Evaluation of Morphological, Qualitative, and Metabolomic Traits during Fruit Ripening in Pomegranate (Punica granatum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Morphological Characterization of Pomegranate Accessions
2.3. Physico–Chemical Traits
2.4. Bioactive Compounds and Antioxidant Activity
2.5. Metabolomic Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Morphological Characterization of Pomegranate Accessions
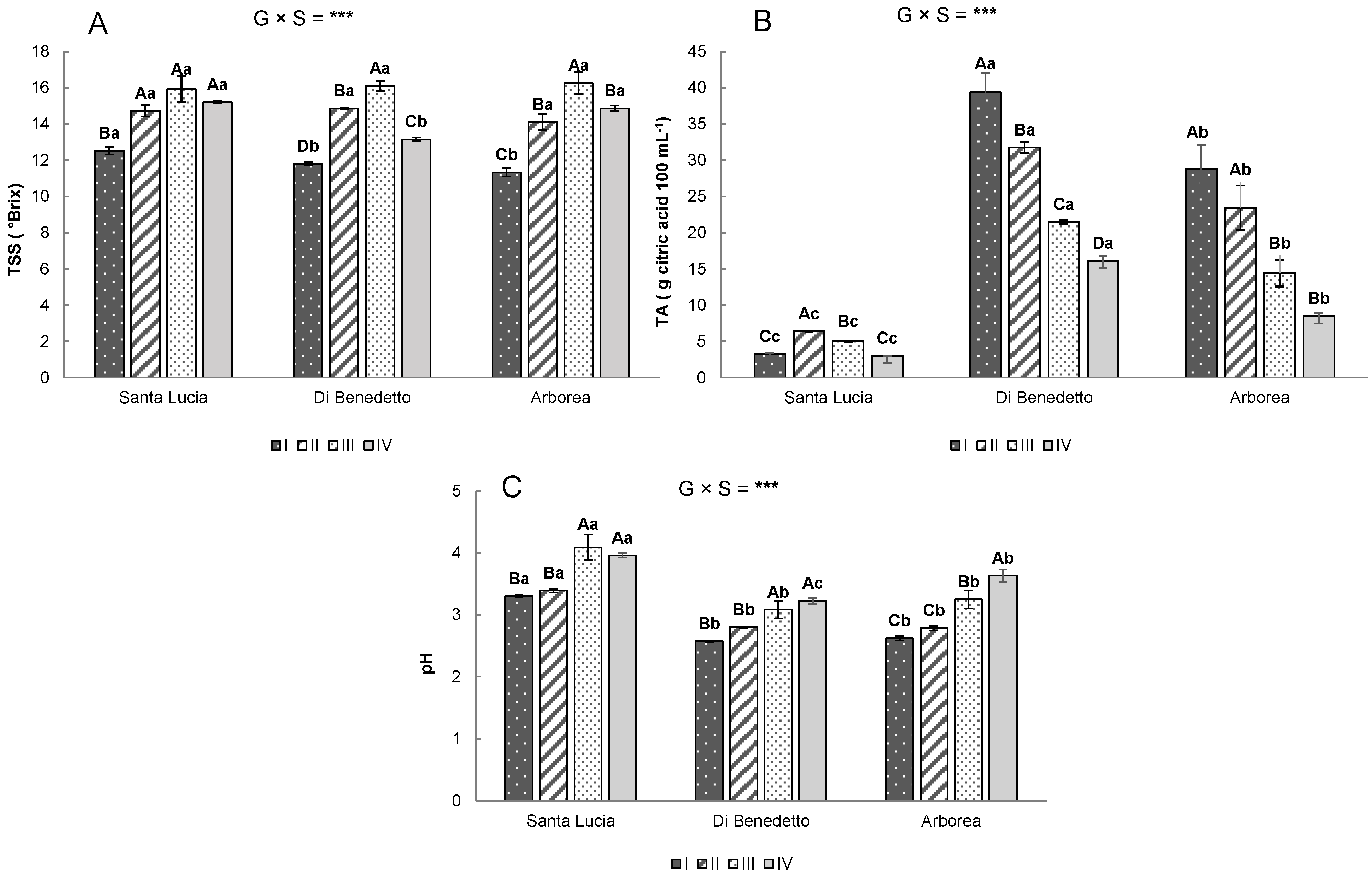
3.2. Physico–Chemical Traits
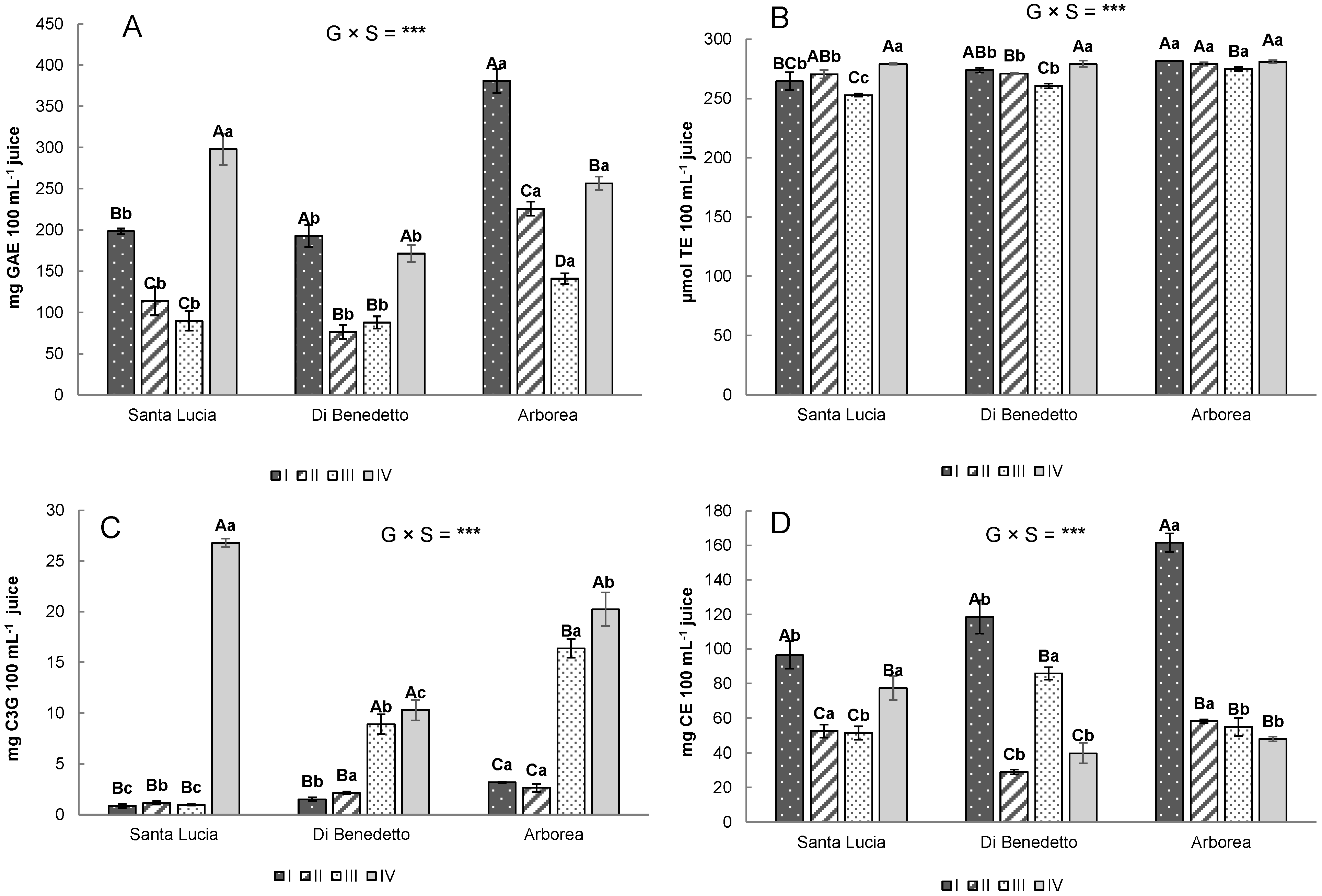
3.3. Bioactive Compounds and Antioxidant Activity
3.4. 1H NMR Analysis
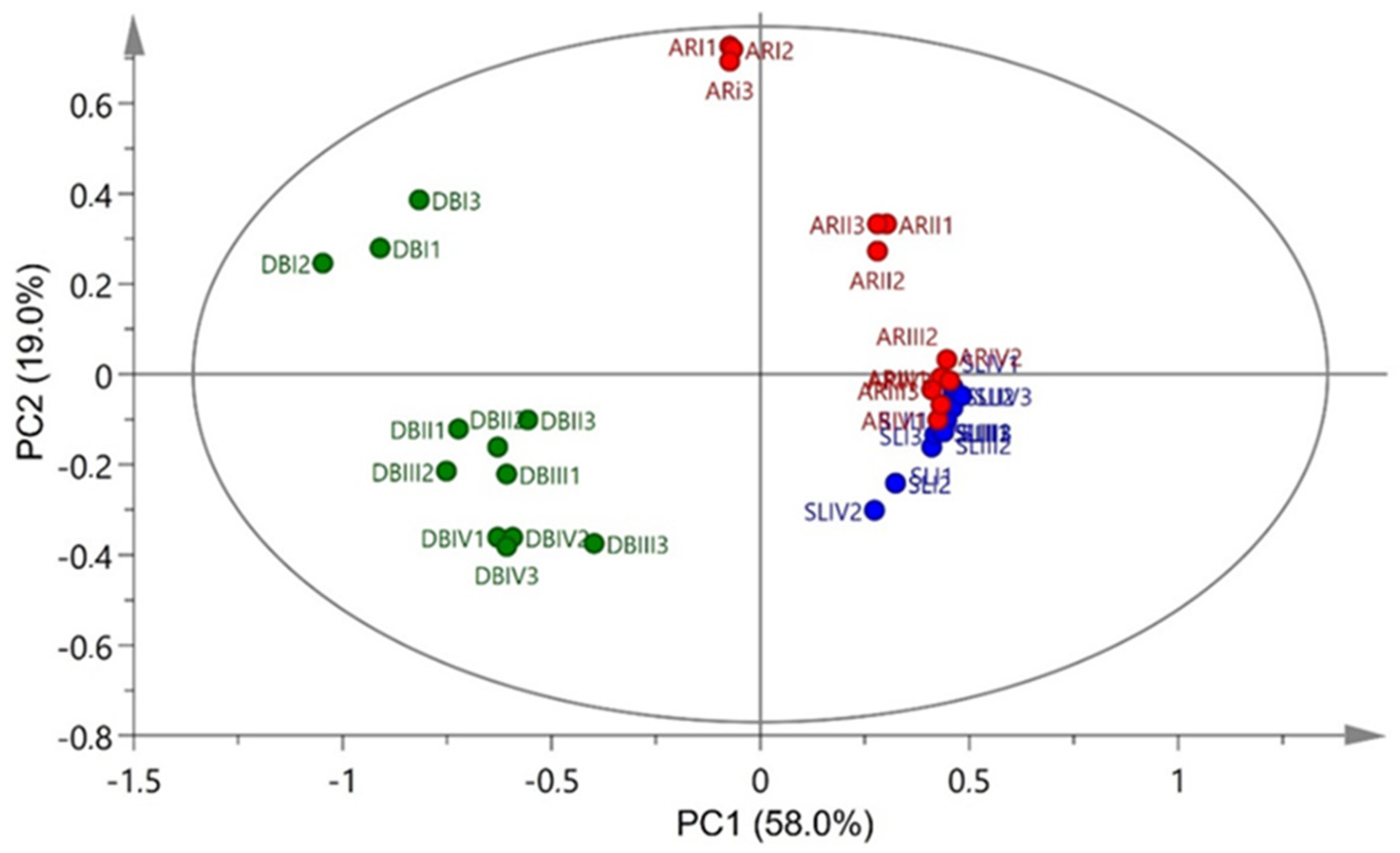
3.5. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akyıldız, A.; Karaca, E.; Ağçam, E.; Dündar, B.; Çınkır, N.İ. Changes in Quality Attributes during Production Steps and Frozen-Storage of Pomegranate Juice Concentrate. J. Food Compost. Anal. 2020, 92, 103548. [Google Scholar] [CrossRef]
- Johanningsmeier, S.D.; Harris, G.K. Pomegranate as a Functional Food and Nutraceutical Source. Annu. Rev. Food Sci. Technol. 2011, 2, 181–201. [Google Scholar] [CrossRef]
- Wu, S.; Tian, L. Diverse Phytochemicals and Bioactivities in the Ancient Fruit and Modern Functional Food Pomegranate (Punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef]
- Saeed, M.; Naveed, M.; BiBi, J.; Kamboh, A.A.; Arain, M.A.; Shah, Q.A.; Alagawany, M.; El-Hack, M.E.A.; Abdel-Latif, M.A.; Yatoo, M.I.; et al. The Promising Pharmacological Effects and Therapeutic/Medicinal Applications of Punica granatum L. (Pomegranate) as a Functional Food in Humans and Animals. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Adiletta, G.; Petriccione, M.; Liguori, L.; Zampella, L.; Mastrobuoni, F.; Di Matteo, M. Overall Quality and Antioxidant Enzymes of Ready-to-eat ‘Purple Queen’ Pomegranate Arils during Cold Storage. Postharvest Biol. Technol. 2019, 155, 20–28. [Google Scholar] [CrossRef]
- Al-Maiman, S.A.; Ahmad, D. Changes in Physical and Chemical Properties during Pomegranate (Punica granatum L.) Fruit Maturation. Food Chem. 2002, 76, 437–441. [Google Scholar] [CrossRef]
