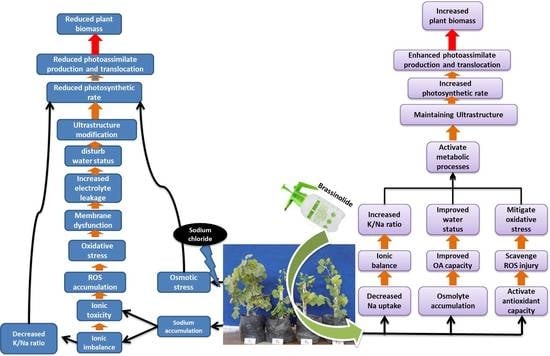
Morpho-Physiological and Anatomical Alterations of Salt-Affected Thompson Seedless Grapevine (Vitis vinifera L.) to Brassinolide Spraying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Treatments and Design
2.2. Analyses of Plant Samples
2.3. Ion Determination
2.4. The Photosynthetic Pigments
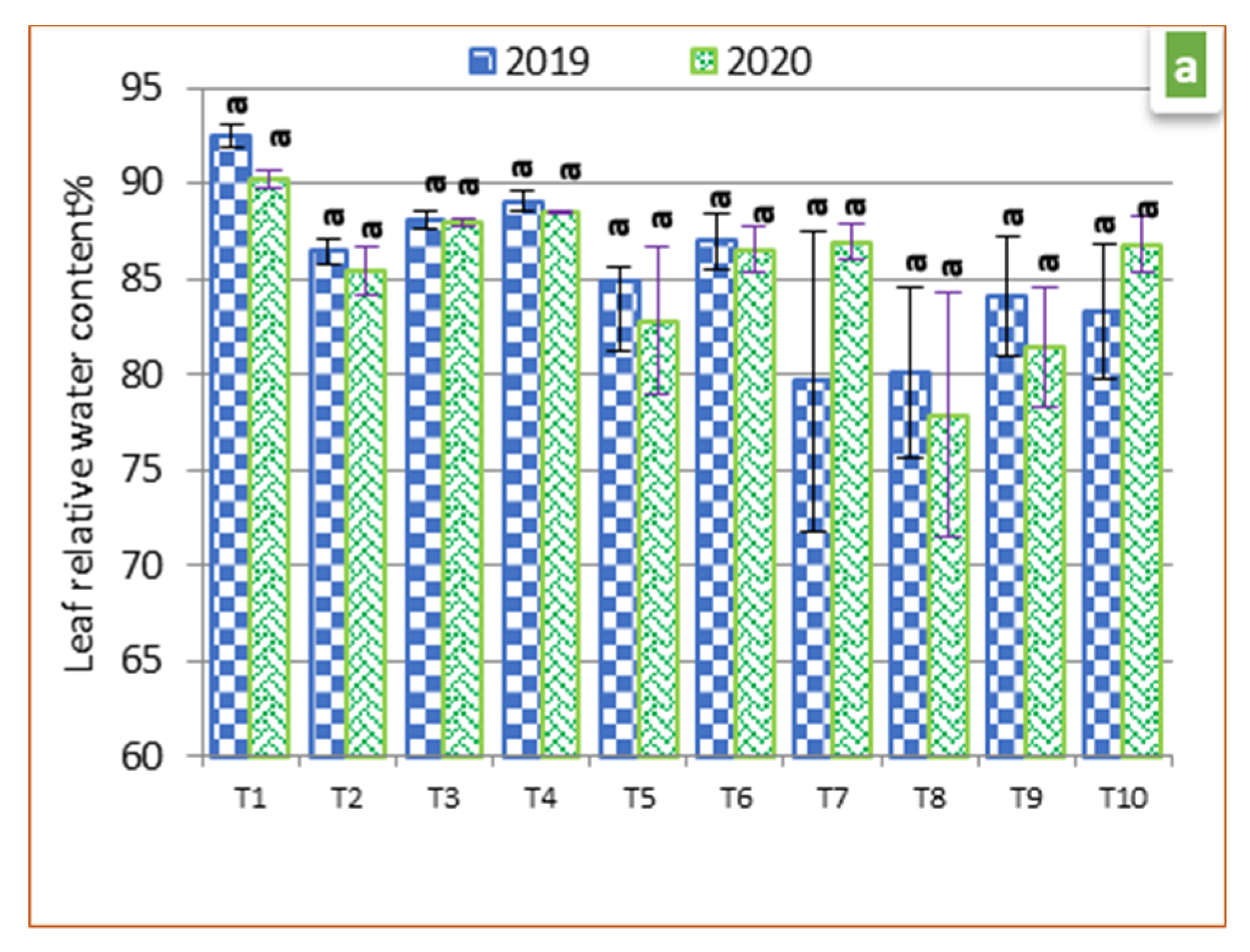
2.5. Leaf Relative Water Content (LRWC)
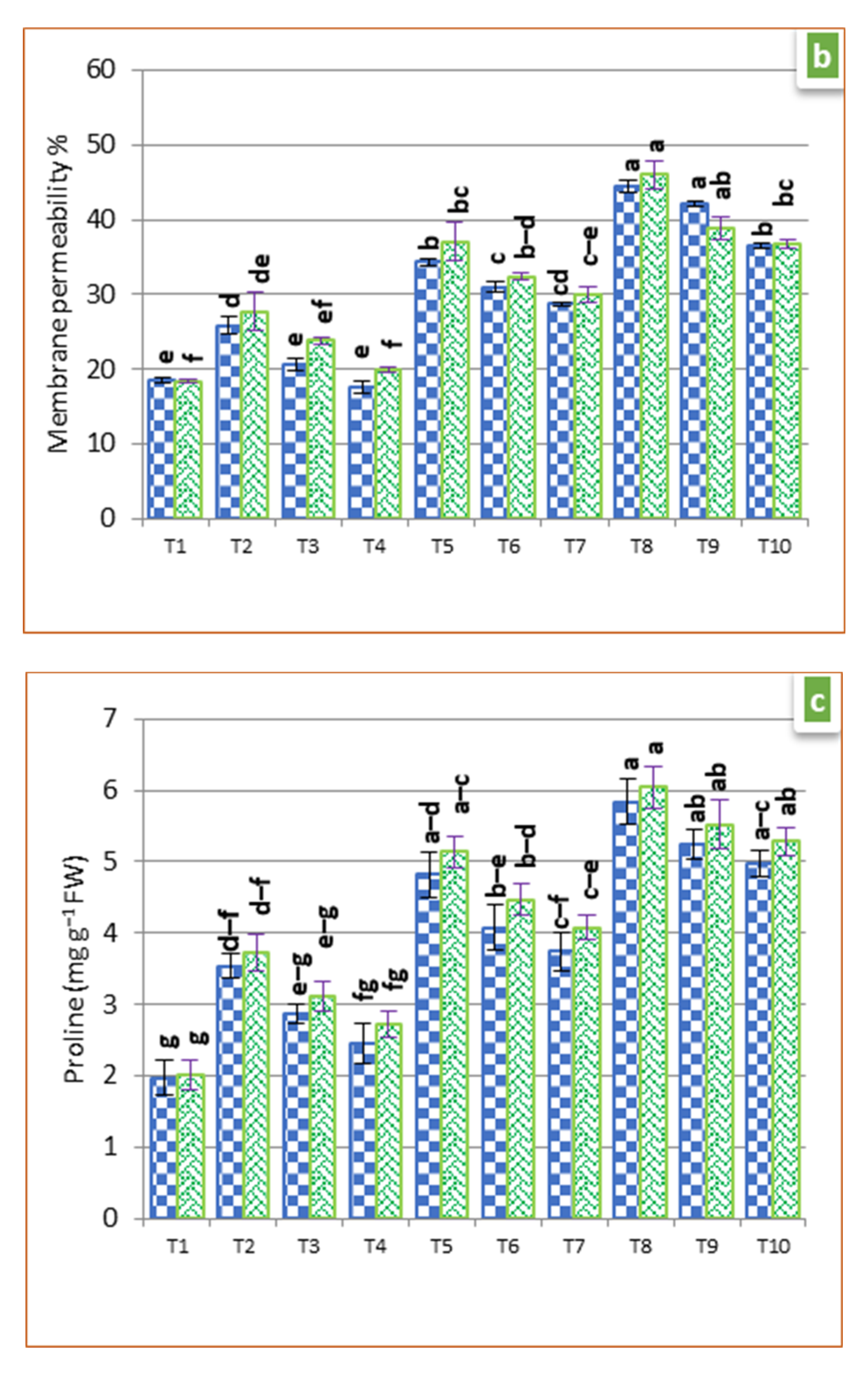
2.6. Membrane Permeability (MP)
2.7. Proline Estimation
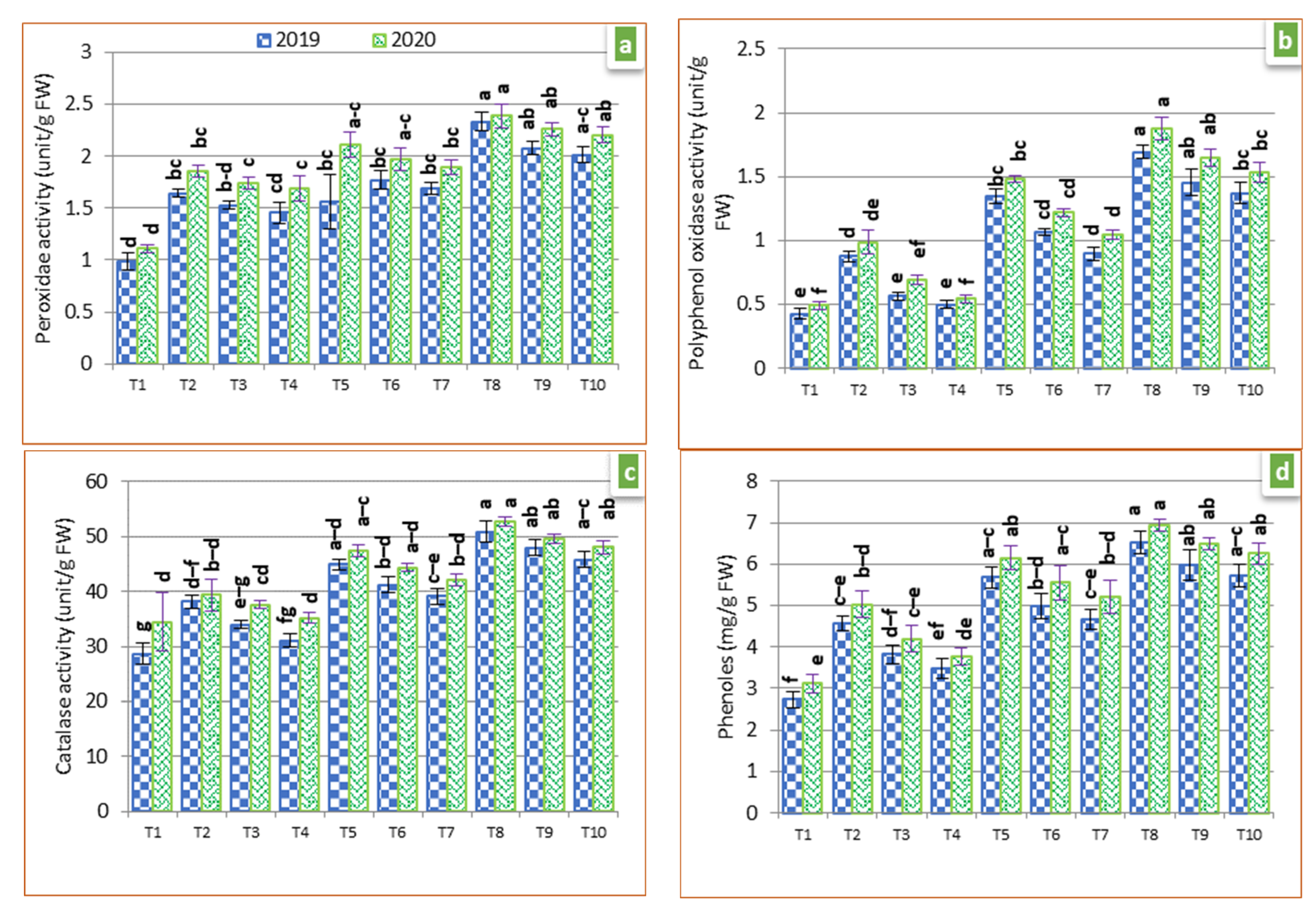
2.8. Antioxidant Enzymes and Phenols Concentration
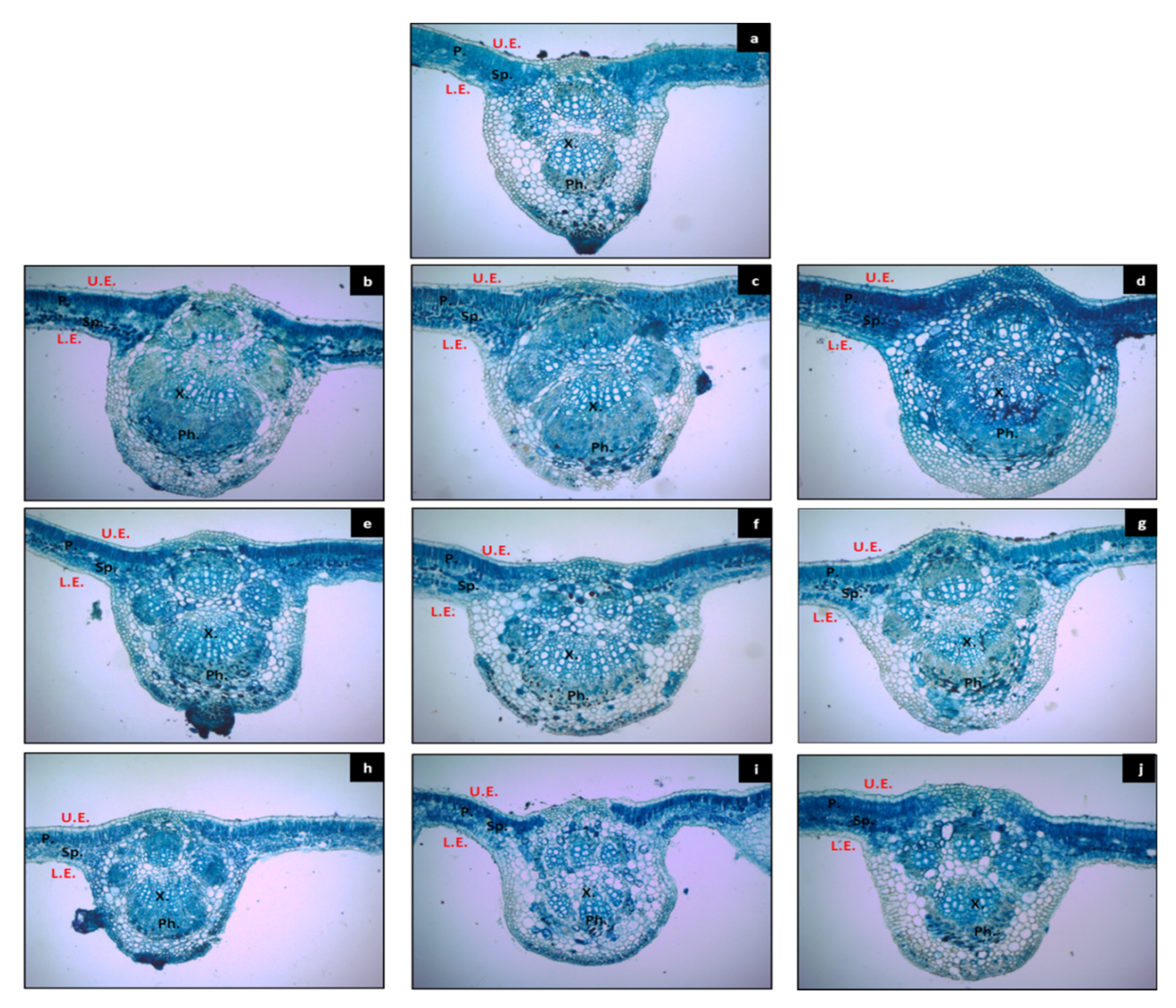
2.9. Anatomical Study
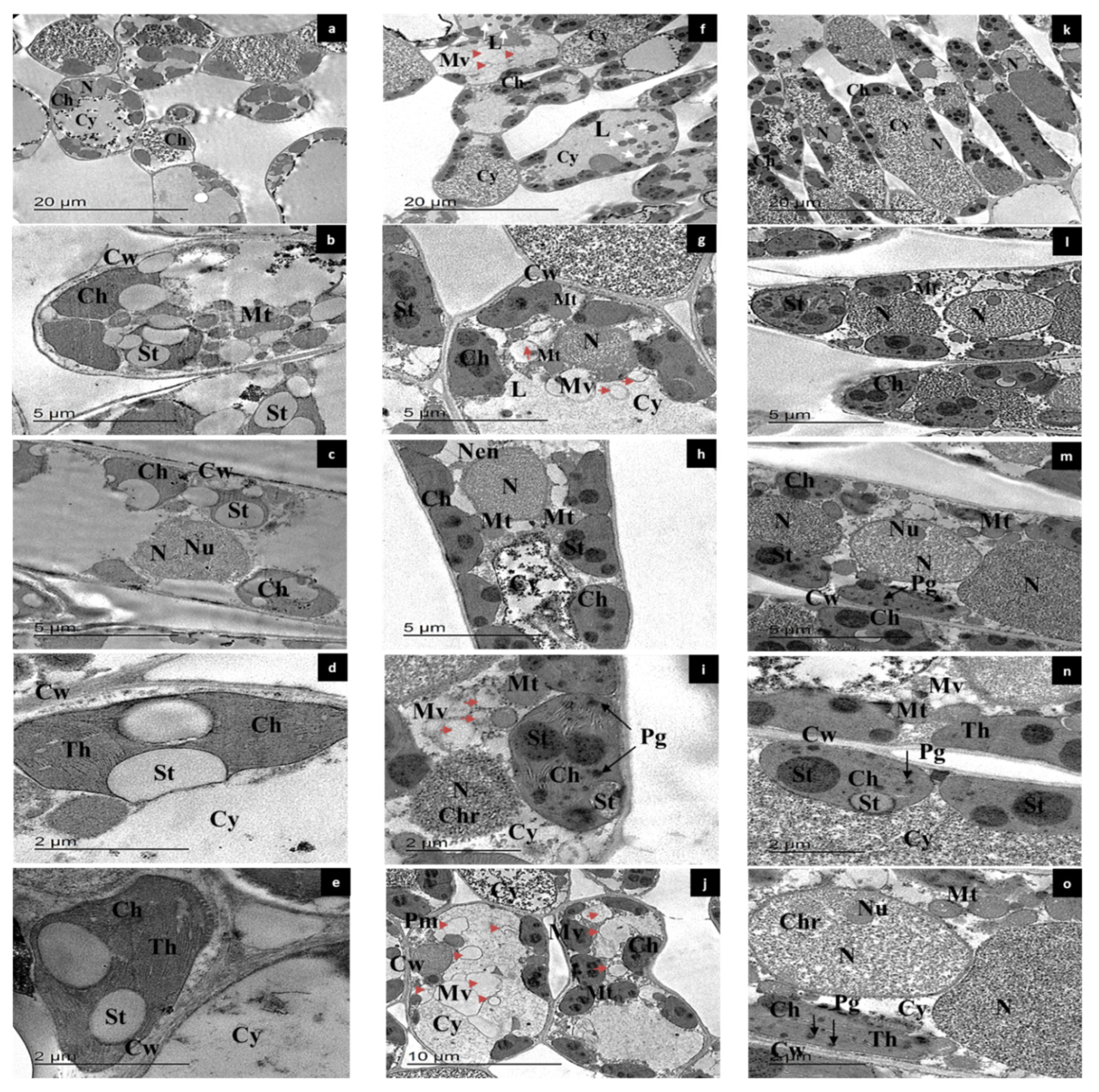
2.10. Transmission Electron Microscopy (TEM)
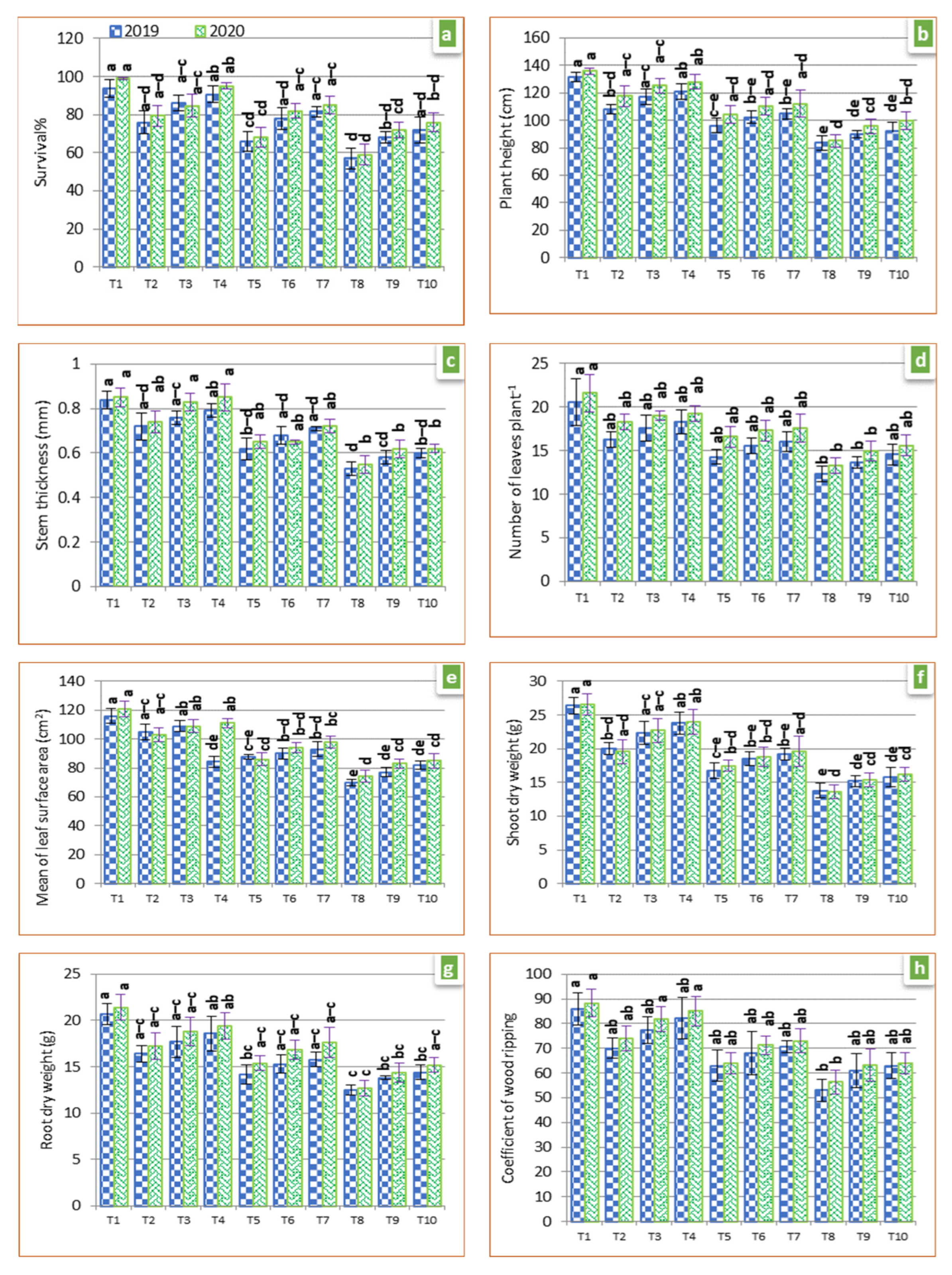
2.11. Growth Parameters
2.12. Statistical Analysis
3. Results
3.1. Mineral Nutrient Concentration
3.2. Photosynthetic Pigments
3.3. Physiological Parameters
3.4. Antioxidant Enzyme Activities and Phenol Concentration
3.5. Leaf Anatomy
3.6. Ultrastructural Characterization of Leaf Mesophyll Cells by TEM
3.7. Plant Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senthilkumar, S.; Vijayakumar, R.M.; Soorianathasundaram, K.; Durga, D. Impact of pre-harvest sprays with gibberellic acid on yield and economics of grape (Vitis vinifera L.) cv. Italia. Ann. Biol. 2018, 34, 170–175. [Google Scholar]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Rahmani, M.; Bakhshi, D.; Qolov, M. Impact of pruning severity and training systems on red and white seedless table grape (Vitis vinifera) qualitative indices. Aust. J. Crop Sci. 2015, 9, 55–61. [Google Scholar]
- Belal, B.E.A. Improvement of physical and chemical properties of Thompson seedless grapes (H4 Strain) by application of brassinolide and gibberellic acid. Egypt J. Hort. 2019, 46, 251–262. [Google Scholar] [CrossRef]
- Zhou-Tsang, A.; Wu, Y.; Henderson, S.W.; Walker, A.R.; Borneman, A.R.; Walker, R.R.; Gilliham, M. Grapevine salt tolerance. Aust. J. Grape Wine Res. 2021, 27, 149–168. [Google Scholar] [CrossRef]
- Farouk, S.; Elhindi, K.M.; Alotaibi, M.A. Silicon supplementation mitigates salinity stress on Ocimum basilicum L. via improving water balance, ion homeostasis, and antioxidant defense system. Ecotoxicol. Environ. Saf. 2020, 206, 111396. [Google Scholar] [CrossRef]
- El-Taher, A.M.; Abd El-Raouf, H.S.; Osman, N.A.; Azoz, S.N.; Omar, M.A.; Elkelish, A.; Abd El-Hady, M.A.M. Effect of salt stress and foliar application of salicylic acid on morphological, biochemical, anatomical, and productivity characteristics of cowpea (Vigna unguiculata L.) plants. Plants 2022, 11, 115. [Google Scholar] [CrossRef]
- Farouk, S.; AL-Huqail, A.A. Sustainable biochar and/or melatonin improve salinity tolerance in borage plants by modulating osmotic adjustment, antioxidants, and ion homeostasis. Plants 2022, 11, 765. [Google Scholar] [CrossRef]
- Gopalakrishnan, T.; Kumar, L. Modeling and mapping of soil salinity and its impact on paddy lands in Jaffna Peninsula, Sri Lanka. Sustainability 2020, 12, 8317. [Google Scholar] [CrossRef]
- Sarwar, M.; Anjum, S.; Ali, Q.; Alam, M.W.; Haider, M.S.; Mehboob, W. Triacontanol modulates salt stress tolerance in cucumber by altering the physiological and biochemical status of plant cells. Sci. Rep. 2021, 11, 24504. [Google Scholar] [CrossRef]
- Kaur, H.; Hussain, S.J.; Kaur, G.; Poor, P.; Alamri, S.; Siddiqui, M.H.; Khan, I.M.R. Salicylic acid improves nitrogen fixation, growth, yield and antioxidant defense mechanisms in chickpea genotypes under salt stress. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol. Environ. Saf. 2017, 137, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Fallah, F.; Nokhasi, F.; Ghaheri, M.; Kahrizi, D.; Beheshti Agha, A.; Ghorbani, T.; Kazemi, E.; Ansarypour, Z. Effect of salinity on gene expression, morphological and biochemical characteristics of Stevia rebaudiana Bertoni under in vitro conditions. Cell Mol. Biol. 2017, 63, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Din, M.; Ullah, A.; Sabagh, A.E.L.; Hossain, A.; Llanes, A.; Liu, L. Copper induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China. Environ. Sci. Pollut. Res. 2020, 27, 5211–5221. [Google Scholar] [CrossRef]
- Mumtaz, S.; Saleem, M.H.; Hameed, M.; Batool, F.; Parveen, A.; Amjad, S.F.; Mahmood, A.; Arfan, M.; Ahmed, S.; Yasmin, H. Anatomical adaptations and ionic homeostasis in aquatic halophyte Cyperus laevigatus L. under high salinities. Saudi J. Biol. Sci. 2021, 28, 2655–2666. [Google Scholar] [CrossRef] [PubMed]
