Modulation of Light and Nitrogen for Quality-Traits Improvement: A Case Study of Altino Sweet Pepper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Yield and Morphological Traits
2.3. Pigments
2.4. Spectrophotometric Determination of TPC, TFC and Antioxidant Capacity
2.5. Vitamin C Content
2.6. Determination of the Polyphenol Profile by High-Performance Liquid Chromatography (HPLC)
2.6.1. Chemicals
2.6.2. Sample Extraction
2.6.3. PAs Identification and Quantification
2.7. Statistical Analysis
3. Results
3.1. Yield and Yield Components
3.2. Fruit Pigments
3.3. Phenolics and Vitamin C in Fruits
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Camacho-Villa, T.C.; Maxted, N.; Scholten, M.; Ford-Lloyd, B. Defining and identifying crop landraces. Plant Genet. Resour. Characterisation Util. 2005, 3, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Azeez, M.A.; Adubi, A.O.; Durodola, F.A. Landraces and crop genetic improvement. In Rediscovery of Landraces as a Resource for the Future; IntechOpen: London, UK, 2008. [Google Scholar]
- Fratianni, F.; Cozzolino, A.; d’Acierno, A.; Nazzaro, F.; Riccardi, R.; Spigno, P. Qualitative aspects of some of some traditional landraces of the tomato “Piennolo” (Solanum lycopersicum L.) of the Campania region, southern Italy. Antioxidants 2020, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Portis, E.; Nervo, G.; Cavallanti, F.; Barchi, L.; Lanteri, S. Multivariate analysis of genetic relationships between Italian pepper landraces. Crop Sci. 2006, 46, 2517–2525. [Google Scholar] [CrossRef]
- Reale, S.; Biancolillo, A.; Gasparrini, C.; Martino, L.D.; Cecco, V.D.; Manzi, A.; Santo, M.D.; D’Archivio, A.A. Geographical discrimination of bell pepper (Capsicum annuum) spices by (HS)-SPME/GC-MS aroma profiling and chemometrics. Molecules 2021, 26, 6177. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.; Tracy, W.; Colley, M.; Culbert, P.; Mazourek, M.; Myers, J.; Zystro, J.; Silva, E.M. Adaptability analysis in a participatory variety trial of organic vegetable crops. Renew. Agric. Food Syst. 2020, 35, 296–312. [Google Scholar] [CrossRef]
- Berni, R.; Cantini, C.; Romi, M.; Hausman, J.F.; Guerriero, G.; Cai, G. Agrobiotechnology goes wild: Ancient local varieties as sources of bioactives. Int. J. Mol. Sci. 2018, 19, 2248. [Google Scholar] [CrossRef] [Green Version]
- Stagnari, F.; Campanelli, G.; Galieni, A.; Platani, C.; Bertone, A.; Ficcadenti, N. Adaptive Responses to Nitrogen and Light Supplies of a Local Varieties of Sweet Pepper from the Abruzzo Region, Southern Italy. Agronomy 2021, 11, 1343. [Google Scholar] [CrossRef]
- Rodríguez, A.; Peña-Fleitas, M.T.; Gallardo, M.; Souza, R.D.; Padilla, F.M.; Thompson, R.B. Sweet pepper and nitrogen supply in greenhouse production: Critical nitrogen curve, agronomic responses and risk of nitrogen loss. Eur. J. Agron. 2020, 117, 126046. [Google Scholar] [CrossRef]