- Aslam, M.N.; Lansky, E.P.; Varani, J. Pomegranate as a Cosmeceutical Source: Pomegranate Fractions Promote Proliferation and Procollagen Synthesis and Inhibit Matrix Metalloproteinase-1 Production in Human Skin Cells. J. Ethnopharmacol. 2006, 103, 311–318. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Fruit Growth Dynamics, Respiration Rate and Physico-Textural Properties during Pomegranate Development and Ripening. Sci. Hortic. 2013, 157, 90–98. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Compounds as Beneficial Phytochemicals in Pomegranate (Punica granatum L.) Peel: A Review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef]
- Wong, T.L.; Strandberg, K.R.; Croley, C.R.; Fraser, S.E.; Nagulapalli Venkata, K.C.; Fimognari, C.; Sethi, G.; Bishayee, A. Pomegranate Bioactive Constituents Target Multiple Oncogenic and Oncosuppressive Signaling for Cancer Prevention and Intervention. Semin 2021, 73, 265–293. [Google Scholar] [CrossRef]
- Farooqi, A.A. Regulation of Deregulated Cell Signaling Pathways by Pomegranate in Different Cancers: Re-Interpretation of Knowledge Gaps. Semin 2021, 73, 294–301. [Google Scholar] [CrossRef]
- Usanmaz, S. Yield and Pomological Characteristics of Three Pomegranate (Punica granatum L.) Cultivars: Wonderful, Acco and Herskovitz. Am. J. Agric. For. 2014, 2, 61. [Google Scholar] [CrossRef]
- Famiani, F.; Battistelli, A.; Moscatello, S.; Cruz-Castillo, J.G.; Walker, R.P. The Organic Acids That Are Accumulated in the Flesh of Fruits: Occurrence, Metabolism and Factors affecting Their Contents—A Review. Rev. Chapingo Ser. Hortic. 2015, 32, 97–128. [Google Scholar] [CrossRef]
- Di Matteo, A.; Di Rauso Simeone, G.; Cirillo, A.; Rao, M.A.; Di Vaio, C. Morphological Characteristics, Ascorbic Acid and Antioxidant Activity during Fruit Ripening of Four Lemon (Citrus Limon (L.) Burm. F.) Cultivars. Sci. Hortic. 2021, 276, 109741. [Google Scholar] [CrossRef]
- Di Rauso Simeone, G.; Di Matteo, A.; Rao, M.A.; Di Vaio, C. Variations of Peel Essential Oils during Fruit Ripening in Four Lemon (Citrus Limon (L.) Burm. F.) Cultivars. J. Sci. Food Agric. 2020, 100, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, R.R.; Caleb, O.J.; Fawole, O.A.; Opara, U.L. Effects of Different Maturity Stages and Growing Locations on Changes in Chemical, Biochemical and Aroma Volatile Composition of ‘Wonderful’ Pomegranate Juice: Maturation and Climate Effects on Pomegranate Juice. J. Sci. Food Agric. 2016, 96, 1002–1009. [Google Scholar] [CrossRef]
