Abstract
Shipping of in vitro micro-cuttings in tubes or jars is a frequently used method as the plants are more likely to quickly reproduce and comply with quarantine regulations in plant germplasm distribution. However, these containers are fragile during transportation. To diminish the risk associated with the long-distance shipping of in vitro plants, a safe and widely applicable packing and conservation technique based on microplate and slow growth was developed in this study. Potato cultivar ZHB and ginger cultivar G-2 were used to optimize the system with microplates (96 wells), vacuum-sealed packaging, and slow-growth techniques. Under regular culture conditions, packing in vacuum-sealed microplates reduced the survival of ZHB and G-2 micro-cuttings to 85.8% and 20.0%, respectively, and regeneration to 61.8% and 0%, respectively. Reducing the temperature to 10 °C maintained the survival of ZHB and G-2 micro-cuttings in the range of 83.3–100% after 60 days. Exposure to darkness decreased the survival of G-2 and inhibited regrowth. Thus, conservation in darkness at 10 °C is suggested. The effects of iron concentration and plant growth retardants were further assessed. The addition of 1/4 MS medium combined with 100 mg/L chlormequat chloride (CCC) resulted in full survival and growth inhibition of plantlets, without malformation identified. Finally, incubation with 1/4 MS medium supplemented with 100 mg/L CCC in vacuum-sealed microplates at 10 °C in the dark resulted in high survival and suppressed germination. Sweet potato HXS was incubated as well to test the broad-spectrum applications of the technique; 100% survival and 6.7% germination was gained. Morphological indices of released cuttings recovered to control levels after two cycles of subculture in MS medium. A 0.1–0.2% genetic variation was detected by SSR and ISSR, suggesting genetic stability of the conserved samples. Finally, micro-cuttings were safely transported to cities located thousands of kilometers away without package and sample damage. Our results enable easy distribution of in vitro plant germplasms.
1. Introduction
Availability of and easy access to diverse plant germplasm, including cultivation crops, their wild relatives, and wild species, are of great importance to human survival and contribute to people’s livelihood via staple and cash crop breeding [1], pharmaceuticals [2], rehabilitation [3], environmental beautification [4,5], and ecological governance and stabilization [3]. Vegetatively propagated plants shipped by wrapping the plant or vegetative mass in express containers for long-distance transportation increases the risk of quarantine and environmental contamination [6]. Furthermore, seasonal availability of scion wood or rooted cuttings may limit their usefulness in germplasm distribution. Thus, a safe and efficient method for plant germplasm transportation, especially for vegetatively propagating plants, is needed. Micropropagation, which exploits the totipotent nature of plant cells to generate new individuals from protoplasts, cells, undifferentiated masses of cells (callus), small pieces of tissue, and/or excised organs, is a time-tested and practical ex situ technique for the short- and medium-term conservation of plant germplasm [2,7,8].
Plant germplasm resources are currently exchanged using in vitro micro-cuttings, which are more likely to massively reproduce and comply with quarantine regulations [9,10,11,12]. In vitro samples are subcultured after arriving at the destination and carry no superficial pathogens or insects. Shipping of micro-cuttings can be a challenge as well. Once the containers or culture bags are damaged during transportation or travel, the germplasms cannot be recovered. Biosafety problems still exist if the plant resources carry obligate pathogens, such as bacteria, viruses, and viroids, and the released micro-cuttings can infect other healthy plants [9,10,13]. Medium liquefaction or combination with explants due to shaking or changes in cabin pressure and failure to maintain sterility within the container, in addition to neglect on shipping docks for extended periods, are additional challenges [14]. According to a report from the National Clonal Germplasm Repository in Corvallis, OR, USA, in vitro micro-cuttings are transported and exchanged in sealed and semi-permeable plastic bags containing firm medium (7–8 g/L agar) and they are folded and packed in crushproof containers, which minimizes the shifting of plants and medium in transit. Weather conditions and arrival date also contribute to loss of shipments. However, appropriate packaging and shipping can increase the viability of the transported cultures for a month or longer under normal conditions [14].
Decreasing the cellular metabolism of micro-cuttings and prolonging the intervals between subcultures by slow-growth techniques [15] may minimize the adverse effects on plant viability associated with delays in customs or quarantine. Slow growth of plant germplasm via medium-term conservation based on in vitro micropropagation reduces costs [16]. This method is usually conducted by reducing the culture temperature, supplying osmotic agents in culture medium, and adding growth inhibitors to or removing growth promoters from the medium [17,18]. To date, several plant germplasms have been successfully conserved using this method. For instance, seven genotypes of wild and elite plants were preserved with seven slow-growth media for 12 months; Tavazza et al. (2015) confirmed that treatments resulted in 65% to 85% of survival and 100% of regrowth in surviving plants [19]. SSR (simple sequence repeats) and ISSR (inter-simple sequence repeats) were used to analyze the genetic stability of slow-growing conserved samples. Tahtamouni et al. (2016) conserved Thymbra spicata supplemented with 0.2 M sucrose storage medium for 3 months, resulting in 100% survival [20]. The growth, oil yield, and carvacrol content of recovered plantlets remained unchanged. Eustoma grandiflorum was successfully conserved in vitro for 90 days by Ramírez-Pérez et al. (2020) [16], without significant changes in vitality. The technique of slow growth prolonged the interval between subcultures significantly and did not alter the genetic stability of conserved samples, which facilitates long-distance shipping and exchange of plant micro-cuttings.
The microplate (96 sample wells) offers limited space for culture and biochemical analysis of organisms. It allows less than 250 μL of medium loading and shorter than 0.5 cm of micro-cutting culture. Space-limited culture ensures less thrashing during shipping and smaller explants, resulting in a slower growth rate. Vacuum sealing can effectively reduce mechanical damage, bumping, and microbe germination of items during transportation [21,22]. It has been widely used in the long-distance shipping of fresh plant fruit [23] or semi-finished bioproducts [22]. In addition, reducing air pressure around plants is another important aspect of slow-growth conservation. When the air pressure is reduced in the culture environment, the growth of in vitro plantlets is reduced as well. The initiation of regrowth under normal conditions revealed no phenotypic modification of the plantlets developing from the inoculums [24]. Space-limited incubation and vacuum-sealed packaging stabilize the substances in the container while reducing the microbe germination and plantlet growth, and thus has a high potency for application in long-distance shipping of plant germplasm.
The present study developed an in vitro incubation system for the long-distance transportation of plant germplasm. Potato and ginger, which are two globally important vegetatively propagated agricultural and horticultural crops, were used to optimize the incubation system based on slow-growth techniques, microplates (96 sample wells), and vacuum-sealed packaging. Recovery and regeneration of the packed micro-cuttings were analyzed under normal in vitro culture conditions. The genetic stability was analyzed using molecular markers SSR and ISSR. The transportation resistance of the present system was tested using automobile transportation and traveling by train. The application of this method was tested in sweet potato in vitro micro-cuttings as well.
