia sclerotiorum. Microb. Genom. 2020, 6, e000345. [Google Scholar] [CrossRef]
- Darshan, K.; Aggarwal, R.; Bashyal, B.M.; Singh, J.; Shanmugam, V.; Gurjar, M.S.; Solanke, A.U. Transcriptome Profiling Provides Insights Into Potential Antagonistic Mechanisms Involved in Chaetomium globosum against Bipolaris sorokiniana. Front. Microbiol. 2020, 11, 2971. [Google Scholar] [CrossRef]
- Broberg, M.; Dubey, M.; Iqbal, M.; Gudmundssson, M.; Ihrmark, K.; Schroers, H.-J.; Funck Jensen, D.; Brandström Durling, M.; Karlsson, M. Comparative genomics highlights the importance of drug efflux transporters during evolution of mycoparasitism in Clonostachys subgenus Bionectria (Fungi, Ascomycota, Hypocreales). Evol. Appl. 2021, 14, 476–497. [Google Scholar] [CrossRef]
- Westermann, A.J.; Barquist, L.; Vogel, J. Resolving host–pathogen interactions by dual RNA-seq. PLOS Pathog. 2017, 13, e1006033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espindula, E.; Sperb, E.R.; Bach, E.; Passaglia, L.M.P. The combined analysis as the best strategy for Dual RNA-Seq mapping. Genet. Mol. Biol. 2020, 42, e20190215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westermann, A.J.; Gorski, S.A.; Vogel, J. Dual RNA-seq of pathogen and host. Nat. Rev. Microbiol. 2012, 10, 618–630. [Google Scholar] [CrossRef]
- Lysøe, E.; Dees, M.W.; Brurberg, M.B. A Three-Way Transcriptomic Interaction Study of a Biocontrol Agent (Clonostachys rosea), a Fungal Pathogen (Helminthosporium solani), and a Potato Host (Solanum tuberosum). Mol. Plant-Microbe Interact. 2017, 30, 646–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hershkovitz, V.; Sela, N.; Taha-Salaime, L.; Liu, J.; Rafael, G.; Kessler, C.; Aly, R.; Levy, M.; Wisniewski, M.; Droby, S. De-novo assembly and characterization of the transcriptome of Metschnikowia fructicola reveals differences in gene expression following interaction with Penicillium digitatumand grapefruit peel. BMC Genom. 2013, 14, 168. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Chen, L.; Sun, Y.; Zhao, L.; Zheng, X.; Yang, Q.; Zhang, X. Investigating Proteome and Transcriptome Defense Response of Apples Induced by Yarrowia lipolytica. Mol. Plant-Microbe Interact. 2017, 30, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Rueda-Mejia, M.P.; Nägeli, L.; Lutz, S.; Hayes, R.D.; Varadarajan, A.R.; Grigoriev, I.V.; Ahrens, C.H.; Freimoser, F.M. Genome, transcriptome and secretome analyses of the antagonistic, yeast-like fungus Aureobasidium pullulans to identify potential biocontrol genes. Microb. Cell 2021, 8, 184–202. [Google Scholar] [CrossRef]
- Laur, J.; Ramakrishnan, G.B.; Labbé, C.; Lefebvre, F.; Spanu, P.D.; Bélanger, R.R. Effectors involved in fungal–fungal interaction lead to a rare phenomenon of hyperbiotrophy in the tritrophic system biocontrol agent–powdery mildew–plant. New Phytol. 2018, 217, 713–725. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-Y.; Gao, T.-T.; Wang, Q. Comparative and Functional Analyses of Two Sequenced Paenibacillus polymyxa Genomes Provides Insights into Their Potential Genes Related to Plant Growth-Promoting Features and Biocontrol Mechanisms. Front. Genet. 2020, 11, 1374. [Google Scholar] [CrossRef]
- Nelkner, J.; Torres Tejerizo, G.; Hassa, J.; Lin, T.W.; Witte, J.; Verwaaijen, B.; Winkler, A.; Bunk, B.; Spröer, C.; Overmann, J.; et al. Genetic Potential of the Biocontrol Agent Pseudomonas brassicacearum (Formerly P. trivialis) 3Re2-7 Unraveled by Genome Sequencing and Mining, Comparative Genomics and Transcriptomics. Genes 2019, 10, 601. [Google Scholar] [CrossRef] [Green Version]