- Yasuor, H.; Ben-Gal, A.; Yermiyahu, U.; Beit-Yannai, E.; Cohen, S. Nitrogen management of greenhouse pepper production: Agronomic, nutritional, and environmental implications. HortScience 2013, 48, 1241–1249. [Google Scholar] [CrossRef] [Green Version]
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Barać, S.; Mastilović, J.; Kevrešan, Ž.; Fallik, E. Effect of shading by coloured nets on yield and fruit quality of sweet pepper. Zemdirbyste 2017, 104, 53–62. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Manojlović, M. Color shade nets improve vegetables quality at harvest and maintain quality during storage. Contemp. Agric. 2018, 67, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, D.; Jifon, J.; Soundy, P. Spectral quality of photo-selective shade nettings improves antioxidants and overall quality in selected fresh produce after postharvest storage. Food Rev. Int. 2018, 34, 290–307. [Google Scholar] [CrossRef]
- Mashabela, M.N.; Selahle, K.M.; Soundy, P.; Crosby, K.M.; Sivakumar, D. Bioactive compounds and fruit quality of green sweet pepper grown under different colored shade netting during postharvest storage. J. Food Sci. 2015, 80, 2612–2618. [Google Scholar] [CrossRef] [PubMed]
- Selahle, K.M.; Sivakumar, D.; Jifon, J.; Soundy, P. Postharvest responses of red and yellow sweet peppers grown under photo-selective nets. Food Chem. 2015, 173, 951–956. [Google Scholar] [CrossRef]
- Amarante, C.V.T.D.; Steffens, C.A.; Argenta, L.C. Yield and fruit quality of ‘Gala’and ‘Fuji’apple trees protected by white anti-hail net. Sci. Hortic. Amst. 2011, 129, 79–85. [Google Scholar] [CrossRef]
- Shahak, Y.; Gal, E.; Offir, Y.; Ben-Yakir, D. Photoselective Shade Netting Integrated with Greenhouse Technologies for improved performance of Vegetable and Ornamental Crops. In Proceedings of the International Workshop on Greenhouse Environmental Control and Crop Production in Semi-Arid Regions, Tucson, AZ, USA, 20–24 October 2008; pp. 75–80. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Extraction of photosynthetic tissues: Chlorophylls and carotenoids. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.2.1–F4.2.6. [Google Scholar] [CrossRef]
- Gouveia, S.; Castilho, P.C. Helichrysum monizii Lowe: Phenolic composition and antioxidant potential. Phytochem. Analysis 2012, 23, 72–83. [Google Scholar] [CrossRef]