- Sofy, M.R.; Elhindi, K.M.; Farouk, S.; Alotaibi, M.A. Zinc and paclobutrazol mediated regulation of growth, upregulating antioxidant aptitude and plant productivity of pea plants under salinity. Plants 2020, 9, 1197. [Google Scholar] [CrossRef]
- Hatami, S.; Pourakbar, L. Effects of manganese on physiological characters of grapevine cultivars under salinity stress. MOJ Eco Environ. Sci. 2020, 5, 62–68. [Google Scholar] [CrossRef]
- Naveed, M.; Sajid, H.; Mustafa, A.; Niamat, B.; Ahmad, Z.; Yaseen, M.; Kamran, M.; Rafique, M.; Ahmar, S.; Chen, J.T. Alleviation of salinity-induced oxidative stress, improvement in growth, physiology and mineral nutrition of canola (Brassica napus L.) through calcium-fortified composted animal manure. Sustainability 2020, 12, 846. [Google Scholar] [CrossRef] [Green Version]
- Saddiq, M.S.; Iqbal, S.; Hafeez, M.B.; Ibrahim, A.M.H.; Raza, A.; Fatima, E.M.; Baloch, H.; Jahanzaib Woodrow, P.; Ciarmiello, L.F. Effect of salinity stress on physiological changes in winter and spring wheat. Agronomy 2021, 11, 1193. [Google Scholar] [CrossRef]
- Inoue, M. Salinization status and salt removal techniques. Geotech. Eng. Manag. 2012, 60, 12–15. [Google Scholar]
- Belkheiri, O.; Mulas, M. The effects of salt stress on growth, water relations and ion accumulation in two halophyte Atriplex Species. Environ. Exp. Bot. 2013, 86, 17–28. [Google Scholar] [CrossRef]
- Shams, M.; Ekinci, M.; Ors, S.; Turan, M.; Agar, G.; Kul, R.; Yildirim, E. Nitric oxide mitigates salt stress effects of pepper seedlings by altering nutrient uptake, enzyme activity and osmolyte accumulation. Physiol. Mol. Biol. Plants 2019, 25, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.B.; Zahra, N.; Zahra, K.; Raza, A.; Khan, A.; Shaukat, K.; Khan, S. Brassinosteroids: Molecular and physiological responses in plant growth and abiotic stresses. Plant Stress 2021, 2, 100029. [Google Scholar] [CrossRef]
- Quamruzzaman, M.; Manik, S.M.N.; Shabala, S.; Zhou, M. Improving performance of salt-grown crops by exogenous application of plant growth regulators. Biomolecules 2021, 11, 788. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alam, P.; Alyemeni, M.N. Modification of osmolytes and antioxidant enzymes by 24-epibrassinolide in chickpea seedlings under mercury (Hg) toxicity. J. Plant Growth Regul. 2018, 31, 309–322. [Google Scholar] [CrossRef]
- Lone, W.A.; Majeed, N.; Yaqoob, U.; John, R. Exogenous brassinosteroid and jasmonic acid improve drought tolerance in Brassica rapa L. genotypes by modulating osmolytes, antioxidants and photosynthetic system. Plant Cell Rep. 2021, 41, 603–617. [Google Scholar] [CrossRef]
- Kolomeichuk, L.V.; Efmova, M.V.; Zlobin, I.E.; Kreslavski, V.D.; Murgan, O.K.; Kovtun, I.S.; Khripach, V.A.; Kuznetsov, V.V.; Allakhverdiev, S.I. 24-Epibrassinolide alleviates the toxic effects of NaCl on photosynthetic processes in potato plants. Photosynth. Res. 2020, 146, 151–163. [Google Scholar] [CrossRef]
- Lv, J.; Zong, X.F.; Ahmad, A.S.; Wu, X.; Wu, C.; Li, Y.P.; Wang, S.G. Alteration in morpho-physiological attributes of Leymus chinensis (Trin.) Tzvelev by exogenous application of brassinolide under varying levels of drought stress. Chil. J. Agric. Res. 2020, 80, 61–71. [Google Scholar] [CrossRef]
- Mazorra Morales, L.M.; Senn, M.E.; Gergoff Grozeff, G.E.; Fanello, D.D.; Carrión, C.A.; Núñez, M.; Bishop, G.J.; Bartoli, C.G. Impact of brassinosteroids and ethylene on ascorbic acid accumulation in tomato leaves. Plant Physiol. Biochem. 2014, 74, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Ali, B.; Hayat, S.; Fariduddin, Q.; Ahmad, A. 24-Epibrassinolide protects against the stress generated by salinity and nickel in Brassica juncea. Chemosphere 2008, 72, 1387–1392. [Google Scholar] [CrossRef]
- Krishna, P. Brassinosteroid mediated stress response. J. Plant Growth Regul. 2003, 22, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Jangid, K.; Kanwar, K.; Panwar, P.; Asiwal, R.C.; Bajya, M.; Bagdi, D.L. Effect of brassinolide in amelioration of salinity adverse effects on growth and yield of wheat. J. Pharmacogn. Phytochem. 2017, 6, 194–197. [Google Scholar]
- Lal, B.; Bagdi, D.L.; Dadarwal, B.K. Role of brassinolide in amelioration of salinity induced adverse effects on growth, yield attributes and yield of wheat. J. Pharmacogn. Phytochem. 2019, 8, 1790–1793. [Google Scholar]
- Su, Q.; Zheng, X.; Tian, Y.; Wang, C. Exogenous brassinolide alleviates salt stress in Malus hupehensis Rehd. by regulating the transcription of NHX-Type Na+(K+)/H+ Antiporters. Front. Plant Sci. 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Babalık, Z.; Demirci, T.; Aşcı, Ö.A.; Baydar, N.G. Brassinosteroids modify yield, quality, and antioxidant components in grapes (Vitis vinifera cv. Alphonse Lavallée). J. Plant Growth Regul. 2020, 39, 147–156. [Google Scholar] [CrossRef]