2. Materials and Methods
2.1. Plant Materials
In vitro materials of 3 cultivated varieties, potato cultivar ‘Zihuabai’ (ZHB), ginger ‘Guizhouxiaohuangjiang-2’ (G-2) (Figure 1A), and sweet potato ‘Hongxinshu’ (HXS), were employed in this study to establish a stable method for plant germplasm medium-term conservation and long-distance shipping. Stock plantlets were cultured in Murashige and Skoog (1962) medium (MS medium) supplemented with 30 g/L sucrose and 7 g/L agar (pH = 5.8) [25]. The cultures were grown at a consistent temperature of 24 ± 2 °C for a 16 h photoperiod with a light intensity of 50 μM s−1 m−2. Subculturing occurred every 3 weeks (Figure 1B).
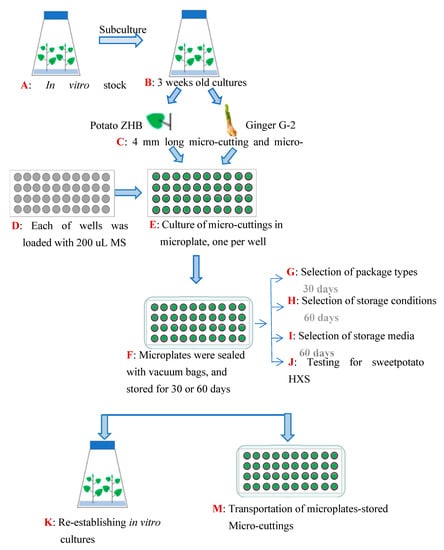
Figure 1.
A flow chart of use of microplates for storage and transportation of in vitro micropropagated plant materials.
2.2. Assessment of Packaging Type
Micro-cuttings, which consisted of one leaf on a 0.4 mm shoot of ZHB and a 0.4 mm long micro-tiller containing one terminal bud of G-2, were harvested (Figure 1C). Each well of sterilized microplates (96 wells, Virya, Shanghai, China) was loaded with 200 μL of MS medium and covered with a lid (Figure 1D). Each microplate was used to ship both samples, one sample per well (Figure 1E). Plates with plant samples were packed in a transparent vacuum-sealing packing bag (sterilized or non-sterilized) (Figure 1F). Vacuum treatment was conducted to test whether evacuating air outside the plate and fastening the plate limited the growth of plant samples. Samples were incubated in a normal culture environment for 30 d as described above. The survival rate (%) and new tissue (newly developed tissues or organs form the original explants; example see results. A newly elongated shoot of ZHB) germination rate (%) were thereafter measured. The survival rate was calculated as the number of samples with living tissue, or total number of samples ∗ 100%. The regrowth of new tissue was measured by dividing the number of samples with newly generated tissue or organs by the total number of samples and multiplied by 100%. Micro-cuttings were then transplanted into MS medium under normal culture conditions for 10 d to test for possible microbial contamination of the unsterilized package (Figure 1G).
2.3. Assessment of Incubation Condition
The plant samples shipped in vacuum-sealed microplates were incubated in a growth chamber under conditions of controlled temperature with photoperiod and light intensity. The cultures were maintained under the following conditions: (1) 25 °C for a 16 h photoperiod with a light intensity of 50 μM s−1 m−2 (25 °C + 16 h light); (2) 25 °C in the dark (25 °C + dark); (3) 10 °C for a 16 h photoperiod with a light intensity of 50 μM s−1 m−2 (10 °C + 16 h light), (4) 10 °C in the dark (10 °C + dark); (5) 4 °C for a 16 h photoperiod with a light intensity of 50 μM/m2/s (4 °C + 16 h light); and (6) 4 °C in the dark (4 °C + dark). Periods were 60 culture days and the survival rate (%) and new tissue germination rate (%) were assessed as described above (Figure 1H).
2.4. Assessment of Culture Medium
The growth of in vitro samples was slowed down to extend the duration of germplasm conservation and long-distance shipping. The effect of ion levels in the culture medium was assessed by decreasing the concentration of major and minor ions to 25%. Then, 1/4 MS medium without or with either daminozide (B9; 60, 80, 100, 120 or 140 mg/L), chlormequat chloride (CCC; 25, 50, 75 or 100 mg/L), paclobutrazol (PP333; 1, 2, 3 and 4 mg/L), or abscisic acid (ABA; 1, 2, 3 and 4 mg/L) was tested. The application of 38 treatments resulted in a combination of 2 plants with 19 media. ZHB and G-2 explants measuring approximately 0.4 cm in length from micropropagated plants were maintained in 300 mL glass jars with 40 mL of medium and stored for 60 d under standard culture conditions as noted above. The survival rate (%) and new tissue regrowth (%) were measured after incubation. Then, the morphology indices, such as plant height (cm) and the number of newly formed leaves, roots, and tillers (for ginger) were also calculated to evaluate the effects of ion density and plant growth regulation (Figure 1I). The most efficient media were then loaded in microplates and used in the following experiments.
2.5. Regrowth Capacity Assessment of Maintained Plants
The micro-cuttings of ZHB and G-2 derived from routinely propagated in vitro plants were maintained in 1/4 MS medium supplemented with 100 mg/L CCC (1/4MS 100 CCC medium) in sterilized microplates (Figure 2a–c). The plates were then vacuum-sealed with non-sterile packages (Figure 2d,e). The cultures were maintained at 10 °C in the dark (Figure 2f). Micro-cuttings were transplanted into MS medium after 60 days of incubation, and incubated under normal culture conditions. The regrowth capacity in terms of percentage of shoots resuming normal growth was evaluated 30 days after sample release (the 1st cycle of culture). The subculture and incubation of recovered ZHB and G-2 plants were repeated (the 2nd cycle of culture). The regrowth capacity and morphology indices were measured as well. In order to test the feasibility of the present method in other plant species, sweet potato HXS in vitro cuttings (0.4 mm long shoot with one 0.2 mm petiole) were harvested and transplanted in sterilized microplates supplemented with 1/4 MS 100 CCC medium. The plates were vacuumized and maintained at 10 °C in the dark for 60 d, followed by analysis of the survival and regeneration (Figure 1J,K).
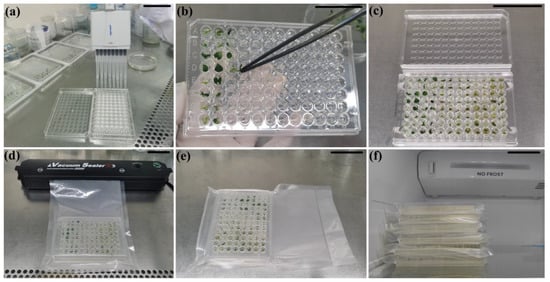
Figure 2.