- Gouveia, S.; Castilho, P.C. Antioxidant potential of Artemisia argentea L’Hér alcoholic extract and its relation with the phenolic composition. Food Res. Int. 2012, 44, 1620–1631. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Gouveia, S.; Castilho, P.C. Phenolic composition and antioxidant capacity of cultivated artichoke, Madeira cardoon and artichoke-based dietary supplements. Food Res. Int. 2012, 48, 712–724. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J. Funct. Foods 2011, 3, 44–49. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Liang, Y.; Liang, K.; Zhang, F.; Xu, T.; Wang, M.; Song, H.; Liu, X.; Lu, B. Determination of phenolic acid profiles by HPLC-MS in vegetables commonly consumed in China. Food Chem. 2019, 276, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, P.; Galieni, A.; Manetta, A.C.; Pace, R.; Guiducci, M.; Pisante, M.; Stagnari, F. Phenolic compounds in grains, sprouts and wheatgrass of hulled and non-hulled wheat species. J. Sci. Food Agric. 2015, 95, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment of Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Valle, A.D.; Dimmito, M.P.; Zengin, G.; Pieretti, S.; Mollica, A.; Locatelli, M.; Cichelli, A.; Novellino, E.; Ak, G.; Yerlikaya, S.; et al. Exploring the nutraceutical potential of dried pepper Capsicum annuum L. on market from Altino in Abruzzo region. Antioxidants 2020, 9, 400. [Google Scholar] [CrossRef]
- Silva, J.M.D.; Fontes, P.C.R.; Milagres, C.D.C.; Garcia, E., Jr. Application of proximal optical sensors to assess nitrogen status and yield of bell pepper grown in slab. J. Soil Sci. Plant Nutr. 2021, 21, 229–237. [Google Scholar] [CrossRef]
- Hassan, S.M.; El-Bebany, A.F.; Salem, M.Z.; Komeil, D.A. Productivity and post-harvest fungal resistance of hot pepper as affected by potassium silicate, clove extract foliar spray and nitrogen application. Plants 2021, 10, 662. [Google Scholar] [CrossRef]
- Ayodele, O.J.; Alabi, E.O.; Aluko, M. Nitrogen fertilizer effects on growth, yield and chemical composition of hot pepper (Rodo). Int. J. Agri. Crop Sci. 2015, 8, 666. [Google Scholar]
- Jovicich, E.D.; Cantiffe, J.; Stoffella, P.J.; Vansickle, J.J. Reduced fertigation of soil-less greenhouse peppers improves fruit yield and quality. Acta Hortic. 2003, 609, 193–199. [Google Scholar] [CrossRef]
- Medina-Lara, F.; Echevarria-Machado, I.; Pacheco-Arjona, R.; Ruiz-Lau, N.; Guzman-Antonio, A.; Martinez-Estevez, M. Influence of nitrogen and potassium fertilization on fruiting and capsaicin content of habanero pepper (Capsicum chinense Jacq). HortScience 2008, 43, 1549–1554. [Google Scholar] [CrossRef] [Green Version]
- D’Egidio, S.; Galieni, A.; Stagnari, F.; Pagnani, G.; Pisante, M. Yield, quality and physiological traits of red beet under different magnesium nutrition and light intensity levels. Agronomy 2019, 9, 379. [Google Scholar] [CrossRef] [Green Version]
- Caruso, G.; Cozzolino, E.; Cuciniello, A.; Maiello, R.; Cenvinzo, V.; Giordano, M.; Pascale, S.D.; Rouphael, Y. Yield and quality of greenhouse organic pepper as affected by shading net in Mediterranean area. Acta Hortic. 2020, 1268, 335–340. [Google Scholar] [CrossRef]
