Abstract
Blue mold is one of the most serious postharvest diseases in apples caused by Penicillium expansum. The purpose of this study is to determine the effect of ε-poly-L-lysine (ε-PL) on the pathogenicity of P. expansum and explore the potential mechanism from the perspective of organic acid. The study investigates the effect of ε-PL treatment on the growth and acid production of P. expansum in vitro and in vivo. When the concentration of ε-PL was 50 mg/L, the growth of P. expansum was inhibited and the decrease in pH value was delayed in the medium. For example, on the third day of culture, P. expansum reduced the pH of the medium from 6.1 to 4.15, and ε-PL inhibited the decrease in the pH value at most 34.4%. When the concentration reached 1000 or 2000 mg/L, the infection of P. expansum in fruits was effectively inhibited. During the growth and infection of P. expansum, gluconic acid is one of the main factors leading to the pH value falling in the local environment. After ε-PL treatment, the accumulation of gluconic acid decreased, the activity of glucose oxidase was suppressed, and then the decline in the local environmental pH slowed down. In addition, after ε-PL treatment, the activities of cell-wall-degrading enzymes, such as cellulase (CL) and polygalacturonase (PG), in the different areas of the P. expansum–apple interaction were also affected by pH change. The results show that ε-PL inhibited the pathogenicity of P. expansum by affecting the accumulation of gluconic acid and slowing the decline in pH in fruit tissues, so as to affect the pathogenicity of P. expansum. This is the first time that the mechanism of ε-PL interfering with the pathogenicity of P. expansum from the perspective of organic acids is clarified.
1. Introduction
Postharvest rot caused by fungal infection results in serious economic losses and affects marketing value, which has become a global problem [,]. Fungi are the main pathogens causing apple decay in the field and during transportation and storage []. Serious fruit and vegetable diseases during postharvest and storage cause heavy losses to the economy, so different strategies for controlling diseases in fruits have been investigated []. Blue mold is one of the most serious postharvest diseases in apple caused by P. expansum. As a pathogenic fungus, P. expansum is capable of producing mycotoxins such as patulin. Therefore, infected postharvest fruit may also be contaminated by patulin, which may cause harm to human and environmental health [,].
ε-poly-L-lysine (ε-PL) has extensive antibacterial activity and low toxicity compared with other chemical antibacterial agents. It was first found in Streptomyces albicans, and it can be decomposed into the essential amino acid lysine in the human body [,]. Recently, research has found that ε-PL can also effectively inhibit the growth and infection of postharvest fungi, such as Penicillium digitatum, Botrytis cinerea, and Penicillium italicum [,,]. Most studies focus on the cell membrane metabolism effects of ε-PL’s antibacterial mechanism. ε-PL is positively charged and combined with negatively charged groups, such as lipoteichoic acid, on the surface of the bacterial cell membrane to change the structure of the cell membrane, resulting in the leakage of intracellular enzymes and soluble proteins, and the dysfunction of the normal respiration and metabolism of bacteria, leading to cell apoptosis []. ε-PL affects the conformation and function of membrane proteins in yeast and causes osmotic stress and plasma wall separation []. Jiao et al. [] reported that ε-PL might attack the cell membranes and induce membrane disintegration and disturbance. ε-PL treatment can destroy the integrity of the cell membrane of B. cinerea, cause the leakage of intracellular soluble carbohydrates and nucleic acids, promote the accumulation of reactive oxygen species, induce the expression of the respiratory burst oxidase homologue gene in ε-PL-treated jujube fruit, and finally inhibit the growth and reproduction of B. cinerea [,,].
In order to infect better, pathogens secrete a series of substances to change the host environment []. In some cases, pathogenicity is closely related to the pH value. The organic acid molecules secreted by certain fungi are multifunctional and can act together with environmental pH to activate virulence factors and enhance pathogenicity. It has been postulated that, during infection, fungi acidify host tissues primarily by adjusting the pH outside the plastid to a value more favorable for cell wall degrading enzymes []. This mechanism ensures that genes encoding cell-wall-degrading enzymes are expressed while promoting the secretion of products at pH conditions optimal for their activity [,]. P. expansum, P. digitatum, P. italicum, B. cinerea and Sclerotinia sclerotiorum utilize tissue acidification to support their attack via the secretion of organic acids [,,,]. Penicillium spp. secrete mainly citric and gluconic acids [,]. S. sclerotiorum and B. cinerea change the host pH by secreting oxalic acid [,]; Phomopsis mangiferae and P. expansum infect mango and apple fruits mainly by secreting gluconic acid [,]. However, there are few reports on the involvement of organic acid in the control mechanism of ε-PL against blue mold caused by P. expansum in apple fruits.
When plant pathogens, such as P. expansum, P. digitatum, and P. italiensis, infect fruit, they cause tissue maceration and rot, accompanied by the secretion of cell-wall-degrading enzymes []. Cell-wall-degrading enzymes disrupt cell cohesion mainly by degrading pectin and cellulose [], in which polygalacturonase (PG) and cellulose (CL) play important roles []. The pretreatment of cell walls with PG appears to promote the ability of other cell-wall-degrading enzymes to attack their substrates. CL primarily hydrolyzes polysaccharides with 1,4-β-glucosidic linkages that play a major role in cellulose degradation [].
The objective of this study is to investigate the changes of pathogenicity and organic acid metabolism of P. expansum after ε-PL treatment and evaluate the level of organic acids and the activity of cell-wall-degrading enzymes in the area where P. expansum interacts with the fruit. This research provides new insights into the mechanism by which ε-PL inhibits the growth and infection of P. expansum.
2. Materials and Methods
2.1. Fungal Culture and Preparation of Spore Suspension
P. expansum was cultivated as described by Zheng et al. []. Periodically, P. expansum was subcultured on potato dextrose agar (PDA) and stored at 4 °C for use. Spore suspensions were prepared from P. expansum cultured at 25 °C for 4 days, the mycelium was removed with sterile double gauze, and a hemocytometer was used to adjust the concentration of the conidial suspension to 1 × 105/mL for use.
2.2. Fruit
Fresh ripe apples (Luochuan Red Fuji) with uniform size and color were selected (the experimental fruits were healthy both physiologically and pathologically) in a supermarket in the Changqing district, Jinan, Shandong Province, and immediately shipped to the laboratory. The fruit were soaked in a 2% (v/v) sodium hypochlorite solution for 2 min for surface disinfestation, rinsed with tap water after soaking, and air dried at room temperature (RT, 25 °C) [].