Manufacturing procedures of microplate shipping of vacuum-packed plant micro-cuttings. (a) Loading each of microplate well with 200 uL 1/4MS 100 CCC medium; (b) micro-cutting culture; (c) cutting cultured plate; (d) vacuumization; (e) vacuum-packed plate; (f) packaged plates stored at 10 °C in the dark (light on state in picture). Bar indicates 5 cm.
2.6. Assessment of Genetic Stability
The morphology indices, including plant height, number of leaves (tillers), length of roots, and number of roots, were measured to assess the generic stability on a morphological level. For the molecular marker test, total genomic DNA was extracted from 100 mg of leaves obtained from ZHB and G-2 plants, which was released from a microplate and subcultured twice using the GeneJET Plant Genomic DNA Purification Mini Kit (Thermo Scientific, Waltham, MA, USA). DNA quality was checked using electrophoresis of the samples on 1% agarose gel and stained with StarStain Red Plus (GenStar, Beijing, China). DNA concentration was determined via spectrophotometry. The molecular analysis was performed using 5 SSR and 5 ISSR primers (Table S1). The amplifications were carried out in 20 μL volumes, containing 2 ng genomic DNA, 1X PCR buffer (Biotools, Madrid, Spain), 200 μM of each dNTP (Roche, South San Francisco, CA, USA), 0.25 U of Taq DNA polymerase (Roche, Basilea, Suiza), and 0.2 μM of forward and reverse primers. The PCRs were carried out using a gene amplification instrument (FastAmp-T96, BIO-DL, Shanghai, China) with initial denaturation at 94 °C for 5 min, followed by 40 cycles of 94 °C for 30 s (94 °C for 50 s for ISSR), annealing temperature at 56 °C for 30 s (58 °C for 50 s for ISSR), 72 °C for 2 min (72 °C for 1 min for ISSR), and a final extension at 72 °C for 10 min. Automatic acquisition and reading was performed using ChampChemi610 (BeijingSaizhi, Beijing, China). All fragments in the size range of 100–2000 bp generated from ZHB were assumed to represent a single dominant locus. Fragments in the range of 100–3000 bp were considered and registered as a single codominant locus. All the reactions were performed in triplicate with 10 plantlets selected randomly (Figure 1L).
2.7. Transportation Tolerance Test
The vacuum-packaged plates carrying ZHB, G-2, and HXS samples were delivered by express mail through automobile transportation without any cushioning materials to test their transportation tolerance. The plates were delivered to four destinations from the starting point at Guizhou University, Guizhou City, Guiyang Province, China. The first location was an ornamental plant germplasm resources nursery on Huangzhuang South Road in Baiyun District, Guangzhou City, Guangdong Province, which is 925 km away from the starting point. The highest temperature at the arrival date was 20 °C and the temperature in the car ranged from around 10–22 °C during a 2-day trip (courier No. YT6303669102525). The next destination was 99-1 Yingchengzi Street, Hunnan District, Shenyang City, Liaoning Province, which is 2293.6 km away from the starting point. The highest temperature at the arrival date was 9 °C and the temperature in the car ranged from around 9–10 °C during a 3.5-day trip (courier No. 75853845991528). The third location was Beijing Agricultural College, Changping District, Beijing, which is 2214 km away from the starting point. The highest temperature at the arrival date was 7 °C and the temperature in the car ranged from around 7–10 °C during a 7-day trip (courier No. 9886429762337). The final destination by car was to Jingfengjiayuan residential quarters, Taoshan District, Qitaihe City, Heilongjiang Province, which is 3681 km away from the starting point. The highest temperature at the arrival date was 7 °C and the temperature in the car ranged from around 7–10 °C during an 8-day trip (courier No. 9886429709918). Furthermore, plates were carried in a coat pocket and suitcase and transported 1426 km by train to Hefei City, Anhui Province to test their ability to withstand shipping stress. The temperature in carriage and car were around 12–16 °C and it was taken 7.5 h by train. The integrity of packaged plates and micro-cuttings was immediately checked after arrival (Figure 1M).
2.8. Experiment Design and Statistical Analysis
For the detailed experiment design, see Figure 1. A complete randomized design was used. At least 10 samples were included in each of the experiments. All experiments were performed in triplicate and conducted at least twice. Data were subjected to one-way ANOVA and the least significant difference (LSD) was calculated at p < 0.05.
3. Results
3.1. The Plant Growth and Contamination Response to Package Types
Vacuum-sealed packaging significantly influenced the survival and new tissue germination of the micro-cuttings of both ZHB and G-2. ZHB plantlets in vacuumized microplates showed a significantly lower survival rate (85.8–87.7%) and regrowth rate (61.8–62.5%) compared to the non-vacuumized counterparts. Similarly, in ginger G-2, vacuumization resulted in low survival (20–30%) and no new tissue germination. When vacuumization was not conducted, all of the ZHB micro-cuttings and 72.4–83.3% G-2 cuttings survived after 30 days of culture under normal subculture conditions, and 94.0–100% of ZHB and 67.0–83.3% of G-2 germinated new tissue (Table 1). Increased germination of in vitro cuttings during long-distance storage and transportation depletes the growth resources of plants, leading to aging. We found that the germinated and elongated potato seedlings pushed the cover and separated the lid and plate (data not shown), which increased the potential risk of contamination, suggesting that vacuum packing was needed. Unsterilized packages did not result in the contamination of the samples or medium if the mother plants were in a sanitary condition (Table 1). Furthermore, high temperature and pressure damaged the plastic membrane. Thus, in the following study, vacuum packages with unsterilized bags were used.

Table 1.
The effect of package types on plant survival, new tissue regrowth, and medium contamination.
3.2. Plant Growth Response to Incubation Conditions
The variation of the culture period and conservation conditions significantly affect the survival and regeneration of micro-cuttings in vacuum-packed plates. Compared to 30-day incubated samples (Table 1), normal culture conditions for 60 d resulted in a significant decrease in the vitality of ZHB (35.7% survival and 20.8% regeneration) and G-2 (0% survival and regrowth), which was even worse in the dark (Table 2). Regardless of illumination conditions, nearly 80% of micro-cuttings developed new tissue before they lost vitality at 25 °C. Such samples were still counted as non-survival and regrowth as those without living cells or new tissue formed on cuttings. In potato ZHB, the conservation resistance of cuttings under encapsulated conditions was significantly improved by low temperature treatments ranging from 4 to 10 °C, and the survival rate was elevated to 81.4% to 100%. Furthermore, the darkness enhanced the stability of the cuttings and significantly less new tissue regrowth was detected. A slight difference was observed with ginger G-2, as the survival declined when samples were incubated in the dark compared to light, but this difference was not significant, and similar tissue regrowth was observed under both treatments. However, no increased survival rate of G-2 occurred at 4 °C (Table 2). The morphology of surviving, regenerated, and dead micro-cuttings is shown in Figure 3. It is worth noting that, during conservation, very limited (less than 0.5%, data not shown) ZHB cuttings developed a callus (Figure 3(b1)), while the majority of regrowing samples developed an elongated stem (Figure 3b). In contrast to potato, the main shoot terminal tended to germinate tillers instead of elongated stems (Figure 3e). Considering that both ZHB and G-2 only survived at 10 °C, the 16 h photoperiod induced an increase in tissue germination in ZHB, which prevented germplasm storage and transportation. Darkness was similar to the real transportation environment. Therefore, the conservation conditions of 10 °C and darkness were selected for the following experiment.