- Kanwar, M.K.; Bajguz, A.; Zhou, J.; Bhardwaj, R. Analysis of brassinosteroids in plants. J. Plant Growth Regul. 2017, 36, 1002–1030. [Google Scholar] [CrossRef]
- Vardhini, B.V.; Anuradha, S.; Rao, S.S.R. Brassinosteroids—A great potential to improve crop productivity. Indian J. Plant Physiol. 2006, 11, 1–12. [Google Scholar]
- Cooper, T.G. The Tools of Biochemistry; A Wiley-Interscience Pub; John Wiley and Sons: New York, NY, USA, 1977. [Google Scholar]
- Motsara, M.R.; Roy, R.N. Guide to Laboratory Establishment for Plant Nutrient Analysis; FAO Fertilizer and Plant nutrition bulletin; Food and Agriculture Organization: Rome, Italy, 2008; 219p. [Google Scholar]
- Lichtenthaler, H.; Wellburn, A. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 603, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dong, Y.J.; He, M.R.; Wang, Z.L.; Chen, W.F.; Hou, J.; Qiu, X.K.; Zhang, J.W. Effects of new coated release fertilizer on the growth of maize. J. Soil Sci. Plant Nutr. 2016, 16, 637–649. [Google Scholar] [CrossRef] [Green Version]
- Augustin, M.A.; Ghazali, H.M.; Hashim, H. Polyphenoloxidase from guava (Psidium guajava L.). J. Agric. Food Chem. 1985, 36, 1259–1265. [Google Scholar] [CrossRef]
- Tian, X.Y.; He, M.R.; Wang, Z.L.; Zhang, J.W.; Song, Y.L.; He, Z.L.; Dong, Y.J. Application of nitric oxide and calcium nitrate enhances tolerance of wheat seedlings to salt stress. Plant Growth Regul. 2015, 77, 343–356. [Google Scholar] [CrossRef]
- Tzortzakis, N.G.; Tzanakaki, K.; Economakis, C. Effect of origanum oil and vinegar on the maintenance of postharvest quality of tomato. Food Nutr. Sci. 2011, 2, 974–982. [Google Scholar] [CrossRef] [Green Version]
- Rizk, M.H.; Rizk, N.A. Effect of Dormex on bud behavior, yield and rate of wood maturity of Thompson seedless grapevine. Egypt J. Appl. Sci. 1994, 9, 525–542. [Google Scholar]
- Boursiac, Y.; Chen, S.; Luu, D.T.; Sorieul, M.; van den Dries, N.; Maurel, C. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 2005, 139, 790–805. [Google Scholar] [CrossRef] [Green Version]
- Farouk, S.; Arafa, S.A. Mitigation of salinity stress in canola plants by sodium nitroprusside application. Span. J. Agric. Res. 2018, 16, e0802. [Google Scholar] [CrossRef] [Green Version]
- Queiros, F.; Rodrigues, J.A.; Almeida, J.M.; Almeida, D.P.; Fidalgo, F. Differential responses of the antioxidant defense system and ultrastructure in a salt-adapted potato cell line. Plant Physiol. Biochem. 2011, 49, 1410–1419. [Google Scholar] [CrossRef]
- López-Gómez, M.; Hidalgocastellanos, J.; Lluch, C.; Herreracervera, J.A. 24-Epibrassinolide ameliorates salt stress effects in the symbiosis Medicago truncatula-Sinorhizobium meliloti and regulates the nodulation in cross-talk with polyamines. Plant Physiol. Biochem. 2016, 8, 212–221. [Google Scholar] [CrossRef]
- Tao, Y.Z.; Zheng, J.; Xu, Z.M.; Zhang, X.H.; Zhang, K.; Wang, G.Y. Functional analysis of ZmDWF1, a maize homolog of Arabidopsis brassinosteroids biosyntheticDWF1/DIMgene. Plant Sci. 2004, 167, 743–751. [Google Scholar] [CrossRef]
- Nomura, T.; Nakayama, N.; Reid, J.B.; Takeuchi, Y.; Yokota, T. Blockage of brassinosteroid biosynthesis and sensitivity cause dwarfism in Pisum sativum. Plant Physiol. 1997, 113, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrichsen, D.M.; Nemhauser, J.; Muramitsu, T.; Maloof, N.N.; Alonso, J.; Ecker, J.R. Three redundant brassinosteroids early response genes encode putative bHLH transcription factors required for normal growth. Genetics 2002, 162, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.G.; Ma, Q.J.; Sun, C.H.; Sun, M.H.; You, C.X.; Hao, Y.J. Overexpression of MdSOS2L1, a CIPK protein kinase, increases the antioxidant metabolites to enhance salt tolerance in apple and tomato. Physiol. Plant. 2016, 156, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Yusuf, M.; Ahmad, A.; Bashir, Z.; Saeed, T.; Fariduddin, Q.; Hayat, S.; Mock, H.P.; Wu, T. Proteomic and physiological assessment of stress sensitive and tolerant variety of tomato treated with brassinosteroids and hydrogen peroxide under low- temperature stress. Food Chem. 2019, 289, 500–511. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol. Biochem. 2020. [Google Scholar] [CrossRef]
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The physiological and molecular mechanism of brassinosteroid in response to stress: A review. Biol. Res. 2018, 51, 46. [Google Scholar] [CrossRef] [Green Version]