2.3. Effect of ε-PL Treatment on the Growth of P. expansum
The effect of ε-PL (Zhengzhou Babonal Bioengineering Co., Ltd., Zhengzhou, China) on the growth of P. expansum was explored referring to the method of Liu et al. []. ε-PL powder was added to the sterilized PDA, and 25 mL of PDA was poured into each Petri dish (90 mm diameter) to achieve final concentrations of 0, 25, 50, 100, and 200 mg/L in the medium. Using a sterile pipette tip, a 7 mm diameter mycelial disk was taken within 24 h of culture and placed in the center of the cooled medium. After sealing the plate, it was placed in a 25 °C incubator. Photographs were taken at 6 days and the colony diameter was measured (cross-diameter method).
2.4. Effect of ε-PL Treatment on Environmental pH Regulation by P. expansum In Vitro
The effect of ε-PL on acid production by P. expansum was tested according to the method of Zhang et al. []. One milliliter of P. expansum spore suspension in sterile water was added to 100 mL of sterile potato dextrose broth (PDB) medium. PDB with a ε-PL concentration of 50 mg/L was prepared and incubated in a sterile shaker at 25 °C and 150 rpm. The pH and gluconic acid (GLA) of the medium or hyphae were measured at 24, 48, 72, and 96 h. There were 3 plates per group, and the experiment was repeated 3 times. The hyphae were filtered and isolated for sampling, and the samples were frozen in liquid nitrogen and stored at −80 °C.
In order to further explore the effect of ε-PL treatment on the acid production of P. expansum, the aniline blue staining method was used to verify the acid production level of ε-PL in vitro. A 1-day-old P. expansum mycelial disk (7 mm diameter) was taken and transferred to PDA medium with ε-PL concentrations of 0, 25, 50, 100, and 200 mg/L, in which the concentration of aniline blue in the PDA medium of each group was 0.1 g/L. There were 3 plates in the each ε-PL concentration group. Plates were transferred to a 25 °C incubator for cultivation. For the acid production of the pathogen, the diameter of the dark blue concentric rings around the fungal colony was measured and recorded. The pH was measured at 0, 0.5, or 1 cm from the outer edge of the P. expansum colony with a hand-held pH meter (Testo 205). The experiment was repeated 3 times with 3 technical replicates each time.
2.5. Effect of ε-PL Treatment on Blue Mold Disease in Apple Fruits
Referring to the method of Jiao et al. [], apples were divided into 4 groups, each group contained 4 replicate subgroups, and each subgroup consisted of 3 apples. The apples were stabbed at the equatorial area with sterile steel nails (width × length × depth = 3 mm × 2 mm × 3 mm). All the apples were inoculated with 10 μL of spore suspension (1 × 105/mL) and then air-dried at RT. After 2 h, 10 μL of sterile ε-PL solutions of different concentrations (0, 1000, and 2000 mg/L) were injected into the apples of each group at the inoculation site. After drying at RT, the fruit were placed in plastic baskets (320 mm × 240 mm × 100 mm), sealed with polyethylene bags, and a relative humidity of 90~95% was maintained with damp gauze, after which the fruit were stored at RT. The diameter of the lesions (cross-diameter method) and pH value were measured during storage, and then tissue samples were taken at 0, 1, or 2 cm from the outer edge of the lesion, referred to as areas A, B, and C, respectively. Samples were frozen with liquid nitrogen and stored at −80 °C. The fruit inoculation experiment was carried out 3 times with 3 replicates, each with 10 fruit per replicate.
2.6. Determination of GOX Activity
The glucose oxidase (GOX) activity of apple tissues inoculated with P. expansum was determined by the micro method. The determination principle of GOX is that GOX catalyzes the production of H2O2, and peroxidase catalyzes the oxidation of 4-aminoantipyrine coupling phenol with H2O2 to produce colored compounds. There is a characteristic absorption peak at 500 nm, and the color depth has a linear relationship with GOX activity. The instructions of the GOX determination kit (Suzhou Bioengineering Company, Suzhou, China) were followed for sample determination, and the absorbance was read at 500 nm with a microplate reader (Molecular Devices CMAX PLUS). GOX activity was expressed in U (1 nmol H2O2 per minute per g of tissue). The test was repeated 2 times with 3 replicates each.
2.7. Determination of Organic Acids
Organic acids were determined according to the method of Wu et al. [] with modifications. After freezing with liquid nitrogen, the sample (apple/mycelium) was ground, 1 g of sample was added into a 10 mL centrifuge tube, 5 mL deionized water was added, shaken for 1 h, then centrifuged at 10,000 rpm and 4 °C for 10 min. The supernatant was removed, 2.5 mL deionized water was added to the precipitate to dissolve it again, the shaking step was repeated, and the two supernatants were combined, about 7.5 mL. The combined supernatant was passed through a filter (0.45 µm), transferred to the injection bottle, and prepared for loading. Three replicates were used for each treatment and the experiment was repeated twice. The organic acid content was expressed in mg/g fresh weight (FW).
High-performance liquid chromatography (HPLC) conditions: Instrument: lc-20t high-performance liquid chromatograph, UV wavelength 210 nm; Chromatographic column: ODS C18 (250 mm × 4.6 mm, 5 μm); Column temperature: 30 °C; Duration: 18 min; Flow rate: 0.5 mL/min; Injection volume: 15 μL; Mobile phase: mobile phase A (0.01 mol/L, pH 2.0 phosphate solution), mobile phase B (methanol), A:B = 95:5.
2.8. Determination of Cell-Wall-Degrading Enzyme Activity
The activities of cell-wall-degrading enzymes, including PG and CL, were measured in the interaction areas of apple fruits. Evenly mixed apple tissue powder was added according to the instructions of the corresponding kit (PG and CL enzyme activity assay kits were purchased from Suzhou Keming Biotechnology Co., Ltd., Suzhou, China), and 0.1 g samples were taken for the determination of the 2 enzyme activities. After the steps were completed, the absorbance values were measured by an enzyme labeling instrument at 540 and 620 nm. The enzyme activity of PG was defined as the decomposition of pectic acid per g of sample to produce 1 mg of galacturonic acid per hour at 40 °C, pH 6.0, and was expressed as U. CL enzyme activity was defined as the catalytic production of 1 μg of glucose per gram of tissue per minute as an activity unit, and was expressed as U. The experiment was repeated twice, 3 replicates each time.
2.9. Data Analysis
The results of all experiments were expressed as mean ± standard deviation (SD). The data were analyzed by a one-way ANOVA and Tukey’s test (SPSS version 15.0, SPSS, Inc., Chicago, IL, USA). Differences were considered statistically significant when p < 0.05. In addition, SPSS software was used for the correlation analysis of the selected variables.