- Caruso, G.; Formisano, L.; Cozzolino, E.; Pannico, A.; El-Nakhel, C.; Rouphael, Y.; Tallarita, A.; Cenvinzo, V.; Pascale, S.D. Shading affects yield, elemental composition and antioxidants of perennial wall rocket crops grown from spring to summer in southern Italy. Plants 2020, 9, 933. [Google Scholar] [CrossRef] [PubMed]
- Galieni, A.; Stagnari, F.; Speca, S.; Pisante, M. Leaf traits as indicators of limiting growing conditions for lettuce (Lactuca sativa). Ann. Appl. Biol. 2016, 169, 342–356. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C. Bell pepper (Capsicum annum L.) crop as affected by shade level: Fruit yield, quality, and postharvest attributes, and incidence of phytophthora blight (caused by Phytophthora capsici Leon.). HortScience 2014, 49, 891–900. [Google Scholar] [CrossRef] [Green Version]
- Aloni, B.; Karni, L.; Zaidman, Z.; Schaffer, A.A. Changes of carbohydrates in pepper (Capsicum annuum L.) flowers in relation to their abscission under different shading regimes. Ann. Bot. 1996, 78, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Shifriss, C.; Pilowsky, M.; Aloni, B. Variation in flower abscission of peppers under stress shading conditions. Euphytica 1994, 78, 133–136. [Google Scholar] [CrossRef]
- Joshi, N.C.; Ratner, K.; Eidelman, O.; Bednarczyk, D.; Zur, N.; Many, Y.; Shahak, Y.; Aviv-Sharon, E.; Achiam, M.; Gilad, Z.; et al. Effects of daytime intra-canopy LED illumination on photosynthesis and productivity of bell pepper grown in protected cultivation. Sci. Hortic. 2019, 250, 81–88. [Google Scholar] [CrossRef]
- González-Real, M.M.; Liu, H.Q.; Baille, A. Influence of fruit sink strength on the distribution of leaf photosynthetic traits in fruit-bearing shoots of pepper plants (Capsicum annuum L.). Environ. Exp. Bot. 2009, 66, 195–202. [Google Scholar] [CrossRef]
- Yoo, H.J.; Kim, J.H.; Park, K.S.; Son, J.E.; Lee, J.M. Light-controlled fruit pigmentation and flavor volatiles in tomato and bell pepper. Antioxidants 2019, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Keyhaninejad, N.; Richins, R.D.; O’Connell, M.A. Carotenoid content in field-grown versus greenhouse-grown peppers: Different responses in leaf and fruit. HortSci 2012, 47, 852–855. [Google Scholar] [CrossRef] [Green Version]
- Filyushin, M.A.; Dzhos, E.A.; Shchennikova, A.V.; Kochieva, E.Z. Dependence of pepper fruit colour on basic pigments ratio and expression pattern of carotenoid and anthocyanin biosynthesis genes. Russ. J. Plant Physiol. 2020, 67, 1054–1062. [Google Scholar] [CrossRef]
- Pizarro, L.; Stange, C. Light-dependent regulation of carotenoid biosynthesis in plants. Cienc. Investig. Agrar. 2009, 36, 143–161. [Google Scholar] [CrossRef]