Table 2.
Survival and new tissue regrowth of cuttings encapsulated in microplates wrapped in vacuum package under various incubation conditions.
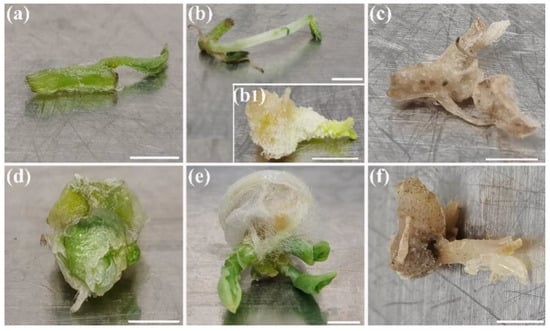
Figure 3.
Survival and new tissue regeneration by micro-cuttings or loss of vitality. (a) Potato ZHB survived cutting without new tissue germination; (b) new tissue germinated ZHB cuttings, (b1) ZHB micro-cutting with callus and bud germination; (c) dead ZHB cuttings; (d–f) ginger G-2 survived cutting, new tissue germinated, and dead cutting, respectively. Images were acquired post 60 d of conservation. Bar indicates 2 mm.
3.3. Plant Growth Response to Slow-Growth Culture Media
Both MS and 1/4 MS media showed 100% survival and total regrowth after 60 days in storage on both potato ZHB and ginger G-2. For ZHB, B9, PP333, and ABA caused partial death of samples in general. CCC-supplemented plants showed the highest survival rate in potato ZHB. Except for MS medium treated with 75 mg/L CCC medium, 25, 50, and 100 mg/L CCC did not decrease the survival (Table 3). In addition, all the plantlets incubated in 25 mg/L and 100 mg/L CCC-supplemented MS medium reformed new tissues. Malformation including leaf bleaching, leathery leaves, tissue necrosis, or swelling of samples were seen on a majority of media except those treated with 75 and 100 mg/L CCC (data not shown). Generally, B9, PP333, and ABA caused malformation on G-2. For G-2, samples incubated in 1/4 MS medium supplemented with 25 and 100 mg/L CCC showed nearly 100% survival and no malformation of micro-cuttings. The addition of ABA led to a significant decrease in survival or regrowth of samples compared to the control (Table 3).

Table 3.
Survival and new tissue regrowth of micro-cuttings cultured in different growth media.
Morphology indices of in vitro plants under each treatment were measured. For ZHB, compared to MS medium, 1/4 MS medium significantly increased the roots but decreased the shoot growth; no obvious changes on other morphological indexes were observed. Following 1/4 MS-medium-based treatment, 60–100 mg of B9 did not significantly affect the regeneration of leaf and root, while 120–140 mg of B9 inhibited it; however, the elongation of the shoot was reduced regardless of dose. Treatment with 25 and 100 mg of CCC suppressed potato growth whereas 50 and 75 mg did not, while 100 mg of CCC generated the shortest plantlets among all CCC treatments. ABA addition resulted in the lowest average number of leaf, root, and stem height on potato ZHB in general, followed by PP333 supplementation (Figure 4). For ginger G-2, decreasing the iron content to 1/4 inhibited rooting and tillering. Regeneration and growth were enhanced by B9 and PP333 depending on the dose. Exposure to 50 and 70 mg of CCC improved the development of roots, leaves, and stems, while 25 and 100 mg doses significantly inhibited all indices, except for the number of tillers. The low regrowth rate under ABA treatment resulted in the shortest and the most undeveloped G-2 plantlets (Figure 5).
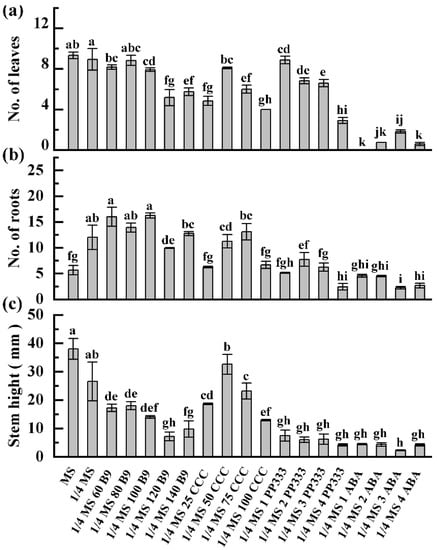
Figure 4.
Growth status of ZHB plantlets incubated in slow-growth media. Data were surveyed 60 d post-culture. ¼ MS 60 B9 refers to ¼ MS medium supplemented with 60 mg/L B9, and so on. (a–c): Average plant height, number of new leaves, and number of new roots of potato ZHB, respectively. Data are presented as means ± SE. Significant differences were analyzed as one-way ANOVA at p < 0.05.
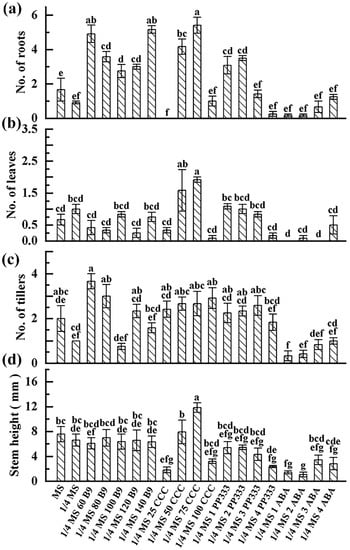
Figure 5.
Growth statues of G-2 plantlets incubated in slow-growth media. Data were surveyed 60 d post-culture. ¼ MS 60 B9 refers to ¼ MS medium supplemented with 60 mg/L B9, and so on. (a–d): Average plant height, number of new leaves, number of new tillers, and number of new roots of ginger G-2, respectively. Data are presented as means ± SE. Significant differences were analyzed as one-way ANOVA at p < 0.05.
High survival, “true to type” morphology, and inhibition of vegetative growth ensure a stronger shipping resistance. Thus, 1/4 MS medium treated with 100 mg/L CCC led to 100% survival, no malformation, and restricted vegetative growth on both ZHB and G-2. Such plantlets were selected for the following experiments.