- Hennessy, R.C.; Glaring, M.A.; Olsson, S.; Stougaard, P. Transcriptomic profiling of microbe–microbe interactions reveals the specific response of the biocontrol strain P. fluorescens In5 to the phytopathogen Rhizoctonia solani. BMC Res. Notes 2017, 10, 376. [Google Scholar] [CrossRef] [Green Version]
- Penttilä, M.; Nevalainen, H.; Rättö, M.; Salminen, E.; Knowles, J. A versatile transformation system for the cellulolytic filamentous fungus Trichoderma reesei. Gene 1987, 61, 155–164. [Google Scholar] [CrossRef]
- Woo, S.L.; Scala, F.; Ruocco, M.; Lorito, M. The Molecular Biology of the Interactions between Trichoderma spp., Phytopathogenic Fungi, and Plants. Phytopathology 2006, 96, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Adnan, M.; Islam, W.; Shabbir, A.; Khan, K.A.; Ghramh, H.A.; Huang, Z.; Chen, H.Y.H.; Lu, G. Plant defense against fungal pathogens by antagonistic fungi with Trichoderma in focus. Microb. Pathog. 2019, 129, 7–18. [Google Scholar] [CrossRef]
- Silva, R.N.; Monteiro, V.N.; Steindorff, A.S.; Gomes, E.V.; Noronha, E.F.; Ulhoa, C.J. Trichoderma/pathogen/plant interaction in pre-harvest food security. Fungal Biol. 2019, 123, 565–583. [Google Scholar] [CrossRef]
- Monfil, V.O.; Casas-Flores, S. Molecular Mechanisms of Biocontrol in Trichoderma spp. and Their Applications in Agriculture. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 32; pp. 429–453. ISBN 978-0-444-59576-8. [Google Scholar]
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma Research in the Genome Era. Annu. Rev. Phytopathol. 2013, 51, 105–129. [Google Scholar] [CrossRef]
- Zeilinger, S.; Reithner, B.; Scala, V.; Peissl, I.; Lorito, M.; Mach, R.L. Signal transduction by Tga3, a novel G protein alpha subunit of Trichoderma atroviride. Appl. Environ. Microbiol. 2005, 71, 1591–1597. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Ramírez, V.; Omero, C.; Chet, I.; Horwitz, B.A.; Herrera-Estrella, A. Trichoderma atroviride G-Protein α-Subunit Gene tga1 Is Involved in Mycoparasitic Coiling and Conidiation. Eukaryot. Cell 2002, 1, 594–605. [Google Scholar] [CrossRef] [Green Version]
- Omann, M.R.; Lehner, S.; Escobar Rodríguez, C.; Brunner, K.; Zeilinger, S. The seven-transmembrane receptor Gpr1 governs processes relevant for the antagonistic interaction of Trichoderma atroviride with its host. Microbiology 2012, 158, 107–118. [Google Scholar] [CrossRef]
- Ruocco, M.; Lanzuise, S.; Vinale, F.; Marra, R.; Turrà, D.; Woo, S.L.; Lorito, M. Identification of a New Biocontrol Gene in Trichoderma atroviride: The Role of an ABC Transporter Membrane Pump in the Interaction with Different Plant-Pathogenic Fungi. Mol. Plant-Microbe Interact. 2009, 22, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K.; Kenerley, C.M. Regulation of Morphogenesis and Biocontrol Properties in Trichoderma virens by a VELVET Protein, Vel1. Appl. Environ. Microbiol. 2010, 76, 2345–2352. [Google Scholar] [CrossRef] [Green Version]
- Dautt-Castro, M.; Estrada-Rivera, M.; Olguin-Martínez, I.; del Carmen Rocha-Medina, M.; Islas-Osuna, M.A.; Casas-Flores, S. TBRG-1 a Ras-like protein in Trichoderma virens involved in conidiation, development, secondary metabolism, mycoparasitism, and biocontrol unveils a new family of Ras-GTPases. Fungal Genet. Biol. 2020, 136, 103292. [Google Scholar] [CrossRef]
- Nygren, K.; Dubey, M.; Zapparata, A.; Iqbal, M.; Tzelepis, G.D.; Durling, M.B.; Jensen, D.F.; Karlsson, M. The mycoparasitic fungus Clonostachys rosea responds with both common and specific gene expression during interspecific interactions with fungal prey. Evol. Appl. 2018, 11, 931–949. [Google Scholar] [CrossRef] [Green Version]
- Lv, B.; Jiang, N.; Hasan, R.; Chen, Y.; Sun, M.; Li, S. Cell Wall Biogenesis Protein Phosphatase CrSsd1 Is Required for Conidiation, Cell Wall Integrity, and Mycoparasitism in Clonostachys rosea. Front. Microbiol. 2020, 11, 1640. [Google Scholar] [CrossRef]