3. Result
3.1. Effect of ε-PL Treatment on the Growth of P. expansum
3.1.1. In Vitro Test
As shown in Figure 1, the diameter of the P. expansum colony was inversely correlated with the concentration of ε-PL, and as the concentration of ε-PL increased, the colony diameter decreased significantly (p < 0.05). When the concentrations of ε-PL were 25, 50, and 100 mg/L, the diameters of the P. expansum colonies were inhibited by 16.1, 30.2, and 55.2% compared with the control on the sixth day, respectively. The growth of P. expansum was completely inhibited when the concentration of ε-PL reached 200 mg/L. The results show that ε-PL treatment effectively inhibited the growth of P. expansum in vitro.
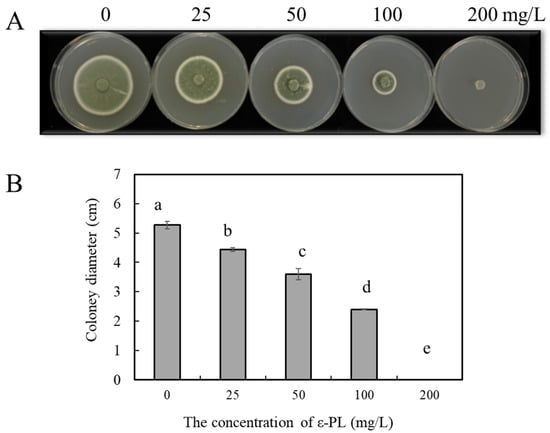
Figure 1.
Effect of ε-PL treatment on the growth of P. expansum in vitro. (A) Colony growth and (B) colony diameter of P. expansum cultured for 6 days. The data are shown as mean ± SD, n = 3. According to Tukey’s test, different letters indicate significant differences (p < 0.05).
3.1.2. In Vivo Test
The effect of ε-PL treatment on the prevention and control of apple blue mold disease is shown in Figure 2A, where the diameter of the lesions in all groups of apple fruits increased with the increase in storage days, and the diameter of the lesions in the treatment group was significantly lower than that in the control group (p < 0.05). On day 9, apple spot diameters in ε-PL concentrations of 1000 and 2000 mg/L groups were suppressed by 10.1% and 18.9% compared with the control group, respectively (Figure 2B) (p < 0.05). In addition, the study found that the larger the concentration of ε-PL, the smaller the diameter of the spot, which indicated ε-PL can suppress blue mold severity.
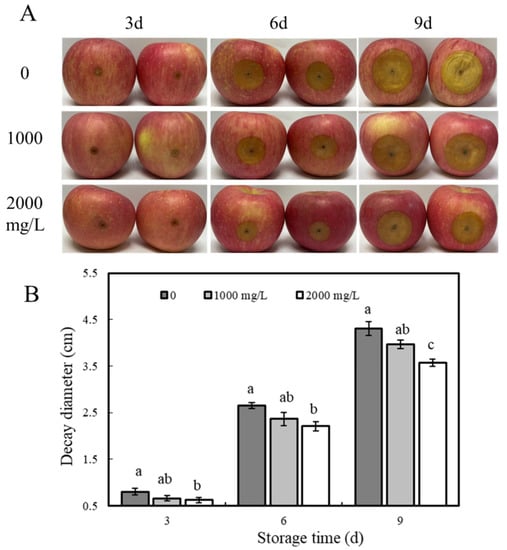
Figure 2.
Effect of ε-PL treatment on the infection of P. expansum in apples after harvest. (A) Apple fruits were wound-inoculated with 10 μL spore suspension of P. expansum at 1 × 104 spores/mL, then treated with ε-PL at different concentrations (0, 1000, and 2000 mg/L) for storage at room temperature. Lesion diameters (B) were recorded after inoculation every 3 days. The vertical bar represents the standard deviation (n = 3). Different letters indicate significant different according to Tukey’s test (p < 0.05).
3.2. Effect of ε-PL Treatment on Environmental pH of P. expansum
3.2.1. Environmental pH Change In Vitro
Acid Production during the Growth of P. expansum
The test measured the organic acids in P. expansum hyphae and PDB supernatant, and the results show that the content of gluconic acid was the highest. The acid-producing levels during the growth of P. expansum treated with ε-PL were determined. As shown in Figure 3A, the pH of the control group medium decreased rapidly, dropping by about 2 pH units after 3 days of incubation, but the pH increased slightly on day 4. After ε-PL treatment, the medium pH did not decrease significantly, and the pH of the control group was significantly lower than that of the treatment group during the whole culture process (p < 0.05). With the increase in culture time, the extracellular gluconic acid content of the P. expansum hyphae gradually increased (p < 0.05), and the gluconic acid content decreased on day 4. The change in gluconic acid content in the treatment group was similar to that in the control group (Figure 3B). In Figure 3C, with the increase in culture time, the intracellular gluconic acid content in the control group continued to accumulate and doubled from day 1 to day 4. The intracellular and extracellular gluconic acid content in the control group was higher than that in the ε-PL treatment group, indicating that the ε-PL treatment effectively inhibited gluconic acid production during the growth of P. expansum.
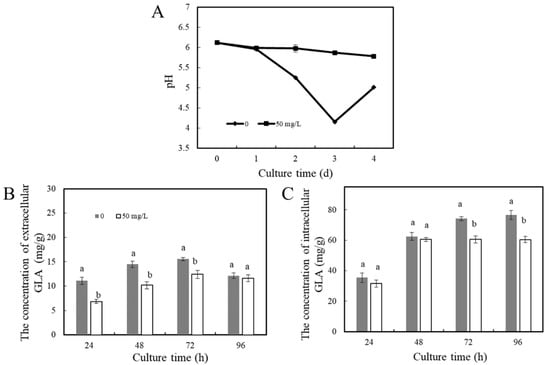
Figure 3.
Effect of ε-PL treatment on the acid production of P. expansum in vitro. (A) pH change of the PDB supernatant during the growth of P. expansum, (B) the intracellular gluconic acid content of mycelia during the growth of P. expansum, and (C) extracellular gluconic acid content during the growth of P. expansum (GLA = gluconic acid). The data provided are the average of three repetitions. The vertical bar represents the standard deviation. According to Tukey’s test, different letters indicate significant differences (p < 0.05).