3.4. Regrowth of Packaged Micro-Cuttings
Taking ¼ MS 100 CCC as the culture medium, ZHB and G-2 micro-cuttings were encapsulated in microplates in vacuum-sealed packages. Sweet potato HXS were used to test the feasibility of this system on different plant germplasms. Rates of 100.0%, 75.3%, and 100% survival were identified for ZHB, G-2, and HXS, respectively, and their regeneration rates were 0% for the first two and 6.7% for HXS (Table 4). Although the survival was lower in G-2, the new tissue regrowth was significantly decreased in both ZHB and G-2 when compared to those incubated in MS medium in the same incubation environment (Table 2). The multiplication, which indicates the quantities of a shoot that a micro-cutting with a single bud would generate, was measured. After releasing them from the plate, an average of 4.4 nodes from ZHB cuttings were harvested after the first cycle of incubation (first 30 days), and significantly higher number of nodes were measured from the other 30-day cultured plantlets (6.9 nodes). Similarly, in the case of sweet potato HXS, an average of 2.4 and 3.6 nodes from the 1st and 2nd cycle of subcultured plantlets, respectively, were identified. Interestingly, for G-2, significantly more shoots were tillered from packed cuttings whereas 4.3 buds were found in the first cycle of culture; the multiplication was decreased to 2.5 on average after the 2nd cycle of incubation (Table 4).

Table 4.
Plant survival, new tissue regrowth, and subculture of packaged micro-cuttings conserved at 10 °C in the dark with 1/4 MS 100 CCC.
Similar patterns of vegetative generation of preserved ZHB and HXS recovered from packed microplates were observed (Figure 6). Generally speaking, the stem height, number of leaves, number of roots, and length of roots were significantly short and few in the 1st cycle of cultured micro-cuttings; however, this inhibition was reversed by further subcuture. The vegetative growth of plantlets was restored to similar levels after another cycle of subculture compared to untreated plants, except for the stem height of sweet potato HXS, which was shorter than in the control after the 2nd cycle of subculture (Figure 6). The average stem height for ginger G-2, similar to ZHB and HXS, was significantly inhibited by the packaging and recovered following successive subculture. However, the growth and differentiation of leaves, roots, and tillers were significantly stimulated by the packaged conservation, and these changes were temporary as well; the growth of cuttings resumed to control levels following the 2nd cycle of subculture (Figure 6). No malformation or changes in morphology of plantlets were observed (Figure S1).
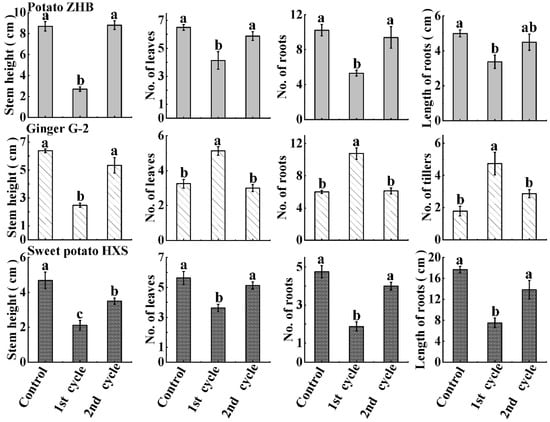
Figure 6.
Growth of ZHB, G-2, and HXS after recovery in MS medium. Statistical analysis of growth indices. Data are presented as means ± SE. Letters indicate significant differences. Significant differences were analyzed via one-way ANOVA at p < 0.05.
3.5. Genetic Stability
ISSR and SSR profiles from recovered ZHB and G-2 plantlets stored in different slow-growth media were compared to those obtained from their respective untreated and normally cultured mother plants. The primers that we used showed different abilities for detecting genetic variation. In both ZHB and G-2, out of 50 primers for each method tested, 5 primers produced strong, clear, and reproducible bands, which differed between the two species (Table 5; Figure 7). In ZHB, 11–14 bands were scorable; in 30 individual samples, SSR primers produced 1860 bands in total (62 bands ∗ 30 samples), and no polymorphic bands were identified (data not shown). Using the ISSR marker, the primers yielded 1470 clean bands (49 bands ∗ 30 samples). Three polymorphic bands were detected, which accounted for about 0.2% genetic variation. In G-2, using the SSR marker, the number of bands for each primer varied between 6 and 9, and a total of 1110 bands (37 bands ∗ 30 samples) were generated from all samples (data not shown). One specific band was detected, which accounted for less than 0.1% of genetic variation. Using SSR, five primers generated 47 bands in a single sample. However, we were unable to detect any signs of genetic variation (Table 5; Figure 7).

Table 5.
The numbers of amplified bands in plantlets regenerated after recovery in MS medium under normal subculture conditions by SSR and ISSR.
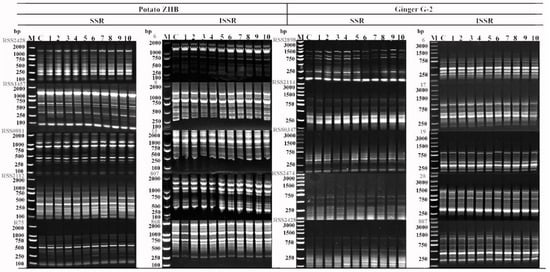
Figure 7.
SSR and ISSR banding patterns in recovered potato ZHB and ginger G-2. Three repeats of 10 randomly selected samples were detected.
3.6. Transportation Feasibility
The transportation resistance of the present device was tested via express automobile transportation and railway transportation in pocket and suitcase, across thousands of kilometers. The packages remained complete and the micro-cuttings were tightly attached to the medium in each cup (Figure 8).
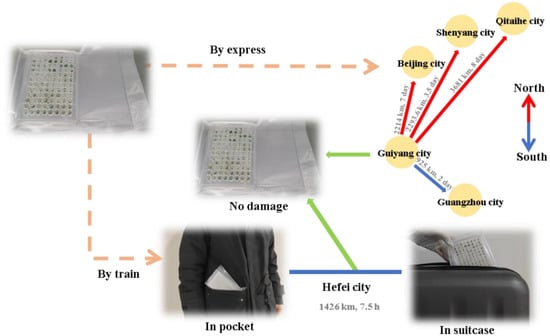
Figure 8.
The transportation and shipping feasibility of micro-cuttings encapsulated in vacuum-packed microplates.
4. Discussion
In vitro plant germplasm resources are currently transported in tubes, jars, or plastic bags that are usually used for plant tissue culture. Different packages have drawbacks and technical constraints that limit their efficiency and decrease the security of the shipped biomaterials. Hence, it is essential to optimize the shipping systems in order to limit the factors that can lead to the loss of plant germplasm and bio-contamination. This can mainly be achieved by transportation-resistant packages and slow-growth systems.