- Anwar, A.; Di, Q.; Yan, Y.; He, C.; Li, Y.; Yu, X. Exogenous 24-epibrassinolide alleviates the detrimental effects of suboptimal root zone temperature in cucumber seedlings. Arch. Agron. Soil Sci. 2019, 65, 1927–1940. [Google Scholar] [CrossRef]
- Mohanabharathi, M.; Sritharan, N.; Senthil, A.; Ravikesavan, R. Physiological studies for yield enhancement in finger millet under drought condition. J Pharm. Phytochem. 2019, 8, 3308–3312. [Google Scholar]
- Hasan, S.A.; Hayat, S.; Ahmad, A. Brassinosteroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere 2011, 84, 1446–1451. [Google Scholar] [CrossRef]
- Gupta, P.; Srivastava, S.; Seth, C.S. 24-Epibrassinolide and sodium nitroprusside alleviate the salinity stress in Brassica juncea L. cv. Varuna through cross talk among proline, nitrogen metabolism and abscisic acid. Plant Soil 2017, 411, 483–498. [Google Scholar] [CrossRef]
- Zhiponova, M.K.; Vanhoutte, I.; Boudolf, V.; Betti, C.; Dhondt, S.; Coppens, F.; Mylle, E.; Maes, S.; González-García, M.P.; Caño-Delgado, A.I.; et al. Brassinosteroid production and signaling differentially control cell division and expansion in the leaf. N. Phytol. 2013, 197, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Karlidag, H.; Yildirim, E.; Turan, M. Role of 24-epibrassinolide in mitigating the adverse effects of salt stress on stomatal conductance, membrane permeability, and leaf water content, ionic composition in salt stressed strawberry (Fragaria x ananassa). Sci. Hortic. 2011, 130, 133–140. [Google Scholar] [CrossRef]
- Miao, S.Q.; Han, Y.F.; Xiao-Zenga, M.A.N. Nodule formation and development in soybeans (Glycine max L.) in response to phosphorus supply in solution culture. Pedosphere 2007, 17, 36–43. [Google Scholar] [CrossRef]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front. Plant Sci. 2016, 7, 609. [Google Scholar] [CrossRef] [Green Version]
- Othman, Y.; Al-Karaki, G.; Al-Tawaha, A.R.; Al-Horani, A. Variation in germination and ion uptake in barley genotypes under salinity conditions. World J. Agric. Sci. 2006, 2, 11–15. [Google Scholar]
- Ghonaim, M.M.; Mohamed, H.I.; Omran, A.A.A. Evaluation of wheat salt stress tolerance using physiological parameters and retrotransposon-based markers. Genet. Resour. Crop Evol. 2020. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Amri, S.M. Exogenous melatonin-mediated modulation of arsenic tolerance with improved accretion of secondary metabolite production, activating antioxidant capacity and improved chloroplast ultrastructure in rosemary herb. Ecotoxicol. Environ. Saf. 2019, 180, 333–347. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Amri, S.M. Exogenous zinc forms counteract NaCl-induced damage by regulating the antioxidant system, osmotic adjustment substances and ions in canola (Brassica napus L. cv Pactol) plants. J. Soil Sci. Plant Nutr. 2019, 19, 887–899. [Google Scholar] [CrossRef]
- Ren, J.; Ye, J.; Yin, L.; Li, G.; Deng, X.; Wang, S. Exogenous melatonin improves salt tolerance by mitigating osmotic, ion, and oxidative stresses in maize seedlings. Agronomy 2020, 10, 663. [Google Scholar] [CrossRef]
- Miranda, R.S.; Mesquita, R.O.; Costa, J.H.; Alvarez-Pizarro, J.C.; Prisco, J.T.; Gomes-Filho, E. Integrative control between proton pumps and SOS1 antiporters in roots is crucial for maintaining low Na+ accumulation and salt tolerance in ammonium-supplied Sorghum bicolor. Plant Cell Physiol. 2017, 58, 522–536. [Google Scholar] [CrossRef]
- Hassanpouraghdam, M.B.; Mehrabani, L.V.; Tzortzakis, N. Foliar application of nano-zinc and iron affects physiological attributes of Rosmarinus officinalis and quietens NaCl salinity depression. J. Soil Sci. Plant Nutr. 2019, 20, 335–345. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. 24-Epibrassinolide alleviates salt induced inhibition of productivity by increasing nutrients and compatible solutes accumulation and enhancing antioxidant system in wheat (Triticum aestivum L.). Acta Physiol. Plant 2013, 35, 729–740. [Google Scholar] [CrossRef]
- Wu, C.J.; Wang, J.; Zhu, J.; Ren, J.; Yang, Y.X.; Luo, T.; Xu, L.X.; Zhou, Q.H.; Xiao, X.F.; Zhou, Y.X.; et al. Molecular characterization of Mg-chelatase CHLI subunit in pea (Pisum sativum L.). Front. Plant Sci. 2022, 13, 821683. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, H.; Zhang, Y.; Li, Z.; Yang, Y.; Guo, Y. The GSK3-like Kinase BIN2 Is a molecular switch between the salt stress response and growth recovery in Arabidopsis thaliana developmental. Cell 2020, 9, 367–380.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Tahjib-UI-Arif, M.; Sohag, A.A.M.; Afrin, S.; Bashar, K.K.; Afrin, T.; Mahamud, A.G.M.S.U.; Polash, M.A.S.; Hossain, M.T.; Sohel, M.A.T.; Brestic, M.; et al. Differential response of sugar beet to long-term mild to severe salinity in a soil–plot Culture. Agriculture 2019, 9, 223. [Google Scholar] [CrossRef] [Green Version]