- Iqbal, M.; Dubey, M.; Broberg, A.; Viketoft, M.; Jensen, D.F.; Karlsson, M. Deletion of the Nonribosomal Peptide Synthetase Gene nps1 in the Fungus Clonostachys rosea Attenuates Antagonism and Biocontrol of Plant Pathogenic Fusarium and Nematodes. Phytopathology 2019, 109, 1698–1709. [Google Scholar] [CrossRef]
- Fatema, U.; Broberg, A.; Jensen, D.F.; Karlsson, M.; Dubey, M. Functional analysis of polyketide synthase genes in the biocontrol fungus Clonostachys rosea. Sci. Rep. 2018, 8, 15009. [Google Scholar] [CrossRef]
- Sun, Z.-B.; Wang, Q.; Sun, M.-H.; Li, S.-D. The Mitogen-Activated Protein Kinase Gene Crmapk Is Involved in Clonostachys chloroleuca Mycoparasitism. Mol. Plant-Microbe Interact. 2020, 33, 902–910. [Google Scholar] [CrossRef]
- Sun, Z.-B.; Sun, M.-H.; Zhou, M.; Li, S.-D. Transformation of the endochitinase gene Chi67-1 in Clonostachys rosea 67-1 increases its biocontrol activity against Sclerotinia sclerotiorum. AMB Express 2017, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Zeng, F.; Gong, X.; Hamid, M.I.; Fu, Y.; Jiatao, X.; Cheng, J.; Li, G.; Jiang, D. A fungal cell wall integrity-associated MAP kinase cascade in Coniothyrium minitans is required for conidiation and mycoparasitism. Fungal Genet. Biol. 2012, 49, 347–357. [Google Scholar] [CrossRef]
- Daguerre, Y.; Edel-Hermann, V.; Steinberg, C. Fungal Genes and Metabolites Associated with the Biocontrol of Soil-borne Plant Pathogenic Fungi. In Fungal Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 33–104. ISBN 978-3-319-25001-4. [Google Scholar]
- Yehuda, H.; Droby, S.; Bar-Shimon, M.; Wisniewski, M.; Goldway, M. The effect of under- and overexpressed CoEXG1-encoded exoglucanase secreted by Candida oleophila on the biocontrol of Penicillium digitatum. Yeast 2003, 20, 771–780. [Google Scholar] [CrossRef]
- Bar-Shimon, M.; Yehuda, H.; Cohen, L.; Weiss, B.; Kobeshnikov, A.; Daus, A.; Goldway, M.; Wisniewski, M.; Droby, S. Characterization of extracellular lytic enzymes produced by the yeast biocontrol agent Candida oleophila. Curr. Genet. 2004, 45, 140–148. [Google Scholar] [CrossRef]
- Grevesse, C.; Lepoivre, P.; Jijakli, M.H. Characterization of the Exoglucanase-Encoding Gene PaEXG2 and Study of Its Role in the Biocontrol Activity of Pichia anomala Strain K. Phytopathology 2003, 93, 1145–1152. [Google Scholar] [CrossRef] [Green Version]
- Friel, D.; Pessoa, N.M.G.; Vandenbol, M.; Jijakli, M.H. Separate and Combined Disruptions of Two Exo-β-1,3-Glucanase Genes Decrease the Efficiency of Pichia anomala (Strain K) Biocontrol against Botrytis cinerea on Apple. Mol. Plant-Microbe Interact. 2007, 20, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Fiori, S.; Fadda, A.; Giobbe, S.; Berardi, E.; Migheli, Q. Pichia angusta is an effective biocontrol yeast against postharvest decay of apple fruit caused by Botrytis cinerea and Monilia fructicola. FEMS Yeast Res. 2008, 8, 961–963. [Google Scholar] [CrossRef] [Green Version]
- Gore-Lloyd, D.; Sumann, I.; Brachmann, A.O.; Schneeberger, K.; Ortiz-Merino, R.A.; Moreno-Beltrán, M.; Schläfli, M.; Kirner, P.; Santos Kron, A.; Rueda-Mejia, M.P.; et al. Snf2 controls pulcherriminic acid biosynthesis and antifungal activity of the biocontrol yeast Metschnikowia pulcherrima. Mol. Microbiol. 2019, 112, 317–332. [Google Scholar] [CrossRef] [Green Version]