The results demonstrate that it is possible to store potato ZHB and ginger G-2 germplasms in space-limited, vacuum-packed, slow-growth, dark conditions. The system was effective in terms of 60-day storage, which requires no additional effort and ensures the vitality of samples. Under normal culture conditions, the samples are not well-conserved in space-limited culture in microplates and vacuum-sealed packages. A mere 85.8% of micro-cuttings survived and the majority of them (62.5%) showed stem germination. In the case of G-2, merely 20% of the cuttings exhibited fresh and live tissues during their 30-day incubation period (Table 1). These cases suggest the disadvantages of long-distance shipping, which decreased the vitality of plant germplasm in vitro [14]. An optimized result was achieved with conserved and packed plates at 10 °C in the dark (Table 2). Lowering the culture temperature and shading from light are important ways to delay the growth of in vitro plants [26]. This strategy successfully resulted in the slow-growth conservation of globe artichoke in vitro [27,28]. It was emphasized that, compared to osmotic stress, storing in cold and dark conditions facilitated the medium-term conservation of globe artichoke [27]. According to De Lacerda et al. (2021) [17], rapidly growing plants P. glomerata and L. filifolia were maintained for up to 360 days with 100% or 50% survival in 5 mL of mineral oil at a temperature of 15 °C, while higher temperatures (20 °C and 25 °C) decreased plant survival. Light is essential for plant growth and development as well as improving cutting survival and regrowth of both ZHB and G-2 in the present study. Considering that increased germination rates were a disadvantage during conservation and shipping, darkness was selected to simulate the real transportation environment. Our findings suggest that the germplasm should be transported during late autumn or late spring but not in extremely cold winter, and the package should be shipped in an opaque express box under relatively ambient air temperature.
To further postpone the germination of sample cuttings during transportation and conservation, we tested the role of ionic concentration in the culture medium and growth retardants on plantlet growth. Subsequently, we used an efficient combination in the packaging system. Reducing the MS medium composition to 1/4 did not affect sample survival or regrowth (Table 3) but resulted in a relatively slower shoot growth (Figure 4 and Figure 5). Our results were consistent with Catană (2010) [7], who reported that 1/4 or 1/10 MS medium for the Caryophyllaceae family facilitated the conservation with optimal parameters depending on the genotype. However, Arbeloaa et al. (2017) [29] reported totally different results involving 138 different fruit trees belonging to 18 species, with generally higher multiplication rates in 1/2 MS medium than in MS medium. Plant growth retardants, including B9, CCC, PP333, and ABA were usually used in plant slow-growth conservation in vitro [20,30,31,32,33]. Compared to B9, PP333, and ABA, 100 mg/L CCC in this study did not inhibit sample germination and regrowth; however, it ensured that all samples were alive and maintained normal morphology of plantlets (Table 3). In addition, compared to those incubated in 1/4 MS blank medium, the regrowth of plantlets was significantly inhibited when 100 mg/L CCC was combined (Figure 4 and Figure 5). Furthermore, the application of 100 mg/L CCC supplemented with 1/4 MS medium in the vacuum-packed plates led to significantly lower new tissue regrowth when compared to MS medium, which indicates the advantages of this medium in delaying the germination of micro-cuttings, though it slightly decreased the survival of G-2 (Table 2 and Table 4). Notably, compared to ZHB, G-2 was more sensitive to the incubation parameters, including temperature, illumination and plant growth regulators selected in this study. Obviously, the packing and conservation systems presented here are not optimal for G-2. Further studies are needed to develop a “special to genotype” system for different kinds of plant genotypes or a broader spectrum adaptive method.
Compared to unpacked micro-cutting controls, variations in morphology were observed after 60 days of storage in vacuum-sealed packages and without the addition of plant growth regulators. Two manners of regrowth of ZHB micro-cuttings were observed, including callus regeneration (though very few) or revival of normal stems. However, no callus was seen in G-2; instead of shoot elongation, which was observed on ZHB, tillering was the main manner of G-2 regrowth in wells of plates (Figure 3). Callus formation may increase the probability of somatic variation [34]. Evidence suggests that calluses carry high levels of genome-wide CHH methylation, particularly across heterochromatic regions [35]. The biotechnological interventions in vitro may cause somatic variation [35,36], suggesting the need for genetic stability assessment [19,37]. The genetic stability is usually estimated via morphological identification and molecular marker assessments [19,37,38,39]. In order to confirm the genetic stability of the present shipping system, we further compared the morphology and reproduction of recovered plantlets, which germinated in vacuum-packed microplates supplemented with 100 mg/L CCC. Molecular markers were analyzed as well.
Cuttings with callus germination or other malformed samples were not seen when the packing system was treated with 100 mg/L CCC after 60 days of conservation (data not shown). Although the analysis of the morphological data of the 1st cycle regenerated plants revealed significant morphological differences, the differences were restored to control levels after the second cycle of subculture (Figure 6). Interestingly, in contrast to ZHB and HXS plants, whose growth was significantly slower in the 1st cycle of subculture, G-2 tillers produced double shoots after release (Figure 6). More particularly, even without the supplementation of a plant growth regulator, G-2 generated tillers during 60-day packing and conservation instead of stem elongation (Figure 3). The number of tillers is used to evaluate the reproductive ability of ginger [40,41]. We found that space-limited incubation and vacuum packing rather than plant growth regulators may have stimulated tillering in the present study. Therefore, this method can be used commercially to accelerate the propagation of in vitro ginger, and the underlying mechanism requires further study.
Molecular markers are frequently used to assess the genetic stability of in-vitro-derived plantlets [19,42,43,44,45,46]. In the present study, SSR and ISSR were used to assess the genetic stability of artichoke plantlets regenerated from a slow-growth packing system used by Tavazza et al. (2015) [19]. Very limited polymorphism was detected in the present study; in potato, about 0.2% genetic variations were detected by ISSR, while for G-2, less than 0.1% genetic variations were identified by SSR but not by ISSR (Table 5). According to Liu and Yang (2012) [42] and Yin et al. (2013) [43], the somaclonal variation rate ranges from 0.37% to 4.2%, suggesting “true-to-type” plants. Our results show that both ZHB- and G-2-regenerated plants were genetically stable using the primers used, indicating that this system did not have a detectable impact on genetic stability in this experimental system.
Ultimately, shipping resistance of the present packing and conservation system was assessed using express automobile transportation or train transportation, which are relatively slow and bumpy for logistics. The destinations were spread across China’s provinces and the temperatures varied from 7 to 22 °C at the time of delivery. The packed plant samples were shipped across thousands of kilometers and reached destinations in 2–8 days without package and sample damage. This indicates that the present methods had positive effects on sample integrity during express transportation under a wide range of temperatures. However, further assessment should be conducted by international sea freight lasting several months.