- Abdelaal, K.A.; EL-Maghraby, L.M.; Elansary, H.; Hafez, Y.M.; Ibrahim, E.I.; El-Banna, M.; El-Esaw, M.I.; Elkelish, A. Treatment of sweet pepper with stress tolerance-inducing compounds alleviates salinity stress oxidative damage by mediating the physio-biochemical activities and antioxidant systems. Agronomy 2020, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Van Hoorn, J.W.; Katerji, N.; Hamdy, A.; Mastrorilli, M. Effect of salinity on yield and nitrogen uptake of four grain legumes and on biological nitrogen contribution from the Soil. Agric. Water Manag. 2001, 51, 87–98. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Alsubaie, Q.D.; Ali, H.M.; Khan, M.N.; Al-Ghamdi, A.; Ibrahim, A.A.; Alsadon, A. Exogenous nitric oxide alleviates sulfur deficiency-induced oxidative damage in tomato seedlings. Nitric Oxide Biol. Chem. 2020, 94, 95–107. [Google Scholar] [CrossRef]
- Szafranska, K.; Reiter, R.J.; Posmyk, M.M. Melatonin improves the photosynthetic apparatus in pea leaves stressed by paraquat via chlorophyll breakdown regulation and it’s accelerated de novo synthesis. Front. Plant Sci. 2017, 8, 179. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Nakamura, Y.; Taliman, N.A.; Sabagh, A.E.; Moghaieb, R.E.; Saneoka, H. Differences in the growth and physiological responses of the leaves of Peucedanum japonicum and Hordeum vulgare exposed to salinity. Agriculture 2020, 10, 317. [Google Scholar] [CrossRef]
- Farouk, S.; Arafa, S.A.; Nassar, R.M.A. Improving drought tolerance in corn (Zea mays L.) by foliar application with salicylic acid. Int. J. Environ. 2018, 7, 104–123. [Google Scholar]
- Wang, M.; Jiang, W.; Yu, H. Effects of exogenous epibrassinolide on photosynthetic characteristics in tomato (Lycopersicon esculentum Mill) seedlings under weak light stress. J. Agric. Food Chem. 2010, 58, 3642–3645. [Google Scholar] [CrossRef]
- Bajguz, A. Effect of brassinosteroids on nucleic acid and protein content in cultured cell of Chlorella vulgaris. Plant Physiol. Biochem. 2000, 38, 209–215. [Google Scholar] [CrossRef]
- Li, G.; Peng, X.; Wei, L.; Kang, G. Salicylic acid increases the contents of glutathione and ascorbate and temporally regulates the related gene expression in salt-stressed wheat seedlings. Gene 2013, 529, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.P.; Zhang, X.; Tang, W.Q.; Oses-Prieto, J.A.; Suzuki, N.; Gendron, J.M.; Chen, H.; Guan, S.; Chalkley, R.J.; Peterman, T.K.; et al. A proteomics study of brassinosteroid response in Arabidopsis. Mol. Cell Proteom. 2007, 6, 2058–2071. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, E.; Turan, E.; Guvenc, M. Effect of foliar salicylic acid application on growth, chlorophyll and mineral content cucumber grown under salt stress. J. Plant Nutr. 2008, 31, 593–612. [Google Scholar] [CrossRef]
- Hayat, S.; Hasan, S.A.; Hayat, Q.; Ahmad, A. Brassinosteroids protect Lycopersicon esculentum from cadmium toxicity applied as shotgun approach. Protoplasma 2010, 239, 3–14. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M. Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L). Protoplasma 2017, 254, 1471–1486. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qin, C.; Maodong, Q.; Dong, X.X.; Ahmad, P.; Abd-Allah, E.F.; Zhang, L. Spermine application alleviates salinity induced growth and photosynthetic inhibition in Solanum lycopersicum by modulating osmolyte and secondary metabolite accumulation and differentially regulating antioxidant metabolism. Plant Physiol. Biochem. 2019, 144, 1–13. [Google Scholar] [CrossRef]
- Morillon, R.; Catterou, M.; Sangwan, R.S.; Sangwan, B.S.; Lassalles, J.P. Brassinolide may control aquaporin activities in Arabidopsis thaliana. Planta 2001, 212, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.J.; Wang, Z.L.; Zhang, J.W.; Liu, S.; He, Z.L.; He, M.R. Interaction effects of nitric oxide and salicylic acid in alleviating salt stress of Gossypium hirsutum L. J. Soil Sci. Plant Nutr. 2015, 15, 561–573. [Google Scholar]
- Hu, Y.; Xia, S.; Su, Y.; Wang, H.; Luo, W.; Su, S.; Xiao, L. Brassinolide increases potato root growth in vitro in a dose-dependent way and alleviates salinity stress. Biomed Res. Int. 2016, 8231873. [Google Scholar] [CrossRef] [Green Version]