- Graziani, G.; Ritieni, A.; Cirillo, A.; Cice, D.; Di Vaio, C. Effects of Biostimulants on Annurca Fruit Quality and Potential Nutraceutical Compounds at Harvest and during Storage. Plants 2020, 9, 775. [Google Scholar] [CrossRef]
- Labbé, M.; Ulloa, P.A.; López, F.; Sáenz, C.; Peña, Á.; Salazar, F.N. Characterization of Chemical Compositions and Bioactive Compounds in Juices from Pomegranates (‘Wonderful’, ‘Chaca’ and ‘Codpa’) at Different Maturity Stages. Chil. J. Agric. Res. 2016, 76, 479–486. [Google Scholar] [CrossRef]
- Tozzi, F.; Legua, P.; Martínez-Nicolás, J.J.; Núñez-Gómez, D.; Giordani, E.; Melgarejo, P. Morphological and Nutraceutical Characterization of Six Pomegranate Cultivars of Global Commercial Interest. Sci. Hortic. 2020, 272, 109557. [Google Scholar] [CrossRef]
- Calani, L.; Beghè, D.; Mena, P.; Del Rio, D.; Bruni, R.; Fabbri, A.; Dall’Asta, C.; Galaverna, G. Ultra-HPLC–MSn (Poly)Phenolic Profiling and Chemometric Analysis of Juices from Ancient Punica granatum L. Cultivars: A Nontargeted Approach. J. Agric. Food Chem. 2013, 61, 5600–5609. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Cavoski, I.; Pacifico, A.; Tedone, L.; Mondelli, D. Morpho-Pomological and Chemical Characterization of Pomegranate (Punica granatum L.) Genotypes in Apulia Region, Southeastern Italy. Sci. Hortic. 2011, 130, 599–606. [Google Scholar] [CrossRef]
- Ferrara, G.; Giancaspro, A.; Mazzeo, A.; Giove, S.L.; Matarrese, A.M.S.; Pacucci, C.; Punzi, R.; Trani, A.; Gambacorta, G.; Blanco, A.; et al. Characterization of Pomegranate (Punica granatum L.) Genotypes Collected in Puglia Region, Southeastern Italy. Sci. Hortic. 2014, 178, 70–78. [Google Scholar] [CrossRef]
- Todaro, A.; Cavallaro, R.; Malfa, S.L.; Continella, A.; Gentile, A.; Fischer, U.A.; Carle, R.; Spagna, G. Anthocyanin profile and antioxidant activity of freshly squeezed pomegranate (Punica granatum L.) juices of Sicilian and Spanish provenances. Ital. J. Food Sci. 2016, 28, 464–479. [Google Scholar]