Environmental pH Change by Aniline Blue Staining Method
The effect of ε-PL treatment on P. expansum acid production was further verified by aniline blue staining. Aniline blue is an alkaline dye that contains two complementary chromophores in which cations can be used to stain. P. expansum is able to secrete acidic substances during growth, so aniline blue can be used to indicate the acid-producing level of P. expansum. As shown in Figure 4A, the diameter of the blue concentric rings on the outside of the edge of the colony formed by the control group was larger and heavier than that in the ε-PL treatment group. In addition, the pH of the color-producing area of the control medium was significantly lower than that of the ε-PL treatment medium (p < 0.05), and the greater the concentration of ε-PL in the central position, the higher the ΔpH compared with the control group. The ΔpH at the ε-PL concentration of 25, 50, 100, and 200 mg/L was 0.03, 0.35, 0.74, and 1.16, respectively. The pH change at 0.5 and 1 cm from the edge of the pathogen is consistent with the central position, and the acidity decreases as the concentration of ε-PL increases (Figure 4C).
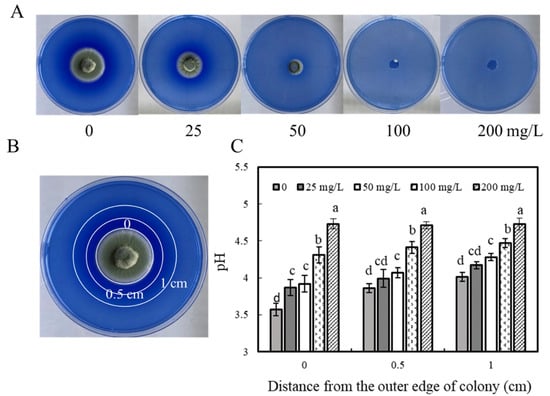
Figure 4.
Effect of ε-PL treatment on aniline blue dye test to determine acidity. (A) The colony status of P. expansum on PDA after adding aniline blue for 6 days; (C) After 6 days of culture, the pH of the PDA medium at 0, 1, or 2 cm from the outer edge of the lesion (B). The vertical bar represents the standard deviation. According to Tukey’s test, different letters indicate significant differences (p < 0.05).
3.2.2. Environmental pH Change In Vivo
Environmental pH Change in P. expansum–Apple Interaction Areas
The effect of ε-PL treatment on the pH of different areas of apple fruits inoculated with P. expansum is shown in Figure 5. From days 3 to 6 of storage, the pH value of fruit area A in the control group decreased. After ε-PL treatment, the change trend of area A was similar to that of the control group, where the pH value of area A on days 3 and 6 was higher than that in the control group (p < 0.05), and there was no significant difference between the control and treatment group on day 9 (Figure 5A). The pH changes in area B and area C of apples still showed the same trend as in area A, both decreasing from days 3 to 6, with an increasing pH on day 9, and the pH of the ε-PL treatment group was higher than that of the control group (Figure 5B,C). The above data showed that ε-PL treatment inhibited the acid production of P. expansum during infection, and ε-PL significantly inhibited the decrease in pH in various areas of the fruit.
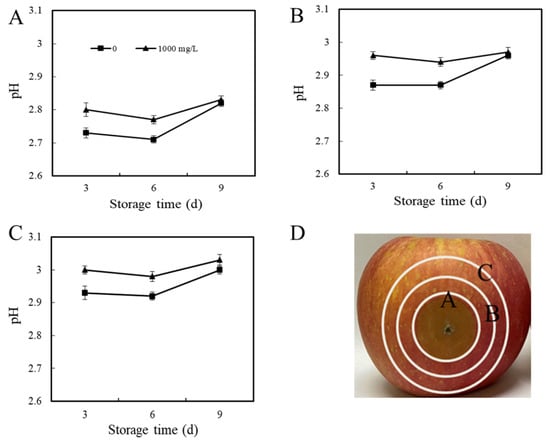
Figure 5.
Effect of ε-PL treatment on pH value of different areas of the P. expansum–apple interaction. The pH of interaction area A (A), area B (B), and area C (C) in fruit were measured on days 3, 6, and 9 (0, 1, or 2 cm, respectively, away from the outer edge of the P. expansum–apple interaction area) with the three interaction areas shown in (D). The vertical bar represents the standard deviation (n = 3). Different letters indicate significant different according to Tukey’s test (p < 0.05).
Organic Acids in P. expansum–Apple Interaction Areas
P. expansum triggers signals for organic acid regulation during infection, leading to changes in the level of organic acids at the fruit interaction area of the fungus. It has been reported that gluconic acid and citric acid might be two of the factors causing the decrease in apple fruit pH. Table 1 shows the determination results of organic acids in different areas of the fruit by HPLC.

Table 1.
Effects of ε-PL treatment on the secretion of organic acids in different areas of the P. expansum–apple interaction. The content of organic acids was measured on days 3, 6, and 9 of apple storage. The results represented the mean ± standard deviation of 3 repetitions of each treatment.
During storage, the gluconic acid content in fruit area A continued to decrease, about 6.55 units on day 9 less than on the 3 day, and after the ε-PL treatment, the gluconic acid content also continued to decrease during storage. On days 3, 6, and 9, the gluconic acid content of area A in the fruit treated with ε-PL decreased by 26.2, 14.8, and 12.0% (p < 0.05), respectively, compared with the control group. The gluconic acid content at the B and C areas of the apple also decreased with the increase in storage time. In addition, on day 3, the gluconic acid content in area C of the control group was 35.5 and 46.6% less than that of areas B and A, respectively. This indicates that the farther away from the area of pathogen infection, the less gluconic acid is accumulated.
The malic acid content measured decreased with longer storage times. As shown in Table 1, on day 3 of storage, the malic acid content in the A, B, and C areas of the apple fruits after treatment with 1000 mg/L ε-PL was 16.4, 9.54, and 12.7% higher than that of the control group (p < 0.05). At the same time, it was observed that on day 3, the malic acid content of the fruit area A of the control group was 9.33 and 9.77% lower than that of the control group B and C, respectively. That is, within a certain range of the P. expansum-apple interaction, the closer to the pathogen infection area, the lower the malic acid content in the tissue.
The citric acid content in the control group of fruit area A decreased slightly on day 6 and gradually recovered on day 9. On days 3, 6, and 9, the citric acid content of area A of the fruit treated with ε-PL decreased by 8.47, 33.3, and 86.7% compared with the control group, respectively. On day 3, the citric acid content of the area B of the fruit treated with ε-PL decreased by 21.2% compared with the control group. There was no significant difference in citric acid content between the control group and the treatment group in area C of the fruit.
