Preharvest Spraying of CaCl2 Alleviates the Scape Bending of Gerbera ‘Harmony’ Flowers by Strengthening the Pectin Crosslinks through Ca2+ Bonds
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design and Treatments
2.2. Scape Bending and Vase Life
2.3. Calcium Content
2.4. Pectin Extraction and Fraction
2.5. WSP and IP Analysis
2.6. Statistical Analysis
3. Results
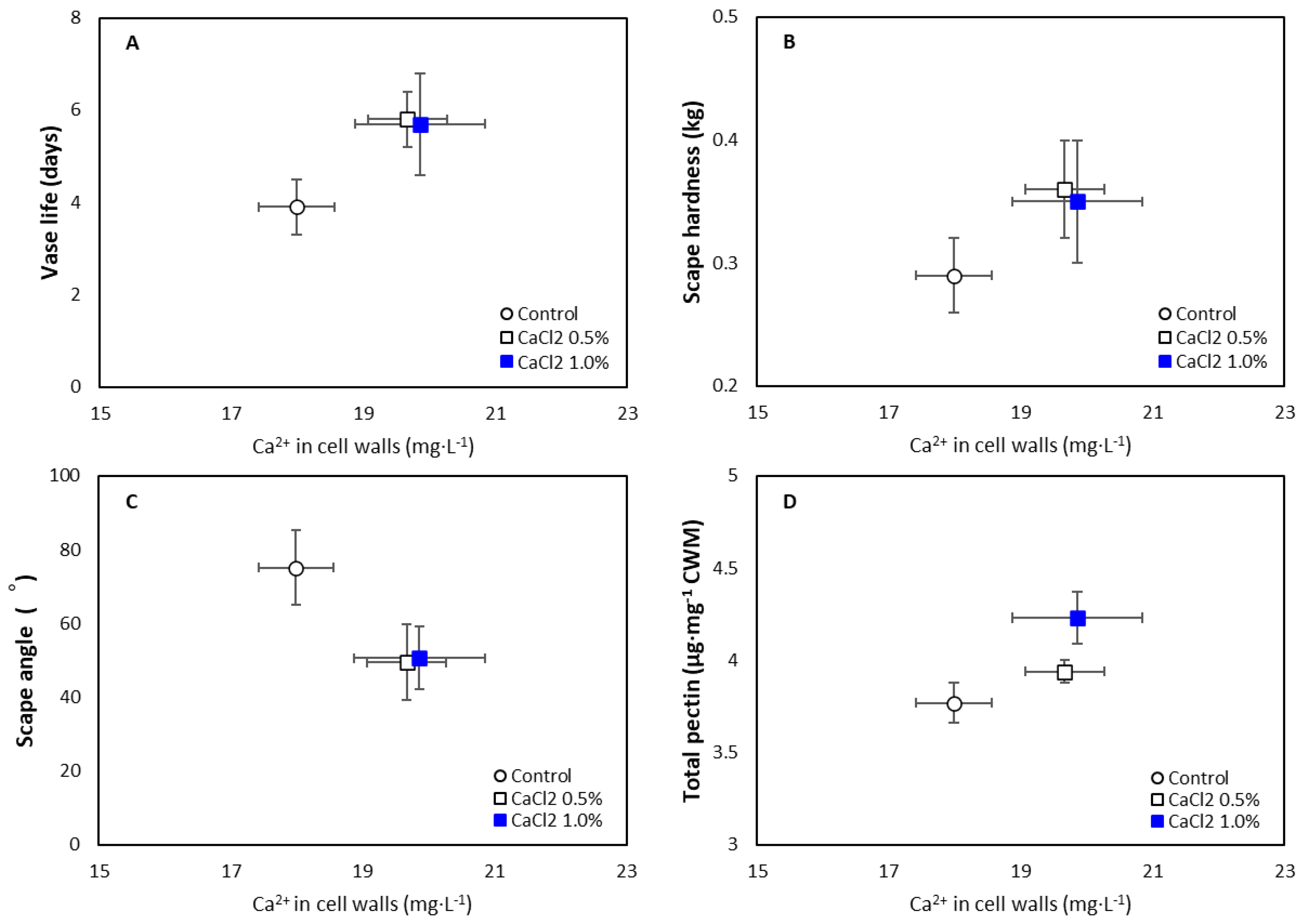
3.1. Effect of Exogenous CaCl2 on Flower Quality
3.2. Effect of Exogenous CaCl2 on Pectin Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darras, A. Overview of the dynamic role of specialty cut flowers in the international cut flower market. Horticulturae 2021, 7, 51. [Google Scholar] [CrossRef]
- Mohammadi, M.; Aelaei, M.; Saidi, M. Pre-harvest spray of GABA and spermine delays postharvest senescence and alleviates chilling injury of cut gerbera flowers during cold storage. Sci. Rep. 2021, 11, 14166. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kim, W.S. Correlation between calcium and pectin contents in cut gerbera during scape bending. Flower Res. J. 2017, 25, 95–100. [Google Scholar] [CrossRef]
- Steinitz, B. The role of sucrose in stabilization of cut gerbera flower stalks. Gartenbauwissenschaft 1982, 47, 77–81. [Google Scholar]
- Marousky, F.J. Vascular structure of the Gerbera scape. Acta Hortic. 1986, 181, 399–406. [Google Scholar] [CrossRef]
- Perik, R.R.J.; Raze, D.; Harkema, H.; Zhong, Y.; van Doorn, W.G. Bending in cut Gerbera jamesonii flowers relates to adverse water relations and lack of stem sclerenchyma development, not to expansion of the stem central cavity or stem elongation. Post. Biol. Technol. 2012, 74, 11–18. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Cell walls: Structure, biogenesis, and expansion. In Plant Physiology, 5th ed.; Taiz, L., Zeiger, E., Eds.; Life Science: Seoul, Korea, 2013; pp. 408–431. [Google Scholar]
- Brummell, D.A. Cell wall disassembly in ripening fruit. Funct. Plant. Biol. 2006, 33, 103–119. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Manganaris, G.A.; Vasilakakis, M.; Mignani, I.; Diamantidis, G.; Tzavella-Klonari, K. The effect of preharvest calcium sprays on quality attributes, physicochemical aspects of cell wall components and susceptibility to brown rot of peach fruits (Prunus persica L. cv. Andross). Sci. Hortic. 2005, 107, 43–50. [Google Scholar] [CrossRef]