- Zoratti, L.; Karppinen, K.; Escobar, A.L.; Haggman, H.; Jaakola, L. Light-controlled flavonoid biosynthesis in fruits. Front. Plant Sci. 2014, 5, 534. [Google Scholar] [CrossRef] [PubMed]
- Amor, F.M.D.; Serrano-Martinez, A.; Fortea, M.I.; Legua, P.; Núñez-Delicado, E. The effect of plant-associative bacteria (Azospirillum and Pantoea) on the fruit quality of sweet pepper under limited nitrogen supply. Sci. Hortic. 2008, 117, 191–196. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, J.; Dawuda, M.M.; Xie, J.; Yu, J.; Li, J.; Zhang, X.; Tang, C.; Wang, C.; Gan, Y. Appropriate ammonium-nitrate ratio improves nutrient accumulation and fruit quality in pepper (Capsicum annuum L.). Agronomy 2019, 9, 683. [Google Scholar] [CrossRef] [Green Version]
- Pérez-López, A.J.; Amor, F.M.D.; Serrano-Martínez, A.; Fortea, M.I.; Núñez-Delicado, E. Influence of agricultural practices on the quality of sweet pepper fruits as affected by the maturity stage. J. Sci. Food Agric. 2007, 87, 2075–2080. [Google Scholar] [CrossRef]
- Ghoname, A.A.; Dawood, M.G.; Riad, G.S.; El-Tohamy, W.A. Effect of nitrogen forms and biostimulants foliar application on the growth, yield and chemical composition of hot pepper grown under sandy soil conditions. Res. J. Agric. Biol. Sci. 2009, 5, 840–852. [Google Scholar]
- Nunez-Ramirez, F.; González-Mendoza, D.; Grimaldo-Juarez, O.; Díaz, L.C. Nitrogen fertilization effect on antioxidants compounds in fruits of habanero chili pepper (Capsicum chinense). Int. J. Agric. Biol. 2011, 13, 827–830. [Google Scholar]
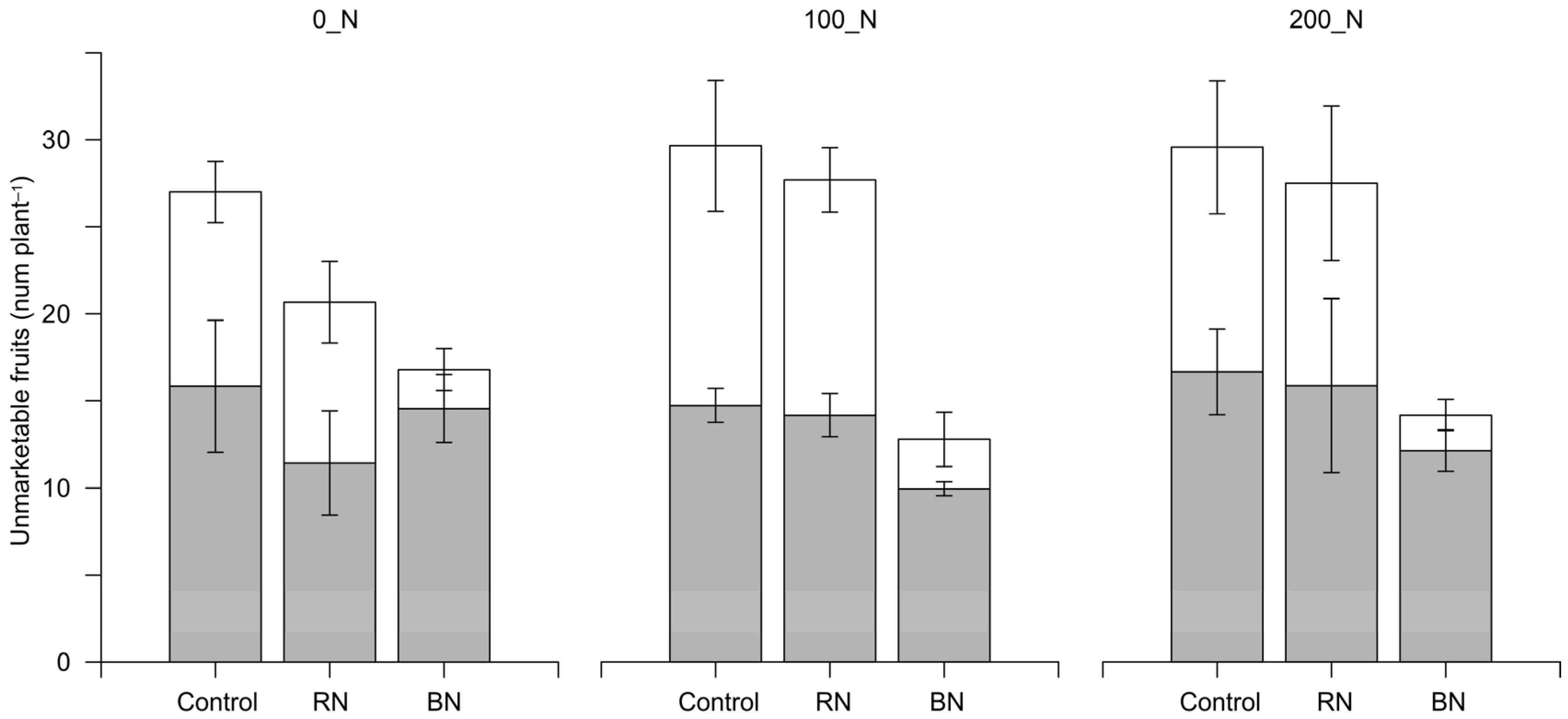
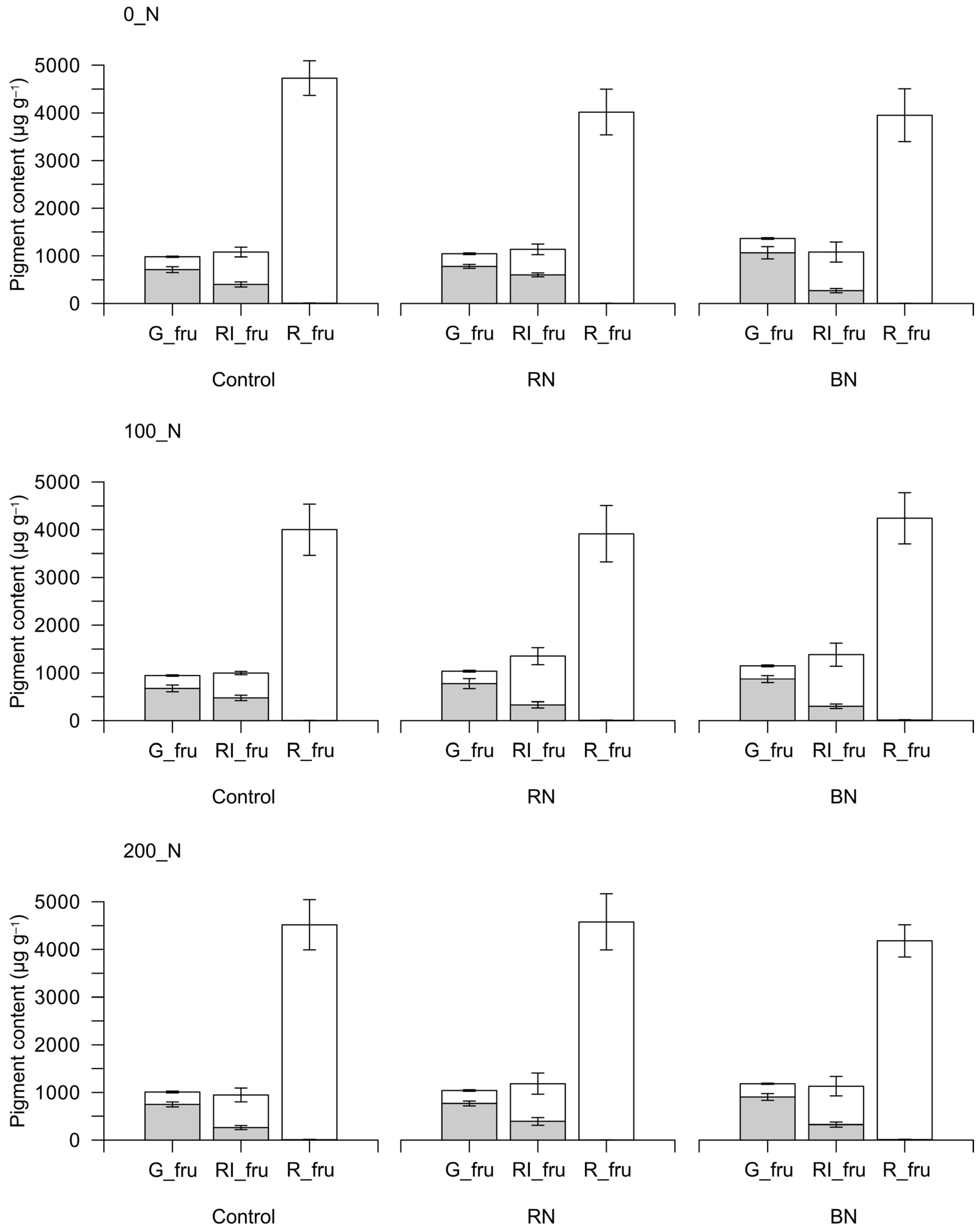
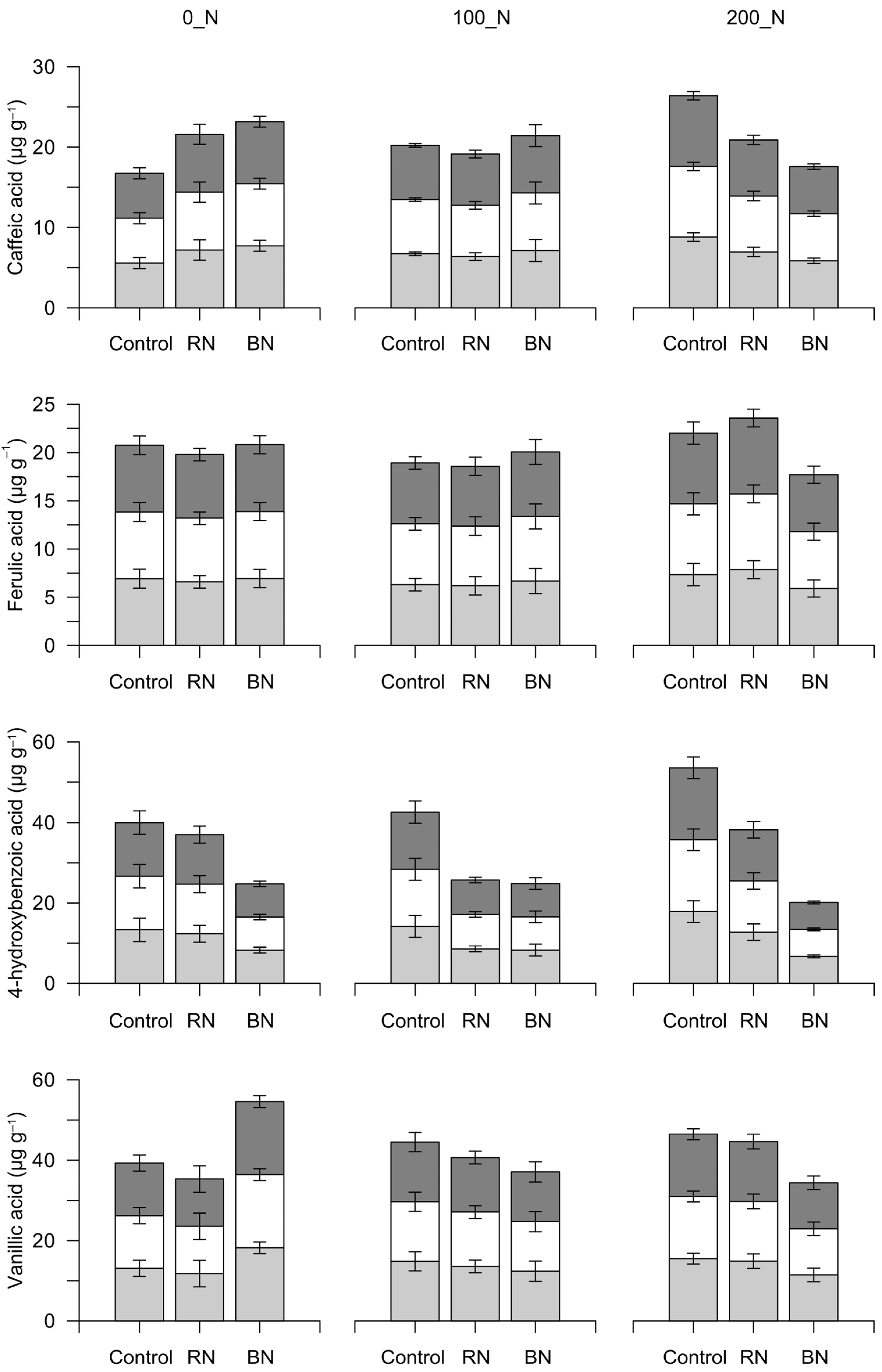
| Effects | Yield (g Plant−1) | Fruit Number (Num Plant−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | RN | BN | o.m. | Control | RN | BN | o.m. | |
| Total | ||||||||
| 0_N | 480.9 | 457.1 | 362.3 | 433.4 | 22.4 | 19.7 | 13.9 | 18.6 |
| 100_N | 549.0 | 583.7 | 443.8 | 525.5 | 27.9 | 25.1 | 16.1 | 23.0 |
| 200_N | 563.1 | 576.7 | 441.1 | 527.0 | 28.6 | 24.9 | 17.0 | 23.5 |
| o.m. | 531.0 | 539.1 | 415.7 | 26.3 | 23.2 | 15.7 | ||
| F-test | ||||||||
| N_rates | n.s. (28.8) | ** (1.0) | ||||||
| Sh | ** (39.1) | ** (1.4) | ||||||
| N_rates × Sh | n.s. (67.8–62.4) | n.s. (2.5–2.3) | ||||||
| Marketable | ||||||||
| 0_N | 364.5 | 372.0 | 303.4 | 346.6 | 16.5 | 15.7 | 11.5 | 14.5 |
| 100_N | 399.5 | 435.3 | 391.1 | 408.6 | 19.8 | 18.2 | 14.0 | 17.3 |
| 200_N | 421.3 | 441.5 | 376.9 | 413.2 | 20.2 | 18.1 | 14.6 | 17.6 |
| o.m. | 395.1 | 416.3 | 357.2 | 18.8 | 17.3 | 13.4 | ||
| F-test | ||||||||
| N_rates | n.s. (25.7) | n.s. (1.0) | ||||||
| Sh | n.s. (31.9) | ** (1.3) | ||||||
| N_rates × Sh | n.s. (55.2–51.9) | n.s. (2.2–2.0) | ||||||
| Unmarketable | ||||||||
| 0_N | 116.4 | 85.1 | 58.9 | 86.8 | 5.9 | 4.0 | 2.4 | 4.1 |