The content of the gluconic acid and citric acid in area A was higher than that of the other two areas. The organic acid data of different areas of the apple showed that the ε-PL treatment affected the accumulation of organic acids in the interaction area of the pathogen, inhibiting the secretion of gluconic acid and citric acid, while the ε-PL treatment promoted the accumulation of malic acid in the fruit tissue to a certain extent.
GOX Activity in P. expansum–Apple Interaction Areas
Glucose oxidase (GOX) is important for the accumulation of gluconic acid. The activity of glucose oxidase in different areas of the apple is shown in Figure 6. In area A, GOX activity in the control group decreased significantly, but on day 9, GOX was slightly increased. The GOX activity after ε-PL treatment was significantly lower than that of the control group, and the change in GOX activity was consistent with the control group. On days 3, 6, and 9, GOX activity in the ε-PL-treated group was 10.1, 32.8, and 38.0% lower, respectively, than that in the control group (Figure 6A). In addition, the data showed that the trend of change in area B and area C was similar to that in area A. From days 3 to 6, GOX activity decreased, and on day 9, GOX activity gradually increased, and the activity in the treatment group was lower than that in the control group (Figure 6B,C). On day 3, the GOX activity in areas B and C in the ε-PL-treated group was reduced by 44.2 and 45.6% compared with the control group (p < 0.05), respectively.
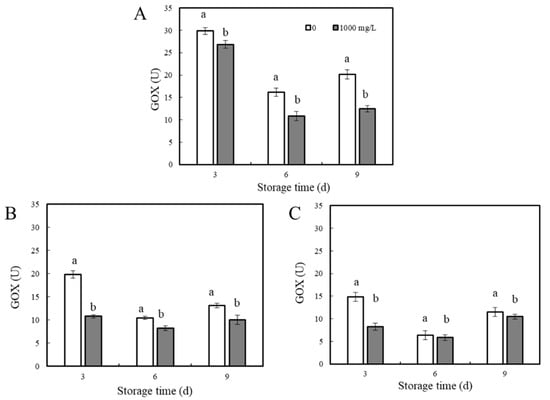
Figure 6.
Effects of ε-PL treatment on GOX activity in different areas of the P. expansum–apple interaction. The GOX activity of interaction area A (A), area B (B) and area C (C) was measured on days 3, 6, and 9, respectively. The vertical bar represents the standard deviation (n = 3). Different letters indicate significant different according to Tukey’s test (p < 0.05).
3.3. Cell-Wall-Degrading Enzyme Activities in P. expansum–Apple Interaction Areas
The PG activity in the various areas of the apples is shown in Figure 7A–C. The changes in PG activity in area A of the control group and ε-PL treatment group were basically the same, and the enzyme activity decreased with the increase in storage time. On days 3, 6, and 9, the enzyme activity of the ε-PL treatment group decreased by 47.1, 28.9, and 62.9%, respectively, compared with the control group (p < 0.05). On day 6 of storage, the PG activity in area B in the ε-PL treatment group was significantly lower than that in the control group (p < 0.05). The change in PG activity in area C was similar to that in area B; it increased first and then decreased during storage. The activity of the ε-PL treatment group was also lower than that in the control group.
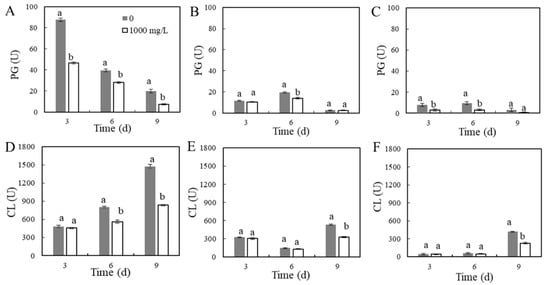
Figure 7.
Effects of ε-PL treatment on cell-wall-degrading enzymes in different areas of the P. expansum–apple interaction. The activities of cell-wall-degrading enzymes were measured on days 3, 6, and 9 of apple storage. Determination of PG activity in area A (A), area B (B), and area C (C) of the apple at different times; determination of CL activity in area A (D), area B (E), and area C (F) of the apple at different times. The vertical bar represents the standard deviation (n = 3). Different letters indicate significant differences according to Tukey’s test (p < 0.05).
The effect of ε-PL treatment on the CL activity in the various areas of the inoculated apples is shown in Figure 7D–F. With the increase in storage time, the CL activity of area A increased during storage. After ε-PL treatment, its activity was significantly lower than that of the control group (p < 0.05). On days 6 and 9 of storage, the CL activity of the ε-PL treatment group was 25.2 and 41.2% lower than that of the control group, respectively. The CL activity of area B in the control group decreased first and then increased. On day 9, the CL activity of the control group was the highest. After ε-PL treatment, on day 9, the CL activity of area B decreased by 38.9% compared with the control group. Additionally, on day 9, the CL activity of area C was the highest, and the enzyme activity of the ε-PL treatment group was 45.7% lower than that of the control group (p < 0.05).
Cell-wall-degrading enzymes play an important role in the infection of P. expansum as one of the pathogenicity factors. The above data showed that ε-PL treatment inhibited the activity of cell-wall-degrading enzymes to a certain extent and further reduced the pathogenicity of P. expansum.
4. Discussion
ε-PL is widely used due to its antimicrobial properties and low toxicity [,]. ε-PL treatment effectively inhibits green mold caused by P. digitatum in citrus [], and gray mold caused by B. cinerea in cherry tomatoes, green peppers, grapes, and strawberries []. The antimicrobial mechanism of ε-PL, which is widely reported, mainly focuses on inducing damage in the fungal cell membrane, leading to cell leakage, disruption of energy and information transmission, and finally leading to cell death []. However, the involvement of organic acid in the control mechanism of ε-PL against blue mold caused by P. expansum in apple fruits has not been reported previously. This study found that, compared with apples inoculated with P. expansum only, the incidence of apple blue mold after ε-PL treatment was reduced, and the accumulation of organic acids in the interaction area between apple and P. expansum also changed.
We investigated the effect of ε-PL on the growth of P. expansum in vivo and in vitro. The results show that ε-PL effectively inhibited the growth of P. expansum colonies in a dose-dependent manner. When the concentration of ε-PL was 200 mg/L, it could completely inhibit the growth of P. expansum on PDA, while in vivo, a larger dose was needed to inhibit the growth of P. expansum. This is somewhat similar to previous studies [,]. The required concentration of ε-PL to inhibit blue mold in vivo is much higher than that in vitro []. There may be several reasons for this phenomenon: (1) After the treatment of the apple with ε-PL, part of the substance is absorbed by the apple fruit tissues, resulting in a loss; (2) ε-PL interacted with the tissue matrix of the apple fruit and changed its original molecular structure; (3) Acidity, protein, salt, and metal ions affect the antimicrobial effect of ε-PL []; and (4) With increasing storage time, ε-PL may degrade, resulting in a decrease in its antimicrobial effect. Therefore, the inhibitory concentration of ε-PL should be adjusted accordingly with the change in environmental conditions.