As far as we know there are very limited published reports describing a safe and highly efficient protocol for long-distance shipping and conservation of in vitro plants, validated by appropriate transportation tests. In conclusion, our results show that it is possible to store and transport in vitro cultures of potato, ginger, and sweet potato for a prolonged period of time by slowing down growth in a vacuum-sealed microplate. This protocol can aid transnational transportation or long-distance shipping of plant germplasm, which usually lasts months, without extreme high or low temperature variation or violent collision. The significantly reduced risk of germplasm loss and biosafety issues have been detected compared to conventional methods of shipping or custom inspection. Our results facilitate the distribution of germplasm to curators and the wider user community.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8070609/s1, Table S1: SSR and ISSR primers and primer sequences; Figure S1: Growth feature of ZHB, G-2, and HXS after recovery in MS medium.
Author Contributions
Project administration, J.L.; methodology, J.L., M.H. and T.H.; formal analysis, J.L. and X.X.; writing—original draft preparation, J.L.; writing—review and editing, W.Z. Investigation, H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Research and application of rapid propagation technology of virus-free Guizhou yellow ginger” project, which received financial support from the Guizhou Modern Seed Industry Group Co., Ltd. and the “Talent introduction” [Guidarenjihezi (2019)73] program supported by Guizhou University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Priyanka, V.; Kumar, R.; Dhaliwal, I.; Kaushik, P. Germplasm conservation: Instrumental in agricultural biodiversity—A review. Sustainability 2021, 13, 6743. [Google Scholar] [CrossRef]
- Chawla, A.; Kumar, A.; Warghat, A.; Singh, S.; Bhushan, S.; Sharma, R.K.; Bhattacharya, A.; Kumar, S. Approaches for conservation and improvement of Himalayan plant genetic resources. Adv. Crop Improv. Tech. 2020, 18, 297–317. [Google Scholar]
- Staub, J.; Chatterton, J.; Bushman, S.; Johnson, D.; Jones, T.; Larson, S.; Robins, J.; Monaco, T. A history of plant improvement by the USDA-ARS forage and range research laboratory for rehabilitation of degraded western U.S. rangelands. Rangelands 2016, 38, 233–240. [Google Scholar] [CrossRef]
- Schiva, T. Bamboo germplasm: Perspectives for ornamental purposes. Acta Hortic. 1999, 486, 255–260. [Google Scholar] [CrossRef]
- Santos, I.R.I.; Salomão, A.N. In vitro germination of zygotic embryos excised from cryopreserved endocarps of queen palm (Syagrus romanzoffiana (Cham.) Glassman). In Vitro Cell. Dev. Biol. Plant 2017, 53, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Chalam, C.V. Elimination of plant viruses by certification and quarantine for ensuring biosecurity. Appl. Plant Virol. 2020, 52, 749–762. [Google Scholar]
- Catană, R.; Mitoi, E.M.; Helepciuc, F.; Holobiuc, I. In vitro conservation under slow growth conditions of two rare plant species from Caryophyllaceae family. Electron. J. Biol. 2010, 6, 86–91. [Google Scholar]
- Reed, B.M.; Sarasan, V.; Kane, M.; Bunn, E.; Valerie, C.P. Biodiversity conservation and conservation biotechnology tools. In Vitro Cell. Dev. Biol. Plant 2011, 47, 1–4. [Google Scholar] [CrossRef]
- Waterworth, P.; Kahn, R.P. Thermotherapy and aseptic bud culture of sugarcane to facilitate the exchange of germplasm and passage through quarantine. Plant Dis. Rep. 1978, 62, 772–776. [Google Scholar]
- Kumar, C.A.; Chandel, K.P.S. Strategy for the production of virus-free plant germplasm under in-vitro conservation and exchange—A brief review. Indian J. Plant Genet. Resour. (IJPGR) 1992, 5, 67–78. [Google Scholar]
- Bekheet, S.A. In vitro culture techniques for conservation of date palm germplasmin in Arab countries. Acta Hortic. 2010, 882, 211–217. [Google Scholar] [CrossRef]
- Negahdar, N.; Hashemabadi, D.; Kaviani, B. In vitro conservation and cryopreservation of Buxus sempervirens L., a critically endangered ornamental shrub. Russ. J. Plant Physiol. 2021, 68, 661–668. [Google Scholar] [CrossRef]
- Filloux, D.; Girard, J.C. Indexing and elimination of viruses infecting yams (Dioscorea spp.) for the safe movement of germplasm. In Proceedings of the 14th Triennial Symposium of the ISTRC, Trivandrum, India, 20–26 November 2006; pp. 1–13. [Google Scholar]
- Reed, B.M.; Paynter, C.; Bartlet, B. Shipping Procudures for Plant Tissue Culture. Available online: https://www.ars.usda.gov/research/publications/publication/?seqNo115=119618# (accessed on 7 January 2022).
- El-Dawayati, M.M.; Zaid, Z.E.; Elsharabasy, S.F. Effect of conservation on steroids contents of callus explants of Date palm cv. sakkoti. Aust. J. Basic Appl. Sci. 2012, 6, 305–310. [Google Scholar]
- Ramírez-Pérez, Y.; Tapia-Campos, E.; Cruz-Gutiérrez, E.J.; Barba-Gonzalez, R. Obtaining a protocol for slow growth for in vitro conservation of eustoma cultivars (Gentianaceae). Acta Hortic. 2020, 1288, 185–188. [Google Scholar] [CrossRef]
- De Lacerda, L.F.; Gomes, H.T.; Bartos, P.M.C.; Vasconcelos, J.M.; Filho, S.C.V.; De Araújo Silva-Cardoso, I.M.; Scherwinski-Pereira, J.E. Growth, anatomy and histochemistry of fast growing species under in vitro conservation through mineral oil and low-temperature combination. Plant Cell Tissue Organ Cult. 2021, 144, 143–156. [Google Scholar] [CrossRef]
- Oseni, O.M.A. Review on plant tissue culture, a technique for propagation and conservation of endangered plant species. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3778–3786. [Google Scholar] [CrossRef]
- Tavazza, R.; Rey, N.A.; Papacchioli, V.; Pagnotta, M.A. A validated slow-growth in vitro conservation protocol for globe artichoke germplasm: A cost-effective tool to preserve from wild to elite genotypes. Sci. Hortic. 2015, 197, 135–143. [Google Scholar] [CrossRef]
- Tahtamouni, R.; Shibli, R.; Al-Abdallat, A.; Al-Qudah, T. Turkish journal of agriculture and forestry analysis of growth, oil yield, and carvacrol in thymbra spicata l. after slow-growth conservation. Turk. J. Agric. For. 2016, 40, 213–221. [Google Scholar] [CrossRef]