| 100_N | 149.5 | 148.4 | 52.7 | 116.8 | 8.1 | 6.9 | 2.1 | 5.7 |
| 200_N | 141.8 | 135.1 | 64.1 | 113.7 | 8.4 | 6.8 | 2.4 | 5.9 |
| o.m. | 135.9 | 122.9 | 58.6 | 7.5 | 5.9 | 2.3 | ||
| F-test | ||||||||
| N_rates | ** (5.4) | * (0.5) | ||||||
| Sh | ** (14.1) | ** (0.6) | ||||||
| N_rates × Sh | n.s. (24.3–20.6) | n.s. (1.0–0.9) | ||||||
| Effects | MFW (g) | L (cm) | D (cm) | Seed (num) | Seed DW (mg) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | RN | BN | o.m. | Control | RN | BN | o.m. | Control | RN | BN | o.m. | Control | RN | BN | o.m. | Control | RN | BN | o.m. | |
| 0_N | 22.5 | 24.3 | 26.5 | 24.4 | 13.1 | 13.7 | 15.0 | 13.9 | 3.1 | 2.5 | 2.6 | 2.8 | 234.0 | 244.7 | 196.7 | 225.2 | 5.51 | 5.42 | 6.20 | 5.71 |
| 100_N | 20.4 | 23.6 | 27.8 | 23.9 | 12.9 | 13.5 | 15.1 | 13.9 | 2.7 | 2.6 | 2.7 | 2.7 | 237.4 | 246.6 | 226.7 | 236.9 | 5.53 | 5.53 | 6.03 | 5.70 |
| 200_N | 20.8 | 25.4 | 25.8 | 24.0 | 12.8 | 13.3 | 14.7 | 13.6 | 2.4 | 2.6 | 2.6 | 2.5 | 215.9 | 235.6 | 198.2 | 216.6 | 6.18 | 6.02 | 6.34 | 6.18 |
| o.m. | 21.2 | 24.4 | 26.7 | 12.9 | 13.5 | 14.9 | 2.7 | 2.6 | 2.7 | 229.1 | 242.3 | 207.2 | 5.74 | 5.66 | 6.19 | |||||
| F test | ||||||||||||||||||||
| N_rates | n.s. (1.9) | n.s. (0.21) | n.s. (0.17) | n.s. (14.4) | n.s. (0.23) | |||||||||||||||
| Sh | ** (0.8) | ** (0.19) | n.s. (0.17) | n.s. (15.4) | * (0.19) | |||||||||||||||
| N_rates × Sh | n.s. (1.4–2.2) | n.s. (0.33–0.34) | n.s. (0.30–0.30) | n.s. (26.6–26.1) | n.s. (0.33–0.36) | |||||||||||||||
| Overall Means | Chl A | Chl B | Chl TOT | Car |
|---|---|---|---|---|
| Green fruits | ||||
| 0_N | 629.6 | 221.1 | 850.7 | 280.5 |
| 100_N | 571.7 | 202.5 | 774.2 | 271.1 |
| 200_N | 594.0 | 213.1 | 807.1 | 271.9 |
| Control | 533.1 | 177.7 | 710.8 | 268.5 |
| RN | 572.6 | 201.3 | 773.9 | 267.7 |
| BN | 689.6 | 257.7 | 947.3 | 287.3 |
| N_rates | n.s. (70.3) | n.s. (20.0) | n.s. (89.7) | n.s. (10.2) |
| Sh | ** (37.8) | ** (18.9) | ** (55.8) | n.s. (12.9) |
| N_rates × Sh | n.s. (65.4–88.2) | n.s. (32.8–33.4) | n.s. (96.6–119.4) | n.s. (22.3–20.9) |
| Early-ripening fruits | ||||
| 0_N | 312.1 | 111.7 | 423.9 | 675.5 |
| 100_N | 271.1 | 98.6 | 369.7 | 875.3 |
| 200_N | 225.7 | 101.5 | 327.2 | 760.4 |
| Control | 283.0 | 97.6 | 380.6 | 628.2 |
| RN | 300.8 | 139.5 | 440.4 | 784.6 |
| BN | 225.1 | 74.8 | 299.9 | 898.4 |
| N_rates | n.s. (60.9) | n.s. (9.4) | n.s. (67.6) | n.s. (200.3) |
| Sh | * (27.4) | ** (14.3) | ** (37.3) | n.s. (111.7) |
| N_rates × Sh | ** (47.5–72.2) | * (24.8–22.4) | ** (64.6–85.7) | n.s. (193.4–255.0) |
| Red fruits | ||||
| 0_N | 1.7 | 2.8 | 4.5 | 4228.3 |
| 100_N | 3.1 | 4.1 | 7.2 | 4045.8 |
| 200_N | 3.0 | 4.5 | 7.5 | 4418.6 |
| Control | 2.4 | 4.0 | 6.3 | 4409.4 |
| RN | 1.9 | 2.1 | 4.0 | 4167.2 |
| BN | 3.4 | 5.3 | 8.8 | 4116.0 |
| N_rates | n.s. (1.9) | n.s. (2.2) | n.s. (3.9) | n.s. (335.2) |