- Magri, A.; Adiletta, G.; Petriccione, M. Evaluation of Antioxidant Systems and Ascorbate-Glutathione Cycle in Feijoa Edible Flowers at Different Flowering Stages. Foods 2020, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Goffi, V.; Magri, A.; Botondi, R.; Petriccione, M. Response of Antioxidant System to Postharvest Ozone Treatment in ‘Soreli’ Kiwifruit. J. Sci. Food Agric. 2020, 100, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Dafny-Yalin, M.; Glazer, I.; Bar-Ilan, I.; Kerem, Z.; Holland, D.; Amir, R. Color, Sugars and Organic Acids Composition in Aril Juices and Peel Homogenates Prepared from Different Pomegranate Accessions. J. Agric. Food Chem. 2010, 58, 4342–4352. [Google Scholar] [CrossRef]
- Adiletta, G.; Petriccione, M.; Liguori, L.; Pizzolongo, F.; Romano, R.; Di Matteo, M. Study of Pomological Traits and Physico-Chemical Quality of Pomegranate (Punica granatum L.) Genotypes Grown in Italy. Eur. Food Res. Technol. 2018, 244, 1427–1438. [Google Scholar] [CrossRef]
- Tezcan, F.; Gültekin-Özgüven, M.; Diken, T.; Özçelik, B.; Erim, F.B. Antioxidant Activity and Total Phenolic, Organic Acid and Sugar Content in Commercial Pomegranate Juices. Food Chem. 2009, 115, 873–877. [Google Scholar] [CrossRef]
- Mirdehghan, S.H.; Rahemi, M. Seasonal Changes of Mineral Nutrients and Phenolics in Pomegranate (Punica granatum L.) Fruit. Sci. Hortic. 2007, 111, 120–127. [Google Scholar] [CrossRef]
- Hasnaoui, N.; Mars, M.; Ghaffari, S.; Trifi, M.; Melgarejo, P.; Hernandez, F. Seed and Juice Characterization of Pomegranate Fruits Grown in Tunisia: Comparison between Sour and Sweet Cultivars Revealed Interesting Properties for Prospective Industrial Applications. Ind. Crop. Prod. 2011, 33, 374–381. [Google Scholar] [CrossRef]
- Martínez, J.J.; Melgarejo, P.; Hernández, F.; Salazar, D.M.; Martínez, R. Seed Characterisation of Five New Pomegranate (Punica granatum L.) Varieties. Sci. Hortic. 2006, 110, 241–246. [Google Scholar] [CrossRef]
- Shulman, Y.; Fainberstein, L.; Lavee, S. Pomegranate Fruit Development and Maturation. Hortic. Sci. 1984, 59, 265–274. [Google Scholar] [CrossRef]
- Nuncio-Jáuregui, N.; Calín-Sánchez, A.; Carbonell-Barrachina, A.; Hernández, F. Changes in Quality Parameters, Proline, Antioxidant Activity and Color of Pomegranate (Punica granatum L.) as Affected by Fruit Position within Tree, Cultivar and Ripening Stage. Sci. Hortic. 2014, 165, 181–189. [Google Scholar] [CrossRef]