- Mansour, M.M.F. Plasma membrane permeability as an indicator of salt tolerance in plants. Biol. Plant 2013, 57, 1–10. [Google Scholar] [CrossRef]
- Hare, P.; Cress, W. Metabolic implications of stress induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Reddy, P.S.; Jogeswar, G.; Rasineni, G.K.; Maheswari, M.; Reddy, A.R.; Varshney, R.K.; Kishor, P.B.K. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol. Biochem. 2015, 94, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Farouk, S.; Omar, M.M. Sweet basil growth, physiological and ultrastructural modification and oxidative defense system under water deficit and silicon forms treatment. J. Plant Growth Regul. 2020, 39, 1307–1331. [Google Scholar] [CrossRef]
- Miller, G.; Honig, A.; Stein, H.; Suzuki, N.; Mittler, R.; Zilberstein, A. Unraveling ∆1-pyrroline-5-carboxylate-proline cycle in plants by uncoupled expression of proline oxidation enzymes. J. Biol. Chem. 2009, 284, 26482–26492. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.P.; Zhu, X.H.; Ding, H.D.; Yang, S.J.; Chen, Y.Y. Foliar application of 24-epibrassinolide alleviates high-temperature-induced inhibition of photosynthesis in seedlings of two melon cultivars. Photosynthetica 2013, 51, 341–349. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, L.; Chen, D.; Liang, M.; Liu, Z.; Shao, H.; Long, X. Salt stress encourages proline accumulation by regulating proline biosynthesis and degradation in Jerusalem artichoke plantlets. PLoS ONE 2013, 8, e62085. [Google Scholar] [CrossRef]
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon 2019, 5, e02952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Shriram, V.; Kavi Kishor, P.B.; Jawali, N.; Shitole, M.G. Enhanced proline accumulation and salt stress tolerance of transgenic indica rice by over-expressing P5CSF129A gene. Plant Biotechnol. Rep. 2010, 4, 37–48. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Reactive oxygen species and antioxidant enzymes involved in plant tolerance to stress. In Abiotic and Biotic Stress in Plants-recent Advances and Future Perspectives; Shanker, A.K., Shanker, C., Eds.; InTech: London, UK, 2016; pp. 463–480. [Google Scholar]
- Arora, N.; Bhardwaj, R.; Sharma, P.; Arora, H.K. Effects of 28-homobrassinolide on growth, lipid peroxidation and antioxidative enzyme activities in seedlings of Zea mays L. under salinity stress. Acta Physiol. Plant 2008, 30, 833–839. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Rahman, M.M.; Das, R.; Hoque, M.M.; Zzaman, W. Effect of freeze drying on antioxidant activity and phenolic contents of Mango (Mangifera indica). Int. Food Res. J. 2015, 22, 613–617. [Google Scholar]
- El-Banna, M.F.; Abdelaal, K.A.A. Response of strawberry plants grown in the hydroponic system to pretreatment with H2O2 before exposure to salinity stress. J. Plant Prod. Mansoura Univ. 2018, 9, 989–1001. [Google Scholar] [CrossRef]
- Nassar, M.A.; Azoz, D.N.; Wessam, S.; Serag El-Din, M. Improved growth and productivity of basil plants grown under salinity stress by foliar application with ascorbic acid. Middle East J. Agric 2019, 8, 211–225. [Google Scholar]
- Guerfel, M.; Baccouri, O.; Boujnah, D.; Chaibi, W.; Zarrouk, M. Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Sci. Hortic. 2009, 15, 15–20. [Google Scholar] [CrossRef]
- Nieman, R.H. Expansion of bean leaves and its suppression by salinity. Plant Physiol. 1965, 40, 156–161. [Google Scholar] [CrossRef]
- Rashid, P.; Karmoker, J.L.; Chakrabortty, S.; Sarker, B.C. The effect of salinity on ion accumulation and anatomical attributes in mungbean (Phaseolus radiate L. cv BARI-3) seedlings. Int. J. Agric. Biol. 2004, 6, 495–498. [Google Scholar]
- Kulaeva, O.N.; Burkhanova, E.; Fedina, A.; Khokhlova, V.; Bokebayeva, G.; Vorbrodt, H.; Adam, G. Effect of brassinosteroids on protein synthesis and plant-cell ultrastructure under stress conditions. In Brassinosteroids. Chemistry, Bioactivity and Applications; Cutler, H.G., Yokota, T., Adam, G., Eds.; American Chemical Society: Washington, DC, USA, 1991; pp. 141–155. [Google Scholar]
- Ibrahim, S.A.; Abo-ELwafa, T.S.A. Alleviation harmful impacts of salinity using some antioxidants substances on Thompson seedless grapevines seedlings. J. Plant Prod. Mansoura Univ. 2018, 9, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Sarker, U.; Oba, S. The response of salinity stress-Induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef]
- Austin, J.R.; Frost, E.; Vidi, P.A.; Kessler, F.; Staehelin, L. Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell 2006, 18, 1693–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arafa, A.A.; Khafagy, M.A.; El-Banna, M.F. The effect of glycinebetaine or ascorbic acid on grain germination and leaf structure of sorghum plants grown under salinity stress. Aust. J. Crop Sci. 2009, 3, 294–304. [Google Scholar]