| Sh | n.s. (1.8) | n.s. (3.8) | n.s. (5.0) | n.s. (416.8) |
| N_rates × Sh | n.s. (3.1–3.2) | n.s. (5.9–5.3) | n.s. (8.6–8.1) | n.s. (721.8–678.1) |
| Effects | TPC (mg GAE 100 g−1) | TFC (mg RUE 100 g−1) | Vit C (mg 100 g−1) | ABTS (mmol TE 100 g−1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | RN | BN | o.m. | Control | RN | BN | o.m. | Control | RN | BN | o.m. | Control | RN | BN | o.m. | |
| Green fruits | ||||||||||||||||
| 0_N | 968.3 | 958.6 | 887.9 | 938.3 | 388.2 | 347.4 | 337.5 | 357.7 | 126.6 | 129.81 | 86.7 | 114.4 | 1.79 | 1.48 | 1.52 | 1.60 |
| 100_N | 981.2 | 871.2 | 866.7 | 906.4 | 383.5 | 328.3 | 369.4 | 360.4 | 123.7 | 88.72 | 91.1 | 101.2 | 1.88 | 1.61 | 1.62 | 1.70 |
| 200_N | 1019.1 | 952.5 | 845.0 | 938.9 | 430.9 | 389.2 | 284.0 | 368.1 | 82.4 | 143.64 | 91.7 | 105.9 | 2.03 | 1.67 | 1.31 | 1.67 |
| o.m. | 989.6 | 927.4 | 866.5 | 400.9 | 355.0 | 330.3 | 110.9 | 120.7 | 89.8 | 1.90 | 1.59 | 1.48 | ||||
| F-test | ||||||||||||||||
| N_rates | n.s. (58.6) | n.s. (45.4) | n.s. (22.3) | n.s. (0.17) | ||||||||||||
| Sh | * (39.1) | n.s. (42.5) | n.s. (13.5) | n.s. (0.18) | ||||||||||||
| N_rates × Sh | n.s. (67.7–80.6) | n.s. (73.7–75.4) | n.s. (23.4–29.4) | n.s. (0.32–0.31) | ||||||||||||
| Early-ripening fruits | ||||||||||||||||
| 0_N | 871.4 | 860.8 | 807.7 | 846.6 | 305.6 | 284.4 | 291.5 | 293.8 | 107.0 | 138.6 | 139.2 | 128.3 | 2.01 | 1.92 | 1.77 | 1.90 |
| 100_N | 992.5 | 954.1 | 851.2 | 932.6 | 369.8 | 275.6 | 323.6 | 323.0 | 108.7 | 104.6 | 120.9 | 111.4 | 2.26 | 1.86 | 1.84 | 1.98 |
| 200_N | 933.0 | 949.4 | 883.2 | 921.8 | 328.4 | 338.4 | 279.1 | 315.3 | 141.1 | 138.3 | 131.8 | 137.1 | 2.04 | 2.04 | 1.87 | 1.98 |
| o.m. | 932.3 | 921.4 | 847.3 | 334.6 | 299.5 | 298.1 | 118.9 | 127.2 | 130.7 | 2.10 A | 1.94 | 1.82 | ||||
| F-test | ||||||||||||||||
| N_rates | n.s. (29.2) | n.s. (16.8) | n.s. (11.5) | n.s. (0.12) | ||||||||||||
| Sh | ** (24.6) | n.s. (19.3) | n.s. (10.1) | * (0.11) | ||||||||||||
| N_rates × Sh | n.s. (42.6–45.4) | n.s. (33.4–32.0) | n.s. (17.5–18.3) | n.s. (0.17–0.18) | ||||||||||||
| Red fruits | ||||||||||||||||
| 0_N | 1069.0 | 1006.1 | 1011.1 | 1028.7 | 358.8 | 310.3 | 278.5 | 315.8 | 160.6 | 156.6 | 160.6 | 159.2 | 2.54 | 2.39 | 2.28 | 2.40 |
| 100_N | 944.6 | 948.6 | 1041.5 | 978.2 | 282.0 | 285.9 | 282.6 | 283.5 | 130.2 | 139.7 | 145.0 | 138.3 | 2.13 | 2.03 | 2.22 | 2.12 |
| 200_N | 1036.9 | 957.3 | 985.1 | 993.1 | 355.7 | 284.8 | 253.7 | 298.1 | 151.9 | 139.4 | 147.7 | 146.3 | 2.26 | 2.11 | 2.20 | 2.19 |
| o.m. | 1016.8 | 970.7 | 1012.5 | 332.1 | 293.7 | 271.6 | 147.6 | 145.2 | 151.1 | 2.31 | 2.17 | 2.23 | ||||
| F-test | ||||||||||||||||
| N_rates | n.s. (25.6) | n.s. (17.6) | n.s. (17.9) | n.s. (0.19) | ||||||||||||
| Sh | n.s. (30.8) | * (18.4) | n.s. (8.6) | n.s. (0.08) | ||||||||||||