Studies have shown that organic acids are important pathogenicity factors in the process of fungal infection []. ε-PL inhibited the growth and acid production of P. expansum. Aniline blue staining further verified that the acid production level of P. expansum decreased after ε-PL treatment. The results of this study show that ε-PL slowed the decrease in the medium pH during the growth of P. expansum, similar to previous studies []. We found that the amount of gluconic acid in cells increased with the increase in culture duration, and the content of gluconic acid in the culture medium increased. We speculate that the secretion of gluconic acid began at the beginning of the spore germination of P. expansum; with the increase in culture time, the intracellular gluconic acid was excreted, resulting in the decrease in environmental pH.
The content of organic acids in the interaction area of the apple with P. expansum was determined by HPLC. Through correlation analysis, it was found that the main factor causing the decline in host environmental pH was gluconic acid (Table S1 shown in the Supplementary Materials). According to the report of Vilanova et al. [], the pH decrease in P. expansum that affected the apples is related to the accumulation of gluconic and fumaric acids. Citric acid and malic acid have little relationship with the decline in apple pH. Previous studies have shown that local tissue pH will decrease after fruit impregnation. S. sclerotiorum and B. cinerea reduce the pH around the host by secreting a large amount of oxalic acid [,]; P. digitatum and P. expansum mainly acidify the host environment by secreting citric acid and gluconic acid []. A large amount of gluconic acid was detected in different areas of the P. expansum–apple interaction in the present study, and the content of gluconic acid in the ε-PL treatment group was significantly lower than that in the control group. Prusky et al. [] proved that the content of organic acids (citric acid and gluconic acid) in the rotten areas of the apple infected by P. expansum and grapefruit infected by P. digitatum was much higher than that in healthy tissues. It has been shown that gluconic acid is the key factor contributing to fungal disease of apples []. In addition, it was also observed that the pathogenicity of P. expansum increased with the decrease in pH in fruit tissue, while the pH in the ε-PL treatment group was higher than that in control group, which inhibited P. expansum to a certain extent. At the same time, other studies have shown that the exogenous addition of gluconic acid and citric acid can increase the infectivity of P. expansum, whereas NaHCO3 treatment inhibits the colonization by P. expansum []. Therefore, we speculate that tissue acidification is an important factor determining pathogenicity. ε-PL treatment inhibits the secretion of gluconic acid of P. expansum and suppressed the decrease in tissue pH, weakening the pathogenicity of P. expansum. However, we do not rule out the possibility that ε-PL inhibits the fungus directly, which results in a lower acid production and less pronounced pH drop. Additionally, this possibility needs to be further verified in the future.
GOX produces H2O2 and D-glucose-1,5-lactone. D-glucose-1,5-lactone spontaneously hydrolyzes to gluconic acid. In our experiment, the activity of the GOX enzyme in ε-PL treated tissue was lower than that in the control group. Maayan et al. [] detected GOX activity in the rotten tissue of the P. mangiferae–mango interaction area, and found that the higher the GOX activity, the more gluconic acid that was accumulated. This is consistent with our results. With the increase in GOX activity in fruit tissues, the accumulation of gluconic acid also increased. It was previously reported that the gene pmgox1 encoding GOX may also be induced in fruits under natural conditions, because fruit ripening will lead to alkalization and increased sugar utilization, thereby enhancing gene expression and the secretion of specific enzymes [].
The PG and CL activities in different areas of the P. expansum–apple interaction were measured. Studies have shown that, as an important pathogenic factor of postharvest pathogens, cell-wall-degrading enzymes are closely related to their pathogenicity [], and they play an important role in the destruction of fruit cells and tissues []. Moreover, the time sequence of different cell-wall-degrading enzymes is different in the process of pathogen infection []. Some studies have documented that the pathogen induces the production of PG and CL when it first invades the host and may act in the early stage of infection []. Our results show that the activity of CL always maintained a high level and reached its highest on day 9. At the same time, the activity of PG decreased with the increase in storage time. Therefore, it is speculated that the increase in CL activity in the later stage of storage might compensate for the decrease in PG activity at that time. Moreover, the experimental results also showed that the activities of two cell-wall-degrading enzymes treated with 1000 mg/L ε-PL were lower than those in the control group. ε-PL treatment might interfere with the acidification of the host during fungal infection, change the pH of tissue, and then affect the activity of cell-wall-degrading enzymes [].
5. Conclusions
In summary, ε-PL significantly inhibited the growth and gluconic acid production of P. expansum in vitro and in apple fruits. In addition, ε-PL hindered the acidification of the host environment, further inhibited the activity of PG and CL, resulting in a reduction in P. expansum infectivity. This is the first time that the mechanism of ε-PL interfering with the pathogenicity of P. expansum from the perspective of organic acids was clarified. These results help in understanding the mechanisms of pH changes on pathogenicity and may provide theoretical and technical guidance for developing new disease control strategies and reduce postharvest losses by disrupting acidification processes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8060468/s1, Table S1: The correlation analysis of pH, organic acid and lesion diameter in the three areas of P. expansum-apple interaction A, B, and C during storage.