- Raese, J.T.; Drake, S.R. Calcium spray materials and fruit calcium concentrations influence apple quality. J. Am. Pom. Soc. 2002, 56, 136–143. [Google Scholar] [CrossRef]
- Perik, R.R.J.; Raze, D.; Ferrante, A.; van Doorn, W.G. Stem bending in cut Gerbera jamesonii flowers: Effects of a pulse treatment with sucrose and calcium ions. Post. Biol. Technol. 2014, 98, 7–13. [Google Scholar] [CrossRef]
- Park, Y.Y.; Cho, M.S. Effects of calcium on the postharvest quality of Gerbera hybrida ‘Tamara’ Flower. J. Kor. Flower Res. Soc. 2004, 12, 178–183. [Google Scholar] [CrossRef]
- Gerasopoulos, D.; Chebli, B. Effect of pre- and post-harvest calcium applications on the vase life of cut gerberas. J. Hortic. Sci. Biol. 1999, 1, 78–81. [Google Scholar] [CrossRef]
- Deljou, M.J.N.; Gholipour, K. Effect of pre and post anthesis foliar application of calcium on postharvest quality of gerbera cut flower. Acta Hortic. 2014, 1034, 539–544. [Google Scholar] [CrossRef]
- Park, S.K.; Lim, J.H.; Choi, S.Y.; Shin, H.K.; Huh, Y.J. A new standard gerbera cultivar ‘Harmony’ with pink and semi-double adaptable to high temperature. Kor. J. Hortic. Sci. Technol. 2013, 31, 255–258. [Google Scholar] [CrossRef][Green Version]
- Laitinen, R.A.E.; Pöllänen, E.; Teeri, T.H.; Elomaa, P.; Kotilainen, M. Transcriptional analysis of petal organogenesis in Gerbera hybrida. Planta 2007, 226, 347–360. [Google Scholar] [CrossRef]
- De Jong, J. Dry storage and subsequent recovery of cut gerbera flowers as an aid in selection for longevity. Sci. Hortic. 1978, 9, 389–397. [Google Scholar] [CrossRef]
- Kim, Y.A.; Choi, S.R.; Kweon, O.K.; Joung, H.Y.; Shin, H.K.; Lee, J.S. Characteristic and vase life in 36 cultivar of cut gerbera flowers. Kor. J. Hortic. Sci. Technol. 2004, 22, 228–235. [Google Scholar]
- Rural Development Administration (RDA) Comprehensive Testing Room Analysis Manual. 2021. Available online: https://lib.rda.go.kr/search/mediaView.do?mets_no=000000314325 (accessed on 1 January 2022).