- Martínez, J.J.; Hernández, F.; Abdelmajid, H.; Legua, P.; Martínez, R.; Amine, A.E.; Melgarejo, P. Physico-Chemical Characterization of Six Pomegranate Cultivars from Morocco: Processing and Fresh Market Aptitudes. Sci. Hortic. 2012, 140, 100–106. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer Acceptance of ‘Brooks’ and ‘Bing’ Cherries Is Mainly Dependent on Fruit SSC and Visual Skin Color. Postharvest Biol. Technol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Orak, H.H.; Yagar, H.; Isbilir, S.S. Comparison of Antioxidant Activities of Juice, Peel, and Seed of Pomegranate (Punica granatum L.) and Inter-Relationships with Total Phenolic, Tannin, Anthocyanin, and Flavonoid Contents. Food Sci. Biotechnol. 2012, 21, 373–387. [Google Scholar] [CrossRef]
- Iwashina, T. Contribution to Flower Colors of Flavonoids Including Anthocyanins: A Review. Nat. Prod. Commun. 2015, 10, 529–544. [Google Scholar] [CrossRef]
- Nassour, R.; Ayash, A.; Al-Tameemi, K. Anthocyanin Pigments: Structure and Biological Importance. J. Chem. Pharm. Sci. 2020, 13, 45–57. [Google Scholar]
- Çam, M.; Hışıl, Y. Pressurised Water Extraction of Polyphenols from Pomegranate Peels. Food Chem. 2010, 123, 878–885. [Google Scholar] [CrossRef]
- Graziani, G.; Gaspari, A.; Di Vaio, C.; Cirillo, A.; Ronca, C.L.; Grosso, M.; Ritieni, A. Assessment of in vitro bioaccessibility of polyphenols from annurca, limoncella, red delicious, and golden delicious apples using a sequential enzymatic digestion model. Antioxidants 2021, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, B.; Marconi, O.; Maranghi, S.; Lutts, S.; Rosati, A.; Famiani, F.; Benincasa, P. Effect of Genotype on the Sprouting of Pomegranate (Punica granatum L.) Seeds as a Source of Phenolic Compounds from Juice Industry by-Products. Plant. Foods Hum. Nutr. 2017, 72, 432–438. [Google Scholar] [CrossRef]
- Legua, P.; Forner-Giner, M.Á.; Nuncio-Jáuregui, N.; Hernández, F. Polyphenolic Compounds, Anthocyanins and Antioxidant Activity of Nineteen Pomegranate Fruits: A Rich Source of Bioactive Compounds. J. Funct. 2016, 23, 628–636. [Google Scholar] [CrossRef]
- Shahkoomahally, S.; Khadivi, A.; Brecht, J.K.; Sarkhosh, A. Chemical and Physical Attributes of Fruit Juice and Peel of Pomegranate Genotypes Grown in Florida, USA. Food Chem. 2021, 342, 128302. [Google Scholar] [CrossRef]