- Rahman, M.S.; Matsumuro, T.; Miyake, H.; Takeoka, Y. Salinity-induced ultrastructural alternations in leaf cells of rice (Oryza sativa L.). Plant Prod. Sci. 2000, 3, 422–429. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.L.; Flowers, T.J.; Wang, S.M. Mechanisms of sodium uptake by roots of higher plants. Plant Soil 2010, 326, 45–60. [Google Scholar] [CrossRef]
- Pareek, A.; Singla, S.L.; Grover, A. Short-term salinity and high temperature stress-associated ultrastructural alterations in young leaf cells of Oryza sativa L. Ann. Bot. 1997, 80, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Rizhsky, L.; Liang, H.J.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.S.; Miyake, H.; Takeoka, Y. Effects of exogenous glycinebetaine on growth and ultrastructure of salt-stressed rice seedlings (Oryza sativa L.). Plant Prod. Sci. 2002, 5, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Arafa, A.A.; Khafagy, M.A.; El-Banna, M.F. Role of glycinebetaine and ascorbic acid in the alleviation of salt-stress induced micro-morphological damages in sweet pepper seedlings. J. Biol. Sci. 2007, 7, 879–887. [Google Scholar] [CrossRef] [Green Version]

| Code | Treatment |
|---|---|
| T1 | Control, irrigated with tap water, 0 salinity (NaCl) without BL application |
| T2 | Irrigated with saline water (1000 mg L−1 NaCl) without BL application |
| T3 | Irrigated with saline water (1000 mg L−1 NaCl) plus 1 mg L−1 BL foliar application |
| T4 | Irrigated with saline water (1000 mg L−1 NaCl) plus 2 mg L−1 BL foliar application |
| T5 | Irrigated with saline water (2000 mg L−1 NaCl) without BL application |
| T6 | Irrigated with saline water (2000 mg L−1 NaCl) plus 1 mg L−1 BL foliar application |
| T7 | Irrigated with saline water (2000 mg L−1 NaCl) plus 2 mg L−1 BL foliar application |
| T8 | Irrigated with saline water (3000 mg L−1 NaCl) without BL application |
| T9 | Irrigated with saline water (3000 mg L−1 NaCl) plus 1 mg L−1 BL foliar application |
| T10 | Irrigated with saline water (3000 mg L−1 NaCl) plus 2 mg L−1 BL foliar application |
| Treatments | Nitrogen% | Phosphorous% | Potassium% | Sodium% | Potassium/Sodium Ratio | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| T1 | 2.53 ± 0.03 a | 2.51 ± 0.02 a | 0.364 ± 0.002 a | 0.362 ± 0.002 a | 0.94 ± 0.01 a | 0.96 ± 0.01 a | 0.29 ± 0.01 f | 0.31 ± 0.01 e | 3.25 ± 0.11 a | 3.11 ± 0.21 a |
| T2 | 1.61 ± 0.03 d,e | 1.60 ± 0.02 d,e | 0.285 ± 0.003 d | 0.280 ± 0.002 d | 0.63 ± 0.01 c | 0.62 ± 0.01 c,d | 0.64 ± 0.01 d | 0.66 ± 0.01 c | 0.98 ± 0.03 c | 0.93 ± 0.01 d |
| T3 | 1.90 ± 0.03 c | 1.92 ± 0.02 c | 0.301 ± 0.002 c | 0.305 ± 0.001 c | 0.88 ± 0.01 a | 0.89 ± 0.01 a | 0.57 ± 0.01 e | 0.65 ± 0.02 c | 1.54 ± 0.02 b | 1.37 ± 0.08 b,c |
| T4 | 2.14 ± 0.03 b | 2.15 ± 0.01 b | 0.324 ± 0.002 b | 0.331 ± 0.001 b | 0.89 ± 0.02 a | 0.89 ± 0.01 a | 0.54 ± 0.01 e | 0.55 ± 0.01 d | 1.65 ± 0.06 b | 1.61 ± 0.03 b |
| T5 | 1.49 ± 0.02 e | 1.52 ± 0.03 e | 0.227 ± 0.001 g | 0.225 ± 0.001 g | 0.49 ± 0.01 d | 0.47 ± 0.02 e | 0.87 ± 0.01 b | 0.89 ± 0.01 a | 0.56 ± 0.02 d | 0.52 ± 0.01 e,f |
| T6 | 1.80 ± 0.03 c | 1.82 ± 0.02 c | 0.243 ± 0.002 f | 0.249 ± 0.001 e,f | 0.67 ± 0.01 b,c | 0.65 ± 0.02 b,c | 0.78 ± 0.01 c | 0.75 ± 0.02 b | 0.85 ± 0.01 c | 0.86 ± 0.02 d,e |
| T7 | 1.89 ± 0.01 c | 1.91 ± 0.02 c | 0.277 ± 0.002 d | 0.281 ± 0.003 d | 0.71 ± 0.01 b | 0.72 ± 0.01 b | 0.66 ± 0.01 d | 0.67 ± 0.01 c | 1.07 ± 0.01 c | 1.07 ± 0.02 c,d |
| T8 | 1.14 ± 0.01 f | 1.11 ± 0.01 f | 0.209 ± 0.001 h | 0.201 ± 0.002 h | 0.34 ± 0.01 e | 0.33 ± 0.01 f | 0.94 ± 0.01 a | 0.96 ± 0.01 a | 0.36 ± 0.01 d | 0.34 ± 0.01 f |
| T9 | 1.64 ± 0.02 d | 1.70 ± 0.01 d | 0.239 ± 0.001 f | 0.241 ± 0.001 f | 0.48 ± 0.01 d | 0.56 ± 0.01 d | 0.81 ± 0.01 b | 0.76 ± 0.01 b | 0.59 ± 0.02 d | 0.73 ± 0.01 d,e |
| T10 | 1.84 ± 0.02 c | 1.85 ± 0.02 c | 0.259 ± 0.001 e | 0.255 ± 0.002 e | 0.69 ± 0.01 b,c | 0.70 ± 0.02 b | 0.79 ± 0.01 b,c | 0.71 ± 0.01 b,c | 0.87 ± 0.03 c | 0.98 ± 0.02 d |
| p value | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Treatments | Chlorophyll a | Chlorophyll b | Total Chlorophyll | Carotenoids | ||||
|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| T1 | 2.18 ± 0.18 a | 2.62 ± 0.12 a | 1.47 ± 0.19 a | 1.68 ± 0.10 a | 3.65 ± 0.37 a | 4.31 ± 0.22 a | 0.185 ± 0.01 | 0.215 ± 0.01 a |
| T2 | 1.47 ± 0.13 a–c | 2.02 ± 0.18 a–c | 0.98 ± 0.09 a,b | 1.19 ± 0.13 b–d | 2.45 ± 0.23 a,b | 3.21 ± 0.30 a–d | 0.135 ± 0.01 | 0.170 ± 0.01 a–c |