| N_rates × Sh | n.s. (53.4–50.6) | n.s. (31.9–31.4) | n.s. (15.0–21.7) | n.s. (0.13–0.22) | ||||||||||||
| Overall Mean | CaA | FA | 4-OHBA | VA |
|---|---|---|---|---|
| Green fruits | ||||
| 0_N | 6.83 | 6.82 | 11.30 | 14.35 |
| 100_N | 6.75 | 6.39 | 10.34 | 13.58 |
| 200_N | 7.20 | 7.03 | 12.44 | 13.93 |
| Control | 7.04 | 6.86 | 15.12 | 14.5 |
| RN | 6.85 | 6.88 | 11.21 | 13.4 |
| BN | 6.91 | 6.51 | 7.74 | 14.0 |
| N_rates | n.s. (0.52) | n.s. (0.56) | n.s. (1.68) | n.s. (1.70) |
| Sh | n.s. (0.71) | n.s. (0.67) | ** (1.86) | n.s. (1.59) |
| N_rates × Sh | n.s. (1.23–1.13) | n.s. (1.16–1.10) | n.s. (3.23–3.12) | n.s. (2.76–2.82) |
| Early-ripening fruits | ||||
| 0_N | 6.20 | 10.66 | 11.50 | 12.95 |
| 100_N | 6.48 | 11.30 | 12.55 | 17.41 |
| 200_N | 6.65 | 11.21 | 13.31 | 13.30 |
| Control | 9.13 | 17.36 | 16.81 | 17.99 |
| RN | 5.66 | 9.13 | 12.40 | 14.69 |
| BN | 4.54 | 6.68 | 8.14 | 10.98 |
| N_rates | n.s. (1.45) | n.s. (3.86) | n.s. (2.00) | n.s. (2.45) |
| Sh | ** (1.07) | ** (1.90) | ** (1.22) | * (2.14) |
| N_rates × Sh | n.s. (1.86–2.10) | n.s. (3.30–4.70) | n.s. (2.12–2.65) | n.s. (3.71–3.90) |
| Red fruits | ||||
| 0_N | 6.19 | 11.32 | 10.89 | 17.77 |
| 100_N | 6.55 | 16.19 | 9.64 | 19.54 |
| 200_N | 6.07 | 11.94 | 9.64 | 18.86 |
| Control | 7.48 | 17.38 | 12.45 | 20.04 |
| RN | 6.39 | 14.78 | 10.93 | 20.71 |
| BN | 4.94 | 7.29 | 6.78 | 15.42 |
| N_rates | n.s. (0.31) | n.s. (1.19) | n.s. (2.59) | n.s. (1.05) |
| Sh | ** (0.46) | ** (1.77) | ** (1.49) | ** (1.18) |
| N_rates × Sh | n.s. (0.80–0.72) | n.s. (3.07–2.77) | n.s. (2.58–3.33) | n.s. (2.05–1.97) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stagnari, F.; Ficcadenti, N.; Manetta, A.C.; Platani, C.; Dattoli, M.A.; Galieni, A. Modulation of Light and Nitrogen for Quality-Traits Improvement: A Case Study of Altino Sweet Pepper. Horticulturae 2022, 8, 499. https://doi.org/10.3390/horticulturae8060499
Stagnari F, Ficcadenti N, Manetta AC, Platani C, Dattoli MA, Galieni A. Modulation of Light and Nitrogen for Quality-Traits Improvement: A Case Study of Altino Sweet Pepper. Horticulturae. 2022; 8(6):499. https://doi.org/10.3390/horticulturae8060499
Chicago/Turabian StyleStagnari, Fabio, Nadia Ficcadenti, Anna Chiara Manetta, Cristiano Platani, Maria Assunta Dattoli, and Angelica Galieni. 2022. "Modulation of Light and Nitrogen for Quality-Traits Improvement: A Case Study of Altino Sweet Pepper" Horticulturae 8, no. 6: 499. https://doi.org/10.3390/horticulturae8060499
APA StyleStagnari, F., Ficcadenti, N., Manetta, A. C., Platani, C., Dattoli, M. A., & Galieni, A. (2022). Modulation of Light and Nitrogen for Quality-Traits Improvement: A Case Study of Altino Sweet Pepper. Horticulturae, 8(6), 499. https://doi.org/10.3390/horticulturae8060499