- Rajasekar, D.; Akoh, C.C.; Martino, K.G.; MacLean, D.D. Physico-Chemical Characteristics of Juice Extracted by Blender and Mechanical Press from Pomegranate Cultivars Grown in Georgia. Food Chem. 2012, 133, 1383–1393. [Google Scholar] [CrossRef]
- Melgarejo, P.; Núñez-Gómez, D.; Legua, P.; Martínez-Nicolás, J.; Almansa, M.S. Pomegranate (Punica granatum L.) a dry pericarp fruit with fleshy seeds. Trend. Food Sci. Technol. 2020, 102, 232–236. [Google Scholar] [CrossRef]
- Hasanpour, M.; Saberi, S.; Iranshahi, M. Metabolic Profiling and Untargeted 1H-NMR-Based Metabolomics Study of Different Iranian Pomegranate (Punica granatum) Ecotypes. Planta Med. 2020, 86, 212–219. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in Organic Acid Profiles During Fruit Development and Ripening: Correlation or Causation? Front. Plant Sci. 2018, 9, 1689. [Google Scholar] [CrossRef]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What Controls Fleshy Fruit Acidity? A Review of Malate and Citrate Accumulation in Fruit Cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Chater, J.M.; Mathon, C.; Larive, C.K.; Merhaut, D.J.; Tinoco, L.W.; Mauk, P.A.; Jia, Z.; Preece, J.E. 1 Juice Quality Traits, Potassium Content, 2 and 1H NMR Derived Metabolites of 14 Pomegranate Cultivars. J. Berry Res. 2019, 9, 209–225. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Rosas-Bautista, A.; Rico-Arzate, E.; Cruz-Narvaez, Y.; Zepeda-Vallejo, L.G.; Lalaleo, L.; Hidalgo-Martínez, D.; Becerra-Martínez, E. Study of Nutritional Quality of Pomegranate (Punica granatum L.) Juice Using 1H NMR-Based Metabolomic Approach: A Comparison between Conventionally and Organically Grown Fruits. LWT 2020, 134, 110222. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Fortes, A.M.; Pais, M.S.; Choi, Y.H.; Verpoorte, R. Monitoring Biochemical Changes during Grape Berry Development in Portuguese Cultivars by NMR Spectroscopy. Food Chem. 2011, 124, 1760–1769. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Mastrobuoni, F.; Di Patre, D.; Zampella, L.; Capuano, L.R.; Scortichini, M.; Petriccione, M. Agronomic, Nutraceutical and Molecular Variability of Feijoa (Acca Sellowiana (O. Berg) Burret) Germplasm. Sci. Hortic. 2015, 191, 1–9. [Google Scholar] [CrossRef]