Author Contributions
Y.L. and W.J. contributed equally. conceptualization, M.F.; methodology, Y.L.; validation, Y.L.; formal analysis, Y.L. and W.J.; investigation, Y.L.; resources, M.F.; data curation, Y.L.; writing—original draft preparation, Y.D. and M.W.; writing—review and editing, W.J.; supervision, M.F. and W.J.; project administration, M.F.; funding acquisition, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Outstanding Youth Science Foundation in Shandong Province (project No. ZR2019YQ16), the National Natural Science Foundation of China (project No. 31871854), the Youth Science and Technology Innovation Team of Shandong Province (project No. 2019KJF010) in China.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, X.Y.; Zhang, G.C.; Li, P.X.; Yang, Q.Y.; Chen, K.P.; Zhao, L.N.; Apaliya, M.T.; Gu, X.; Zhang, H.Y. Mechanisms of glycine betaine enhancing oxidative stress tolerance and biocontrol efficacy of Pichia caribbica against blue mold on apples. Biol. Control 2017, 108, 55–63. [Google Scholar] [CrossRef]
- Li, J.K.; Lei, H.H.; Song, H.M.; Lai, T.F.; Xu, X.B.; Shi, X.Q. 1-methylcyclopropene (1-MCP) suppressed postharvest blue mold of apple fruit by inhibiting the growth of Penicillium expansum. Posthavest. Biol. Technol. 2017, 125, 59–64. [Google Scholar] [CrossRef]
- Simonato, B.; Lorenzini, M.; Zapparoli, G. Effects of post-harvest fungal infection of apples on chemical characteristics of cider. LWT 2021, 138, 110620. [Google Scholar] [CrossRef]
- Žebeljan, A.; Vico, I.; Duduk, N.; Žiberna, B.; Krajnc, A.U. Dynamic changes in common metabolites and antioxidants during Penicillium expansum-apple fruit interactions. Physiol. Mol. Plant Pathol. 2019, 106, 166–174. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Liu, Y. Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 2013, 167, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Xu, W.; Zheng, X.; Zhang, X.; Abdelhai, M.H.; Zhao, L.; Li, H.; Diao, J.; Zhang, H. Exploring the effect of β-glucan on the biocontrol activity of Cryptococcus podzolicus against postharvest decay of apples and the possible mechanisms involved. Biol. Control 2018, 121, 14–22. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Bhayani, K.; Ghosh, T.; Bajaj, S.; Trivedi, N.; Mishra, S. Stability of phycobiliproteins using natural preservative ε-Polylysine (ε-PL). Ferment. Technol. 2018, 7, 149. [Google Scholar] [CrossRef]
- Ge, Y.H.; Wei, M.L.; Li, C.Y.; Chen, Y.R.; Lv, J.Y.; Meng, K.; Wang, W.H.; Li, J.R. Reactive oxygen species metabolism and phenylpropanoid pathway involved in disease resistance against Penicillium expansum in apple fruit induced by ε-poly-L-lysine. J. Sci. Food Agric. 2018, 98, 5082–5088. [Google Scholar] [CrossRef]
- Shu, C.; Cui, K.B.; Li, Q.Q.; Cao, J.K.; Jiang, W.B. Epsilon-poly-l-lysine (ε-PL) exhibits multifaceted antifungal mechanisms of action that control postharvest Alternaria rot. Int. J. Food Microbiol. 2021, 348, 109224. [Google Scholar] [CrossRef]
- Jiao, W.X.; Liu, X.; Chen, Q.M.; Du, Y.M.; Li, Y.Y.; Yue, F.L.; Dong, X.Q.; Fu, M.R. Epsilon-poly-l-lysine (ε-PL) exhibits antifungal activity in vivo and in vitro against Botrytis cinerea and mechanism involved. Postharvest Biol. Technol. 2020, 168, 111270. [Google Scholar] [CrossRef]
- Liu, K.W.; Zhou, X.J.; Fu, M.R. Inhibiting effects of epsilon-poly-lysine (ε-PL) on Pencillium digitatum and its involved mechanism. Postharvest Biol. Technol. 2017, 123, 94–101. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.L.; Li, C.Z.; Vittayapadung, S.; Cui, H.Y. Antibacterial mechanism of ε-Poly-lysine against Listeria monocytogenes and its application on cheese. Food Control 2018, 91, 76–84. [Google Scholar] [CrossRef]
- Li, H.; He, C.; Li, G.; Zhang, Z.; Li, B.; Tian, S.P. The modes of action of epsilon-polylysine (ε-PL) against Botrytis cinerea in jujube fruit. Postharvest Biol. Technol. 2019, 147, 1–9. [Google Scholar] [CrossRef]
- Qin, G.Z.; Zong, Y.Y.; Chen, Q.L.; Hua, D.L.; Tian, S.P. Inhibitory effect of boron against Botrytis cinerea on table grapes and its possible mechanisms of action. Int. J. Food Microbiol. 2010, 138, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Brito, N.; Espino, J.J.; Gonzalez, C. Endo-β-1,4-xylanase xyn11A required for virulence in Botrytis cinereal. Mol. Plant Microbe Interact. 2006, 19, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Prusky, D.; McEvoy, J.L.; Leverentz, B.; Conway, W.S. Local modulation of host pH by Colletotrichum species as a mechanism to increase virulence. Mol. Plant Microbe Interact. 2001, 14, 1105–1113. [Google Scholar] [CrossRef] [Green Version]
- Bateman, D.F.; Beer, S.V. Simultaneous production and synergistic action of oxalic acid and polygalacturonase during pathogenesis by Sclerotium rolfsii. Phytopathology 1965, 55, 204–211. [Google Scholar]
- Eshel, D.; Miyara, I.; Ailing, T.; Dinoor, A.; Prusky, D. pH regulates endoglucanase expression and virulence of Alternaria alternata persimmon fruit. Mol. Plant Microbe Interact. 2002, 15, 774–779. [Google Scholar] [CrossRef] [Green Version]
- Vilanova, L.; Wisniewski, M.; Norelli, J.; Viñas, I.; Torres, R.; Usall, J.; Phillips, J.; Droby, S.; Teixidó, N. Transcriptomic profiling of apple in response to inoculation with a pathogen (Penicillium expansum) and a non-pathogen (Penicillium digitatum). Plant Mol. Biol. 2014, 32, 566–583. [Google Scholar] [CrossRef]
- Prusky, D.; Yakoby, N. Pathogenic fungi: Leading or led by ambient pH? Mol. Plant Pathol. 2003, 4, 509–516. [Google Scholar] [CrossRef]