- Campbell, A.D.; Huysamer, M.; Stotz, H.U.; Greve, L.C.; Labavitch, J.M. Comparison of ripening processes in intact tomato fruit and excised pericarp discs. Plant Physiol. 1990, 94, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Selvendran, R.R.; O’Neill, M.A. Isolation and analysis of cell wall from plant material. Methods Biochem. Anal. 1987, 32, 25–153. [Google Scholar] [CrossRef] [PubMed]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef]
- Sissel, T.; Amihud, B.; Abraham, H.H. Calcium regulation of senescence in rose petals. Physiol. Plant 1999, 107, 214–219. [Google Scholar] [CrossRef]
- Li, C.; Tao, J.; Zhao, D.; You, C.; Ge, J. Effect of calcium sprays on mechanical strength and cell wall fractions of herbaceous peony (Paeonia Lactiflora Pall.) inflorescence stems. Int. J. Mol. Sci. 2012, 13, 4704–4713. [Google Scholar] [CrossRef]
- Jona, R.; Accati, E.; Mayak, S. Senescence processes as reflected in change in polysaccharidic cell wall components. Acta Hortic. 1980, 113, 153–158. [Google Scholar] [CrossRef]
- Agusti, M.; Juan, M.; Martinez-Fuentes, A.; Mesejo, C.; Almela, V. Calcium nitrate delays climacteric of persimmon fruit. Ann. Appl. Biol. 2004, 144, 65–69. [Google Scholar] [CrossRef]
| Treatment (CaCl2) | Vase Life (Days) | Scape Bending (%) | Scape Angle (°) |
|---|---|---|---|
| Control | 3.9 b z | 50.0 | 75.1 b |
| 0.5% | 5.8 a | 23.3 | 49.5 a |
| 1% | 5.7 a | 26.7 | 50.6 a |
| Significance | ** | - | * |
| Treatment (CaCl2) | Scape Hardness (kg) | Calcium Content (Ca2+mg·L−1) | ||
|---|---|---|---|---|
| Start | End | Start | End | |
| Control | 0.34 b z | 0.29 b | 17.99 b | 16.93 b |
| 0.5% | 0.38 a | 0.36 a | 19.67 a | 18.38 a |
| 1% | 0.38 a | 0.35 a | 19.86 a | 18.48 a |
| Significance | ** | ** | ** | * |
| Treatment (CaCl2) | Pectin (μg·mg−1 CWM) | |||||||
|---|---|---|---|---|---|---|---|---|
| WSP | IP | Total Pectin | IP/Total Pectin | |||||
| Start | End | Start | End | Start | End | Start | End | |
| Control | 0.91 c z | 1.63 | 2.74 b | 2.15 c | 3.65 c | 3.78 c | 0.75 a | 0.57 b |
| 0.5% | 1.09 b | 1.53 | 2.77 a | 2.41 b | 3.86 b | 3.94 b | 0.72 a | 0.61 a |
| 1% | 1.36 a | 1.66 | 2.83 a | 2.58 a | 4.19 a | 4.23 a | 0.68 b | 0.61 a |
| Significance | *** | ns | * | *** | *** | *** | * | ** |
| Variable | VL | SB | SA | SH | Ca2+ | WSP | IP | Total Pectin |
|---|---|---|---|---|---|---|---|---|
| CaCl2 | 0.36 * | −0.40 | −0.30 * | 0.50 ** | 0.47 ** | 0.96 *** | 0.24 * | 0.86 *** |
| VL | −0.38 | 0.52 | 0.35 * | 0.33 * | 0.31 * | 0.52 * | 0.48 * | |
| SB | 0.87 ** | −0.31 ** | −0.42 ** | −0.16 | −0.06 | −0.15 | ||
| SA | 0.38 ** | −0.31 | −0.27 * | −0.09 | −0.26 * | |||
| SH | 0.55 * | 0.45 ** | 0.11 | 0.39 ** | ||||
| Ca2+ WSP | 0.42 ** | 0.10 | 0.38 ** | |||||
| 0.10 | 0.80 ** | |||||||
| IP | 0.68 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Kim, W.S. Preharvest Spraying of CaCl2 Alleviates the Scape Bending of Gerbera ‘Harmony’ Flowers by Strengthening the Pectin Crosslinks through Ca2+ Bonds. Horticulturae 2022, 8, 523. https://doi.org/10.3390/horticulturae8060523
Park J, Kim WS. Preharvest Spraying of CaCl2 Alleviates the Scape Bending of Gerbera ‘Harmony’ Flowers by Strengthening the Pectin Crosslinks through Ca2+ Bonds. Horticulturae. 2022; 8(6):523. https://doi.org/10.3390/horticulturae8060523
Chicago/Turabian StylePark, Jiwon, and Wan Soon Kim. 2022. "Preharvest Spraying of CaCl2 Alleviates the Scape Bending of Gerbera ‘Harmony’ Flowers by Strengthening the Pectin Crosslinks through Ca2+ Bonds" Horticulturae 8, no. 6: 523. https://doi.org/10.3390/horticulturae8060523
APA StylePark, J., & Kim, W. S. (2022). Preharvest Spraying of CaCl2 Alleviates the Scape Bending of Gerbera ‘Harmony’ Flowers by Strengthening the Pectin Crosslinks through Ca2+ Bonds. Horticulturae, 8(6), 523. https://doi.org/10.3390/horticulturae8060523