- Petriccione, M.; Pagano, L.; Forniti, R.; Zampella, L.; Mastrobuoni, F.; Scortichini, M.; Mencarelli, F. Postharvest Treatment with Chitosan Affects the Antioxidant Metabolism and Quality of Wine Grape during Partial Dehydration. Postharvest Biol. Technol. 2018, 137, 38–45. [Google Scholar] [CrossRef]

| Stages of Ripening (S) | Harvest Date | Fruit Description |
|---|---|---|
| I | 3 September | Immature fruits with green epicarp |
| II | 23 September | Unripe fruit with a light red epicarp |
| III | 22 October | Semi-ripe fruit with a deeper red epicarp |
| IV | 3 November | Ripe fruit with an intense red epicarp |
| Fruit Weight (g) | Equatorial Diameter (mm) | Polar Diameter (mm) | |||||||
| Stages | Santa Lucia | Di Benedetto | Arborea | Santa Lucia | Di Benedetto | Arborea | Santa Lucia | Di Benedetto | Arborea |
| I | 165.82 ± 5.05 Cb | 190.06 ± 7.20 Da | 168.71 ± 9.08 Bb | 72.97 ± 1.04 Cb | 78.13 ± 1.28 Ca | 74.25 ± 1.29 Bb | 61.70 ± 0.46 Ca | 62.75 ± 1.07 Ca | 62.14 ± 1.16 Ca |
| II | 267.78 ± 22.24 Ba | 242.69 ± 8.40 Cb | 193.88 ± 15.94 Bc | 84.67 ± 1.95 Ba | 81.88 ± 1.52 Cb | 75.88 ± 1.59 Bc | 70.72 ± 1.30 Ba | 65.69 ± 0.90 Cb | 65.81 ± 1.32 Bb |
| III | 324.00 ± 35.22 Aa | 317.19 ± 19.59 Ba | 290.50 ± 13.24 Ab | 89.00 ± 3.36 Aa | 90.31 ± 2.04 Ba | 87.13 ± 1.51 Aa | 77.19 ± 2.32 Aa | 74.50 ± 1.60 Ba | 76.56 ± 1.26 Aa |
| IV | 323.22 ± 16.63 Aa | 392.19 ± 14.87 Aa | 295.25 ± 10.54 Ab | 89.19 ± 1.53 Ab | 97.63 ± 1.25 Aa | 86.50 ± 1.18 Ac | 75.03 ± 1.27 Aab | 78.38 ± 1.11 Aa | 73.38 ± 1.21 Ab |
| Significance | |||||||||
| G × S | *** | *** | *** | ||||||
| Seed Weight (g) | Juice Content (%) | ||||||||
| Stages | Santa Lucia | Di Benedetto | Arborea | Santa Lucia | Di Benedetto | Arborea | |||
| I | 82.20 ± 3.75 Ca | 77.59 ± 3.72 Ca | 76.99 ± 4.82 Ba | 54.50 ± 1.32 Ba | 51.50 ± 5.50 Ba | 37.50 ± 9.85 Db | |||
| II | 124.38 ± 13.42 Ba | 104.69 ± 5.57 Bb | 85.109 ± 6.54 Bb | 68.75 ± 2.53 Aa | 53.50 ± 6.96 Bb | 48.00 ± 10.03 Cb | |||
| III | 141.03 ± 14.96 ABa | 124.79 ± 8.22 Aa | 135.119 ± 7.51 Aa | 66.75 ± 3.66 Aa | 68.25 ± 7.17 Aa | 61.50 ± 11.87 Ba | |||
| IV | 147.31 ± 7.96 Aa | 136.89 ± 7.60 Aa | 141.129 ± 6.60 Aa | 63.25 ± 4.90 Ab | 70.00 ± 8.91 Aa | 69.50 ± 12.96 Aa | |||