| T3 | 1.76 ± 0.19 a–c | 2.36 ± 0.14 a,b | 1.12 ± 0.10 a,b | 1.42 ± 0.17 a–c | 2.88 ± 0.29 a,b | 3.78 ± 0.31 a,b | 0.154 ± 0.01 | 0.192 ± 0.01 a,b |
| T4 | 1.88 ± 0.24 a,b | 2.44 ± 0.09 a,b | 1.24 ± 0.16 a,b | 1.53 ± 0.09 a,b | 3.12 ± 0.40 a,b | 3.97 ± 0.19 a,b | 0.164 ± 0.01 | 0.198 ± 0.01 a,b |
| T5 | 1.20 ± 0.16 b,c | 1.52 ± 0.12 c,d | 0.79 ± 0.09 b | 0.95 ± 0.06 c,d | 1.99 ± 0.26 a,b | 2.47 ± 0.18 c,d | 0.122 ± 0.01 | 0.138 ± 0.01 c |
| T6 | 1.40 ± 0.12 a–c | 1.88 ± 0.11 b–d | 0.94 ± 0.09 a,b | 1.16 ± 0.07 b–d | 2.34 ± 0.22 a,b | 3.04 ± 0.18 b–d | 0.134 ± 0.01 | 0.163 ± 0.01 b,c |
| T7 | 1.52 ± 0.10 a–c | 2.07 ± 0.16 a–c | 0.99 ± 0.07 a,b | 1.23 ± 0.09 a–d | 2.51 ± 0.18 a,b | 3.31 ± 0.25 a,c | 0.141 ± 0.01 | 0.176 ± 0.01 a–c |
| T8 | 1.08 ± 0.10 c | 1.35 ± 0.11 d | 0.71 ± 0.08 b | 0.82 ± 0.09 d | 1.79 ± 0.19 a,b | 2.18 ± 0.21 d | 0.141 ± 0.02 | 0.128 ± 0.01 c |
| T9 | 1.18 ± 0.10 b,c | 1.47 ± 0.07 c,d | 0.78 ± 0.07 b | 0.91 ± 0.03 d | 1.96 ± 0.17 a,b | 2.39 ± 0.10 c,d | 0.121 ± 0.01 | 0.140 ± 0.01 c |
| T10 | 1.26 ± 0.13 b,c | 1.57 ± 0.06 c,d | 0.84 ± 0.08 b | 0.98 ± 0.03 c,d | 2.11 ± 0.21 a,b | 2.55 ± 0.10 c,d | 0.127 ± 0.01 | 0.147 ± 0.01 b,c |
| p value | ** | *** | ** | *** | ** | *** | NS | *** |
| Treatments | Dimension of Midrib (μm) | Thickness of Leaf Blade (μm) | Upper Epidermis Thickness (μm) | Palisade Tissue Thickness (μm) | Spongy Tissue Thickness (μm) | Lower Epidermis Thickness (μm) | Dimension of Compound Vascular Bundle (μm) | ||
|---|---|---|---|---|---|---|---|---|---|
| Thickness | Width | Thickness | Width | ||||||
| T1 | 75.83 ± 1.11 d | 61.57 ± 0.57 d | 15.84 ± 0.20 e,f | 1.62 ± 0.10 b | 5.87 ± 0.09 d,e | 6.53 ± 0.09 d | 1.82 ± 0.09 d | 45.04 ± 0.22 d | 42.76 ± 0.57 d |
| T2 | 82.41 ± 0.90 b,c | 70.88 ± 1.19 b,c | 17.71 ± 0.21 e | 2.32 ± 0.09 a | 4.75 ± 0.21 e,f | 8.14 ± 0.24 b,c | 2.48 ± 0.09 a,b | 60.58 ± 1.00 a,b | 55.24 ± 1.22 b,c |
| T3 | 85.88 ± 0.65 a,b | 75.48 ± 0.79 b | 30.75 ± 0.35 b | 1.62 ± 0.1 b | 19.42 ± 0.32 a | 7.48 ± 0.12 b–d | 2.22 ± 0.09 b–d | 60.88 ± 0.91 a,b | 58.60 ± 0.14 a,b |
| T4 | 89.34 ± 1.63 a | 76.47 ± 0.79 b | 33.76 ± 1.01 a | 2.214 ± 0.25 a | 19.35 ± 0.52 a | 9.91 ± 0.32 a | 2.27 ± 0.11 b,c | 66.82 ± 1.01 a | 57.81 ± 0.91 a,b |
| T5 | 75.48 ± 0.79 d | 62.12 ± 0.85 c,d | 21.64 ± 0.40 d | 2.43 ± 0.06 a | 6.45 ± 0.05 d | 9.93 ± 0.32 a | 2.83 ± 0.09 a | 53.70 ± 2.45 c | 47.27 ± 0.85 c,d |
| T6 | 68.80 ± 0.79 e | 79.59 ± 0.91 a,b | 27.21 ± 0.79 c | 1.42 ± 0.06 b | 16.13 ± 0.37 b | 7.28 ± 0.38 c,d | 2.38 ± 0.06 b | 46.03 ± 0.91 d | 55.24 ± 0.65 b,c |
| T7 | 79.00 ± 1.51 c,d | 71.87 ± 1.94 b | 28.18 ± 0.91 b,c | 1.62 ± 0.1 b | 16.64 ± 0.50 b | 7.64 ± 0.38 b–d | 2.27 ± 0.08 b,c | 56.03 ± 1.84 b,c | 53.06 ± 1.23 b,c |
| T8 | 65.09 ± 0.48 e,f | 58.41 ± 0.70 d | 14.37 ± 0.16 f | 1.52 ± 0.11 b | 4.25 ± 0.20 f | 6.37 ± 0.21 d | 2.22 ± 0.09 b–d | 41.97 ± 0.91 d | 32.86 ± 0.57 e |
| T9 | 61.62 ± 0.65 f | 56.18 ± 1.18 d | 23.63 ± 0.12 d | 1.45 ± 0.04 b | 14.04 ± 0.12 c | 6.26 ± 0.09 d | 1.87 ± 0.06 c,d | 43.56 ± 0.70 d | 41.33 ± 0.95 d,e |
| T10 | 82.66 ± 2.47 b,c | 87.36 ± 5.26 a | 20.98 ± 0.63 d | 2.53 ± 0.11 a | 6.76 ± 0.26 d | 8.85 ± 0.52 a,b | 2.83 ± 0.09 a | 58.16 ± 2.56 b,c | 67.56 ± 5.89 a |
| p value | *** | *** | *** | *** | *** | *** | *** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Banna, M.F.; AL-Huqail, A.A.; Farouk, S.; Belal, B.E.A.; El-Kenawy, M.A.; Abd El-Khalek, A.F. Morpho-Physiological and Anatomical Alterations of Salt-Affected Thompson Seedless Grapevine (Vitis vinifera L.) to Brassinolide Spraying. Horticulturae 2022, 8, 568. https://doi.org/10.3390/horticulturae8070568
El-Banna MF, AL-Huqail AA, Farouk S, Belal BEA, El-Kenawy MA, Abd El-Khalek AF. Morpho-Physiological and Anatomical Alterations of Salt-Affected Thompson Seedless Grapevine (Vitis vinifera L.) to Brassinolide Spraying. Horticulturae. 2022; 8(7):568. https://doi.org/10.3390/horticulturae8070568
Chicago/Turabian StyleEl-Banna, Mostafa F., Arwa Abdulkreem AL-Huqail, Saad Farouk, Bassam E. A. Belal, Mosaad A. El-Kenawy, and Ahmed F. Abd El-Khalek. 2022. "Morpho-Physiological and Anatomical Alterations of Salt-Affected Thompson Seedless Grapevine (Vitis vinifera L.) to Brassinolide Spraying" Horticulturae 8, no. 7: 568. https://doi.org/10.3390/horticulturae8070568
APA StyleEl-Banna, M. F., AL-Huqail, A. A., Farouk, S., Belal, B. E. A., El-Kenawy, M. A., & Abd El-Khalek, A. F. (2022). Morpho-Physiological and Anatomical Alterations of Salt-Affected Thompson Seedless Grapevine (Vitis vinifera L.) to Brassinolide Spraying. Horticulturae, 8(7), 568. https://doi.org/10.3390/horticulturae8070568