- Manteau, S.; Abouna, S.; Lambert, B.; Legendre, L. Differential regulation by ambient pH of putative virulence factor secretion by the phytopathogenic fungus Botrytis cinerea. FEMS Microbiol. Ecol. 2003, 43, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Prusky, D.; Mcevoy, J.L.; Saftner, R.; Conway, W.S.; Jones, R. Relationship between host acidification and virulence of Penicillium spp. on apple and citrus fruit. Phytopathology 2004, 94, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollins, J.A.; Dickman, M.B. pH signaling in Sclerotinia sclerotiorum: Identification of a pacC/RIM1 homolog. Appl. Environ. Microbiol. 2001, 67, 75–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidzon, M.; Alkan, N.; Kobiler, I.; Kobiler, I.; Prusky, D. Acidification by gluconic acid of mango fruit tissue during colonization via stem end infection by Phomopsis mangiferae. Postharvest Biol. Technol. 2010, 55, 71–77. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, Y.; Li, M.; Yang, C.; Shi, C.; Zheng, C.J. Studies of postharvest berry abscission of “Kyoho” table grapes during cold storage and high oxygen atmospheres. Postharvest Biol. Technol. 2007, 43, 95–101. [Google Scholar] [CrossRef]
- Nakano, T.; Ito, Y. Molecular mechanisms controlling plant organ abscission. Plant Biotechnol. 2013, 30, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, R.; Ma, W.; Huang, X.; Li, M.; Chen, Q. Yong Enhanced saccharification of SO2 catalyzed steam-exploded corn stover by polyethylene glycol addition. Biomass Bioenergy 2011, 35, 2053–2058. [Google Scholar] [CrossRef]
- McCleary, D.; Mangan, R.; Daly, S.; Fort, R.; Ivory, N. Novel substrates for the measurement of endo-1,4-β-glucanase (endo-cellulase). Carbohyd. Res. 2014, 385, 9–17. [Google Scholar] [CrossRef]
- Zheng, B.B.; Zhao, L.; Jiang, X.H.; Cheronoac, S.; Liu, J.J.; Ogutuad, C.; Ntini, C.; Zhang, X.J.; Han, Y.P. Assessment of organic acid accumulation and its related genes in peach. Food Chem. 2020, 334, 127567. [Google Scholar] [CrossRef]
- Askarne, L.; Talibi, I.; Boubaker, H.; Boudyach, E.H.; Msanda, F.; Saadi, B.; Serghini, M.A.; Ait Ben Aoumar, A. In vitro and in vivo antifungal activity of several Moroccan plants against Penicillium italicum, the causal agent of citrus blue mold. Crop Prot. 2012, 40, 53–58. [Google Scholar] [CrossRef]
- Liu, X.; Jiao, W.X.; Du, Y.M.; Chen, Q.M.; Su, Z.B.; Fu, M.R. Chlorine dioxide controls green mold caused by Penicillium digitatum in citrus fruits and the mechanism involved. J. Agric. Food Chem. 2020, 68, 13897–13905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nie, J.Y.; Jing, L.I.; Zhang, H.; Li, Y.; Saqib, F.; Syed, A.S.B.; Wang, J. Evaluation of sugar and organic acid composition and their levels in highbush blueberries from two regions of china. J. Integr. Agric. 2020, 19, 2352–2361. [Google Scholar] [CrossRef]
- Wu, J.Y.; Fan, J.B.; Li, Q.H.; Jia, L.T.; Xu, L.L.; Wu, X.; Wang, Z.W.; Li, H.Y.; Qi, K.J.; Qiao, X.; et al. Variation of organic acids in mature fruits of 193 pear (Pyrus spp.) cultivars. J. Food Compos. Anal. 2022, 109, 104483. [Google Scholar] [CrossRef]
- Wei, M.L.; Ge, Y.H.; Li, C.Y.; Chen, Y.R.; Wang, W.H.; Duan, B.; Li, X. Antifungal activity of ε-poly-L-lysine on Trichothecium roseum in vitro and its mechanisms. Physiol. Mol. Plant Pathol. 2018, 103, 23–27. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Jiao, X.; Li, K.H.; Sun, Y.J.; Zhou, W.L.; Shen, Y.L.; Qian, J.; Chang, A.P.; Wang, J.Q.; et al. Characterization of the biosynthetic pathway of nucleotide sugar precursor UDP-glucose during sphingan WL gum production in Sphingomonas sp. WG. J. Biotechnol. 2019, 302, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, W.S.; Yuan, S.Z.; Li, Q.Q.; Sang, W.N.; Cao, J.K.; Jiang, W.B. Methyl p-coumarate inhibits black spot rot on jujube fruit through membrane damage and oxidative stress against Alternaria alternata. Postharvest Biol. Technol. 2018, 145, 230–238. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.Y.; Chen, T.; Xu, Y.; Tian, S.P. Antifungal effects of hinokitiol on development of Botrytis cinerea in vitro and in vivo. Postharvest Biol. Technol. 2020, 159, 111038. [Google Scholar] [CrossRef]
- Dou, Y.; Routledge, M.N.; Gong, Y.Y.; Godana, E.A.; Dhanasekaran, S.; Yang, Q.Y.; Zhang, X.Y.; Zhang, H.Y. Efficacy of epsilon-poly-L-lysine inhibition of postharvest blue mold in apples and potential mechanisms. Postharvest Biol. Technol. 2021, 171, 111346. [Google Scholar] [CrossRef]
- Bo, T.; Liu, M.; Zhong, C.; Zhang, Q.; Su, Q.Z.; Tan, Z.L.; Jia, S.R. Metabolomic analysis of antimicrobial mechanisms of ε-poly-L-lysine on Saccharomyces cerevisiae. J. Agric. Food Chem. 2014, 62, 4454–4465. [Google Scholar] [CrossRef]
- Vilanova, L.; Viñas, I.; Torres, R.; Usall, J.; Buron-Moles, G.; Teixidó, N. Acidification of apple and orange hosts by Penicillium digitatum and Penicillium expansum. Int. J. Food Microbiol. 2014, 178, 39–49. [Google Scholar] [CrossRef]
- Hadas, Y.; Goldberg, I.; Pines, O.; Prusky, D. Involvement of gluconic acid and glucose oxidase in the pathogenicity of Penicillium expansum in apples. Phytopathology 2008, 97, 384–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prusky, D.; Lichter, A. Mechanisms modulating fungal attack in post-harvest pathogen interactions and their control. Eur. J. Plant Pathol. 2008, 121, 281–289. [Google Scholar] [CrossRef]
- Hématy, K.; Cherk, C.; Somerville, S. Host-pathogen warfare at the plant cell wall. Curr. Opin. Plant Biol. 2009, 12, 406–413. [Google Scholar] [CrossRef] [PubMed]
- D’Ovidio, R.; Mattei, B.; Roberti, S.; Bellincampi, D. Polygalacturonases, polygalacturonase-inhibiting proteins and pectic oligomers in plant–pathogen interactions. BBA-Proteins Proteom. 2004, 1696, 237–244. [Google Scholar] [CrossRef]
- Xu, M.Q.; Yang, Q.Y.; Boateng, N.A.S.; Ahima, J.; Dou, Y.; Zhang, H.Y. Ultrastructure observation and transcriptome analysis of Penicillium expansum invasion in postharvest pears. Postharvest Biol. Technol. 2020, 165, 111198. [Google Scholar] [CrossRef]
- Luca, S.; Carla, C.; Serena, R.; Renato, D.; Francesco, F. An endo-polygalacturonase (pg) of fusarium moniliforme escaping inhibition by plant polygalacturonase-inhibiting proteins (pgips) provides new insights into the pg-pgip interaction. FEMS Microbiol. Lett. 2004, 1, 117–124. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).