- Fernando, I.; Fei, J.; Stanley, R. Measurement and analysis of vibration and mechanical damage to bananas during longdistance interstatetransport by multitrailer road trains. Postharvest Biol. Technol. 2019, 158, 11097. [Google Scholar] [CrossRef]
- Nikulina, E.O.; Ivanova, G.V.; Kolman, O.I.; Ivanova, A.N.; Perestoronin, D.Y. Research of the influence of vacuum packaging on the quality and safety of meat semi-finished products. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 667, p. 032066. [Google Scholar]
- Jiang, Q.J.; Jin, W.W.; Zhang, W.Y.; Zhang, Z.C.; You, L.F.; Bi, Y.Q.; Yuan, L.M. Analysis of vibration acceleration levels and quality deterioration of Chinese bayberry fruit in semi–vacuum package by express delivery. J. Food Process. Eng. 2021, 44, e13899. [Google Scholar] [CrossRef]
- Anca, B.; Adriana, P.V.; Rodica, Z.; Luiza, M.; Mihaela, P. The results in the field of the in vitro conservation of the cultivars when using classic and modern conservation methods. An. Univ. Oradea-Fasc. Biol. 2007, 14, 23–26. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Monticelli, S.; Gentile, A.; Forni, C.; Caboni, E. Slow growth in â Pisanaâ apricot and in â Cerinaâ. apple, two Italian cultivars. Acta Hortic. 2020, 1285, 117–124. [Google Scholar] [CrossRef]
- Bekheet, S.; Usama, I.A. In vitro conservation of globe artichoke (Cynara scolymus L.) Germplasm. Int. J. Agric. Biol. 2007, 9, 404–407. [Google Scholar]
- Benelli, C.; Carlo, A.D.; Previati, A.; Roncasaglia, R. Recenti acquisizioni sulla conservazione in vitro in crescita rallentata. In Proceedings of the IX Giornate Scientifiche SOI Italus Hortus Conference, Florence, Italy, 10–12 March 2010; p. 91. [Google Scholar]
- Arbeloaa, A.; Marí n, J.A.; Andreu, P.; Garcí a, E.; Lorente, P. In vitro conservation of fruit trees by slow growth storage. Acta Hortic. 2017, 1155, 13. [Google Scholar] [CrossRef] [Green Version]
- Žiauka, J.; Kuusienė, S. Different inhibitors of the gibberellin biosynthesis pathway elicit varied responses during in vitro culture of aspen (Populus tremula L.). Plant Cell Tissue Organ Cult. 2010, 102, 221–228. [Google Scholar]
- Ciobanu, I.B.; Constantinovici, D.; Creţu, L. Influence of genotype and cultivation conditions on vitroplantlets evolution of solanum tuberosum L. local varieties. An. Ştiinţifice Ale Univ. Al. I. Cuza Iaşi. 2011, 2, 13–20. [Google Scholar]
- Indrayanti, R.; Putri, R.E.; Sedayu, A.; Adisyahputra. Effect of paclobutrazol for in vitro medium-term storage of banana variant cv. Kepok (Musa acuminata × Balbisiana Colla). In Proceedings of the 9th International Conference on Global Resource Conservation (ICGRC 2018), Malang, Indonesia, 7–8 March 2018; pp. 020001–020009. [Google Scholar]
- Buldakov, S.A. Use of growth inhibitor chlormequat chloride in potato culture in vitro. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2021; Volume 285, p. 03003. [Google Scholar]
- Kumar, S.; Kumari, R.; Baheti, T.; Thakur, M.; Ghani, M. Plant regeneration from axillary bud, callus and somatic embryo in carnation (Dianthus caryophyllus) and assessment of genetic fidelity using RAPD–PCR analysis. Indian J. Agric. Sci. 2016, 86, 1482–1488. [Google Scholar]
- Lizamore, D.; Bicknell, R.; Winefield, C. Elevated transcription of transposable elements is accompanied by het-siRNA-driven de novo DNA methylation in grapevine embryogenic callus. BMC Genom. 2021, 22, 676. [Google Scholar] [CrossRef]
- Kaeppler, S.M.; Kaeppler, H.F.; Rhee, Y. Epigenetic aspects of somaclonal variation in plants. Plant Mol. Biol. 2000, 43, 179–188. [Google Scholar] [CrossRef]
- Li, J.W.; Chen, H.Y.; Li, X.Y.; Zhang, Z.B.; Blystad, D.R.; Wang, Q.C. Cryopreservation and evaluations of vegetative growth, microtuber production and genetic stability in regenerants of purple-fleshed potato. Plant Cell Tissue Organ Cult. 2016, 128, 641–653. [Google Scholar] [CrossRef]
- Mendes, M.; Verde, D.; Ramos, A.; Gesteira, A.; Souza, A. In vitro conservation of citrus rootstocks using paclobutrazol and analysis of plant viability and genetic stability. Sci. Hortic. 2021, 286, 110231. [Google Scholar] [CrossRef]
- Parab, A.R.; Lynn, C.B.; Subramaniam, S. Assessment of genetic stability on in vitro and ex vitro plants of Ficus carica var. black jack using issr and damd markers. Mol. Biol. Rep. 2021, 48, 7223–7231. [Google Scholar] [CrossRef] [PubMed]
- Kandiannan, K.; Prasath, D.; Sasikumar, B. Biennial harvest reduces rhizome multiplication rate and provides no yield advantage in ginger (zingiber officinale roscoe.). J. Spices Aromat. Crops 2016, 25, 79–83. [Google Scholar]
- Marsh, L.; Hashem, F.; Smith, B. Organic ginger (Zingiber officinale Rosc.) development in a short temperate growing season: Effect of seedling transplant type and mycorrhiza application. Am. J. Plant Sci. 2021, 12, 14. [Google Scholar] [CrossRef]
- Liu, X.M.; Yang, G.C. Adventitious shoot regeneration of oriental lily (Lilium orientalis) and genetic stability evaluation based on ISSR marker variation. In Vitro Cell. Dev. Biol. Plant 2012, 48, 172–179. [Google Scholar] [CrossRef]
- Yin, Z.F.; Zhao, B.; Bi, W.L.; Chen, L.; Wang, Q.C. Direct shoot regeneration from basal leaf segments of Lilium and assessment of genetic stability in regenerants by ISSR and AFLP markers. In Vitro Cell. Dev. Biol. Plant 2013, 49, 333–342. [Google Scholar] [CrossRef]
- Yin, Z.F.; Bi, W.L.; Chen, L.; Zhao, B.; Volk, G.M.; Wang, Q.C. An efficient, widely applicable cryopreservation of Lilium shoot tips by droplet vitrification. Acta Physiol. Plant 2014, 36, 1683–1692. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Yaakob, Z.; Anuar, N. Factors affecting in vitro regeneration of Ficus carica L. and genetic fidelity studies using molecular marker. J. Plant Biochem. Biotechnol. 2020, 30, 304–316. [Google Scholar] [CrossRef]
- Gautam, N.; Bhattacharya, A. Molecular marker based assessment of genetic homogeneity within the in vitro regenerated plants of Crocus sativus L.–a globally important high value spice crop. S. Afr. J. Bot. 2021, 140, 461–467. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).