| Significance | |||||||||
| G × S | *** | *** | |||||||
| L* | a* | b* | |||||||
| Stages | Santa Lucia | Di Benedetto | Arborea | Santa Lucia | Di Benedetto | Arborea | Santa Lucia | Di Benedetto | Arborea |
| I | 48.62 ± 0.87 Cc | 53.21 ± 1.41 Bb | 56.56 ± 0.99 Ca | 5.10 ± 1.37 Cab | 30.42 ± 16.52 Aa | −0.78 ± 1.53 Dc | 27.55 ± 0.76 Ab | 32.73 ± 1.05 Ba | 34.89 ± 0.94 Aa |
| II | 58.53 ± 1.22 Ba | 59.25 ± 1.15 Aa | 59.56 ± 1.03 Ba | 13.52 ± 1.63 Bb | 28.81 ± 1.08 Aa | 7.78 ± 1.71 Cc | 68.82 ± 36.12 Aa | 35.58 ± 0.72 Aa | 36.93 ± 0.84 Aa |
| III | 64.19 ± 1.31 Aa | 52.06 ± 1.12 Bb | 65.02 ± 0.71 Aa | 15.81 ± 2.28 Ba | 116.87 ± 76.83 Aa | 15.01 ± 1.41 Ba | 30.98 ± 0.81 Ab | 26.23 ± 1.10 Cc | 35.17 ± 0.33 Aa |
| IV | 44.70 ± 1.21 Db | 45.55 ± 1.11 Cb | 61.21 ± 0.93 Ba | 33.13 ± 0.78 Ab | 36.41 ± 1.18 Aa | 20.51 ± 1.16 Ac | 22.28 ± 0.97 Aa | 23.67 ± 0.86 Ca | 79.92 ± 44.40 Aa |
| Significance | |||||||||
| G × S | *** | ns | ns | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirillo, A.; Magri, A.; Scognamiglio, M.; D’Abrosca, B.; Fiorentino, A.; Petriccione, M.; Di Vaio, C. Evaluation of Morphological, Qualitative, and Metabolomic Traits during Fruit Ripening in Pomegranate (Punica granatum L.). Horticulturae 2022, 8, 384. https://doi.org/10.3390/horticulturae8050384
Cirillo A, Magri A, Scognamiglio M, D’Abrosca B, Fiorentino A, Petriccione M, Di Vaio C. Evaluation of Morphological, Qualitative, and Metabolomic Traits during Fruit Ripening in Pomegranate (Punica granatum L.). Horticulturae. 2022; 8(5):384. https://doi.org/10.3390/horticulturae8050384
Chicago/Turabian StyleCirillo, Aurora, Anna Magri, Monica Scognamiglio, Brigida D’Abrosca, Antonio Fiorentino, Milena Petriccione, and Claudio Di Vaio. 2022. "Evaluation of Morphological, Qualitative, and Metabolomic Traits during Fruit Ripening in Pomegranate (Punica granatum L.)" Horticulturae 8, no. 5: 384. https://doi.org/10.3390/horticulturae8050384
APA StyleCirillo, A., Magri, A., Scognamiglio, M., D’Abrosca, B., Fiorentino, A., Petriccione, M., & Di Vaio, C. (2022). Evaluation of Morphological, Qualitative, and Metabolomic Traits during Fruit Ripening in Pomegranate (Punica granatum L.). Horticulturae, 8(5), 384. https://doi.org/10.3390/horticulturae8050384