- Sui, Y.; Sun, Z.; Zou, Y.; Li, W.; Jiang, M.; Luo, Y.; Liao, W.; Wang, Y.; Gao, X.; Liu, J.; et al. The Rlm1 transcription factor in Candida oleophila contributes to abiotic stress resistance and biocontrol efficacy against postharvest gray mold of kiwifruit. Postharvest Biol. Technol. 2020, 166, 111222. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Li, B.; He, C.; Chen, Y.; Tian, S. Influence of Oxidative Stress on Biocontrol Activity of Cryptococcus laurentii against Blue Mold on Peach Fruit. Front. Microbiol. 2017, 8, 151. [Google Scholar] [CrossRef] [Green Version]
- de Werra, P.; Péchy-Tarr, M.; Keel, C.; Maurhofer, M. Role of gluconic acid production in the regulation of biocontrol traits of Pseudomonas fluorescens CHA0. Appl. Environ. Microbiol. 2009, 75, 4162–4174. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Yu, S.-M.; Jeong, S.C.; Lee, Y.H. High-throughput analysis of genes involved in biocontrol performance of Pseudomonas fluorescens NBC275 against Gray mold. J. Appl. Microbiol. 2020, 128, 265–279. [Google Scholar] [CrossRef] [Green Version]
- Murata, K.; Suenaga, M.; Kai, K. Genome Mining Discovery of Protegenins A–D, Bacterial Polyynes Involved in the Antioomycete and Biocontrol Activities of Pseudomonas protegens. ACS Chem. Biol. 2021. [Google Scholar] [CrossRef]
- Chaudhary, T.; Dixit, M.; Gera, R.; Shukla, A.K.; Prakash, A.; Gupta, G.; Shukla, P. Techniques for improving formulations of bioinoculants. 3 Biotech 2020, 10, 199. [Google Scholar] [CrossRef]
- Berninger, T.; González López, Ó.; Bejarano, A.; Preininger, C.; Sessitsch, A. Maintenance and assessment of cell viability in formulation of non-sporulating bacterial inoculants. Microb. Biotechnol. 2018, 11, 277–301. [Google Scholar] [CrossRef] [Green Version]
- Behle, R.; Birthisel, T. Formulations of Entomopathogens as Bioinsecticides. In Mass Production of Beneficial Organisms; Morales-Ramos, J.A., Rojas, M.G., Shapiro-Ilan, D.I., Eds.; Academic Press: San Diego, CA, USA, 2014; Chapter 14; pp. 483–517. ISBN 978-0-12-391453-8. [Google Scholar]
- Butu, M.; Rodino, S.; Butu, A. Biopesticide formulations-current challenges and future perspectives. In Advances in Bio-Inoculant Science; Rakshit, A., Meena, V.S., Abhilash, P.C., Sarma, B.K., Singh, H.B., Fraceto, L., Parihar, M., Singh, A.K., Eds.; Woodhead Publishing: Sawston, UK, 2022; Chapter 3; pp. 19–29. ISBN 978-0-12-823355-9. [Google Scholar]
- Jangir, M.; Sharma, S.; Sharma, S. Development of next-generation formulation against Fusarium oxysporum and unraveling bioactive antifungal metabolites of biocontrol agents. Sci. Rep. 2021, 11, 22895. [Google Scholar] [CrossRef]
- Melin, P.; Schnürer, J.; Håkansson, S. Formulation and stabilisation of the biocontrol yeast Pichia anomala. Antonie Van Leeuwenhoek 2011, 99, 107–112. [Google Scholar] [CrossRef]
- Cumagun, C.J.R. Advances in Formulation of Trichoderma for Biocontrol. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 39; pp. 527–531. ISBN 978-0-444-59576-8. [Google Scholar]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.-P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Sriram, S.; Roopa, K.P.; Savitha, M.J. Extended shelf-life of liquid fermentation derived talc formulations of Trichoderma harzianum with the addition of glycerol in the production medium. Crop Prot. 2011, 30, 1334–1339. [Google Scholar] [CrossRef]
- Montesinos, E. Development, registration and commercialization of microbial pesticides for plant protection. Int. Microbiol. 2003, 6, 245–252. [Google Scholar] [CrossRef]
- Popp, J.; Pető, K.; Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 2013, 33, 243–255. [Google Scholar] [CrossRef]
- (EFSA), E.F.S.A.; Alvarez, F.; Arena, M.; Auteri, D.; Borroto, J.; Brancato, A.; Carrasco Cabrera, L.; Castoldi, A.F.; Chiusolo, A.; Colagiorgi, A.; et al. Peer review of the pesticide risk assessment of the active substance sheep fat. EFSA J. 2022, 20, e07073. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).